SUMMARY
Several inherited metabolic disorders are associated with an accumulation of reactive acyl-CoA metabolites that can non-enzymatically react with lysine residues to modify proteins. While the role of acetylation is well-studied, the pathophysiological relevance of more recently discovered acyl modifications, including those found in inherited metabolic disorders, requires more investigation. We recently showed that sirtuin 4 (SIRT4) removes glutaryl, 3-hydroxy-3-methylglutaryl, 3-methylglutaryl, and 3-methylglutaconyl modifications from lysine residues. Thus, we used SIRT4 knockout mice, which can accumulate these novel post-translational modifications, as a model to investigate their physiological relevance. Since SIRT4 is localized to mitochondria and previous reports have shown SIRT4 influences metabolism, we thoroughly characterized glucose and lipid metabolism in male and female SIRT4KO mice across different genetic backgrounds. While only minor perturbations in overall lipid metabolism were observed, we found SIRT4KO mice consistently had elevated glucose- and leucine-stimulated insulin levels in vivo and developed accelerated age-induced insulin resistance. Importantly, elevated leucine-stimulated insulin levels in SIRT4KO mice were dependent upon genetic background since SIRT4KO mice on a C57BL/6NJ genetic background had elevated leucine-stimulated insulin levels but not SIRT4KO mice on the C57BL/6J background. Taken together, the data suggest that accumulation of acyl modifications on proteins in inherited metabolic disorders may contribute to the overall metabolic dysfunction seen in these patients.
Keywords: post-translational modifications, sirtuin, insulin resistance, insulin secretion, leucine, SIRT4, NNT, C57BL/6J, C57BL/6NJ, MGylation, MGcylation, HMGylation, acylation
INTRODUCTION
Inherited metabolic disorders of amino acid and fatty acid oxidation often result in an accumulation of reactive acyl-CoA species (Pougovkina et al., 2014). These reactive metabolites can non-enzymatically modify proteins and alter their functions (Wagner et al., 2017). Indeed, fibroblasts from patients with short-chain acyl-CoA dehydrogenase, propionyl-CoA carboxylase, and malonyl-CoA dehydrogenase deficiencies show strikingly elevated levels of lysine propionylation, butyrylation, and malonylation, respectively (Pougovkina et al., 2014). Furthermore, mice with defective glutaryl-CoA dehydrogenase, which converts glutaryl-CoA to crotonyl-CoA, show a large increase in protein glutarylation in the liver (Tan et al., 2014). Finally, a mouse model of 3-hydroxy-3-methylglutaryl-CoA lyase (HMGCL) deficiency shows elevated 3-hydroxy-3-methylglutarylation (HMGylation) of lysine residues in liver tissue (Wagner et al., 2017). Interestingly, liver tissue from HMGCL knockout mice also have elevated 3-methylglutaryl-CoA and 3-methylglutaconyl-CoA, with a corresponding increase in lysine 3-methylglutarylation (MGylation) and 3-methylglutaconylation (MGcylation) (Wagner et al., 2017). While the primary genetic defect in these inherited metabolic disorders likely contributes the most to their phenotypes, aberrant protein modification due to accumulated reactive acyl-CoA species could also contribute to the overall clinical presentation.
Compared to protein phosphorylation and acetylation, little is known about the biological effects of the more recently identified protein glutarylation, HMGylation, MGylation, and MGcylation. Previously, we found that glutarylation of carbamoyl phosphate synthase 1 (CPS1) can inhibit its activity, leading to impaired urea cycle activity, and elevated ammonia levels in HeLa cells (Tan et al., 2014). Glutarylation and HMGylation also inhibits malate dehydrogenase 2 (MDH2), a key enzyme in the tricarboxylic acid cycle (Wagner et al., 2017). Further, we found that increased acylation of methylcrotonyl-CoA carboxylase (MCCC) resulted in lower MCCC activity and impaired leucine metabolism (Anderson et al., 2017). Given the paucity of information in the literature regarding the physiological effects of these post-translational modifications (PTMs), we set out to further investigate the relevance of glutarylation, HMGylation, MGylation, and MGcylation in vivo.
We recently reported that sirtuin 4 (SIRT4) can target lysine glutarylation, HMGylation, MGylation, and MGcylation for removal, and mice lacking SIRT4 have elevations in these PTMs in liver tissue (Anderson et al., 2017). Thus, to further investigate the role of these PTMs in metabolic and organismal homeostasis, we characterized several physiological parameters in SIRT4 knockout mice. We focused on metabolic measurements since SIRT4 has consistently shown effects on glucose and lipid metabolism. For example, pancreatic beta-cell lines with Sirt4 knocked down (Ahuja et al., 2007) and islets isolated from SIRT4KO mice (Haigis et al., 2006) show elevated glucose-stimulated insulin secretion. Further, islets isolated from SIRT4KO mice also have elevated amino acid-stimulated insulin secretion (Haigis et al., 2006). Furthermore, several studies have shown that loss of SIRT4 is associated with an elevation in fatty acid oxidation genes (Laurent et al., 2013a; Laurent et al., 2013b; Nasrin et al., 2010). Interestingly, SIRT4KO mice are protected against diet-induced obesity but surprisingly still develop diet-induced insulin resistance (Laurent et al., 2013b). Given these observations, we set out to thoroughly characterize glucose and lipid metabolism in SIRT4KO mice. Most studies on SIRT4KO mice thus far have been limited to male mice with a 129S1/Sv genetic background, rather than the C57BL/6 background, which is more commonly used for metabolic studies. Therefore, we characterized both male and female SIRT4KO mice on a variety of genetic backgrounds to better define the role of SIRT4 in whole body glucose and lipid metabolism.
METHODS
Animals
SIRT4KO mice from the 129S1/Sv-Oca2+ Tyr+ Kitl+ strain were obtained from the Jackson Laboratory (#012756). These mice were then backcrossed for 3 generations onto the C57BL/6J (Jackson Laboratory, #000664) background to obtain SIRT4KO mice on a mixed genetic background (SIRT4KO/mixed). SIRT4KO mice on a pure C57BL/6J background (SIRT4KO/J) were generated by backcrossing the mixed background mice for another 7 generations onto the C57BL/6J background for a total of 10 generations. SIRT4KO mice on a C57BL/6NJ background (SIRT4KO/NJ) mice were generated by backcrossing the mixed background mice for another 4 generations on the C57BL/6J background and then 3 more generations to the closely related C57BL/6NJ mice (Jackson Laboratory, #005304) to re-introduce a functional Nnt gene. Mice were group-housed on a 12-hour light/dark cycle with free access to water and PicoLab Rodent Diet 20 (LabDiet #5053, St. Louis, MO). Age, sex, genotype, and number of animals used per study are provided in the appropriate figure legends. All in vivo procedures were performed on healthy animals in accordance with the Duke Institutional Animal Care and Use Program.
Body composition measurements
Fat mass and lean mass were measured by the University of Massachusetts Mouse Metabolic Phenotyping Center. Conscious mice were non-invasively assessed for fat and lean mass using 1H-MRS (Echo Medical Systems, Houston, TX).
