Abstract
There has been a dramatic increase in the emergence of antibiotic resistant bacterial strains, which has made antibiotic choices for infection control increasingly limited and more expensive. In the U.S. alone, antibiotic resistant bacteria cause at least 2 million infections and 23,000 deaths a year resulting in a $55–70 billion per year economic impact. Antibiotics are critical to the success of surgical procedures including orthopaedic prosthetic surgeries, and antibiotic resistance is occurring in nearly all bacteria that infect people, including the most common bacteria that cause orthopaedic infections, such as Staphylococcus aureus (S. aureus). Most clinical cases of orthopaedic surgeries have shown that patients infected with antibiotic resistant bacteria, such as methicillin resistant S. aureus (MRSA), are associated with increased morbidity and mortality. This paper reviews the severity of antibiotic resistance at the global scale, the consequences of antibiotic resistance, and the pathways bacteria used to develop antibiotic resistance. It highlights the opportunities and challenges in limiting antibiotic resistance through approaches like the development of novel, non-drug approaches to reduce bacteria functions related to orthopaedic implant-associated infections.
Keywords: antibiotic resistance, S. aureus, orthopaedic implant, infection, antibiotic alternative, multidrug resistance
1. Antibiotic resistance – a serious global problem
Antibiotics have revolutionized medicine, including improving orthopaedic surgical and implant outcomes, in many respects and have transformed human health and well-being for the better. Before the use of antibiotics, the fatality rate for Staphylococcus aureus (S. aureus) bacteremia was high and most wound infections were treated by amputation; for instance, ~70% of amputations in World War I were result of wound infections.1 The introduction of antibiotics has dramatically improved the fate of infected patients and has changed the way various diseases and surgical procedures are treated. The ability of antibiotics to treat and cure infection has dramatically reduced the number of incidences of infection, significantly improving the quality of life for numerous patients, reducing childhood mortality, increasing life expectancy, and saving numerous lives.
Antibiotics were first studied in the late 1800s and it was in early 1900s that penicillin was discovered. The value of using penicillin during and after orthopaedic surgeries was first highly appreciated during World War II when treating casualties from the War. The success of penicillin was followed by the development of a variety of new antibiotics. Currently, cephalosporins (e.g., cefazolin, cefalotin), aminoglycosides (e.g., gentamicin, tobramycin, amikacin), glycopeptide antibiotics (e.g., vancomycin, teicoplanin), and quinolones (e.g., ciprofloxacin, ofloxacin) have been extensively used in orthopaedic surgeries to prevent or treat infections.
Unfortunately, the discovery and increasingly widespread use (especially the misuse) of antibiotics have led to the rapid appearance of antibiotic resistant strains today; more and more infections are caused by microorganisms that fail to respond to conventional treatments. Meanwhile, the discovery and development of antibiotics have been declining rapidly over the past several decades; for instance, 16, 14, 10, and 7 new antibiotics were approved during 1983–1987, 1988–1992, 1993–1997, and 1998–2002, respectively, while only 5 and 2 were approved during 2003–2007 and 2008–2012, respectively.2 This decline was due to decreasing antibiotic research and development in major pharmaceutical companies;2 investment in new antibiotic development has been hampered by the uncertain lifecycles (associated with antibiotic resistance development) of new antibiotic drugs and government regulations affecting the pace of translational exploitation.3
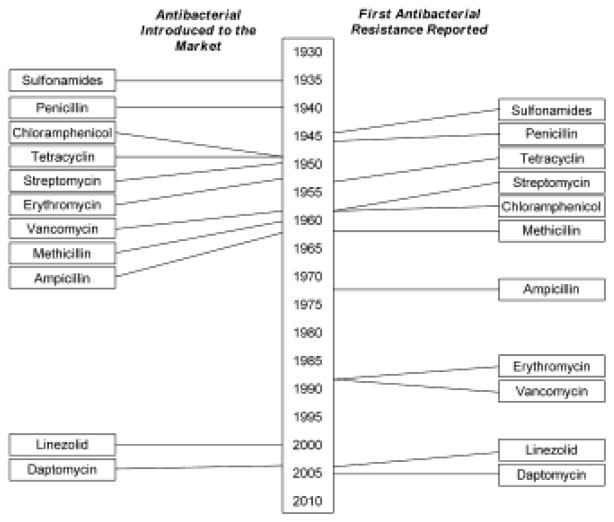
The approximate timeline for the introduction of multiple major antibiotics and the subsequent emergence of clinically significant bacteria antibiotic resistance is shown in Fig. 1.4 Following the introduction of sulfonamides and penicillin around 1937 and 1940, resistance to sulfonamides and penicillin were reported within a few years (around 1945). Resistance to tetracycline, streptomycin, and chloramphenicol was found in the 1950s. Methicillin was introduced in 1959 and methicillin resistant S. aureus (MRSA) was identified in 1961. MRSA has since become widespread in hospitals and, relatively recently, in numerous communities worldwide leading to the consideration of antibiotic resistance as a real threat to human health. Linezolid and daptomycin were introduced in the 2000s and their resistance was reported within five years. In fact, a report was given to the United Nations in 2016 concerning the significant consequences of antibiotic resistance to human health; this report represents only the second time in the history of the United Nations that threats to human health have been presented.
Fig. 1.
Timeline showing the time between the introduction of an antibacterial and the development of clinically significant resistance. Reprinted with permission from 4.
Currently, the most notorious antibiotic resistant bacteria is S. aureus, and antibiotic resistant microorganisms including Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species have been identified as the so-called “ESKAPE” microorganisms which have caused significant morbidity and mortality.5 In the U.S., the Centers for Disease Control and Prevention (CDC) has also classified multidrug resistant (MDR) microorganisms into three different levels (i.e., threat levels of urgent, serious, and concerning).6 Among these MDR microorganisms, many of them have been reported in orthopaedic implant-associated infections including MRSA, vancomycin-resistant S. aureus (VRSA), multidrug-resistant Acinetobacter, extended spectrum β-lactamase producing enterobacteriaceae (ESBLs), and multidrug-resistant Pseudomonas aeruginosa. Their resistance has a broad effect on treating and preventing infections (Table 1).7 The consequences of antibiotic resistance are very serious, and could present a significant impact on morbidity and mortality and lead to financial burdens for patients and public health systems, as described below:
Table 1.
