Abstract
Rationale
Mitochondria play a dual role in the heart, responsible for meeting energetic demands and regulating cell death. Paradigms have held that mitochondrial fission and fragmentation are the result of pathologic stresses such as ischemia, are an indicator of poor mitochondrial health, and lead to mitophagy and cell death. However, recent studies demonstrate that inhibiting fission also results in decreased mitochondrial function and cardiac impairment, suggesting that fission is important for maintaining cardiac and mitochondrial bioenergetic homeostasis.
Objective
The purpose of this study is to determine whether mitochondrial fission and fragmentation can be an adaptive mechanism used by the heart to augment mitochondrial and cardiac function during a normal physiologic stress such as exercise.
Methods and Results
We demonstrate a novel role for cardiac mitochondrial fission as a normal adaptation to increased energetic demand. During submaximal exercise, “physiologic” mitochondrial fragmentation results in enhanced, rather than impaired mitochondrial function, and is mediated in-part by β1-adrenergic receptor signaling. Similar to pathologic fragmentation, physiologic fragmentation is induced by activation of Drp1; however, unlike pathologic fragmentation, membrane potential is maintained and regulators of mitophagy are downregulated. Inhibition of fission with P110, Mdivi-1 or in mice with cardiac specific Drp1 ablation, significantly decreases exercise capacity.
Conclusions
These findings demonstrate the requirement for physiological mitochondrial fragmentation to meet the energetic demands of exercise as well as providing additional support for the evolving conceptual framework, where mitochondrial fission and fragmentation play a role in the balance between mitochondrial maintenance of normal physiology and response to disease.
Subject Terms: Basic Science Research, Exercise, Metabolism, Physiology
Keywords: Mitochondria, mitochondrial fission, bioenergetics, exercise, β–adrenergic receptor blocker, physiological process
INTRODUCTION
Cardiac muscle is especially sensitive to alterations in energetic homeostasis due to the high ATP demands of rhythmic contraction 1. The heart initially responds to increased demands by using local ATP stores and anaerobic metabolism, however, persistent increases are met by sustained aerobic ATP production through increased mitochondrial oxidative phosphorylation and changes in mitochondrial dynamics (fission, fusion, mitophagy, biogenesis) 2,3. In the heart, mitochondrial fission and subsequent fragmentation are usually the result of pathological stressors such as ischemia and are an indicator of dysfunctional mitochondria 4,5. However, recent studies have shown that inhibiting fission also results in cardiac impairment 6–9, suggesting that fission is also important for maintaining normal mitochondrial homeostasis. Song et al. have shown that conditional Drp1 knockout (−/−) mice have preserved mitochondrial function, but increased mitophagy and eventual heart failure10. Others have shown that disruption of fission results in the accumulation of dysfunctional mitochondria11. In contrast, the Drp-1 inhibitor mdivi-1 decreases mitophagy and rescues pressure overload-induced heart failure12. It has also been proposed that, in cardiomyocytes, fission does not substantially alter mitochondrial morphology but is mainly required to facilitate clearance of dysfunctional mitochondria through asymmetric division and mitophagy 8,10. Since these studies have all relied on transgenic manipulation of dynamic regulators, such as Drp1, or were done in the context of pathology such as ischemia, the role for mitochondrial fission as a normal cardiac physiologic adaption is still unknown.
Several studies in skeletal muscle suggest, but do not confirm, a role for mitochondrial dynamics in maintaining energetic homeostasis. Kitaoka et al., investigated simulated resistance exercise in gastrocnemius muscle and showed increased Drp1 phosphorylation at Serine 616, suggesting activation of mitochondrial fission 13. Picard et al., subjected mice to voluntary treadmill exercise for 3h and although they found no change in regulators of mitochondrial dynamics, they did find increased mitochondrial interactions, suggesting active remolding of skeletal muscle mitochondria14. Finally, Feng et al., showed that during early exercise, expression of fission mediator Fis1 is increased in skeletal muscle, suggesting a role for “physiologic” mitochondrial fragmentation in maintaining bioenergetics during normal physiologic stress 15. Although these studies support the role of mitochondrial dynamics in skeletal muscle energetic adaptation to exercise, they do not confirm whether mitochondrial dynamic processes, such as fission and fusion, are necessary components for exercise performance. Skeletal muscle is also different from cardiac muscle both in sarcomere morphology and function and in mitochondrial organization and density16,17. Only one study has been performed in cardiac muscle, by Dworatzek et al,. who showed that 8 weeks of exercise conditioning increased cardiac mitochondrial number, and decreased their size18. In the current study, we sought to determine whether mitochondrial fission and fragmentation occur in the heart as a physiological adaptive response to acute submaximal exercise, whether it is required to meet the increased bioenergetic demands of normal levels of exercise, and the similarities and differences between exercise-induced and pathologic-induced fragmentation.
METHODS
Data and material availability
Microarray data has been submitted to the NCBI Gene Expression Omnibus (GEO) database in MIAME format. Request for data can be addressed to: Dr. Daniel Bernstein, 750 Welch Road Suite 325, Palo Alto, CA 94304. danb@stanford.edu.
Please refer to the Materials and Methods section in the Online Data Supplement for a detailed description of the experimental method.
Animal protocols
Male 8–10 wk. old FVB/NJ mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were subjected to exercise on a treadmill equipped for simultaneous measurement of VO2 and VCO2 (Columbus Instruments, Columbus, OH), and distance ran and respiratory exchange ratio (RER) were determined using our established protocols 19. Mice were exercised using two different protocols. First, sub-maximal exercise was performed for 1 h at a speed of 20 m/min with a constant grade of 5°. Exercise capacity using this protocol was assessed by running mice to exhaustion at a speed of 20m/min at a constant grade of 5° and recording total distance ran. Second, maximal exercise capacity was assessed using a protocol that incrementally increased the degree of elevation by 2° and the speed by 2 m/min every 3 min. Exercise time was determined as the time until exhaustion or 10 seconds while resting on the shock grid. Exercise distance was calculated as the total vertical distance traveled (meters) = Treadmill speed (meters/min) X Percent grade (Sin θ) X Exercise time (min). Work (Joules) was calculated as the body weight (kg) X total vertical distance traveled (meters) X 9.8.
β-adrenergic signaling and mitochondrial dynamics modulation
To assess subtype-specific β-AR signaling, we utilized β2-AR knockout (−/−) mice generated on an FVB background in our laboratory 20. To assess mitochondrial fission, mice were treated with the Drp1-Fis1 inhibitor P110, provided by Dr. Daria Mochly-Rosen, (0.5 mg/kg) or Mdivi-1 (25 mg/kg) by intraperitoneal injection, followed immediately by exercise testing. Either saline, TAT47–57 peptide (0.35 mg/kg) or 2% DMSO in saline were used as control treatments. To assess the role of β1-AR signaling, mice were treated with the β1-AR antagonist metoprolol (5mg/kg) by IP injection 90 minutes prior to maximal exercise challenge. To assess cardiac specific mitochondrial fission, Drp1loxp/loxp 21 C57B/6NJ mice were provided by Dr. Katsuyoshi Mihara and Dr. Masatoshi Nomura and were crossed onto the myh6-MER-Cre-Mer C57B/6NJ mouse line (Jackson Laboratory, Bar Harbor, ME) and gene recombination was induced by administration of tamoxifen (20mg/kg) IP for 5 days. On day 7 mice were used for experimentation. Male aged-matched Drp1+/+Cre+/+ and Drp−/−Cre−/− were treated with tamoxifen and used as controls.
Isoproterenol, doxorubicin and inhibitor dosing
HL-1 cells and cardiac myocytes were treated with 10 μM isoproterenol (ISO) for 1 h. HL-1 cells were pre-treated with 300 nM of the subtype specific inhibitors CGP 12177 (β1 antagonist) and ICI 118551 (β2 antagonist) and 1μM of L748,337 (β3 antagonist) and propranolol (β1&β2 antagonist) for 30 min before ISO exposure. Cells were also pretreated with 30μM of the mitochondrial division inhibitor Mdivi-1 for 30 min before ISO exposure.