Plasma Analytes
Mice were typically fasted for 6 hours starting at 8:30 am unless otherwise indicated. Blood samples were collected from the saphenous vein using heparinized capillary tubes and centrifuged at 4600 RCF for 9 minutes at 4 °C to obtain plasma. Plasma free fatty acids were measured using the Wako HR Series NEFA-HR(2) kit (Wako Diagnostics, #999-3469, #995-34791, #991-34891, #993-35191). Plasma glycerol and triglycerides were measured with the Serum Triglyceride Determination Kit (Sigma-Aldrich, #TR0100). Plasma β-hydroxybutyrate was measured using the Wako Autokit 3-HB (Wako Diagnostics, #417-73501, #413-73601). Plasma cholesterol was measured using the Wako Cholesterol E reagent (Wako Diagnostics, #999-02601). Lipoprotein profiles were generated by the Vanderbilt University Mouse Metabolic Phenotyping Center. Blood glucose was measured using a Nova Max Plus glucometer (Nova Diabetes Care) and plasma insulin levels were measured using the Stellux Chemi Rodent Insulin ELISA (Alpco, 80-INSMR-CH01).
Metabolic Assays
Lipid tolerance tests were performed by fasting the mice for 6 hours starting at 8:00 am and then orally gavaging the mice with 5 μL/g extra virgin olive oil. Plasma samples were collected over the next 4 hours for triglyceride measurements. Glucose and leucine tolerance tests were performed by fasting the mice for 6 hours starting at 8:30 am and then orally gavaging the mice with 1.5 mg/g D-glucose or 0.3 mg/g L-leucine respectively. Blood glucose was measured at regular intervals over the next 2 hours. Plasma samples were obtained at 0, 7, 15, and 60 minutes post-gavage for insulin measurements. Insulin tolerance tests were performed by first fasting the mice for 6 hours starting at 8:30 am and then injecting insulin (Humulin R U-100, Lilly, #0002-8215-17) intraperitoneally. Blood glucose was measured at regular intervals over the next 2 hours.
Islet perifusion
Islet perifusions were performed as described (Luciani et al., 2007). Notably, circulating free fatty acids can affect glucose-stimulated insulin secretion (Dobbins et al., 1998; Dobbins et al., 2002), which can alter the way islets respond to glucose in the fed versus fasted state (Stein et al., 1996). To help control for these effects, all of the islets isolated for our study were collected from non-fasted male mice at the beginning of the light cycle. To further minimize any differences in physiological conditions at the time of islet harvest, all islets were cultured overnight in RPMI 1640 (Sigma, 11875) with 10% FBS and penicillin/streptomycin and then pre-incubated in Krebs-Ringer buffer containing 3 mM glucose for 1 hour prior to perifusion. Between nutrient stimulations, islets were washed with Krebs-Ringer buffer.
Statistics
Two-way ANOVAs with age/time, genotype, and the interaction between age/time and genotype as the factors tested were performed using the GraphPad Prism software. P values less than 0.05 indicating the effect of genotype by two-way ANOVA are indicated in the figures. Asterisks indicate p<0.05 between wild-type and SIRT4KO by a Bonferroni multiple comparisons test.
RESULTS
Prior studies on SIRT4KO mice were performed on largely male mice originating from the 129S1/Sv-Oca2+ Tyr+ Kitl+ strain. However, since mice on a C57BL/6 background are considered to be more susceptible to metabolic perturbations, we backcrossed these SIRT4KO mice onto a C57BL/6J background for three generations. By performing a genome scan for SNPs, we found that out of 256 SNPs tested, our SIRT4KO mice matched 86.5% of the C57BL/6J reference SNPs. When comparing to SNPs from 129S1/SvImJ mice, the recommended control from Jackson Laboratory, our SIRT4KO mice matched only 41.2% of the SNPs tested. Thus, these SIRT4KO mice were considered to be on a mixed genetic background (SIRT4KO/mixed) but more closely related to C57BL/6J mice than 129S1/SvImJ mice.
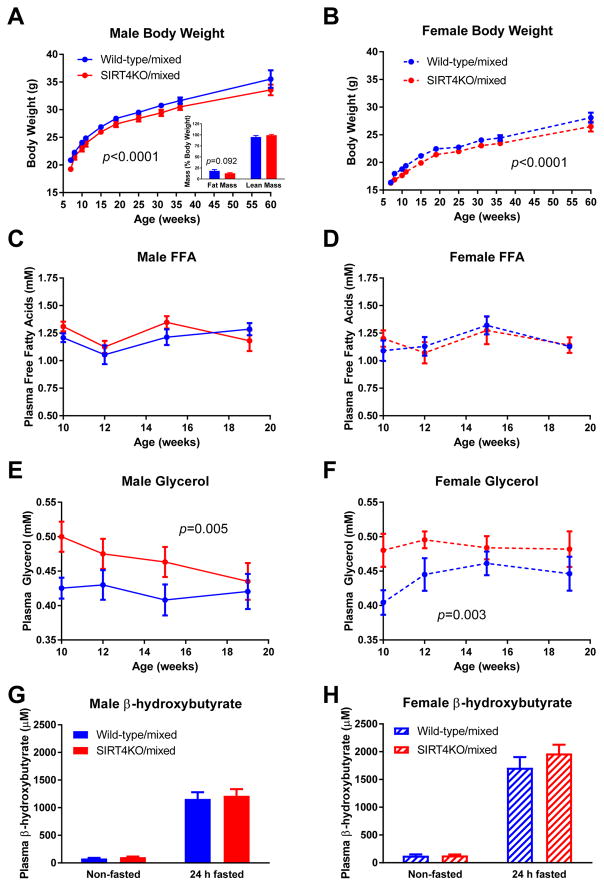
Our comprehensive metabolic characterization of SIRT4KO/mixed mice began with body weight measurements in male and female mice. Both SIRT4KO/mixed male and female mice consistently weighed an average of 4% less than their littermate controls from 7 weeks of age through over 1 year of age (Figure 1A–B). In males, reduced body weight was associated with a trend for reduced fat mass in SIRT4KO/mixed mice compared to wild-type controls (Figure 1A, inset). These findings are consistent with prior studies showing elevated fat oxidation in primary hepatocytes isolated from SIRT4KO mice (Laurent et al., 2013a) and reduced lipogenesis in white adipose tissue from SIRT4KO mice (Laurent et al., 2013b). To further characterize lipid metabolism, we measured circulating lipid levels in SIRT4KO/mixed mice in the postabsorptive state after a 6-hour fast. We found no differences in plasma free fatty acids in male or female SIRT4KO/mixed male mice (Figure 1C–D). Interestingly, both male and female mice with a loss of SIRT4 had elevated plasma glycerol levels, particularly in the younger ages tested (Figure 1E–F). Plasma β-hydroxybutyrate levels were not affected by a loss of SIRT4 in male or female mice in the non-fasted or 24-hour fasted state (Figure 1G–H).
Fig. 1.
Mixed background SIRT4KO mice have decreased body weight. (A) Male (n≥12/10 wild-type/SIRT4KO at each time point) and (B) female (n≥8/12 wild-type/SIRT4KO at each time point) SIRT4KO mice were weighed at various time points over more than a year. (A, inset) Percent fat mass and lean mass were measured at 60 weeks of age (n=11/10 wild-type/SIRT4KO). Mice were fasted for 5–6 hours and then plasma samples collected for free fatty acid and glycerol measurements at various ages in male (C and E; n=13/8) and female (D and F; n=7/10) SIRT4KO mice. Plasma β-hydroxybutyrate levels were assessed in non-fasted and 24-hour fasted male (G; n=12/10) and female (H; n=9/12) SIRT4KO mice. Closed blue and red circles with a solid line indicate male wild-type and SIRT4KO mice on a mixed genetic background. Closed blue and red circles with a dashed line indicate female wild-type and SIRT4KO mice on a mixed genetic background. Data are shown as average±SEM. P values less than 0.05 by two-way ANOVA are indicated. Asterisks indicate p<0.05 between wild-type and SIRT4KO by Bonferroni’s multiple comparisons test.