Effects of antibiotic resistance. Reprinted from reference,7 Clinical Microbiology and Infection, 22, Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance, 416–422. Copyright (2016), with permission from Elsevier.
| The effect | Examples |
|---|---|
| Morbidity and mortality | All-cause |
| Attributable to infection | |
| Increased length of hospital stay | |
| Increased length of mechanical ventilation | |
| Increased need for intensive care and invasive devices | |
| Excess surgery | |
| Functional decline and need for post-acute care | |
| Need for contact isolation | |
| Loss of work | |
| Increased resource utilization and cost | Hospital, intensive-care unit and post-acute care beds |
| Additional nursing care, support services, diagnostic tests and imaging | |
| Additional use of isolation rooms and consumables (gloves, gowns) | |
| Cost of targeted infection control programs including screening, isolation | |
| Guideline alterations | Loss of narrow-spectrum antibiotic classes |
| Altered empiric therapy regimens | |
| Use of agents with reduced efficacy | |
| Use of agents with increased toxicity | |
| Reduced hospital activity | Unit closures |
| Cancellation of surgery |
Antibiotic resistance likely compromises the safety and efficacy of surgical procedures like implantation and transplantation that require the protection of antibiotics. It is estimated that between 38.7% and 50.9% of microorganisms causing surgical site infections are resistant to standard prophylactic antibiotics in the U.S.7
Antibiotic resistance has a direct effect on treating infections. Patients (not orthopaedic specific) with infections caused by MDR microorganisms are generally at increased risk of worse clinical outcomes and death, and consume more health-care resources compared with similar infections caused by antibiotic susceptible strains.8 Approximately a two-fold increase in morbidity, mortality, and cost for patients with resistant versus susceptible infections has been reported in patients (not orthopaedic specific) who had clinical cultures positive for Enterobacter species.9 A two-fold higher risk of death was attributed to infections caused by carbapenem-resistant K. pneumoniae compared to infections caused by carbapenem-susceptible strains in adult patients with K. pneumoniae bacteremia.10 Meanwhile, hospitals spend, on average, an additional $10,000–40,000 to treat a patient infected by resistant bacteria versus susceptible strains.11 According to the CDC, in the U.S. alone, antibiotic resistant bacteria cause at least 2 million infections, 23,000 deaths a year,6 and $55–70 billion per year in economic impact.12,13 In Europe, approximately 25,000 people die annually due to MDR bacterial infections, along with a 1.5 billion per year cost in the economy.14,15
Antibiotic resistance may have negative effects on domesticated animals such as pets and farm animals.16
2. Molecular mechanisms of antibiotic resistance
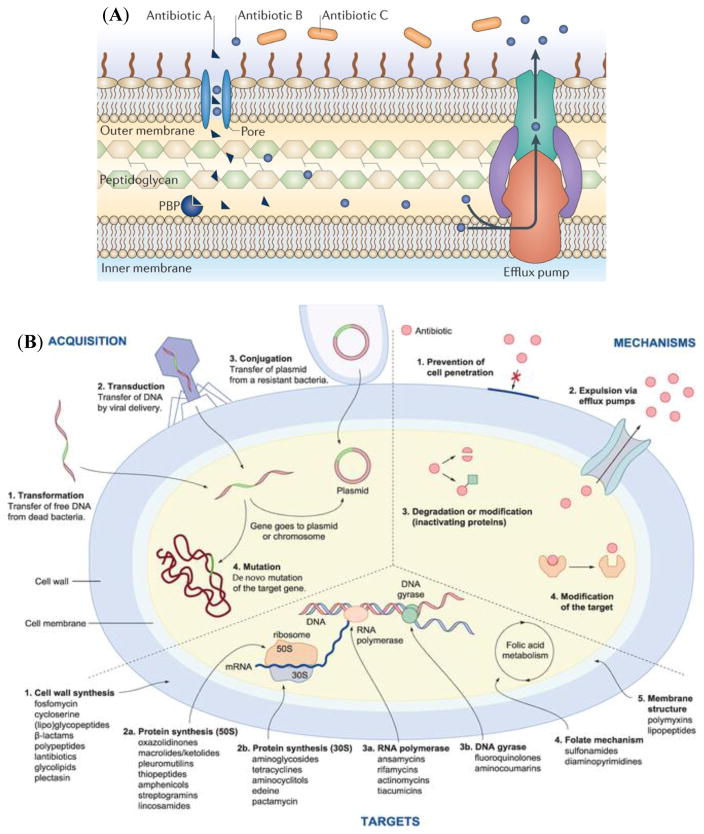
Antibiotic resistance is defined as the ability of microorganisms to resist the effects of drugs (e.g., antibiotics) – that is, the germs are not killed and their growth is not stopped.17 Resistance to a specific antibiotic is relative to the microorganisms to be tested and their previous antibiotic exposures. The molecular mechanisms of antibiotic resistance have been extensively reviewed.14,18 There are two distinct types of antibiotic resistance: intrinsic and acquired. Microorganisms can be intrinsically resistant to certain antibiotics as a result of inherent structural or functional characteristics (Fig. 2A).14 For instance, a particular antibiotic may be structurally unable to penetrate the outer membrane of certain microorganisms or the antibiotic entering the membrane is removed by efflux pumps.
Fig. 2.
(A) Intrinsic mechanisms of resistance. The figure shows an overview of intrinsic resistance mechanisms. The example shown is of β-lactam antibiotics targeting a penicillin-binding protein (PBP). Antibiotic A can enter the cell via a membrane-spanning porin protein, reach its target and inhibit peptidoglycan synthesis. Antibiotic B can also enter the cell via a porin, but unlike Antibiotic A, it is efficiently removed by efflux. Antibiotic C cannot cross the outer membrane and so is unable to access the target PBP. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Microbiology,14 copyright (2015). (B) The four resistance acquisition pathways, the four main mechanisms of resistance, and the five main targets for antibiotics. Reprinted with permission from 19.