C2C12 or isolated adult cardiac myocytes were exposed to 100μM doxorubicin. After exposures, cells were either washed with PBS or used directly for morphology or respiration measurement. Mice were exposed to 10mg/kg of doxorubicin or saline control by tail vain injection. After 30min exposure, cardiac tissue was harvested for protein analysis.
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Normally distributed data comparing two groups were analyzed by Student’s t test. Normally distributed data comparing multiple groups were analyzed by ANOVA with multiple comparisons analysis (Tukeys). Non-parametric data comparing two groups were analyzed using the Mann-Whitney U test. Statistical analysis of microarray data was performed using Stanford microarray database software and the Significance Analysis of Microarrays (SAM), Database for Annotation, Visualization, and Integrated Discovery, Hi-Throughput GOMiner and TIGR TM4 software. Multiple comparisons were accounted for by False Discovery Rate (FDR) analysis and genes were considered significantly altered with a p value less than 0.01 by Fisher’s exact test. To control for multiple comparisons, we required a false discovery rate of 5%. Microarray data has been submitted to the NCBI Gene Expression Omnibus (GEO) database in MIAME format.
RESULTS
Exercise induces “physiologic” mitochondrial fragmentation
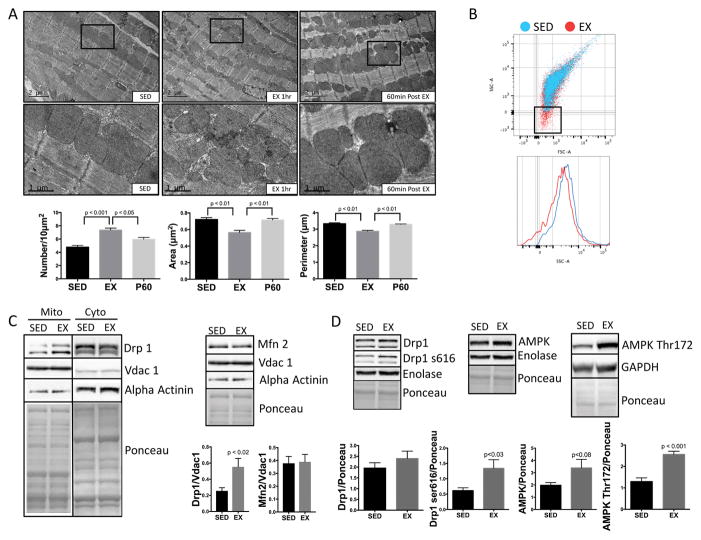
In mice subjected to submaximal treadmill exercise for 1h 19, cardiac mitochondria demonstrated significant fragmentation: increase in number and decrease in area and perimeter by quantitative electron microscopy (Fig. 1A). We confirmed exercise-induced mitochondrial fragmentation using flow cytometry side scatter area (SSA), showing that mitochondria from exercised mice were smaller in size vs. sedentary controls (Fig. 1B). SSA results were validated by calibrating with beads of known size (2μm, 1μm, 0.5μm, 0.2μm) (Online Fig. I). Evidence of mitochondrial fission can be confirmed by translocation of the mitochondrial fission mediator Drp1 from the cytosol to the mitochondrial fraction22. Purity of our mitochondrial fraction was assessed by low abundance of the cytosolic protein alpha actinin and robust abundance of mitochondrial Vdac1. Exercise induced mitochondrial translocation of the pro-fission mediator Drp1 (Fig. 1C) and phosphorylation of the fission activating site Drp1 Serine 616 (Fig 1D). The pro-fission AMP-activated protein kinase (AMPK) was activated, as evidenced by phosphorylation at the activating site Thr172 (Fig. 1D); total AMPK protein expression was unchanged (increased, but did not reach statistical significance). In contrast, the pro-fusion mediator Mfn 2 was unchanged (Fig. 1C). After one-hour of recovery from exercise, mitochondrial architecture begins to revert to sedentary morphology (Fig. 1A).
Figure 1. Exercise induces physiologic mitochondrial fragmentation.
(A) Electron micrographs from LV myocardium show mitochondrial fragmentation after 1h of exercise. Mitochondrial number was increased and area and perimeter decreased vs. controls. Mitochondrial morphology returned to the sedentary state after 60 minutes of post exercise rest (n=4 biological replicates/group, at least 10 fields/subject were averaged for each biological replicate). (B) Mitochondrial size was assessed by Flow cytometry as side scatter area (SSA). Isolated mitochondria from EX mice were smaller than SED (n= 4/group). (C) Pro-fission Drp1 is increased in mitochondrial fractions while pro-fusion Mfn 2 is unchanged (n=8/group). (D) Activating Drp1 phosphorylation site serine 616 and AMPK phosphorylation site Thr172 were also increased with exercise in whole heart fractions, along with a marginal but non-significant increase in total AMP-kinase (n=5/group).
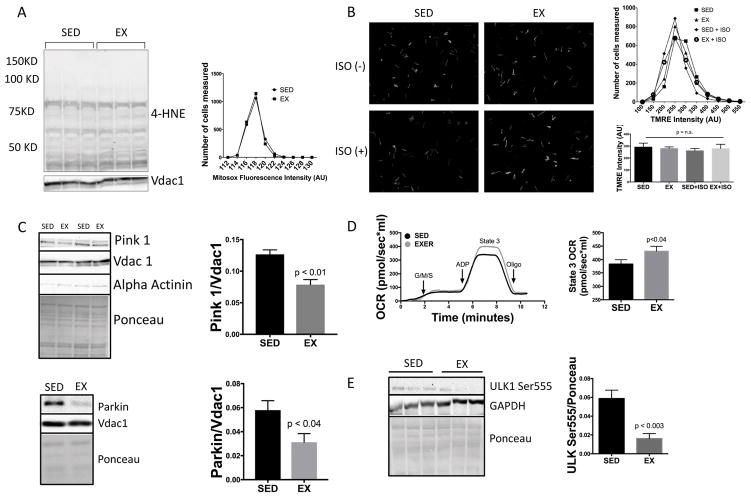
To highlight differences between physiologic and pathologic mitochondrial fragmentation we compared exercise to a model of pathologic fragmentation, doxorubicin cardiotoxicity. Doxorubicin has been shown to induce mitochondrial fission and fragmentation associated with mitochondrial dysfunction23,24. During pathologic fragmentation (Online Fig. IIA), mitochondrial oxidative stress is increased (Online Figure IIB) 25. In contrast, in exercise-induced physiologic fragmentation, levels of oxidative stress (4-HNE lipid peroxidation and MitoSox) were unchanged (Fig. 2A). In pathologic fragmentation, membrane potential (Δψm) is decreased, increasing PINK1 accumulation on the mitochondrial membrane, resulting in translocation of Parkin, accumulation of LC3 (Online Figure IIC) and activation of mitophagy 26–31. In contrast, during physiologic fragmentation, Δψm was preserved (Fig. 2B). Furthermore, Δψm remained normal even when isoproterenol (ISO) was added to simulate the hypersympathetic state during exercise. Preservation of Δψm prevented mitochondrial PINK1 accumulation and recruitment of Parkin (Fig. 2C), typical of pathologic fragmentation and markers of mitophagy7,32. Finally, pathologic fragmentation is associated with decreased mitochondrial function (Online Figure IIB), whereas physiologic fragmentation is associated with enhanced mitochondrial function (Fig. 2D). We have previous shown similar deleterious effects of pathologic fragmentation in models of diabetes33 and ischemia-reperfusion34.
Figure 2. Exercised-induced physiologic mitochondrial fragmentation is associated with inhibition of mitophagy and enhanced mitochondrial function.
(A) Oxidative stress (4-HNE & MitoSOX) was not increased, despite fragmentation (n=3/group). (B) Membrane potential (TMRE) was maintained in cardiac myocytes isolated from exercised mice, both before and after sympathetic stimulation (ISO 10μM) vs. sedentary controls (n=3/group). (C) Pro-mitophagy Parkin and Pink1 are both decreased in mitochondrial fractions (n=3–5/group). (D) Mitochondrial respiration is enhanced with 1hr of exercise as measured by Oroboros oxygraph normalized to 50μg of mitochondria (n=5/group). (E) Pro autophagy ULK-1 is dephosphorylated (inactive) at Ser555 with 1hr of exercise (n=5/group).
