Abstract
The specific immune-reaction between the anti-citrinin antibody immobilized on the surface of magnetic/silica core–shell (MSCS) and the citrinin–Rho123–BSA conjugate brings the Rho123 fluorophore as an acceptor and the QDs as a donor in close spatial proximity and causes FRET for occurring upon photo-excitation of the QDs. The novelties of this study include: (1) immobilization of the MSCS; (2) large amount of the immobilized QDs, and (3) immobilization of a large amount of Rho123 on the BSA macromolecule. Cd/Te QDs were synthesized by the simultaneous reduction of cadmium chloride and tellurium in the presence of sodium borohydride. Magnetic nanoparticles were synthesized using FeSO4 and FeCl3. The prepared magnetic nanoparticles shelled by silica using tetraethoxysilane in the presence of ammonia. Transmission electron microscopy (TEM) analysis was used for investigating shape and monodispersity of the nanoparticles. EDC/NHS was used as a cross linking agent for immobilization of the QDs, conjugation of citrinin to amino groups of BSA, labeling of BSA with Rho123 and also for immobilization of the amino-functionalized MSCS on the immobilized QDs. Immobilization of the anti-citrinin antibody on the surface of the amino-functionalized MSCS was performed by Schiff-base mechanism. By using these three effective strategies, sensitivity of the designed nanobiosensor was incredibly enhanced as a very low limit of detection (up to 0.1 pM). The feasibility of this technique was tested by the detection of citrinin in the spiked human serum. Results showed that there was a linear correlation between the decreased fluorescence intensity of the Rho123 and increased fluorescence intensity of the QDs with increasing concentration of citrinin in the spiked samples in the range of 1–6 pM. According to obtained results, we conclude that this highly sensitive detection scheme is a easy, quick and impressive method that can be used in optical-based nanosensors.
Keywords: Citrinin, Quantum dots, Magnetic/silica core–shell, Rhodamine 123, Fluorescence Resonance Energy Transfer
1. Introduction
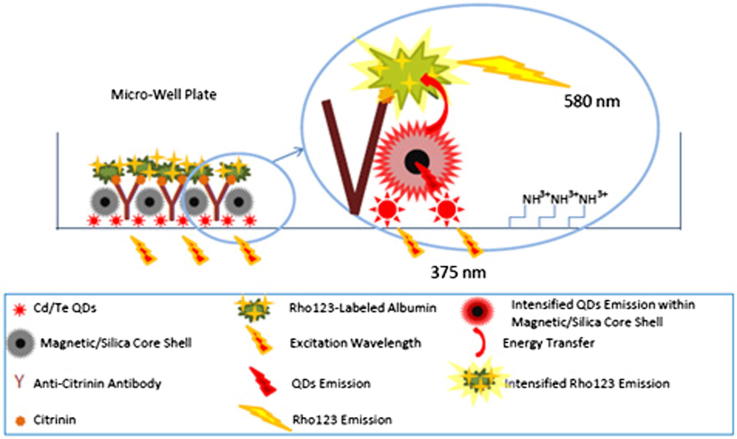
Mycotoxins are toxic secondary metabolites produced by species of filamentous fungi that may contaminate feed and food products including milk, oil seeds, dried fruits, nuts, spices and cereals (rice, corn, wheat, barley, etc). They are major problems for animal and human health. Several surveys reported that they are highly toxic, teratogenic, estrogenic, mutagenic, immunosuppressive and carcinogenic (Tavakoli et al., 2013, Mozaffari Nejad et al., 2013, Mozaffari Nejad et al., 2014; Eslami et al., 2015, Heshmati and Mozaffari Nejad, 2015). Citrinin is produced by Penicillium citrinum, Penicillium expansum and Penicillium verrucosum and some species of Monascus and Aspergillus (Bragulat et al., 2008). In the previous studies, different kinds of nanoparticles such as gold (Kamelipour et al., 2014), QDs (Rad et al., 2012, Zekavati et al., 2013, Shamsipur et al., 2012, Shanehsaz et al., 2013) were used to develop nanobiosensors and to detect a variety of analytes. However, in the current study, we decided to use MSCS to intensify signals to make a highly sensitive nanobiosensor (Khaksarinejad et al., 2015). In this study, a highly sensitive competitive immunoassay for the determination of citrinin was designed based on intensified fluorescence signal using magnetic/silica core–shell. As briefly illustrated in Scheme 1, the QDs nanoparticles were immobilized on the micro-well plate based on self-assembly mono-layer technique. 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were used as zero-valent cross-linker to bind carboxylic groups of the QDs and amino groups of the micro-well plate. By this strategy, a large amount of QDs could participate in the designed nanobiosensor. Then, the amino-functionalized magnetic/silica core–shells, as the second layer, were immobilized on the surface of the immobilized QDs. Because of the close proximity of the immobilized QDs and the immobilized magnetic/silica core–shell, the emission of the QDs passes through the silica layer of the MSCS and interestingly intensified. In fact, magnetic/silica core–shells act as nano-mirrors to multiply the emission light of the QDs. Anti-citrinin antibody, as a third layer, was then immobilized on the level of the immobilized amino-functionalized MSCS using glutaraldehyde, as a cross-linker, based on Schiff-base interaction. NaBH4 was used as a reducing agent to reduce Schiff-base interaction. Bovine