Meanwhile, microorganisms may have acquired resistance that can be obtained via chromosomal mutations or, more commonly, by acquiring an antibiotic resistance gene from another bacterium via mobile plasmids or transposons (so called horizontal gene transfer).19 According to Riedl et al., currently, there are five main targets (i.e., cell wall synthesis, protein synthesis, RNA polymerase and DNA gyrase, folate mechanism, and membrane structure) for antibiotics, and antibiotic resistance can essentially be acquired through four different pathways (i.e., transformation, transduction, conjugation, and mutation) and expressed by four different mechanisms (i.e., prevention of cell penetration, expulsion via efflux pumps, inactivating proteins, and modification of the target) (Fig. 2B).19 For instance, MRSA is resistant to numerous penicillin-like β-lactam antibiotics primarily due to its expression of the mecA gene which encodes the low affinity penicillin binding protein PBP 2a.20 The Antibiotic Resistance Genes Database currently lists the existence of more than 23,000 potential resistance genes of about 380 different types from available bacterial genome sequences.21 Fortunately, the number of functional resistance genes is much smaller.
Biofilm formation is also believed to be one key means of antibiotic resistance in orthopaedic implant-associated infections. Bacterial biofilms are inherently resistant to antibiotics22 because (i) certain antibiotics fail to penetrate the full depth of the biofilm, (ii) some cells within biofilms are slow-growing or nongrowing probably as a result of nutrient limitation, and (iii) some cells within biofilms may adopt a distinct and protected biofilm phenotype. For instance, biofilm forming S. epidermidis strains (126 out of 342), compared to those non-producing isolates, presented a significantly higher prevalence of resistance to ciprofloxacin and sulfamethoxazole as well as the four aminoglycosides.23
A variety of factors including human activities may influence the presence of antibiotic resistance and antibiotic resistance genes are omnipresent in natural environments. Numerous types of anthropogenic activity (such as antibiotic use in agriculture and antibiotic presence in waste disposal) have created major environmental reservoirs for antibiotic resistance genes.24 Animals, wind, water, etc., may disseminate antibiotic resistance genes throughout the environment.25 As a result, wastewater treatment plants have been found to be rich in antibiotic resistance genes and resistant microorganisms,26 and bacteriophages (viruses that infect bacteria), found in wastewater, are reservoirs of antibiotic resistance genes.27
It is noteworthy to mention that antibiotic resistance mechanisms have existed for a long time. Antibiotic resistance genes and resistance-encoding integrons have been found in the gut flora of people who are not exposed to antibiotics and who have been apparently isolated from modern civilization.28,29 Furthermore, genes encoding resistance to β-lactam, tetracycline, and glycopeptide antibiotics have been identified from the 30,000-year-old DNA found in Beringian permafrost sediments.30
3. Antibiotic resistance profiles in orthopaedic implant-associated infections
Orthopaedic implant-associated infections are often treated with multiple surgical procedures along with systemic and/or local antibiotic treatment. It is well known that Staphylococci (especially S. aureus and S. epidermidis) are the most common causative microorganisms involved in orthopaedic implant-associated infections. S. aureus has long been recognized as exhibiting high levels of antibiotic resistance, while numerous other microorganisms have been observed to exhibit increasing antibiotic resistance in the recent years, including S. epidermidis23,31 and a number of less frequently seen Staphylococcal species.32,33
Numerous clinical studies have been reported about orthopaedic implant-associated infections, along with increasing clinical studies focusing on antibiotic resistance and its prevalence in orthopaedic implant-associated infections.32–38 In primary and revision periprosthetic joint infections (PJIs), the most common infecting organisms were S. aureus and coagulase-negative Staphylococci (CNS),34–36 and most strains were resistant to at least one antibiotic (Table 2).32–37 Alarmingly, in some cases,36 CNS resistance to both methicillin and gentamicin seems to be much higher than S. aureus and has been increasing, which is a concern for future antibiotic prophylaxis. Antibiotic resistant Staphylococci were also found to present in orthopaedic patients with loosened or failed hip prostheses even without clinical manifestations of infection.37 The role of Staphylococci and their antibiotic resistance prevalence may have been underestimated in previously considered “aseptic” implant loosening failures.37 In addition, compared to isolates that were not associated with orthopaedic implants, S. aureus strains isolated from orthopaedic implant-associated infections were significantly more frequently resistant to ciprofloxacin and four aminoglycosides (i.e., amikacin, gentamicin, netilmicin, and tobramycin).38
Table 2.
Prevalence and antibiotic resistance in orthopaedic surgeries.