We next sought to determine whether the absence of autophagy in our studies was reflective of a true failure to activate autophagy or was related to an increase in autophagic flux by treating with chloroquine. Although LC3-I, LC3-II and P62 levels were similar between chloroquine treated exercised and sedentary mice, we could not see enhanced LC3/P62 accumulation with chloroquine alone, therefore we cannot exclude a change in autophagic flux in our model. To evaluate an additional endpoint for autophagy activity, we assessed the activation state of the pro-autophagy protein ULK136 and found dephosphorylation at the activating site Ser555, another confirmation of the inactivation of autophagy (Fig. 2E). A previously published exercise study showed evidence for activation of autophagy, but this study utilized a more extreme level of exercise, beyond VO2 max and anaerobic threshold37. This suggests there may be tiered levels of mitochondrial fission during exercise, where a low grade of fission (“physiologic fragmentation”) may be beneficial during sub-maximal exercise (as normally experienced by exercising humans). In contrast, a higher grade of fission during extreme exercise (as experienced during laboratory exercise stress testing or marathon running) might be at least partly detrimental. To address this question, we compared tissues from mice subjected to sub-maximal (1hr exercise) and those from mice subjected to extreme/maximal (run to exhaustion, beyond anaerobic threshold) exercise. We found a similar increase in total mitochondrial number in both exercise groups compared with sedentary mice (Online Fig. IIIA). Similar to sub-maximal exercise, this was associated with Drp1 translocation to the mitochondrial fraction (Online Fig. IIIB). These data confirm that mitochondrial fission also occurs with extreme/maximal exercise. Again, we found no change in LC3 or P62 with submaximal or maximal exercise (Online Fig. IIIC). Similarly, Parkin was not increased, however, the decrease in Parkin translocation to the mitochondria that was seen with sub-maximal exercise (Fig. 2C) was not seen with extreme/maximal exercise (Online Fig. IIIB). Together, these data suggest that mitophagy and autophagy do not occur during either sub-maximal or extreme/maximal exercise. It still remains possible that a tiered level of exercise-induced fission may occur, where normal physiologic levels of exercise induce physiologic fragmentation and extreme/maximal exercise induces features of both physiologic and pathologic fragmentation, however, we have not been able to induce pathologic fission with either form of exercise.
Physiologic mitochondrial fragmentation is required for maximal exercise performance
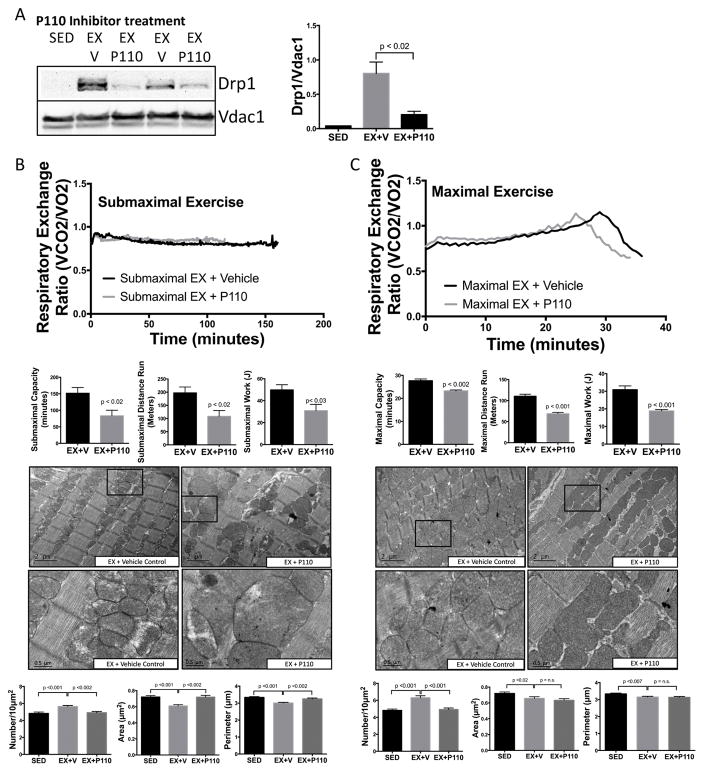
To determine whether physiologic fragmentation is necessary to achieve maximal exercise performance, mice were treated with P110 19, which inhibits fission by blocking the interaction between Drp1 and Fis1 preventing Drp1s mitochondrial translocation (Fig. 3A). 38 Mice were then subjected to both submaximal and maximal exercise capacity tests. P110 treatment resulted in a 45% decrease in submaximal exercise time and 45% decrease in submaximal exercise distance (Fig. 3B). To best control for any differences in weight, we determined total work (J) and found that P110 treated mice had a 38% decrease in submaximal exercise work. Similarly, P110 treated mice subjected to a maximal exercise capacity test had a 16% decrease in maximal exercise time, 37% decrease in distance and 38% decrease in total work (Fig. 3C). P110 treated mice also reached anaerobic threshold (RER>1.0) earlier than vehicle treated mice, demonstrating that blocking mitochondrial fission during exercise results in earlier utilization of oxidative phosphorylation-independent (anaerobic) ATP production. During early stage exercise (first 5–10 minutes), P110 did not affect respiratory exchange ratio (RER) (Online Fig. IV), evidence that physiologic fragmentation becomes important only after a more prolonged increase in energy demand. Both submaximal and maximal exercise capacity tests induced mitochondrial fragmentation. Submaximal exercise-induced fragmentation was completely blocked by P110 treatment, while maximal exercise-induced fragmentation was partially blocked (Fig. 3B, 3C), further demonstrating the requirement for physiologic mitochondrial fission to reach maximal exercise capacity. Since fission can also occur through Fis-1 independent mechanisms 39, we confirmed the role of Drp-1 in physiologic fragmentation using the Drp1 GTPase inhibitor Mdivi-1. Similar to P110, mice treated with Mdivi-1 had decreased maximal exercise capacity (Online Fig. V).
Figure 3. Mitochondrial fragmentation is required for submaximal and maximal exercise capacity.
(A) (A) The Drp1-inhibitor P110 (0.5 mg/kg i.p.) decreased Drp1 mitochondrial translocation vs. TAT vehicle (V) control (n=3/group). (B) Blocking Drp1 decreased submaximal and maximal (C) exercise time (minutes), distance (meters) and work (J). P110 treatment also prevented mitochondrial fragmentation, as evidenced by failure to increase mitochondrial number during both (B) submaximal and (C) maximal exercise. (n=5/group).