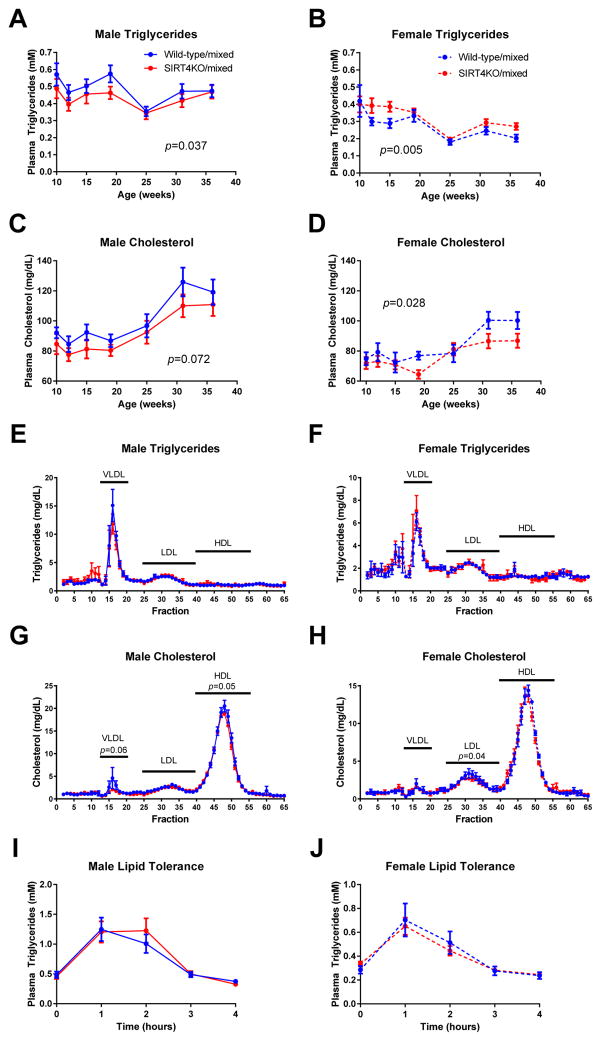
We next assessed plasma triglycerides and cholesterol levels over time. After a 6-hour fast, male SIRT4KO/mixed mice had slightly lower plasma triglycerides but this reduction was normalized over time (Figure 2A). In contrast, triglycerides in female SIRT4KO/mixed mice were subtly higher than littermate controls over time (Figure 2B). Interestingly, we observed a trend towards lower plasma cholesterol levels in both male and female SIRT4KO/mixed mice compared to wild-type controls (Figure 2C–D). Given the subtle changes in triglyceride and cholesterol levels seen when measuring these lipids in whole plasma, we used a more sensitive assay by fractionating the plasma and generating a lipoprotein profile on male and female SIRT4KO mice. Consistent with the measurements made in whole plasma, the lipoprotein profiles indicate that indeed the differences in triglycerides and cholesterol between wild-type and SIRT4KO mice are subtle. Measuring triglycerides in fractionated plasma revealed that SIRT4 has little effect on triglycerides incorporated into very low density lipoprotein (VLDL) particles, low density lipoprotein (LDL) particles, or high density lipoprotein (HDL) particles (Figure 2E–F). Similar to the cholesterol measurements in whole plasma, cholesterol measurements in fractionated plasma indicate that loss of SIRT4 leads to a small decrease in plasma cholesterol in both males and females. However, males had decreased HDL-cholesterol and females had decreased LDL-cholesterol (Figure 2G–H). In addition to these measurements in the 6-hour fasted, postabsorptive state, we also assessed postprandial triglyceride clearance by orally administering a lipid bolus and measuring triglyceride clearance over time. The lipid tolerance test showed that both male and female SIRT4KO/mixed mice had normal triglyceride clearance (Figure 2I–J). Collectively, these data reveal an interesting sexual dimorphism in plasma lipids in SIRT4KO/mixed mice, whereby SIRT4KO/mixed male mice had slightly lower and females had slightly higher plasma triglycerides and males had lower HDL-cholesterol whereas females had lower LDL-cholesterol. Overall, however, the effects of SIRT4 on plasma lipid levels in both male and female SIRT4KO/mixed mice are subtle.
Fig. 2.
Mixed background SIRT4KO mice have subtly decreased plasma lipids. Mice were fasted for 5–6 hours and then plasma collected for triglyceride measurements in male (A; n≥13/8 wild-type/SIRT4KO) and female (B; n≥7/10 wild-type/SIRT4KO) mice. Plasma cholesterol was measured in male (C; n≥10/5 wild-type/SIRT4KO) and female (D; n≥4/9 wild-type/SIRT4KO) SIRT4KO mice after a 5–6 hour fast. Lipoprotein profiles were generated by fractionating pooled plasma samples collected from 6-hour fasted mice. Data are the average of 3 pools of plasma each containing equal volumes of plasma from at least 5 mice. Triglycerides and cholesterol were measured in each fraction from male (E and G) and female (F and H) SIRT4KO mice. Oral lipid tolerance tests were performed in male (I; n=17/17 wild-type/SIRT4KO) and female (J; n=13/19 wild-type/SIRT4KO) SIRT4KO mice and wild-type controls by fasting the mice for 5–6 hours and then gavaging them with 5 μL/g extra virgin olive oil. Plasma triglycerides were measured every hour for the next 4 hours. Closed blue and red circles with a solid line indicate male wild-type and SIRT4KO mice on a mixed genetic background. Closed blue and red circles with a dashed line indicate female wild-type and SIRT4KO mice on a mixed genetic background. Data are shown as average±SEM. P values less than 0.05 by two-way ANOVA are indicated. Asterisks indicate p<0.05 between wild-type and SIRT4KO by Bonferroni’s multiple comparisons test.