| Infection | Microorganism | Antibiotic resistance profile | Ref. |
|---|---|---|---|
| 1131 Staphylococcal strains isolated from patients undergoing revision of surgical wounds and treatment of infected prostheses | 193 were identified as Staphylococci other than S. aureus and S. epidermidis. S. hominis, S. haemolyticus, S. capitis, S. warneri, and S. cohnii were relatively prevalent, being 4.2%, 3.7%, 2.7%, 2.6%, and 1.6%, respectively. | These 193 species were often (e.g. 51–66%) resistant to penicillin and had significantly different patterns of resistance toward other antibiotics like ampicillin, clindamycin, erythromycin, gentamicin, tobramycin, and vancomycin. | 32 |
| Seven knee PJIs | Escherichia coli (E. coli) was isolated in six cases and Klebsiella pneumoniae in one case. | E. coli was resistant to ciprofloxacin but susceptible to gentamicin and Klebsiella pneumoniae was resistant to ciprofloxacin and gentamicin but susceptible to cotrimoxazole. | 33 |
| 4009 primary hip and knee arthroplasties with an overall infection rate of 0.87% | The most common infecting organisms were coagulase-negative Staphylococci or CNS (35%) and S. aureus (25%). | 92% of the CNS strains were cefazolin-resistant and 9.1% of the S. aureus strains were methicillin-resistant. Overall, 53% of the organisms was cefazolin-resistant. | 34 |
| ~800 orthopaedic clinical isolates from infections associated with prosthetic implants | 34% were S. aureus, 32% S. epidermidis, 8% Pseudomonas, 5% Enterococcus, 2% Escherichia, 2% Streptococcus, and 13% other CNS. | ~80% of both S. aureus and S. epidermidis were resistant to cephalosporins (penicillin drugs) and ~40% had methicillin/oxacillin resistance. | 35 |
| 72 revision PJIs | S. aureus (36%) and CNS (35%) were the most common infective organisms. | S. aureus and CNS were resistant to methicillin (20% vs. 72%) and gentamicin (4% vs. 40%). | 36 |
| 12 patients undergoing one-stage revision of aseptic implant loosening (had no clinical manifestations of infection) | All patients were positive for bacterial growth. Staphylococci were the overwhelming majority and CNS was cultured from nine patients with eight S. epidermidis isolates and one S. warneri. | Staphylococcal isolates were resistant to methicillin (8 isolates), macrolides, lincosamides, and group B streptogramins (4), aminoglycosides (4), cotrimoxazole (3), ciprofloxacin (3), fusidic acid (3), and rifampin (1). Five out of ten were MDR. | 37 |
| 19 patients who had implant failure accompanied with ongoing PJIs | Staphylococci were the only microorganisms. S. epidermidis and S. aureus were cultured from 11 and four patients, respectively, in addition to S. warneri (2 isolates), S. lugdunensis (1), S. simulans (1), and S. captitis (1). | These Staphylococci were resistant to methicillin (12 isolates), macrolides, lincosamides, and group B streptogramins (6), aminoglycosides (5), cotrimoxazole (4), ciprofloxacin (3), fusidic acid (2), and rifampin (3). Seven Staphylococcal isolates were MDR. | 37 |
Besides S. aureus and S. epidermidis, some less frequently seen species like S. hominis, S. haemolyticus, S. capitis, S. warneri, S. cohnii, Escherichia coli (E. coli), and Klebsiella pneumoniae were observed and were often found to be antibiotic resistant.32,33 These usually less observed species, most of which have been thought to play a commensal role, may potentially become pathogenic, especially in immunocompromised patients including patients with orthopaedic implants. In view of their differences in prevalence and antibiotic resistance, we would suggest that these less observed species also be monitored for their prevalence, acquisition of antibiotic resistance, virulence, and pathogenic properties, and may need to be treated individually.
Meanwhile, older infected patients, who frequent present with compromised health, may also have different antibiotic resistance profiles compared to younger patients. In a study of 163 patients with orthopaedic implant-associated infections, the Staphylococci found in the older patients (age 60 and older) were more frequently methicillin resistant or MDR compared to those associated with infections in younger patients.39 For instance, S. epidermidis strains resistant to methicillin had a significantly higher prevalence in older patients than in younger patients (91% vs. 66%, p = 0.006), and the corresponding MDR prevalence was significantly higher in older patients as well (94% vs. 72%, p = 0.011).39 The observed compromised health status and poor bone quality in older infected patients might have contributed to their higher antibiotic resistance prevalence compared to younger patients.
4. Antibiotic resistance linked to less optimal clinical outcomes in orthopaedic implant-associated infections
S. aureus is one of the most common causes of orthopaedic implant-associated infections, with both methicillin-susceptible and -resistant strains. The incidence of MRSA and other antibiotic resistance has increasingly been reported worldwide over the past decade. One question has been raised but has not been clearly answered: Does antibiotic resistance influence the clinical outcome of orthopaedic associated infections?
Quite a few case studies have shown that antibiotic resistant bacteria have contributed to worse clinical outcomes compared to those infected by antibiotic susceptible bacteria (Table 3).40–45 It was found that patients infected with MRSA had significantly longer hospital stays,40 were at a significantly higher risk of treatment failure,40,41 had significantly more surgical procedures,42,45 had significantly more co-morbidities,45 had significantly poorer clinical outcomes,41 and had significantly lower satisfactory outcomes42 compared to those infected with methicillin susceptible S. aureus or MSSA. Similar tends were reported in infected children (less than 18 years old). Children with bone and joint infections infected with MRSA had a significantly longer duration of febrile days and hospital stays43,44 and antibiotic treatment44 compared to those infected with MSSA.
Table 3.
Poorer clinical outcomes of orthopaedic patients infected by MRSA compared to those infected by MSSA.
| Time | Patient | Outcome | Ref. |
|---|---|---|---|
| 1995–2004 | 43 patients with periprosthetic joint infections | Significantly longer hospital stay (15 vs. 10 days). Significantly higher risk of treatment failure |
40 |
| 1997–2001 | 70 patients with periprosthetic joint infections | Successfully treated only 48% and 18% of hip and knee replacements, respectively, in MRSA infected patients compared to 81% and 89% in MSSA infected cases | 41 |
| 1998–2004 | 31 patients with delayed deep infection after total knee arthroplasty | Significantly higher mean number of surgical procedures per patient. Significantly lower proportion of patients with satisfactory outcomes | 42 |
| 2000–2002 | 59 children with musculoskeletal infections | Significantly longer febrile days and hospital stays. | 43 |
| 2004–2008 | 74 children with bone and joint infections | Significantly longer duration of febrile days, hospital stays, and antibiotic treatment | 44 |
| 2005–2011 | 30 vertebral osteomyelitis patients (16 cases of MRSA and 14 MSSA) | Significantly higher rate of patients to undergo surgical procedure within three months (56.3% vs. 14.3%) | 45 |
However, there are a few studies, likely underpowered, showing higher but not significantly different morbidity or mortality rates between infections caused by antibiotic resistant and susceptible microorganisms in orthopaedic implant-associated infections. In a recent large multicenter study during 2003–2010, 342 PJI patients were identified with S. aureus, and similar failure rates were observed for MRSA and MSSA (46 vs. 44%).46 Interestingly, during antibiotic treatment and after the first 30 days, MRSA cases were more than twice as likely to fail as those infected with MSSA, while after antibiotic treatment, MSSA cases failed more than MRSA cases.46 In another study of 98 patients with PJIs caused by S. aureus during 2000 to 2006, the treatment failure rate was higher, but not significant (p=0.38), in infections caused by MRSA compared to those caused by MSSA, and the treatment failure rates were 29.4% and 19.7 % in MRSA- and MSSA-infected patients, respectively.47 Similarly, higher although not significant (p=0.242) recurrent infection rates were found in MRSA infected patients compared to MSSA infected patients among 61 S. aureus infected hip and knee patients from 1998–2011.48 MRSA infected patients showed significantly higher erythrocyte sedimentation rates, C-reactive proteins and neutrophil percentages during their initial visits.48
Therefore, from the available case studies, we can conclude that orthopaedic implant-associated infections caused by microorganisms resistant to antibiotics likely have a less optimal outcome compared to those caused by antibiotic susceptible microorganisms.