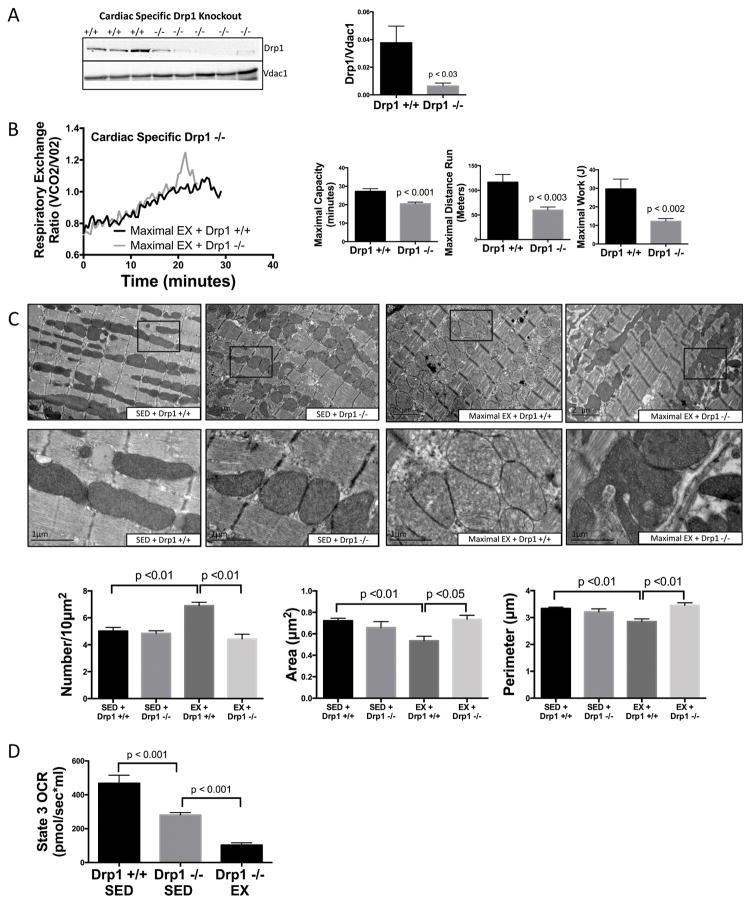
Finally, to confirm the role of cardiac, as opposed to skeletal muscle, mitochondrial fragmentation in adaptation to exercise, we utilized conditional cardiac-specific Drp1-knockout (Drp1 −/−) mice 6,21. Ablation of Drp1 was induced with tamoxifen (20mg/kg) for 5 days and mice were subjected to a maximal exercise capacity test on day 7. Drp1+/+Cre+/+ and Drp−/−Cre−/− were used as controls and were also exposed to tamoxifen for 5 days and subjected to an exercise capacity test on day 7. Drp1 was absent in cardiac tissue but not in skeletal muscle (Fig. 4A, Online Fig. VIA) and was the only mitochondrial dynamic regulator decreased, as Mfn 1 & 2, LC3, and Parkin remained unchanged (Online Fig. VIB). Although long-term knockout of Drp1 can have deleterious effects6,10, this brief period of ablation did not affect mitochondrial ROS, membrane potential or baseline cardiac function (ejection fraction & cardiac output) (Online Fig. VIC&D). Similar to P110 treatment, cardiac-specific Drp1 −/− mice subjected to a maximal exercise capacity test resulted in a 24% decrease in exercise time, 38% decrease in exercise distance, 58% decrease in total work and a shorter time to reach anaerobic threshold (Fig. 4C). As expected, maximal exercise induced mitochondrial fragmentation in exercised Drp1 +/+ mice, which was blocked in exercised Drp1 −/− mice (Fig. 4D). Of interest, exercised Drp1−/− mice exhibited large, highly abnormal mitochondria, some extremely elongated and others with multiple finger-like projections (“puzzle-piece mitochondria”), which we speculate are related to an incomplete stimulus for fission (Fig. 4C & Online Fig. VII). To confirm that these abnormal mitochondria were a result of exercise and not due to the gene knockout itself, we compared sedentary Drp1+/+ mice to sedentary Drp1−/− mice and found no change in mitochondrial morphology, confirming that this abnormal mitochondrial phenotype occurs only in response to exercise (Fig. 4C). As expected, sedentary Drp1 −/− had a 40% reduction in state 3 mitochondrial respiration, similar to that previously described6. Interestingly, exercised Drp1 −/− mice had a dramatic 65% reduction in state 3 mitochondrial respiration at the end of 1hr exercise (20m/min) vs sedentary Drp1 −/−, indicating a markedly compromised response to energetic stress (Fig. 4D).
Figure 4. Cardiac mitochondrial fragmentation is required for maximal exercise capacity.
(A) Cardiac-specific conditional Drp1 −/− mice were generated by crossing Drp1loxp/loxp mice with myh6-MER-Cre-Mer mice and treating with tamoxifen (20mg/kg, i.p.) for 5 days. (B) Drp1 −/− decreased maximal exercise time (min), distance (m) and work (J) and (C) prevented exercise-induced mitochondrial fragmentation, but resulted in very abnormal appearing mitochondria, both elongated (arrow) or hyper-segmented (bottom panel). No changes in mitochondrial morphology were observed between sedentary Drp1 +/+ and Drp1 −/− (n=3–5 biological replicates/group, at least 10 fields/subject were averaged for each biological replicate). (D) Reduced mitochondrial function was observed in sedentary and exercised Drp1 −/− mice by Oroboros oxygraph normalized to 50μg of mitochondria (n=5/group).
β1-AR signaling induces physiological mitochondrial fragmentation and enhances mitochondrial function
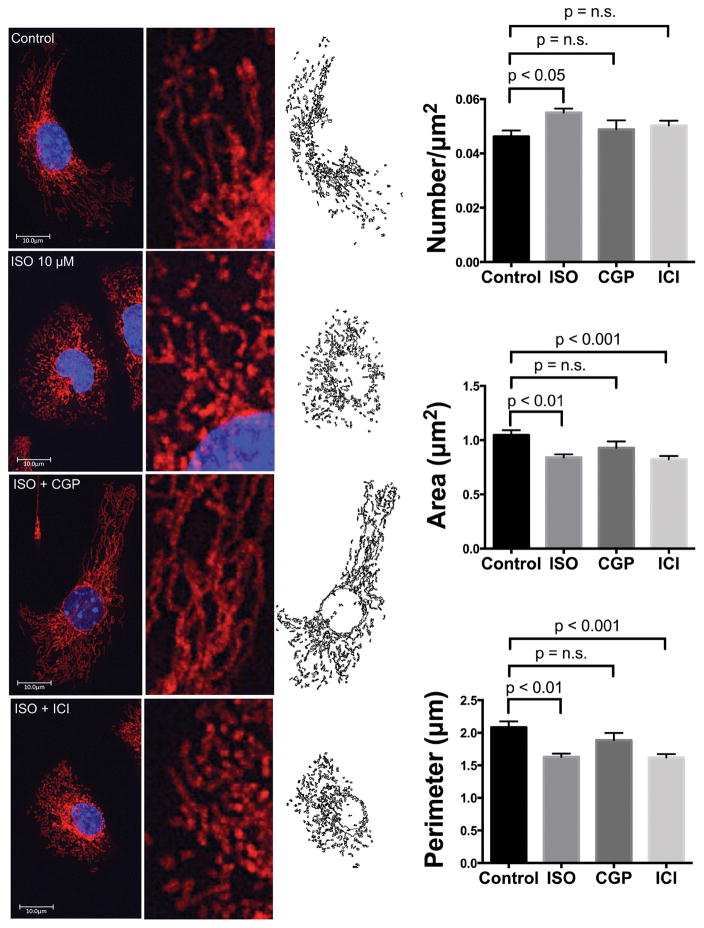
The sympathetic nervous system is a principal mechanism for meeting the hemodynamic demands of exercise, increasing heart rate and contractility, both of which increase energetic demands. This response is mediated by β-adrenergic receptor (β-AR) signaling 40, largely through protein kinase A (PKA)-mediated increases in intracellular calcium ([Ca2+]i) a regulator of both mitochondrial ATP production and mitochondrial dynamics41. To examine the role of β-AR signaling in mitochondrial fragmentation, HL-1 cardiomyocytes were labeled with MitoTracker and treated with a physiologic dose (10μM) of the β-AR agonist isoproterenol (ISO) for 1h, which resulted in increased fragmentation (Fig. 5). The β1-AR antagonist CGP12177 (300nM) blocked ISO-induced fragmentation whereas the β2-AR antagonist ICI118,551 (300nM) did not, demonstrating β1-AR subtype-specific regulation of mitochondrial dynamics.
Figure 5. β1-AR signaling induces mitochondrial fragmentation in HL-1 cells.
Cells were treated with ISO (10μM) for 1h and imaged by confocal microscopy. ISO increased mitochondrial fragmentation (decreased perimeter and area and increased number), and this was blocked by CGP12177 (β1-antagonist) but not by ICI118551 (β2-antagonist) (n=13–25 cells/group). Cells were quantified using a custom macro in Image J.