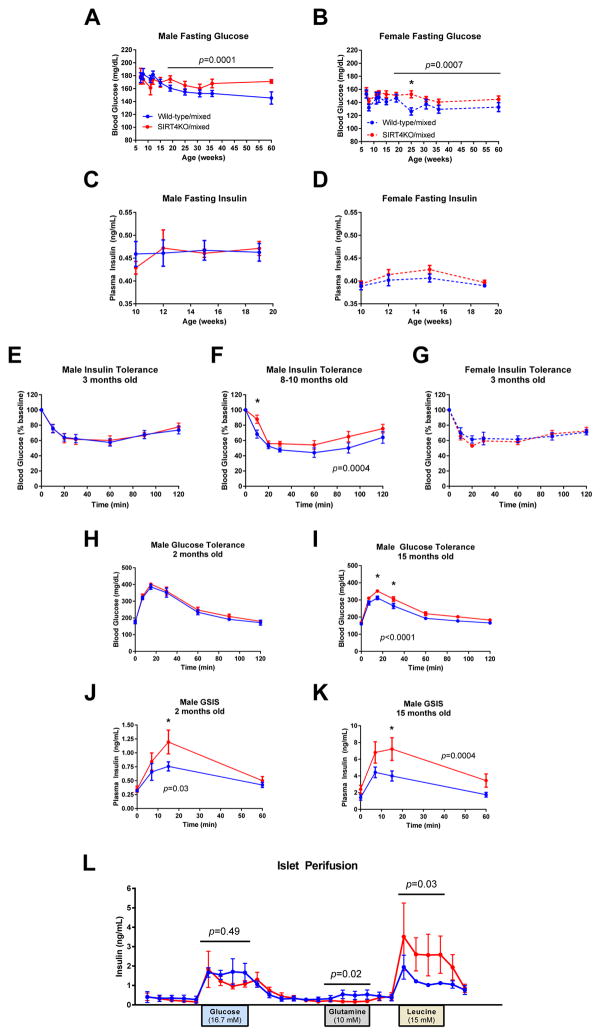
We next assessed glucose metabolism in these SIRT4KO mice on a mixed genetic background. In both male and female SIRT4KO/mixed mice, we observed no difference in 6-hour fasted blood glucose levels in mice younger than 15 weeks of age. However, as these mice aged past 15 weeks, the SIRT4KO/mixed mice had higher blood glucose levels compared to littermate controls (Figure 3A–B). Plasma insulin levels were unchanged in young male and female SIRT4KO/mixed mice (Figure 3C–D). In contrast, when aged to 15 months old, SIRT4KO/mixed males had higher fasting insulin levels than their wild-type controls (Figure 3K, t=0). These data suggest that SIRT4KO/mixed mice may develop age-induced insulin resistance faster than their wild-type controls. Indeed, 3-month old SIRT4KO/mixed male and female mice had normal insulin tolerance (Figure 3E and 3G), but when males were aged to 8 months of age, they developed insulin resistance (Figure 3F). Consistent with this, when young 9-week old SIRT4KO/mixed males were given an oral gavage of glucose, they had normal glucose tolerance (Figure 3H). However, when SIRT4KO/mixed males aged, they developed glucose intolerance (Figure 3I). Interestingly, SIRT4KO/mixed males required higher insulin levels to maintain normal glucose levels during a glucose tolerance test. In young SIRT4KO/mixed males, the elevated glucose-stimulated insulin levels were able to maintain normal glucose tolerance (Figure 3H and 3J), but when SIRT4KO mice aged, they were unable to maintain normal glucose tolerance despite having elevated glucose-stimulated insulin levels (Figure 3I and 3K). Taken together, these data demonstrate that male SIRT4KO/mixed mice develop age-induced insulin resistance at a faster rate than their littermate controls.
Fig. 3.
Mixed background SIRT4KO mice develop accelerated age-induced insulin resistance. Blood glucose levels were measured in 5- to 6-hour fasted male (A; n>12/8 wild-type/SIRT4KO) and female (B; n>7/10 wild-type/SIRT4KO) mice. Plasma insulin levels were measured in 5- to 6-hour fasted male (C; n>12/8 wild-type/SIRT4KO) and female (D; n>7/10 wild-type/SIRT4KO) mice. Insulin tolerance was measured by intraperitoneally injecting 6-hour fasted mice with insulin and then monitoring blood glucose over the next 2 hours in 3-month-old male (E; 0.7 U/kg, n=14/14), 8- to 10-month-old male (F; 0.9 U/kg, n=14/14), and 3-month-old female (G; 0.6 U/kg, n=7/7) wild-type and SIRT4KO mice. Glucose tolerance and glucose-stimulated insulin levels were measured by orally gavaging 5- to 6-hour fasted mice with 1.5 mg/g glucose and then monitoring blood glucose and plasma insulin over the next 2 hours in 2-month-old male (H and J; n=8/8) and 15-month-old male (I and K; n=16/15) wild-type and SIRT4KO mice. (L) Pancreatic islets were isolated from 18-month-old wild-type and SIRT4KO mice and then 100 islets from each mouse were subject to a perifusion. Islets were washed with Krebs-Ringer buffer containing 3 mM glucose in between nutrient stimulations (n=4/4 wild-type/SIRT4KO). Closed blue and red circles with a solid line indicate male wild-type and SIRT4KO mice on a mixed genetic background. Closed blue and red circles with a dashed line indicate female wild-type and SIRT4KO mice on a mixed genetic background. Data are shown as average±SEM. P values less than 0.05 by two-way ANOVA are indicated. Asterisks indicate p<0.05 between wild-type and SIRT4KO by Bonferroni’s multiple comparisons test.
To determine if elevated glucose-stimulated insulin levels in SIRT4KO/mixed mice could be due to a direct effect of SIRT4 on insulin secretion from pancreatic islets, we isolated islets from SIRT4KO/mixed and wild-type control mice and performed islet perifusion studies. In contrast to previous studies conducted on SIRT4KO mice on pure genetic backgrounds (Anderson et al., 2017; Haigis et al., 2006), we did not observe higher glucose-stimulated insulin secretion from SIRT4KO/mixed islets ex vivo (Figure 3L). Since islets isolated from SIRT4KO mice on a 129/Sv background have been reported to show elevated amino acid-stimulated insulin secretion (Haigis et al., 2006), we also tested glutamine and leucine-stimulated insulin secretion in islets isolated from our SIRT4KO mice on a mixed genetic background. Unlike previous reports (Haigis et al., 2006), we did not observe enhanced glutamine-stimulated insulin secretion (Figure 3L). However, we observed a potent increase in leucine-stimulated insulin secretion in our SIRT4KO/mixed islets (Figure 3L), which is consistent with other studies done on isolated islets regardless of genetic background (Anderson et al., 2017; Haigis et al., 2006). Thus, it appears that the genetic background of SIRT4KO mice can influence the effect of SIRT4 on insulin secretion.
Since genetic background appeared to be important for the effects of SIRT4 on glucose metabolism, we further backcrossed the mixed background SIRT4KO mice onto a pure C57BL/6J background, a commonly used strain for metabolic studies. During the course of our backcrossing, a peptide-binding study reported that SIRT4 has the potential to bind to nicotinamide nucleotide transhydrogenase (NNT) (Rauh et al., 2013). NNT is a protein on the inner mitochondrial membrane that couples translocation of protons across the inner membrane to the interconversion of NADH and NADPH. Nnt is a gene known to be mutated in C57BL/6J mice and can exert effects on body weight and glucose metabolism (Fisher-Wellman et al., 2016; Freeman et al., 2006; Nicholson et al., 2010). After just 3 generations of backcrossing to the C57BL/6J background, our mixed background SIRT4KO mice all expressed the mutated version of Nnt (data not shown). However, to be thorough, we fully backcrossed our SIRT4KO/mixed mice to the C57BL/6J background and then also crossed these mice to the closely related C57BL/6NJ mice, which express functional Nnt. This allowed us to compare the effects of SIRT4 on glucose metabolism in C57BL/6 mice with (SIRT4KO/NJ) or without (SIRT4KO/J) functional NNT.