5. Challenges, opportunities, and obligations related to bacteria resistant orthopaedic implant-associated infections
It is widely acknowledged that antibiotic resistance is one of the biggest threats facing healthcare today.6 Due to antibiotic resistance and reduced availability of new antibiotics, many routine surgical treatments such as hip and knee replacements are becoming increasingly challenging and could be life threatening.49 There is little question that the excessive and inappropriate use of antibiotics are the most important causes of antibiotic resistance.50 This situation needs immediate action which must limit the spread of antibiotic resistance, stimulate the development of new antibiotics and alternatives, and prolong the effectiveness of current and new antibiotics. There is no doubt that both orthopaedic surgeons and orthopaedic researchers may play important roles in such actions in reducing the threat of antibiotic resistance, and the actions we take will only be sustained if based on a sound understanding of the relative roles of many factors, particularly patients and implants, microorganisms, orthopaedic surgeons and staff, and clinical settings (Fig. 3), in the emergence, spread, and persistence of antibiotic resistance.
Fig. 3.
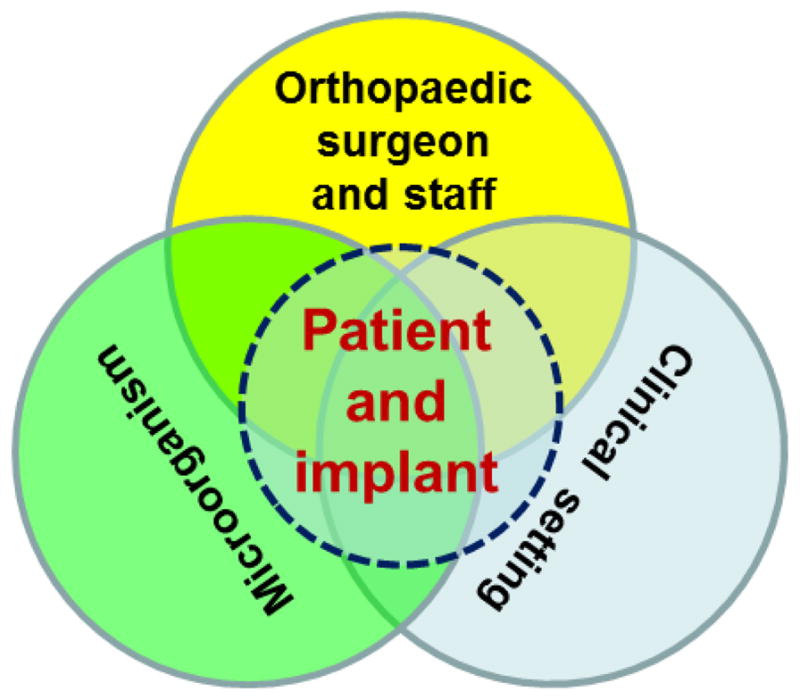
Major factors that contribute to the emergence, spread, and persistence of antibiotic resistance in orthopaedic implant-associated infections.
Facing increasing challenges in antibiotic resistance, orthopaedic surgeons are presented with multiple opportunities or obligations to alleviate the crisis. These include:
Obligations for an honest and open discussion of orthopaedic implant infections. It has been proposed that the current healthcare system in some regions of the world (such as the U.S.) actually provides a financial incentive to attribute a failed orthopaedic implant to anything but infection, even when infection is involved. This incentive to attribute implant failures to causes other than infection may stem from changes in healthcare reimbursement policy where hospitals must now cover all costs (rather than insurance companies) if infection occurs within a certain number of days post implantation. It is not until we have an open and honest discussion about the prevalence of orthopaedic implant infections that appropriate resources will be allocated to combat such infections.
Obligations to establish resistance surveillance, monitoring, and data-sharing, which will assist orthopaedic surgeons in developing better evidence-based databases for more appropriate antibiotic use.
Obligations to develop national and international principles and guidelines for the use of antibiotics based on clinical evidence and apply them in practice, combined with effective teamwork, communication, and accountability. Antibiotics like rifampin has been commonly used in combination with other antibiotics to treat S. aureus infections, and has seemed to be promising in treating orthopaedic implant-associated infections; however, data supporting this practice are limited and more definitive data are lacking.51 Guidelines for prophylaxis and treatment of orthopaedic infections have been established,52–54 and if implemented worldwide, they could have immense impact on healthcare policy and practices. These principles and guidelines will enable orthopaedic surgeons to be more effective in preventing infections and reducing the use of antibiotics. Preventing patients from developing acute infections after orthopaedic surgeries will eliminate the need for extended antibiotic use for possible chronic or recurrent infections.
Obligations to explore personalized medicine. In current practice, the treatment of patients for orthopaedic infections is completed sometimes without even knowing bacteria antibiotic resistant profiles and is not based on a thorough review of patient history.
Obligations to establish and follow stricter hygiene (especially hand hygiene) measures, which will lead to a further reduction in the transmission of antibiotic resistance or microorganisms. One of the most common vehicles for resistance transmission is the human hand, which can become easily contaminated in contact with the patient or the clinical settings near the patient.
Obligations to further promote practicing and advocating antimicrobial stewardship – structured guidance and support for responsible selection and utilization of antibiotics – orthopaedic surgeons may better train the next generation of orthopaedic surgeons. – Obligations to broaden their coordination with international efforts in reducing antibiotic resistance. Antibiotic resistance is a global problem, and has been exacerbated by the ease of international travel and trade, and increasing global population densities.