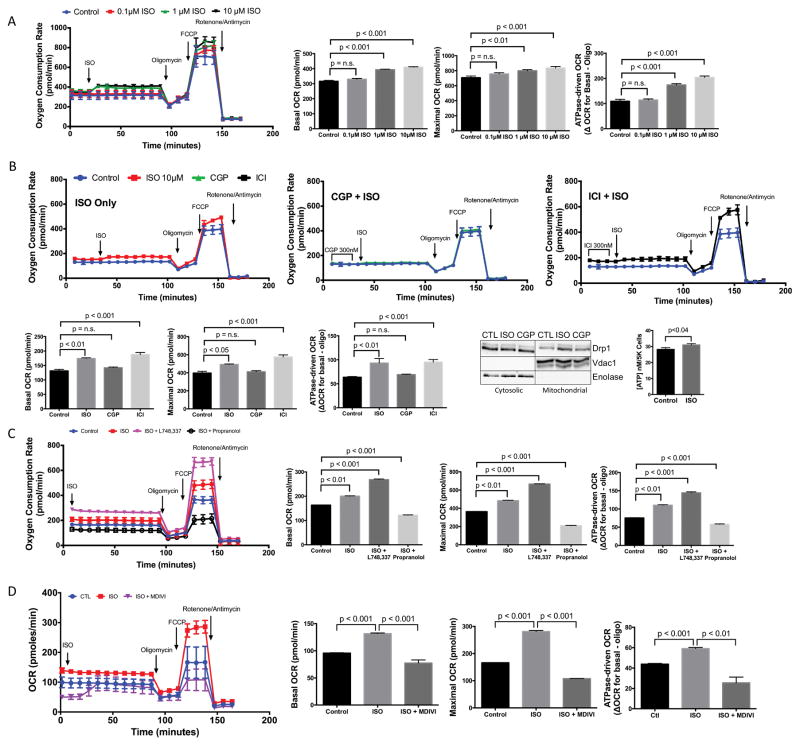
To assess whether this in vitro model of physiologic mitochondrial fragmentation is associated with enhanced mitochondrial function, respiration was assessed by Seahorse oximetry. ISO induced a dose-dependent increase in basal respiration (before oligomycin), maximum respiratory capacity (after FCCP) and ATPase-driven respiration (ΔOCR Basal-Oligo) (Fig. 6A). CGP12177 blocked the effects of ISO on respiration, whereas ICI118551 intriguingly increased basal, maximal and ATPase-driven respiration above ISO alone (Fig. 6B), likely a result of the elimination of β2-AR-Gi inhibitory signaling 42. Similar to exercise, ISO stimulated Drp1 mitochondrial translocation, and this was blocked by CGP12177 (Fig. 6B). Confirming the role of β1-AR signaling in energy production, ISO significantly increased ATP production (Fig. 6B).
Figure 6. β1-AR signaling enhances mitochondrial respiration in HL-1 cells, which is opposed by both β2-AR and β3-AR signaling.
(A) ISO increased basal, maximal and ATPase-driven respiration in a dose-dependent manner starting at 1μM (n=5/group). (B) This increase was blocked by CGP and further increased by ICI (n=4/group). ISO increased Drp1 mitochondrial translocation, which was blocked by CGP. ISO also significantly increased ATP production (n=7/group). (C) ISO enhanced respiration was synergistically increased with the β3AR antagonist L748,337 and blocked with propranolol (β1/β2 antagonist) (n=5/group). (D) Inhibition of mitochondrial fission with Mdivi-1 blocked ISO-induced increased in mitochondrial respiration (n=5/group).
We also assessed the role of β3-adrenergic receptor signaling, since it is also stimulated by ISO and has been shown to play a role in modulating mitochondrial dynamics in skeletal muscle and brown adipose tissue43. We pretreated cells with 1μM of the β3-AR antagonist L748,33744,45 or the β1/β2 antagonist propranolol46 for 30 minutes before ISO exposure. We found that inhibition of β3-AR signaling (L748,337 + ISO) resulted in a synergistic increase in respiration, similar to that seen with the β2-AR antagonist ICI118551, while activation of only the β3-AR (propranolol + ISO) resulted in a dramatic decrease in respiration (Fig. 6C). These results demonstrate that β3-AR signaling opposes β1-AR induced physiologic fragmentation. Previous reports have shown that β3-ARs, similar to β2-ARs, can couple to the inhibitory protein, Gi47 and also promote pro-mitofusion pathways43.
To confirm the connection between β-AR signaling and mitochondrial fission-enhanced respiration, we pretreated cells with 30μM of the mitochondrial division inhibitor Mdivi-1 for 30 minutes before ISO exposure and assessed respiration. Pretreatment with Mdivi-1 resulted in a significant decrease in respiration (Fig. 6D), confirming the connection between βAR signaling, mitochondrial fission and enhanced mitochondrial respiration.
Since HL-1 cells are an immortalized cell line, we next determined whether similar changes in mitochondrial morphology and function were observed in a primary cardiac myocyte culture. Cardiac myocytes were isolated from adult mice and treated with 10μM ISO for 1h. Electron microscopy again showed significant mitochondrial fragmentation with increased number and decreased area and perimeter (Online Fig. VIIIA). Mitochondrial function, assessed by Oroboros oxygraph, showed a significant increase in both basal and maximal oxygen consumption (Online Fig. VIIIB). Thus, mitochondrial fragmentation induced by physiologic-level β1-AR signaling increases mitochondrial function, additional evidence that mitochondrial fragmentation can be associated with enhanced and not degraded mitochondrial function in the heart.
Deletion of the β2-AR in vivo results in Drp1-mediated mitochondrial fission and enhanced exercise capacity
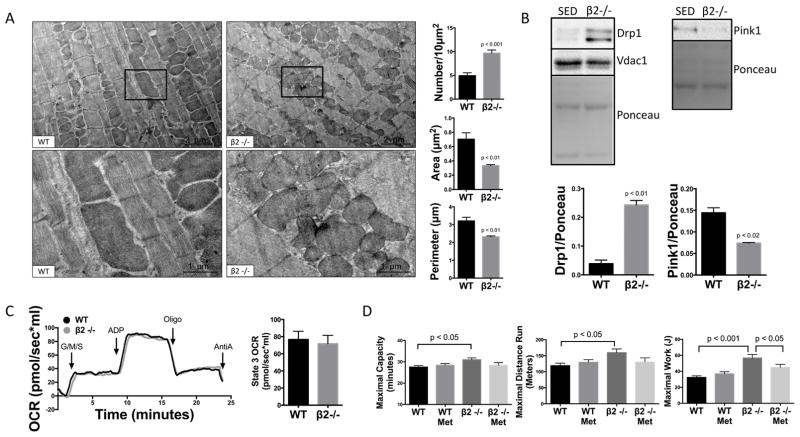
To provide additional in vivo evidence for β1-AR mediated mitochondrial fragmentation, we evaluated β2-AR knockout mice (β2−/−). These mice are a model of chronic physiologic-level β1-AR signaling, without alterations in β1-receptor density or function, and without the counterbalancing effects of β2-ARs and β2-AR-Gi signaling20,42. Importantly, we have previously shown that β2−/− mice have normal cardiac structure and function and normal survival 20. Consistent with our in vitro results, β2-AR−/− mice show marked mitochondrial fragmentation (Fig. 7A), Drp1 translocation, and downregulation of mitophagy regulators PINK1 (Fig. 7B). Of 35 mitochondrial-related genes identified as up- or down-regulated using a genome-wide screen (Online Table I), the only regulator of mitochondrial dynamics changed was beclin-1, and this mitophagy mediator was downregulated, suggesting that transcriptional regulation of mitochondrial dynamic regulators is not the principal mechanism underlying chronic β-AR-induced changes in mitochondrial dynamics. Importantly, despite marked fragmentation in the β2−/−, mitochondrial function was not impaired at baseline (Fig. 7C) and β2/− mice have enhanced exercise running time, distance and Work (Fig. 7D) vs. WT. To confirm the role of enhanced β1-AR signaling, we blocked β1-AR signaling 90 minutes prior to exercise challenge by treating with the β1-specific antagonist metoprolol (5mg/kg) IP. The enhanced exercise work in the β2−/− was significantly decreased with β1-AR blockade (Fig. 7D), additional evidence that mitochondrial fission and fragmentation play a major role in cardiac exercise adaptation.