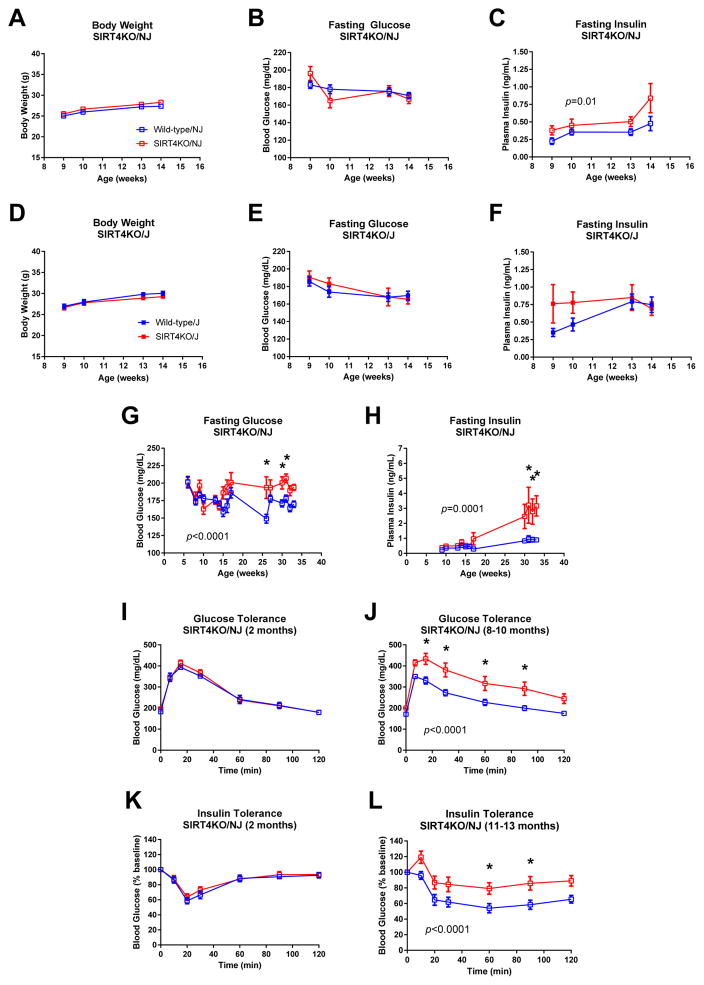
We first measured body weight in SIRT4KO/NJ and SIRT4KO/J males compared to their respective littermate controls. Since SIRT4KO/mixed males showed a robust difference in glucose metabolism, we focused our studies on male mice. Unlike mixed background SIRT4KO mice (Figure 1A), SIRT4KO males on a pure C57BL/6NJ or C57BL/6J background showed no differences in body weight compared to their wild-type controls (Figure 4A and 4D). After a 6-hour fast, blood glucose levels also were not different between male SIRT4KO/NJ and SIRT4KO/J compared to their respective wild-type controls (Figure 4B and 4E). Notably, plasma insulin levels were consistently higher in SIRT4KO/NJ mice compared to controls (Figure 4C). Conversely, while SIRT4KO/J mice had hyperinsulinemia early in life, this effect was normalized by 13 weeks of age (Figure 4F). Thus, the presence of functional NNT appears to worsen fasting hyperinsulinemia in male SIRT4KO/NJ mice.
Fig. 4.
SIRT4KO mice on a pure C57BL/6NJ background develop accelerated age-induced insulin resistance. Body weight was measured in SIRT4KO/NJ (A; n≥9/11 wild-type/SIRT4KO) and SIRT4KO/J (D; n≥13/7 wild-type/SIRT4KO) mice and their respective littermate controls. Blood glucose levels were measured in 5- to 6-hour fasted SIRT4KO/NJ (B; n≥9/11 wild-type/SIRT4KO) and SIRT4KO/J (E; n≥13/7 wild-type/SIRT4KO) mice and their respective littermate controls. Plasma insulin levels were measured in 5- to 6-hour fasted SIRT4KO/NJ (C; n=9/11 wild-type/SIRT4KO) and SIRT4KO/J (F; n=13/7 wild-type/SIRT4KO) mice and their respective littermate controls. Blood glucose (G; n≥6/4 wild-type/SIRT4KO) and plasma insulin (H; n≥6/4 wild-type/SIRT4KO) was measured in male SIRT4KO/NJ mice fasted for 5–6 hours. Glucose tolerance was measured by orally gavaging 5- to 6-hour fasted mice with 1.5 mg/g glucose and then monitoring blood glucose over the next 2 hours in 2-month-old (I; n=9/11) and 8- to 10-month-old (J; n=11/11) male wild-type and SIRT4KO/NJ mice. Insulin tolerance was measured by intraperitoneally injecting 5- to 6-hour fasted mice with insulin and then monitoring blood glucose over the next 2 hours in 2-month-old (K; 1 U/kg, n=9/5) and 11- to 13-month-old (L; 1.4 U/kg, n=13/11) male wild-type and SIRT4KO/NJ mice. Panels G–L were reproduced with permission from Anderson and Huynh et al. (Anderson et al., 2017). Open blue and red squares indicate male wild-type and SIRT4KO mice on a C57BL/6NJ background. Closed blue and red squares indicate male wild-type and SIRT4KO mice on a C57BL/6J background. Data are shown as average±SEM. P values less than 0.05 by two-way ANOVA are indicated. Asterisks indicate p<0.05 between wild-type and SIRT4KO by Bonferroni’s multiple comparisons test.
Since male SIRT4KO/NJ mice had consistent hyperinsulinemia, we continued characterizing glucose metabolism in these mice to determine the consequences of chronically elevated insulin levels. Male SIRT4KO/NJ mice developed hyperglycemia at older ages (Figure 4G), similar to the SIRT4KO/mixed mice (Figure 3A). Further, the hyperinsulinemia seen in young SIRT4KO/NJ mice was also exacerbated with age (Figure 4H). Similar to SIRT4KO/mixed males (Figure 3H), SIRT4KO/NJ males had normal glucose tolerance at 2 months of age (Figure 4I) but developed glucose intolerance by 8 months of age (Figure 4J). Accordingly, SIRT4KO/NJ males had normal insulin sensitivity at 2 months of age (Figure 4K) and were insulin resistant by 10 months of age (Figure 4L). Collectively, these data show that male SIRT4KO mice on a mixed genetic background and male SIRT4KO mice on a pure C57BL/6NJ background both develop accelerated age-induced insulin resistance.
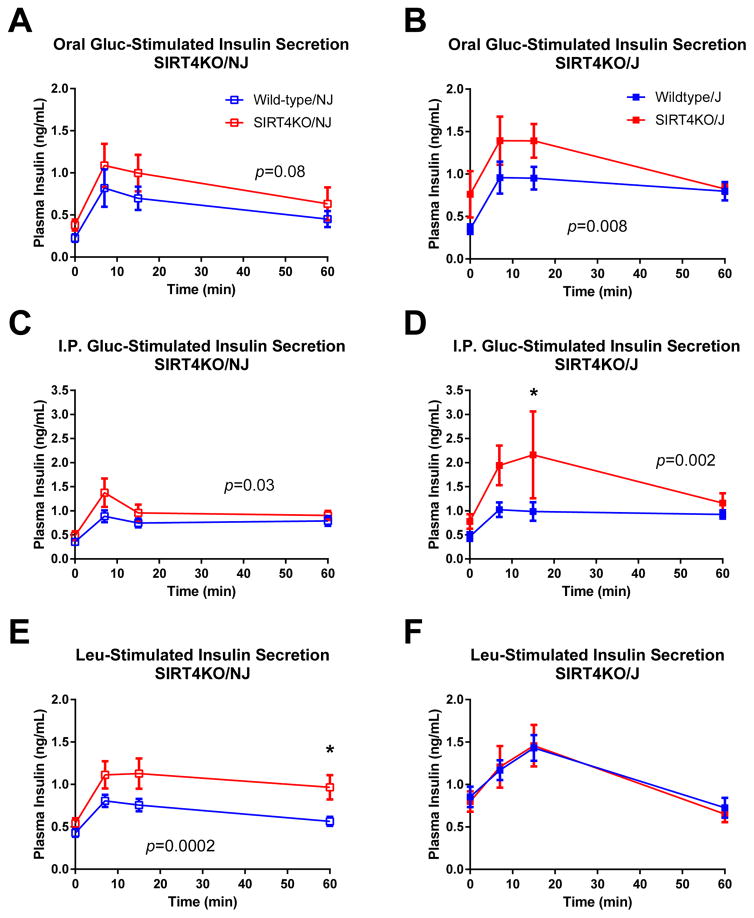
We next assessed insulin secretion in SIRT4KO/NJ and SIRT4KO/J mice. After an oral gavage of glucose, both SIRT4KO/NJ and SIRT4KO/J males had elevated glucose-stimulated insulin levels (Figure 5A–B). Since it has been reported that NNT may alter the incretin effect on insulin secretion (Fergusson et al., 2014), we also measured glucose-stimulated insulin levels after an intraperitoneal (IP) injection of glucose in order to bypass the incretin effect. Similar to the mice given oral glucose, male SIRT4KO/NJ and SIRT4KO/J mice displayed higher glucose-stimulated insulin levels after intraperitoneal glucose compared to controls (Figure 5C–D). Taken together, the data show that elevated glucose-stimulated insulin levels in SIRT4KO males are independent of genetic background, Nnt status, or the incretin effect.