serum albumin (BSA) was used as a support to immobilize citrinin and Rho123. EDC/NHS activated carboxylic group of citrinin binds to amino groups of BSA and Rho123 molecules bind to carboxylic group of BSA using EDC and NHS. In this step, citrinin and Rho123 bind to BSA to make a Rho123-labeled citrinin. For this purpose, a large number of Rho123 are located in a close proximity to the immobilized QDs and MSCS when citrinin binds to the antigen binding site of anti-citrinin antibody. In fact, BSA was used just for conjugating a molecule of citrinin with a large number of Rho123 to enhance the obtained signal. Consequently, the signal of the designed nanobiosensor interestingly intensified by these three simple but effective factors: (a) a large number of the immobilized QDs on the bottom of micro-well plate (b) immobilized MSCS in a close proximity of the immobilized QDs and (c) a large number of Rho123 which are conjugated with carboxylic groups of BSA.
Scheme 1.
Excitation wavelength at 375 nm excites the immobilized QDs on the surface of the amino-functionalized micro-well plate. MSCS was then immobilized on the QDs nanoparticles. Proximity of the QDs and the MSCS leads to intensify the emission of the QDs. Emission of the QDs passes through the magnetic/silica core–shell and effectively intensified. Very close proximity of the MSCS and the immobilized Rho123 in a range of Förster distance leads to effective energy transfer (FRET) from the intensified emission of the QDs to the Rho123. In the presence of free citrinin in a sample, the Rho123-labeled citrinin–albumin leaves the binding sites of the antibody, which leads to a significant reduction of the Rho123 emission. Reduction in the Rho123 emission is linearly relative to free citrinin concentration in a sample.
2. Materials and apparatus
2.1. Materials
Citrinin, anti-citrinin antibody, cadmium chloride (CdCl2), sodium borohydride (NaBH4), tellurium powder (Te), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) purchased from Sigma chemical company (St. Louis, Mo, http://www.sigmaaldrich.com). Thioglycolic acid (TGA), FeSO4, FeCl3, tetraethoxysilane (TEOS), (3-aminopropyl) tetraethoxysilane (APTES) and all materials were obtained from Merck chemical Co. (Germany). All materials were used as supplied without further purification. All solutions were prepared using double-distilled water. Shimadzu fluorescence spectrometer (Japan) was used for recording all fluorescence spectra. Moreover, all optical measurements were conducted under ambient conditions. A Malvern dynamic light scattering (DLS) apparatus (UK) was used for studding synthesized QDs size distribution. Experiments are done in triplicate.
2.2. Preparation and immobilization of TGA-capped Cd/Te QDs
The Cd/Te QDs was synthesized and characterized in accordance with previous relevant studies. (Rad et al., 2012, Zekavati et al., 2013). Briefly, Te powder (0.1 g) was reduced by NaBH4 (0.280 g) in 10 mL of distilled water under continuous N2 bubbling and vigorous stirring. Change in the color of the solution (from violet to white) was observed after 5 h. In order to remove the white precipitate of sodium tetraborate, the solution was filtered using an ultra-filter. Then, the fresh prepared oxygen-free NaHTe aqueous solution was added into a CdCl2 2.5 H2O (0.358 g) in 200 mL nitrogen-saturated double-distilled water at pH 10 in the presence of TGA (200 μL). TGA was used as a stabilizing and capping agent. The mixture was refluxed under a nitrogen atmosphere while vigorously stirred. Different sizes of QDs could be achieved by prolonging the refluxing time. In order to remove excess substances, the prepared solution was washed three times with absolute ethanol using sigma high-speed centrifuge (15 min, 10,000×g). The produced precipitate was re-dispersed in 250 mL double-distilled water and kept in a dark place, oxygen-free condition. The QDs with maximum excitation of 375 nm and emission wavelength of 535 nm were used in this study. The QDs nanoparticles were immobilized on the amino-functionalized micro-well plate based on self-assembly mono-layer technique. EDC and NHS (1:1 molar ratio) were used as zero-valent cross-linker to bind carboxylic groups of the QDs and amino groups of the micro-well plate. Then the micro-wells were washed with excess of water to remove any un-reacted materials. Spectrofluorometric study was used to confirm the immobilization of the QDs on the micro-well plate. The fluorescence intensity of the micro-wells was monitored at 530 nm while excited at 375 nm.