Figure 7. Ablation of the β2-AR results in physiological mitochondrial fission and enhanced exercise capacity.
(A) β2−/− mice, a model of unopposed physiologic-level β1-AR signaling, show dramatic mitochondrial fragmentation with increased number and decreased area and perimeter versus WT littermates (n=3–5/group). (B) Drp1 mitochondrial translocation was increased and PINK1 was decreased in sedentary β2−/− (n=3/group). (C) Despite marked mitochondrial fragmentation, β2−/− mice had normal baseline respiration by Oroboros oxygraph normalized to 50μg of mitochondria (n=4/group). (D) β2−/− mice have enhanced exercise running time, distance and work, which is blocked with β1-AR inhibition by metoprolol (n=8/group).
DISCUSSION
Mitochondrial fission has traditionally been associated with pathologic stress and with impaired mitochondrial function, however, more recent studies have suggested a role for mitochondrial fission in normal cardiac homeostatic regulation 6,8,10. In the current study, we demonstrate that cardiac mitochondrial fission and fragmentation is not limited to pathologic states and is a component of normal physiologic regulation of mitochondrial function during physiologic stress, such as submaximal exercise. This is consistent with recent reports that have suggested that fission is necessary for normal mitochondrial quality control 5, however as these studies have relied on genetic manipulation, e.g. Drp-1 ablation, or were done in a pathological context, our study is the first to demonstrate a role for mitochondrial fragmentation in normal cardiac physiology. As a positive mediator of mitochondrial energetics, physiologic fragmentation is essential for reaching maximal exercise performance. When mitochondrial fission and fragmentation were blocked with the Drp1 inhibitors P110, Mdivi-1, or by short-term conditional cardiac-specific Drp1 knockout, exercise capacity was significantly decreased, there was an earlier shift to oxygen-independent mechanisms for ATP generation, as evidenced by time to anaerobic threshold, and there was a dramatic reduction in ATP synthase-dependent state 3 respiration. A similar effect of mitochondrial fission on bioenergetics has been reported by Benard et al., who show that Drp1 silencing in HeLa cells decreases state 3 respiration, rate of ATP synthesis and survival during glucose starvation 48. The reduction in mitochondrial function seen with Drp1 silencing may be due to its role in mitochondrial remodeling, opening the cristae and expanding the intermembrane space 48,49. Prior studies in other cell systems support the concept that fission, fusion and mitophagy are rapid enough processes to be operative within the time frame of physiologic stressors, such as exercise 50–52. One of the limitations of our study is that there is no method available to directly measure mitochondrial function during exercise in vivo, therefore our measurements were affected by the time delay required to isolate mitochondria from exercised mouse hearts. Thus, our finding of slightly increased mitochondrial function may be an underestimate of the degree to which mitochondrial function is augmented during exercise. Our critical finding here is that we see mitochondrial fission without a decrease in function.
The mitochondrial dynamic response to pathological stressors such as ischemia/reperfusion injury (limited oxygen availability), doxorubicin toxicity (oxidative mitochondrial stress), or diabetes (metabolic stress) have been published by our group and others, all showing activation of mitochondrial fission and fragmentation, increased ROS production, and the initiation of clearance of dysfunctional mitochondria through mitophagy/autophagy4,23,24,33,34. This is in contrast to physiologic stressors such as submaximal exercise (increased metabolic demand) where ROS is not increased and mitochondrial membrane potential is maintained, preventing the activation of PINK1/Parkin, and suppression of mitophagy. Interestingly, pro-autophagy ULK-1 is inactivated with submaximal exercise, despite AMPK activation53. ULK-1 inactivation may be mediated by phosphatases that are activated during exercise, e.g. dual-specificity protein phosphatase 1, which targets ULK154, is expressed in the heart55 and has been shown to become activated with exercise56.
One prior study by He et al. has shown that autophagy is activated in the heart during exercise37. In contrast, our model of submaximal exercise did not induce mitophagy or autophagy, supporting the concept that these processes are inactivated during submaximal exercise in the absence of mitochondrial dysfunction. One difference between our model and that of He et al. is that we exercised mice below their anaerobic threshold at 80% of their maximum capacity for 1hr at a consistent speed of 20m/min, without pre-training; whereas their study subjected mice first to two days of exercise training (8–10m/min for 5–10min at 10° incline) and then to maximum exercise on day 3. Periods of exercise training have been shown to induce autophagy in other studies57. To examine whether the difference in autophagy in our study from that of He et al. was due to levels of exercise stress, we exercised mice to maximal capacity, showing that this also induces fission, without an increase in Parkin (although unlike in our studies with sub-maximal exercise, Parkin did not decrease), and still without evidence of autophagy (Online Fig. IIIC). Thus, although there may be a tiered level of mitochondrial fission which occurs with exercise, where sub-maximal exercise induces physiologic fragmentation without mitophagy/autophagy to enhance respiration, and extreme/maximal exercise induces a higher level of fission, we still do not see activation of autophagy in any of our models. Kruse et al., have shown similar findings in skeletal muscle from patients subjected to submaximal exercise (1hr at 70% VO2max), where Drp1 is activated immediately after exercise, but LC3 is decreased. Critically, LC3 is only increased after a 3hr post exercise period58, suggesting that autophagy may be a feature of recovery from exercise. There are several other differences that could explain the divergence in our results from those of He et al. Our submaximal exercise study was done on an FVB/NJ background, while that of He et al., was done on a C57Bl/6 background, which has been shown to have reduced total exercise capacity compared to FVB/NJ59, so that similar exercise protocols could have led to increased stress in the C57Bl/6 mice. Importantly, we have also found that autophagy-related proteins increase rapidly (as early as 5 mins) in the period of recovery from submaximal exercise (data not shown), thus making it necessary to harvest cardiac tissue immediately to assess the true state of autophagy during exercise. Finally, we cannot rule out the possibility of changes in autophagic flux during acute submaximal exercise, since we were unable to demonstrate an increase in autophagic markers after chloroquine treatment in the absence of major additional stressors such as ischemia or starvation. Despite this limitation, we still see no evidence of activation of mitophagy, as PINK1 and Parkin levels decrease with acute exercise and most importantly mitochondrial membrane potential and function are preserved.
We further show that physiologic fragmentation is mediated in part by increased β1-AR signaling, activating Drp-1, and increasing mitochondrial respiration. We confirmed this by co-treating cells with Mdivi-1 and ISO, which resulted in the blockade of the ISO-stimulated mitochondrial respiration. These data provide further evidence that physiologic mitochondrial fragmentation is associated with enhanced, rather than degraded, mitochondrial function. Interestingly, treatment with the β2-AR antagonist ICI or the β3-AR antagonist L748,337 results in a greater increase in mitochondrial respiration above ISO alone. ICI blocks both β2-AR-Gs as well as β2-AR-Gi signaling, thus enhancing β1-AR-Gs signaling60. The β3-AR has also been shown to couple to Gi47. Thus, treatment with ISO + ICI or L748,337 results in greater stimulation of β1-ARs, resulting in the observed increased respiration above ISO alone. Finally, β3-AR receptor signaling is known to activate mitochondrial fusion pathways in brown adipose tissue43, which could potentially oppose β1-AR-induced mitochondrial fission in the heart. However, β3-ARs are expressed at very low levels in ventricular myocytes of mice61 and humans62. Thus, the effects of β3-AR signaling may not be as important in the exercising heart as in other tissues.