Fig. 5.
Leucine-stimulated insulin levels are elevated in SIRT4KO/NJ mice but not SIRT4KO/J mice. Oral glucose-stimulated insulin levels were measured by gavaging 5- to 6-hour fasted mice with 1.5 mg/g glucose and then monitoring plasma insulin over the next 2 hours in 2-month-old male SIRT4KO/NJ (A; n=9/11 wild-type/SIRT4KO) and SIRT4KO/J (B; n=13/7 wild-type/SIRT4KO). Intraperitoneal glucose-stimulated insulin levels were measured by intraperitoneally injecting 5- to 6-hour fasted mice with 1.5 mg/g glucose and then monitoring plasma insulin over the next 2 hours in 2-month-old male SIRT4KO/NJ (C; n=9/10 wild-type/SIRT4KO) and SIRT4KO/J (D; n=13/7 wild-type/SIRT4KO). Oral leucine-stimulated insulin levels were measured by gavaging 5- to 6-hour fasted mice with 0.3 mg/g leucine and then monitoring plasma insulin over the next 2 hours in 4-month-old male SIRT4KO/NJ (E; n=15/15 wild-type/SIRT4KO) and SIRT4KO/J (F; n=18/12 wild-type/SIRT4KO). Panels A and E were reproduced with permission from Anderson and Huynh et al. (Anderson et al., 2017). Open blue and red squares indicate male wild-type and SIRT4KO mice on a C57BL/6NJ background. Closed blue and red squares indicate male wild-type and SIRT4KO mice on a C57BL/6J background. Data are shown as average±SEM. P values less than 0.05 by two-way ANOVA are indicated. Asterisks indicate p<0.05 between wild-type and SIRT4KO by Bonferroni’s multiple comparisons test.
Given the robust effect that leucine had on insulin secretion in islets isolated from male SIRT4KO mice on a mixed background (Figure 3L), we tested whether leucine could stimulate insulin secretion in vivo in SIRT4KO/NJ and SIRT4KO/J mice. Leucine-stimulated insulin levels were elevated in male SIRT4KO/NJ compared to their wild-type controls (Figure 5E). Interestingly, leucine-stimulated insulin levels were clearly not different between male SIRT4KO/J mice and their controls, which lack functional NNT (Figure 5F). Therefore, elevated leucine-stimulated insulin levels in SIRT4KO mice in vivo are dependent upon genetic background and potentially functional NNT.
DISCUSSION
SIRT4 has recently been shown to target several novel PTMs, including glutarylation, HMGylation, MGylation, and MGcylation (Anderson et al., 2017), which have been shown to accumulate in several mouse models of inherited metabolic disorders (Tan et al., 2014; Wagner et al., 2017). Since mice with a lack of SIRT4 also show increases in these PTMs and these PTMs have been identified on metabolic enzymes (Anderson et al., 2017), we explored the possibility that an accumulation of these PTMs could affect glucose and lipid metabolism in SIRT4KO mice. We measured an array of metabolic parameters in SIRT4KO mice on three different genetic backgrounds and, in many cases, measurements were done on both male and female mice. Given the notion that sirtuins may be involved in the aging process, many of our studies were performed over time as the mice aged. Notably, few metabolic studies have reported data from SIRT4KO mice on a C57BL/6 background, which is the most common genetic background used to study glucose and lipid metabolism in mice. Overall, we found that lipid metabolism was only subtly affected by SIRT4, but glucose metabolism was dysregulated and SIRT4KO mice consistently developed accelerated age-induced insulin resistance across different genetic backgrounds.
Previous metabolic studies on SIRT4 suggest that lipid metabolism is a major target of SIRT4. Several studies have shown that loss of SIRT4 activates pathways involved in fatty acid oxidation (Ho et al., 2013; Laurent et al., 2013a; Laurent et al., 2013b; Nasrin et al., 2010). Functionally, knockdown of SIRT4 led to elevated fatty acid oxidation in primary myotubes (Nasrin et al., 2010) and increased fatty acid oxidation in primary hepatocytes isolated from SIRT4KO mice (Laurent et al., 2013a). Further, SIRT4KO mice on a 129/Sv background have a decreased respiratory exchange ratio during and after exercise, suggesting higher overall lipid oxidation (Laurent et al., 2013b). Lipogenesis and fat mass in white adipose tissue were also decreased in SIRT4KO mice (Laurent et al., 2013b). Despite these reported effects of SIRT4 on lipid metabolism, our data here show that overall lipid metabolism is only subtly altered in SIRT4KO mice. While we observed a slight sexual dimorphism between male and female mice, we did not observe any striking differences in circulating triglycerides, cholesterol, or β-hydroxybutyrate, and postprandial triglyceride clearance was also unchanged. Similar to previous reports on 129/Sv SIRT4KO mice (Laurent et al., 2013b), we observed a trend towards decreased fat mass in our mixed background SIRT4KO mice. These SIRT4KO/mixed mice also displayed a slight decrease in body weight, but importantly, food intake and energy expenditure were not measured in these mice and this will be important for future studies. Nevertheless, this reduction in body weight in SIRT4KO/mixed mice seems to depend on the 129/Sv genetic background, since our SIRT4KO/NJ and SIRT4KO/J mice did not have differences in body weight. Therefore, we conclude that loss of SIRT4 does not have a major effect on overall lipid metabolism in mice.
We observed striking and consistent effects of SIRT4 on glucose metabolism. Recently, we reported that SIRT4KO mice on a pure C57BL/6NJ background exhibit accelerated age-induced fasting hyperinsulinemia, fasting hyperglycemia, glucose intolerance, and insulin resistance (Anderson et al., 2017; N.B. some data are reprinted with permission in Figures 4 and 5 for ease of comparison). Consistent with these previous observations, we now show that SIRT4KO mice on a mixed background also display this same acceleration of age-induced insulin resistance. Thus, regardless of genetic background, loss of SIRT4 promotes the development of insulin resistance with aging. These data are in accordance with the notion that sirtuins play a role in preventing diseases associated with aging (Anderson et al., 2014; McDonnell et al., 2015; Nakagawa and Guarente, 2014; Watroba and Szukiewicz, 2016).