Orthopaedic researchers are also presented with tremendous opportunities in searching for strategies to avoid or inhibit antibiotic resistance. Such opportunities include:
Developing advanced diagnostic methods, which would need to be simple, quick, accurate, and low cost, to detect and profile antibiotic resistance genes or microorganisms. Diagnostic tests are crucial to the management of infectious diseases and combatting the rise in antibiotic resistance,55 and the introduction of rapid and accurate diagnostics tests into orthopaedic surgeons’ offices will likely influence their prescribing of a more rational use of antibiotics. As an example, differentiating between Gram-positive and Gram-negative bacteria would be expected to significantly facilitate the appropriate use of antibiotics.
Searching for new antibiotic targets and antibiotics with multiple modes of actions against bacteria, and rejuvenating or repurposing current and old antibiotics. Antibiotics with multiple modes of antimicrobial action would help reduce antibiotic resistance. Screening for new antibiotics from natural sources could broaden the possibilities for treating infections. New antibiotics do not have to have equal effectiveness against Gram-positive and Gram-negative resistant microorganisms.
Developing advanced antibiotic cocktails that may have synergistic antimicrobial effects or are less likely induce resistance. Scientific and clinical evidence should be used for specific combinations of antibiotics rather than the ad hoc combinations that are sometimes chosen by prescribers. Such cocktail approaches (e.g., a fluoroquinolone plus a macrolide) have been applied with success in the treatment of diseases like HIV infection.
Discovering non-antibiotic drugs and effective vaccines, bacteriophages, or immunotherapeutic approaches. Multiple antibiotic alternatives have been studied,56–59 and some drugs, which may not exhibit direct bacteriostatic or bactericidal activities, may stimulate or recruit the host’s innate immune system56,60 and they may be used in conjunction with antibiotics.61 Many of these alternative strategies are promising but, unfortunately, are still in their early development stages.
Expansion of research into non-biomolecule approaches to keep bacteria from attaching to implant surfaces and thus allowing the immune system to clear such microorganisms more easily. Specifically, research in the area of nanotechnology has led to the generation of nanoscale surface features that can both decrease bacteria attachment and increase osseointegration without the use of antibiotics or biomolecules.62–65 Such approaches can provide a quick and effective FDA approval process to reduce orthopaedic implant infections that do not involve antibiotics.
Further examining the mechanisms of antibiotic resistance. Antibiotic resistance has been in existence for a long time and new resistance mechanisms continue to occur. A better mechanistic and structural understanding will allow researchers to tackle the origin of resistance.
Along with the opportunities and obligations described above come the challenges facing orthopaedic surgeons and researchers, among all parties involved, in dealing with increasing antibiotic resistance:
How to collaborate with pharmaceutical companies in the discovery and development of new antibiotics or alternatives? The investment and interest of pharmaceutical companies in the discovery and development of new antibiotics is decreasing,2 since finding new antibiotics is challenging and the return on investment is poor compared to other therapeutic areas (e.g., drugs for long-term chronic diseases). Note that actions like the Generating Antibiotics Incentives Now Act (GAIN Act) signed to law in 2012 are positive but may not be attractive enough for pharmaceutical companies.
How to effectively eliminate antibiotic resistance transmission? It is important to break the transmission chains of antibiotic resistance genes because, even if we are able to make new antibiotics or alternative treatments, bacteria are likely to develop resistance within short time periods.
How to control antibiotic waste? Antibiotic waste should not simply be dumped into the environment, which houses various microorganisms that have been contributing to the emergence and spread of antibiotic resistance.
How to efficiently publicize scientific findings, either from laboratories or bedside, and make them readily accessible and understood by the general public? The public plays an important role in antibiotic resistance emergence and spreading. However, a recent study found that the public was aware of the contributions of antibiotic misuse and overuse to resistance, but many do not consider antibiotic resistance to be an important health problem.66
Screening for resistance may come with issues. Screening all patients on admission for various potential resistant microorganisms may not be cost-effective at this point, while screening for only one microorganism while ignoring others may seem unwise. Also, isolation of patients infected with resistant microorganisms has been effective in reducing the spread of resistance but it could be problematic with regard to children, for whom extended isolation may pose unique challenges to families.
New antibiotic resistance mechanisms are emerging, and infections caused by multidrug-resistant Gram-negative bacteria are increasingly seen and are particularly difficult to treat. New antibiotics or alternative treatments will have to tackle this challenge as well.
Antibiotic alternatives may reduce the use of antibiotics; however, the role of non-antibiotic drugs in developing antibiotic resistance has not been examined yet and is not clear.
The majority of research to date focuses on infections formed by a single species of bacteria; however, most infections are polymicrobial (consisting of two or more bacterial species)33–35,67 which is more difficult to treat.68 It is not clear whether polymicrobial infections have higher antibiotic resistance compared to monomicrobial infections and the role of each individual microorganism in contributing to antibiotic resistance is also unknown.
Lack of funding nationally and globally; the U.S. has pledged a significant increase in funding in research related to antibiotic resistance but, compared to the magnitude of resistance, the funding is not sufficient and globally, we continue to lag behind. Every month, it appears that a new microorganism threat faces humans that we are ill-equipped to handle and we will continue to be ill-equipped to handle unless research moves ahead of such outbreaks.
In summary, in orthopaedic infections, antibiotic resistant microorganisms likely lead to less than optimal clinical outcomes compared to those that are susceptible to antibiotics. The increasing occurrence of antibiotic resistance has presented unique opportunities, obligations, and challenges to orthopaedic researchers and surgeons.
Acknowledgments
BL acknowledges financial support from Department of Defense office of the Congressionally Directed Medical Research Programs (CDMRP), WV NASA EPSCoR, AO Foundation (Project S-13–15L), Osteosynthesis and Trauma Care Foundation, and Orthopaedic Research and Education Foundation. BL also acknowledges the WVU Core Facilities supported by GM103488/RR032138, RR020866, GM104942, OD016165, and GM103434. The authors acknowledge Suzanne Danley for proofreading.
Footnotes
Author Contributions Statement: Both BL and TJW have contributed to the design, drafting, and revising of this perspective review paper. Both authors have read and approved the final submitted manuscript.