Another model of fragmentation in the absence of pathology is the β2-AR knockout, where β1-AR signaling is not increased, but is unopposed by β2-ARs, including inhibitory Gi-signaling42. These mice have marked mitochondrial fragmentation without evidence of mitochondrial functional impairment, normal cardiac structure and function, and enhanced exercise capacity, which is blocked by β1-AR inhibition, confirming our in vitro findings of β1-AR subtype-specific regulation of mitochondrial dynamics, and providing further evidence of the process of physiologic fragmentation. One limitation of this part of our study is the inability in vivo to selectively block the effects of β1-AR stimulation on mitochondrial fission as opposed to the global effects of β1-AR stimulation on cardiac function. Thus, the β1-AR receptor-mediated increase in fission that we clearly show in vitro may be only one of the pathways mediating this process in vivo, e.g. as evidenced by the lack of an effect of metoprolol on total exercise capacity in WT mice (Fig 8D), and the normal exercise capacity we have previously shown in the β1-AR −/− mice63. Other pathways that may play a role in exercise-induced fission include the β3-AR and AMPK signaling pathway. However, in the setting of the β2-AR −/−, on a background of increased fission and fragmentation at rest, the important role of the β1-AR receptor in mediating mitochondrial fission is demonstrated.
Mitochondrial fragmentation can be viewed as an active process (cleavage of individual mitochondria) or a morphologic outcome (the presence of more numerous and smaller mitochondria), and the two are not necessarily equivalent. The bizarre mitochondrial morphology (Fig. 4C), which we have dubbed “puzzle piece mitochondria” induced by exercise in Drp1−/− mice suggests that mitochondrial fission was being actively stimulated by exercise, but that the process was interrupted before mitochondrial division. This is further evidence for a more complex role for mediators such as Drp1.
In conclusion, we have shown that mitochondrial fragmentation is a component of the cardiac response to physiologic increases in energetic demand, as encountered during normal levels of exercise. In contrast to pathologic fragmentation, physiologic mitochondrial fragmentation enhances mitochondrial function and deactivates mitophagy, the first demonstration of a positive role for fission in normal, non-pathological, non-genetically manipulated cardiac physiology (Online Table II). Whether physiologic fragmentation can be harnessed as a preventive or treatment of cardiovascular disease remains to be determined, although some of the cardiovascular benefit of exercise could be mediated by this mechanism.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Mitochondrial fission/fragmentation occurs in response to pathological stimuli.
Pathological mitochondrial fission/fragmentation is associated with decreased mitochondrial function, and the elimination of damaged mitochondria through mitophagy and cell death.
What New Information Does The Article Contribute?
Mitochondrial fission/fragmentation occurs in the heart in response to normal physiological stimuli such as routine exercise.
Physiological mitochondrial fission/fragmentation is associated with enhanced mitochondrial function and does not activate mitophagy.
Physiological mitochondrial fission/fragmentation is an adaptation to enhance energy delivery to meet increased demands and is required for reaching maximal exercise capacity.
Mitochondrial dynamics (fission, fusion, mitophagy, biogenesis) are important mediators of tissue homeostasis. In the heart, pathological stress, such as ischemia, induces mitochondrial fission/fragmentation, and is associated with decreased mitochondrial function, increased oxidative stress and activation of mitophagy. In contrast, the role of mitochondrial dynamics in normal cardiac physiology is not well understood.. We studied the role of mitochondrial dynamics in normal physiologic stress, such as occurs during routine exercise. We found that mitochondrial fission/fragmentation occurs in response to exercise, but is associated with enhanced rather than degraded mitochondrial function, absence of oxidative stress, and inhibition of mitophagy. This “physiologic fragmentation” is mediated in part by β1-adrenergic stimulation and activation of pro-fission Drp1. Physiologic fragmentation is required for achieving maximal exercise capacity: pharmacologic inhibition or genetic ablation of Drp1 significantly reduces exercise capacity. Thus, mitochondrial fission/fragmentation is not only a pathologic process, but a physiologic one that is activated during exercise to maintain cardiac energetic homeostasis. Given the known benefits of exercise on cardiovascular health, these findings suggest that mitochondrial fission may be a promising pathway that can be targeted to simulate the beneficial effects of exercise.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the National Institute of Health to DB (HL061535), RG (P01 HL112730), MC (K99HL129474), and by a T32 Post-Doctoral Fellowship to MC and KB (HL094274). Electron microscopy studies were supported in part by the ARRA (1S10RR026780-01) from the National Center for Research Resources (NCRR).
Nonstandard Abbreviations and Acronyms
- AMPK
5′ AMP-activated protein kinase
- ATP
Adenosine triphosphate
- AR
Adrenergic Receptor
- CQ
Chloroquine
- Drp1
Dynamin related protein 1
- Drp1 −/−
Drp1 knockout
- EX
Exercise
- Mfn
Mitofusin
- IP
Intraperitoneal
- ISO
Isoproterenol
- RER
Respiratory exchange ratio
- SED
Sedentary
- SQSTM1
Sequestosome 1
- PhosULK 1
Phospho-UNC-51-like kinase 1 (Ser555)
- Δψm
Mitochondrial membrane potential
Footnotes
AUTHOR CONTRIBUTIONS
Experiments were performed by MC, GF, KN, MZ, KB, GJ, HU, ES and AS. Experimental design and conception was performed by MC, RG and DB. The manuscript was written by MC and DB and all authors provided editorial review.
DISCLOSURES
The authors declare no financial interests.
References
- 1.Matsuoka S, Sarai N, Jo H, Noma A. Simulation of ATP metabolism in cardiac excitation-contraction coupling. Prog Biophys Mol Biol. 2004;85:279–299. doi: 10.1016/j.pbiomolbio.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Wallace DC, Fan W, Procaccio V. Mitochondrial Energetics and Therapeutics. Annu Rev Pathol Mech Dis. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wibom R, Hultman E, Johansson M, Matherei K, Constantin-Teodosiu D, Schantz PG. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol. 1992;73:2004–2010. doi: 10.1152/jappl.1992.73.5.2004. [DOI] [PubMed] [Google Scholar]
- 4.Disatnik M-H, Ferreira JCB, Campos JC, Gomes KS, Dourado PMM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circulation Research. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 7.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Höke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. The EMBO Journal. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorn GW, Song M, Walsh K. Functional implications of mitofusin 2-mediated mitochondrial-SR tethering. Journal of Molecular and Cellular Cardiology. 2015;78:123–128. doi: 10.1016/j.yjmcc.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, Aoki H, Katada S, Nakada K, Nomura M, Mizushima N, Mihara K, Ishihara N. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Molecular and Cellular Biology. 2015;35:211–223. doi: 10.1128/MCB.01054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., II Mitochondrial Fission and Fusion Factors Reciprocally Orchestrate Mitophagic Culling in Mouse Hearts and Cultured Fibroblasts. Cell Metabolism. 2015;21:273–285. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO Journal. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS ONE. 2012;7:e32388. doi: 10.1371/journal.pone.0032388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitaoka Y, Ogasawara R, Tamura Y, Fujita S, Hatta H. Effect of electrical stimulation-induced resistance exercise on mitochondrial fission and fusion proteins in rat skeletal muscle. Appl Physiol Nutr Metab. 2015;40:1137–1142. doi: 10.1139/apnm-2015-0184. [DOI] [PubMed] [Google Scholar]