Despite the consistent effect of SIRT4 on the development of insulin resistance across genetic backgrounds, the mechanism by which SIRT4KO mice develop age-related insulin resistance may differ. We found that loss of SIRT4 led to elevated glucose-stimulated insulin levels in vivo regardless of genetic background. However, islets isolated from SIRT4KO/mixed mice did not show elevated glucose-stimulated insulin secretion ex vivo, whereas we previously showed that islets from SIRT4KO/NJ mice did (Anderson et al., 2017). Therefore, elevated in vivo glucose-stimulated insulin levels in SIRT4KO/mixed mice may be due to lower insulin clearance rather than higher insulin secretion. Further, SIRT4KO/NJ mice had increased leucine-stimulated insulin levels in vivo, while SIRT4KO/J mice clearly did not. This could perhaps explain why SIRT4KO/NJ mice had sustained hyperinsulinemia throughout life and SIRT4KO/J mice did not. Importantly, this suggests that functional NNT could play a role in leucine-stimulated insulin levels in vivo and that SIRT4 may regulate this function of NNT. While NNT has not been found to be modified by lysine glutarylation, HMGylation, MGylation, or MGcylation, SIRT4 deacetylation activity was found against a peptide substrate representing Lys-397 of NNT (Rauh et al., 2013). While these data suggest SIRT4 might directly deacylate and potentially modulate NNT activity, more rigorous studies are needed to test this hypothesis. When considered with a prior study using SIRT4KO mice on a pure 129/Sv background (Haigis et al., 2006), which express functional NNT, we conclude that NNT is required for loss of SIRT4 to produce both increased glucose and leucine-stimulated insulin secretion.
We recently reported that ablation of SIRT4 in C57BL/6NJ mice led to a build-up of novel PTMs that resulted in impaired leucine metabolism and likely contributed to elevated leucine-stimulated insulin secretion (Anderson et al., 2017). Since this preceded insulin resistance in these mice, we hypothesize that inappropriate hyperinsulinemia contributes to the development of insulin resistance in these SIRT4KO/NJ mice. This is consistent with previous studies showing that hyperinsulinemia can drive the development of obesity and diabetes (Attane et al., 2016; Gray et al., 2010; Mehran et al., 2012; Rajan et al., 2016). It would be interesting now to see what role NNT might play in this. Given the fact that SIRT4KO/J mice did not show elevated leucine-stimulated insulin levels in vivo, it would be intriguing to monitor SIRT4KO/J mice at older ages to see if they develop milder insulin resistance with aging than their SIRT4KO/NJ counterparts.
While our study suggests that NNT status is likely a key difference between SIRT4KO/J and SIRT4KO/NJ mice that accounts for the metabolic differences between these mice, other genetic differences are known between C57BL/6J and C57BL/6NJ mice. Aside from differences in Nnt, the Jackson Laboratory also reports that C57BL/6NJ mice have a missense mutation in the Cyfip2 gene, which encodes cytoplasmic fragile X mental retardation 1 interacting protein 2 (https://www.jax.org/strain/005304). Further, the Jackson Laboratory reports five additional SNP differences: three are not located in known genes and two result in intronic variants in the myotubularin related protein 3 (Mtmr3) and neural precursor cell expressed, developmentally down-regulated gene 9 (Nedd9) genes respectively. Of these genes, only Nnt has been shown to influence insulin secretion and glucose metabolism (Fisher-Wellman et al., 2016; Fontaine and Davis, 2016; Toye et al., 2005) and only NNT has been shown to potentially interact with SIRT4 (Rauh et al., 2013). Thus, it is most likely that the different NNT status between SIRT4KO/J and SIRT4KO/NJ mice is responsible for the metabolic differences that we observed. However, little is known about the metabolic effects of the other genes mentioned above and it is possible that these other genes could contribute to differences between SIRT4KO/J and SIRT4KO/NJ mice.
We attribute the metabolic differences between SIRT4KO and wild-type mice mainly to an accumulation of SIRT4-targeted PTMs in SIRT4KO mice. Indeed, we previously reported elevated PTMs on a specific protein in the leucine oxidation pathway, methylcrotonyl-CoA carboxylase (MCCC), in SIRT4KO/NJ liver tissue. This corresponded with decreased MCCC activity and reduced flux through leucine oxidation in liver mitochondria (Anderson et al., 2017). Since SIRT4 is expressed across several metabolic tissues, we predict that SIRT4-targeted PTMs accumulate in many tissues in SIRT4KO mice, and indeed, we observe decreased leucine flux in heart mitochondria as well. However, to solidify the role of MGylation, MGcylation, and HMGylation on metabolic regulation, future studies should be focused on directly measuring these SIRT4-targeted PTM levels in other metabolic tissues, particularly pancreatic islets. Furthermore, SIRT4 may have metabolic effects independent of its deacylation activities. For example, SIRT4 was reported to bind to SIRT3 and prevent SIRT3 from binding to its substrates (Luo et al., 2017). Thus, more studies are needed to better understand the role of SIRT4 in regulating metabolic processes.
These studies on SIRT4KO mice on different genetic backgrounds underscore the important effects that genetic background can have on metabolic phenotypes. However, regardless of the differences in glucose metabolism due to genetic background, our extensive characterization of metabolism in SIRT4KO mice shows that, on a whole, loss of SIRT4 leads to inappropriately elevated insulin secretion and accelerated insulin resistance over time. These studies further emphasize the idea that sirtuins protect against the diseases of aging.
Our data also suggest that the novel PTMs targeted by SIRT4 could play a role in regulating glucose metabolism in inherited metabolic disorders and contribute to overall metabolic dysfunction. Although human tissues have not been tested yet, elevated hepatic glutarylation is observed in a mouse model of GCDH deficiency (Tan et al., 2014) and more MGylation, MGcylation, and HMGylation is seen in liver tissue from a mouse model of HMGCL deficiency (Wagner et al., 2017). Patients with either GCDH deficiency (Zinnanti et al., 2007) or HMGCL deficiency (Gibson et al., 1988) both present with hypoglycemia. While we have not been able to find information on insulin secretion in these patients, there is the potential for HMGCL deficiency to affect insulin secretion, which may contribute to hypoglycemia. Out of seven tissues tested, HMGCL expression and activity was found to be second highest in human pancreatic tissue (Puisac et al., 2010). Thus, with deficient HMGCL, it is possible that PTMs accumulate on pancreatic proteins and cause elevated insulin secretion, contributing to hypoglycemia. In addition, NNT mutations can lead to familial glucocorticoid deficiency in humans (Meimaridou et al., 2012). Similar to C57BL/6J mice with NNT loss of function mutations, patients with familial glucocorticoid deficiency caused by NNT mutations also have reduced insulin levels, developing hypoinsulinemia (Gupta et al., 2011) and in some cases even progressing to insulin-dependent diabetes (Scott et al., 2017). SIRT4 has the potential to bind NNT (Rauh et al., 2013) and our data show that mice with a loss of SIRT4 develop increased insulin secretion, which appears to be dependent on having functional NNT. Taken together, these observations suggest a role for SIRT4 to negatively regulate NNT activity, which in turn decreases insulin secretion. It would be interesting now to explore whether SIRT4 can contribute to the phenotypes seen in familial glucocorticoid deficiency. Collectively, our data suggest a novel role for SIRT4 and the PTMs it targets in contributing to metabolic dysfunction seen in several different inherited metabolic disorders.
SYNOPSIS.
Loss of sirtuin 4 in mice leads to elevated glucose- and leucine-stimulated insulin levels in vivoand accelerated age-induced insulin resistance across different genetic backgrounds.