Disclosure: The authors report no conflicts of interest in this work.
References
- 1.Hirsch EF. The Treatment of Infected Wounds, Alexis Carrel's contribution to the care of wounded soldiers during World War I. J trauma. 2008;64:S209–10. doi: 10.1097/TA.0b013e31816b307d. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Benjamin DK, Jr, et al. 10 x '20 Progress--development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685–94. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Economics. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 4.O'Connell KM, Hodgkinson JT, Sore HF, et al. Combating multidrug-resistant bacteria: current strategies for the discovery of novel antibacterials. Angew Chem Int Ed Engl. 2013;52:10706–33. doi: 10.1002/anie.201209979. [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. [accessed on January 16, 2017];Antibiotic resistance threats in the United States. 2013 http://www.cdc.gov/drugresistance/threat-report-2013/
- 7.Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22:416–22. doi: 10.1016/j.cmi.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. [accessed on January 16, 2017];Global Report on Antimicrobial Resistance, 2014. 2014 http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1.
- 9.Cosgrove SE, Kaye KS, Eliopoulous GM, et al. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch Intern Med. 2002;162:185–90. doi: 10.1001/archinte.162.2.185. [DOI] [PubMed] [Google Scholar]
- 10.Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–6. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 11.OECD. Antimicrobial Resistance in G7 countries and beyond: Economic Issues, Policies and Options for Action. 2015 Available at: http://www.oecd.org/els/health-systems/antimicrobial-resistance.htm.
- 12.Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–84. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 13.Report to the President on Combating Antibiotic Resistance. Executive Office of the President, President’s Council of Advisors on Science and Technology United States; Sep, 2014. [accessed on January 16, 2017]. http://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST/pcast_carb_report_sept2014.pdf. [Google Scholar]
- 14.Blair JMA, Webber MA, Baylay AJ, et al. Molecular mechanisms of antibiotic resistance. Nat Rev Micro. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 15.Walker DFT. Annual Report of the Chief Medical Officer: Volume Two, 2011: Infections and the Rise of Antimicrobial Resistance. Department of Health; 2011. [Google Scholar]
- 16.Bengtsson B, Greko C. Antibiotic resistance--consequences for animal health, welfare, and food production. Upsala J Med Sci. 2014;119:96–102. doi: 10.3109/03009734.2014.901445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.About Antimicrobial Resistance. Centers for Disease Control and Prevention; [Accessed on May 22, 2017]. https://www.cdc.gov/drugresistance/about.html. [Google Scholar]
- 18.Lin J, Nishino K, Roberts MC, et al. Mechanisms of antibiotic resistance. Front Microbiology. 2015;6:34. doi: 10.3389/fmicb.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chellat MF, Raguz L, Riedl R. Targeting Antibiotic Resistance. Angew Chem Int Ed Engl. 2016;55:6600–26. doi: 10.1002/anie.201506818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Pop M. ARDB--Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009;37:D443–7. doi: 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 23.Arciola CR, Campoccia D, Gamberini S, et al. Antibiotic resistance in exopolysaccharide-forming Staphylococcus epidermidis clinical isolates from orthopaedic implant infections. Biomaterials. 2005;26:6530–5. doi: 10.1016/j.biomaterials.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Doyle MP, Busta FF, Cords B, et al. Antimicrobial resistance: implications for the food system. Compr Rev Food Sci Food Saf. 2006;5:7. [Google Scholar]
- 25.Allen HK, Donato J, Wang HH, et al. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8:251–9. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 26.Szczepanowski R, Linke B, Krahn I, et al. Detection of 140 clinically relevant antibiotic-resistance genes in the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to selected antibiotics. Microbiology. 2009;155:2306–19. doi: 10.1099/mic.0.028233-0. [DOI] [PubMed] [Google Scholar]
- 27.Subirats J, Sanchez-Melsio A, Borrego CM, et al. Metagenomic analysis reveals that bacteriophages are reservoirs of antibiotic resistance genes. Int J Antimicrob Agents. 2016;48:163–7. doi: 10.1016/j.ijantimicag.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Bartoloni A, Pallecchi L, Rodriguez H, et al. Antibiotic resistance in a very remote Amazonas community. Int J Antimicrob Agents. 2009;33:125–9. doi: 10.1016/j.ijantimicag.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Pallecchi L, Bartoloni A, Paradisi F, et al. Antibiotic resistance in the absence of antimicrobial use: mechanisms and implications. Expert Rev Anti-Infect Ther. 2008;6:725–32. doi: 10.1586/14787210.6.5.725. [DOI] [PubMed] [Google Scholar]
- 30.D/'Costa VM, King CE, Kalan L, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–61. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 31.Cherifi S, Byl B, Deplano A, et al. Comparative epidemiology of Staphylococcus epidermidis isolates from patients with catheter-related bacteremia and from healthy volunteers. J Clin Microbiol. 2013;51:1541–7. doi: 10.1128/JCM.03378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arciola CR, Campoccia D, An YH, et al. Prevalence and antibiotic resistance of 15 minor staphylococcal species colonizing dic implants. Int J Artif Organs. 2006;29:395–401. doi: 10.1177/039139880602900409. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Pastor JC, Vilchez F, Pitart C, et al. Antibiotic resistance in orthopaedic surgery: acute knee prosthetic joint infections due to extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae. Eur J Clin Microbiol. 2010;29:1039–41. doi: 10.1007/s10096-010-0950-y. [DOI] [PubMed] [Google Scholar]
- 34.Ravi S, Zhu M, Luey C, et al. Antibiotic resistance in early periprosthetic joint infection. ANZ J Surgery. 2016;86:1014–8. doi: 10.1111/ans.13720. [DOI] [PubMed] [Google Scholar]