- 14.Picard M, Gentil BJ, McManus MJ, White K, St Louis K, Gartside SE, Wallace DC, Turnbull DM. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. Journal of Applied Physiology. 2013;115:1562–1571. doi: 10.1152/japplphysiol.00819.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Z, Bai L, Yan J, Li Y, Shen W, Wang Y, Wertz K, Weber P, Zhang Y, Chen Y, Liu J. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: regulatory effects of hydroxytyrosol. Free Radical Biology and Medicine. 2011;50:1437–1446. doi: 10.1016/j.freeradbiomed.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Clemmens EW, Entezari M, Martyn DA, Regnier M. Different effects of cardiac versus skeletal muscle regulatory proteins on in vitro measures of actin filament speed and force. The Journal of Physiology. 2005;566:737–746. doi: 10.1113/jphysiol.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S-Y, Gifford JR, Andtbacka RHI, Trinity JD, Hyngstrom JR, Garten RS, Diakos NA, Ives SJ, Dela F, Larsen S, Drakos S, Richardson RS. Cardiac, skeletal, and smooth muscle mitochondrial respiration: are all mitochondria created equal? Am J Physiol Heart Circ Physiol. 2014;307:H346–52. doi: 10.1152/ajpheart.00227.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dworatzek E, Mahmoodzadeh S, Schubert C, Westphal C, Leber J, Kusch A, Kararigas G, Fliegner D, Moulin M, Ventura-Clapier R, Gustafsson J-A, Davidson MM, Dragun D, Regitz-Zagrosek V. Sex differences in exercise-induced physiological myocardial hypertrophy are modulated by oestrogen receptor beta. Cardiovascular Research. 2014;102:418–428. doi: 10.1093/cvr/cvu065. [DOI] [PubMed] [Google Scholar]
- 19.Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol. 1997;272:H1053–61. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- 20.Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y-I, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 22.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marechal X, Montaigne D, Marciniak C, Marchetti P, Hassoun SM, Beauvillain JC, Lancel S, Neviere R. Doxorubicin-induced cardiac dysfunction is attenuated by ciclosporin treatment in mice through improvements in mitochondrial bioenergetics. Clin Sci. 2011;121:405–413. doi: 10.1042/CS20110069. [DOI] [PubMed] [Google Scholar]
- 24.Gharanei M, Hussain A, Janneh O, Maddock H. Attenuation of doxorubicin-induced cardiotoxicity by mdivi-1: a mitochondrial division/mitophagy inhibitor. PLoS ONE. 2013;8:e77713. doi: 10.1371/journal.pone.0077713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson DC, Fauconnier J, Yamada T, Lacampagne A, Zhang SJ, Katz A, Westerblad H. Mitochondrial production of reactive oxygen species contributes to the -adrenergic stimulation of mouse cardiomycytes. The Journal of Physiology. 2011;589:1791–1801. doi: 10.1113/jphysiol.2010.202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 27.Campello S, Strappazzon F, Cecconi F. Mitochondrial dismissal in mammals, from protein degradation to mitophagy. Biochim Biophys Acta. 2014;1837:451–460. doi: 10.1016/j.bbabio.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb RA. Cell Death Pathways in Acute Ischemia/Reperfusion Injury. Journal of Cardiovascular Pharmacology and Therapeutics. 2011;16:233–238. doi: 10.1177/1074248411409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong S-B, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 31.Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012;13:378–385. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, Chan DC. Mitochondrial dynamics-fusion, fission, movement, and mitophagy-in neurodegenerative diseases. Human Molecular Genetics. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chennamsetty I, Coronado MJ, Contrepois K, Keller MP, Carcamo-Orive I, Sandin J, Fajardo G, Whittle AJ, Fathzadeh M, Snyder M, Reaven G, Attie AD, Bernstein D, Quertermous T, Knowles JW. Nat1 Deficiency Is Associated with Mitochondrial Dysfunction and Exercise Intolerance in Mice. Cell Rep. 2016;17:527–540. doi: 10.1016/j.celrep.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning Involves Selective Mitophagy Mediated by Parkin and p62/SQSTM1. PLoS ONE. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ju J-S, Varadhachary AS, Miller SE, Weihl CC. Quantitation of “autophagic flux” in mature skeletal muscle. Autophagy. 2010;6:929–935. doi: 10.4161/auto.6.7.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi X, Qvit N, Su Y-C, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 41.Dorn GW, II, Maack C. Journal of Molecular and Cellular Cardiology. Journal of Molecular and Cellular Cardiology. 2013;55:42–49. doi: 10.1016/j.yjmcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 43.Soriano FX, Liesa M, Bach D, Chan DC, Palacín M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes. 2006;55:1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 44.Cernecka H, Sand C, Michel MC. The odd sibling: features of β3-adrenoceptor pharmacology. Mol Pharmacol. 2014;86:479–484. doi: 10.1124/mol.114.092817. [DOI] [PubMed] [Google Scholar]
- 45.Treinys R, Zablockaitė D, Gendvilienė V, Jurevičius J, Skeberdis VA. β3-Adrenergic regulation of L-type Ca2+ current and force of contraction in human ventricle. J Membr Biol. 2014;247:309–318. doi: 10.1007/s00232-014-9635-2. [DOI] [PubMed] [Google Scholar]
- 46.Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. British Journal of Pharmacology. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soeder KJ, Snedden SK, Cao W, Rocca Della GJ, Daniel KW, Luttrell LM, Collins S. The beta3-adrenergic receptor activates mitogen-activated protein kinase in adipocytes through a Gi-dependent mechanism. J Biol Chem. 1999;274:12017–12022. doi: 10.1074/jbc.274.17.12017. [DOI] [PubMed] [Google Scholar]
- 48.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 49.Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. The EMBO Journal. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherman S, Nachmias D, Elia N. A simple, straightforward correlative live-cell-imaging-structured-illumination-microscopy approach for studying organelle dynamics. Microsc Res Tech. 2015;78:777–783. doi: 10.1002/jemt.22540. [DOI] [PubMed] [Google Scholar]
- 51.Giedt RJ, Pfeiffer DR, Matzavinos A, Kao C-Y, Alevriadou BR. Mitochondrial dynamics and motility inside living vascular endothelial cells: role of bioenergetics. Ann Biomed Eng. 2012;40:1903–1916. doi: 10.1007/s10439-012-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plucińska G, Paquet D, Hruscha A, Godinho L, Haass C, Schmid B, Misgeld T. In vivo imaging of disease-related mitochondrial dynamics in a vertebrate model system. J Neurosci. 2012;32:16203–16212. doi: 10.1523/JNEUROSCI.1327-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Zhou J-Y, Kho D, Reiners JJ, Wu GS. Role for DUSP1 (dual-specificity protein phosphatase 1) in the regulation of autophagy. Autophagy. 2016;12:1791–1803. doi: 10.1080/15548627.2016.1203483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bermudez O, Pages G, Gimond C. The dual-specificity MAP kinase phosphatases: critical roles in development and cancer. Am J Physiol, Cell Physiol. 2010;299:C189–202. doi: 10.1152/ajpcell.00347.2009. [DOI] [PubMed] [Google Scholar]
- 56.Connolly PH, Caiozzo VJ, Zaldivar F, Nemet D, Larson J, Hung S-P, Heck JD, Hatfield GW, Cooper DM. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J Appl Physiol. 2004;97:1461–1469. doi: 10.1152/japplphysiol.00316.2004. [DOI] [PubMed] [Google Scholar]
- 57.Fiuza-Luces C, Delmiro A, Soares-Miranda L, González-Murillo Á, Martínez-Palacios J, Ramírez M, Lucia A, Morán M. Exercise training can induce cardiac autophagy at end-stage chronic conditions: insights from a graft-versus-host-disease mouse model. Brain Behav Immun. 2014;39:56–60. doi: 10.1016/j.bbi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Kruse R, Pedersen AJT, Kristensen JM, Petersson SJ, Wojtaszewski JFP, Højlund K. Intact initiation of autophagy and mitochondrial fission by acute exercise in skeletal muscle of patients with Type 2 diabetes. Clin Sci. 2017;131:37–47. doi: 10.1042/CS20160736. [DOI] [PubMed] [Google Scholar]
- 59.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92:2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 60.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 61.Myagmar B-E, Flynn JM, Cowley PM, Swigart PM, Montgomery MD, Thai K, Nair D, Gupta R, Deng DX, Hosoda C, Melov S, Baker AJ, Simpson PC. Adrenergic Receptors in Individual Ventricular Myocytes: The Beta-1 and Alpha-1B Are in All Cells, the Alpha-1A Is in a Subpopulation, and the Beta-2 and Beta-3 Are Mostly Absent. Circulation Research. 2017;120:1103–1115. doi: 10.1161/CIRCRESAHA.117.310520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michel MC, Harding SE, Bond RA. Are there functional β3-adrenoceptors in the human heart? British Journal of Pharmacology. 2011;162:817–822. doi: 10.1111/j.1476-5381.2010.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rohrer DK, Schauble EH, Desai KH, Kobilka BK, Bernstein D. Alterations in dynamic heart rate control in the beta 1-adrenergic receptor knockout mouse. Am J Physiol. 1998;274:H1184–93. doi: 10.1152/ajpheart.1998.274.4.H1184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.