Acknowledgments
We would like to thank Jason Kim at the University of Massachusetts Mouse Metabolic Phenotyping Center (supported by NIH grant 2U2C-DK093000) for providing body composition measurements and Carla Harris at the Vanderbilt University Mouse Metabolic Phenotyping Center (supported by grant U24-DK059637) for generating the lipoprotein profiles. We would also like to acknowledge funding support from the American Heart Association grants 12SDG8840004 and 12IRG9010008 (MDH), The Ellison Medical Foundation (MDH), the National Institutes of Health and the NIA grant R01AG045351 and R56AG052568 (MDH), and the Duke Pepper Older Americans Independence Center (OAIC) Program in Aging Research supported by the National Institute of Aging (P30AG028716-01). The work was partially supported by a Canadian Institutes for Health Research grant to JDJ. FKH was supported by a Canadian Diabetes Association/American Diabetes Association Post-doctoral Fellowship (PF-3-13-4342-FH). The funding sources had no role in the conduct or presentation of this research.
Footnotes
COMPLIANCE WITH ETHICS GUIDELINES
Conflict of Interest
Frank K. Huynh, Xiaoke Hu, Zhihong Lin, James D. Johnson, and Matthew D. Hirschey declare that they have no conflicts of interest.
Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed. Animal studies were approved by the Duke Institutional Animal Care and Use Program (Protocol #A091-17-04).
Author Contributions
Conceptualization, F.K.H. and M.D.H.; Investigation, F.K.H., X.H., and Z.L.; Writing - Original Draft, F.K.H.; Writing - Review & Editing, All authors; Supervision, J.D.J. and M.D.H.; Project Administration, M.D.H.; Funding Acquisition, M.D.H.
References
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Green MF, Huynh FK, Wagner GR, Hirschey MD. SnapShot: Mammalian Sirtuins. Cell. 2014;159:956–956. e951. doi: 10.1016/j.cell.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Huynh FK, Fisher-Wellman K, Stuart JD, Peterson BS, Douros JD, Wagner GR, Thompson JW, Madsen AS, Green MF, et al. SIRT4 Is a Lysine Deacylase that Controls Leucine Metabolism and Insulin Secretion. Cell Metab. 2017;25:838–855. doi: 10.1016/j.cmet.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attane C, Peyot ML, Lussier R, Poursharifi P, Zhao S, Zhang D, Morin J, Pineda M, Wang S, Dumortier O, et al. A beta cell ATGL-lipolysis/adipose tissue axis controls energy homeostasis and body weight via insulin secretion in mice. Diabetologia. 2016;59:2654–2663. doi: 10.1007/s00125-016-4105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson G, Ethier M, Guevremont M, Chretien C, Attane C, Joly E, Fioramonti X, Prentki M, Poitout V, Alquier T. Defective insulin secretory response to intravenous glucose in C57Bl/6J compared to C57Bl/6N mice. Mol Metab. 2014;3:848–854. doi: 10.1016/j.molmet.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Wellman KH, Ryan TE, Smith CD, Gilliam LA, Lin CT, Reese LR, Torres MJ, Neufer PD. A Direct Comparison of Metabolic Responses to High-Fat Diet in C57BL/6J and C57BL/6NJ Mice. Diabetes. 2016;65:3249–3261. doi: 10.2337/db16-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman H, Shimomura K, Cox RD, Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a link between insulin secretion, glucose metabolism and oxidative stress. Biochem Soc Trans. 2006;34:806–810. doi: 10.1042/BST0340806. [DOI] [PubMed] [Google Scholar]
- Gray SL, Donald C, Jetha A, Covey SD, Kieffer TJ. Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic beta-cell leptin signaling. Endocrinology. 2010;151:4178–4186. doi: 10.1210/en.2010-0102. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Ho L, Titus AS, Banerjee KK, George S, Lin W, Deota S, Saha AK, Nakamura K, Gut P, Verdin E, et al. SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging (Albany NY) 2013;5:835–849. doi: 10.18632/aging.100616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, de Boer VC, Finley LW, Sweeney M, Lu H, Schug TT, Cen Y, Jeong SM, Li X, Sauve AA, et al. SIRT4 represses peroxisome proliferator-activated receptor alpha activity to suppress hepatic fat oxidation. Mol Cell Biol. 2013a;33:4552–4561. doi: 10.1128/MCB.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, Dephoure N, Fischer F, Boanca G, Vaitheesvaran B, et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell. 2013b;50:686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani DS, Ao P, Hu X, Warnock GL, Johnson JD. Voltage-gated Ca(2+) influx and insulin secretion in human and mouse beta-cells are impaired by the mitochondrial Na(+)/Ca(2+) exchange inhibitor CGP-37157. Eur J Pharmacol. 2007;576:18–25. doi: 10.1016/j.ejphar.2007.07.055. [DOI] [PubMed] [Google Scholar]
- Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, Hao DL, Chen HZ, Liu DP. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. 2017;38:1389–1398. doi: 10.1093/eurheartj/ehw138. [DOI] [PubMed] [Google Scholar]
- McDonnell E, Peterson BS, Bomze HM, Hirschey MD. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab. 2015;26:486–492. doi: 10.1016/j.tem.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16:723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nurnberg P, Mann NP, Banerjee R, Saka HN, et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44:740–742. doi: 10.1038/ng.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Guarente L. SnapShot: sirtuins, NAD, and aging. Cell Metab. 2014;20:192–192. e191. doi: 10.1016/j.cmet.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Nasrin N, Wu X, Fortier E, Feng Y, Bare OC, Chen S, Ren X, Wu Z, Streeper RS, Bordone L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285:31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Reifsnyder PC, Malcolm RD, Lucas CA, MacGregor GR, Zhang W, Leiter EH. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity (Silver Spring) 2010;18:1902–1905. doi: 10.1038/oby.2009.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pougovkina O, Te Brinke H, Wanders RJ, Houten SM, de Boer VC. Aberrant protein acylation is a common observation in inborn errors of acyl-CoA metabolism. J Inherit Metab Dis. 2014;37:709–714. doi: 10.1007/s10545-014-9684-9. [DOI] [PubMed] [Google Scholar]
- Rajan S, Shankar K, Beg M, Varshney S, Gupta A, Srivastava A, Kumar D, Mishra RK, Hussain Z, Gayen JR, et al. Chronic hyperinsulinemia reduces insulin sensitivity and metabolic functions of brown adipocyte. J Endocrinol. 2016;230:275–290. doi: 10.1530/JOE-16-0099. [DOI] [PubMed] [Google Scholar]
- Rauh D, Fischer F, Gertz M, Lakshminarasimhan M, Bergbrede T, Aladini F, Kambach C, Becker CF, Zerweck J, Schutkowski M, et al. An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat Commun. 2013;4:2327. doi: 10.1038/ncomms3327. [DOI] [PubMed] [Google Scholar]
- Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GR, Bhatt DP, O’Connell TM, Thompson JW, Dubois LG, Backos DS, Yang H, Mitchell GA, Ilkayeva OR, Stevens RD, et al. A Class of Reactive Acyl-CoA Species Reveals the Non-enzymatic Origins of Protein Acylation. Cell Metab. 2017;25:823–837. e828. doi: 10.1016/j.cmet.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watroba M, Szukiewicz D. The role of sirtuins in aging and age-related diseases. Adv Med Sci. 2016;61:52–62. doi: 10.1016/j.advms.2015.09.003. [DOI] [PubMed] [Google Scholar]