- 35.Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–9. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 36.Malhas AM, Lawton R, Reidy M, et al. Causative organisms in revision total hip & knee arthroplasty for infection: Increasing multi-antibiotic resistance in coagulase-negative Staphylococcus and the implications for antibiotic prophylaxis. Surgeon. 2015;13:250–5. doi: 10.1016/j.surge.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Bogut A, Niedzwiadek J, Strzelec-Nowak D, et al. Infectious prosthetic hip joint loosening: bacterial species involved in its aetiology and their antibiotic resistance profiles against antibiotics recommended for the therapy of implant-associated infections. New Microbiol. 2014;37:209–18. [PubMed] [Google Scholar]
- 38.Campoccia D, Montanaro L, Baldassarri L, et al. Antibiotic resistance in Staphylococcus aureus and Staphylococcus epidermidis clinical isolates from implant orthopedic infections. Int J Artif Organs. 2005;28:1186–91. doi: 10.1177/039139880502801117. [DOI] [PubMed] [Google Scholar]
- 39.Morgenstern M, Erichsen C, von Ruden C, et al. Staphylococcal orthopaedic device-related infections in older patients. Injury. 2016;47:1427–34. doi: 10.1016/j.injury.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 40.Salgado CD, Dash S, Cantey JR, et al. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Rel Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 41.Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Rel Res. 2002:116–24. doi: 10.1097/00003086-200211000-00021. [DOI] [PubMed]
- 42.Joshy S, Gogi N, Thomas B, et al. Delayed onset of deep infection after total knee arthroplasty: comparison based on the infecting organism. J Orthop Surg. 2007;15:154–8. doi: 10.1177/230949900701500205. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Aguilar G, Avalos-Mishaan A, Hulten K, et al. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J. 2004;23:701–6. doi: 10.1097/01.inf.0000133044.79130.2a. [DOI] [PubMed] [Google Scholar]
- 44.Kini AR, Shetty V, Kumar AM, et al. Community-associated, methicillin-susceptible, and methicillin-resistant Staphylococcus aureus bone and joint infections in children: experience from India. J Pediatr Orthop Part B. 2013;22:158–66. doi: 10.1097/BPB.0b013e32835c530a. [DOI] [PubMed] [Google Scholar]
- 45.Inoue S, Moriyama T, Horinouchi Y, et al. Comparison of clinical features and outcomes of staphylococcus aureus vertebral osteomyelitis caused by methicillin-resistant and methicillin-sensitive strains. SpringerPlus. 2013;2:283. doi: 10.1186/2193-1801-2-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013;56:182–94. doi: 10.1093/cid/cis746. [DOI] [PubMed] [Google Scholar]
- 47.Senneville E, Joulie D, Legout L, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis. 2011;53:334–40. doi: 10.1093/cid/cir402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryu DJ, Kang JS, Moon KH, et al. Clinical Characteristics of Methicillin-resistant Staphylococcus aureus Infection for Chronic Periprosthetic Hip and Knee Infection. Hip Pelvis. 2014;26:235–42. doi: 10.5371/hp.2014.26.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance 2016 [Google Scholar]
- 50.Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 51.Perlroth J, Kuo M, Tan J, et al. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med. 2008;168:805–19. doi: 10.1001/archinte.168.8.805. [DOI] [PubMed] [Google Scholar]
- 52.Parvizi J, Shohat N, Gehrke T. Prevention of periprosthetic joint infection: new guidelines. Bone Joint J. 2017;99-b:3–10. doi: 10.1302/0301-620X.99B4.BJJ-2016-1212.R1. [DOI] [PubMed] [Google Scholar]
- 53. [Accessed on April 2, 2017]; http://www.who.int/gpsc/ssi-prevention-guidelines/en/
- 54. [Accessed on April 2, 2017]; https://www.cdc.gov/hicpac/SSI/001_SSI.html.
- 55.O’Neill J. Review on Antimicrobial Resistance. 2015. Rapid diagnostics: stopping unnecessary use of antibiotics. [Google Scholar]
- 56.Li B, Jiang B, Boyce BM, et al. Multilayer polypeptide nanoscale coatings incorporating IL-12 for the prevention of biomedical device-associated infections. Biomaterials. 2009;30:2552–8. doi: 10.1016/j.biomaterials.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li B, Jiang B, Dietz MJ, et al. Evaluation of local MCP-1 and IL-12 nanocoatings for infection prevention in open fractures. J Orthop Res. 2010;28:48–54. doi: 10.1002/jor.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li B, McKeague AL. Emerging ideas: Interleukin-12 nanocoatings prevent open fracture-associated infections. Clin Orthop Rel Res. 2011;469:3262–5. doi: 10.1007/s11999-010-1690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, Hamza T, Tidwell JE, et al. Unique antimicrobial effects of platelet-rich plasma and its efficacy as a prophylaxis to prevent implant-associated spinal infection. Adv Healthc Mater. 2013;2:1277–84. doi: 10.1002/adhm.201200465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finlay BB, Hancock REW. Can innate immunity be enhanced to treat microbial infections? Nat Rev Micro. 2004;2:497–504. doi: 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]
- 61.Boyce BM, Lindsey BA, Clovis NB, et al. Additive effects of exogenous IL-12 supplementation and antibiotic treatment in infection prophylaxis. J Orthop Res. 2012;30:196–202. doi: 10.1002/jor.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Webster TJ, Patel AA, Rahaman MN, et al. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta biomater. 2012;8:4447–54. doi: 10.1016/j.actbio.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 63.Taylor EN, Webster TJ. Multifunctional magnetic nanoparticles for orthopedic and biofilm infections. Int J Nanotechn. 2010;8:21–35. [Google Scholar]
- 64.Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomed. 2011;6:1463–73. doi: 10.2147/IJN.S22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomed. 2012;7:2767–2781. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carter RR, Sun J, Jump RL. A Survey and Analysis of the American Public's Perceptions and Knowledge About Antibiotic Resistance. Open forum infectious diseases. 2016;3:ofw112. doi: 10.1093/ofid/ofw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolcott R, Costerton J, Raoult D, et al. The polymicrobial nature of biofilm infection. Clin Microbiol Infec. 2013;19:107–112. doi: 10.1111/j.1469-0691.2012.04001.x. [DOI] [PubMed] [Google Scholar]
- 68.Hall BB, Fitzgerald RH, Jr, Rosenblatt JE. Anaerobic osteomyelitis. J Bone Joint Surg Am. 1983;65:30–5. [PubMed] [Google Scholar]