2.3. Synthesis of amino-functionalized magnetic/silica core–shell
Synthesis of magnetic/silica core–shell was based on our experiences with some modification (Khaksarinejad et al., 2015). To synthesize magnetic nanoparticles, FeSO4 and FeCl3 (1:2 molar ratio) were dissolved in 250 mL of double distilled water and was kept under nitrogen flow while vigorously stirred. Then, ten mL of NH4OH solution was added to the mixture and vigorously stirred. The prepared magnetic nanoparticles were collected using a magnetic bar. Trinatrium citrate (100 mg) was used as a stabilizing agent to stabilize the magnetic nanoparticles. The citrate-stabilized magnetic nanoparticles solution was ultra-sonicated (400 W, 60%, 15 min, Hielscher ultrasonic, Germany. http://www.hielscher.com). TEOS (200 μL) was then added drop by drop while vigorously stirred and then NH4OH (50 μL) was added to the solution. pH of the mixture was adjusted to 9–10 using diluted NH4OH. Characteristics of the MSCS were shown by TEM. To prepare amino-functionalized MSCS, APTES (25 μL) was added to the prepared MSCS solution while vigorously stirred. The reaction was kept for 3 h in this condition. Un-reacted materials were removed by washing three times with double-distilled water using a high-speed centrifuge at 10,000×g for 30 min. Transmission electron microscopy images was used to confirm the magnetic nanoparticles and MSCS preparation and also estimation the core and shell sizes. Rho123 was used to confirm the immobilization of the MSCS. Glutaraldehyde was used as a cross-linker between amino group of the silica shell and the Rho123 molecule. Before glutaraldehyde treatment, the micro-well plate was treated with EDC/NHS-activated adipic acid to block all the amino groups of the micro-well plate. Then diluted Rho123 was added to the micro-wells and kept overnight at a dark place. NaBH4 was used to reduce Schiff-base interactions and also to reduce the un-reacted aldehyde groups to hydroxyl moieties. Then the micro-wells were washed with excess of water to remove any un-reacted materials. Fluorescence intensity of the immobilized Rho123 at 580 nm was then monitored in a spectrofluorometer upon exciting at 510 nm. In order to show the intensifying properties of the immobilized MSCS on the emission of the immobilized QDs, the fluorescence intensity of the QDs/magnetic/silica-treated micro-well plate was compared with the fluorescence intensity of the QDs-treated micro-well.
2.4. Immobilization of anti-citrinin antibody on the surface of the immobilized amino-functionalized magnetic/silica core–shell
The immobilized MSCS was treated with diluted glutaraldehyde (1% v/v) and kept overnight in a dark place. Excess glutaraldehyde was removed by extreme washing with double-distilled water. Anti-citrinin antibody (10 μg) was dissolved in 1 mL PBS (25 mM, pH 6.5) by gentle pipetting and was then added to the micro-well plate. NaBH4 was used as a reducing agent to reduce Schiff-base interaction. Micro-well was washed with PBS to remove any un-reacted materials. Immobilization of the anti-citrinin antibody was confirmed by reading the optical density of the solution of the antibody at 280 nm in a Shimadzu spectrophotometer. Any reduction in the optical density of the antibody solution at 280 nm shows the immobilization rate of the antibody (Khaksarinejad et al., 2015).
2.5. Preparation of citrinin–Rho123–BSA conjugate
Citrinin (0.1 mg) was dissolved in 1 mL absolute ethanol and then treated with EDC/NHS (1:1 molar ratio) to functionalize carboxylic group of citrinin. BSA (0.1 g) was dissolved in 20 mL phosphate buffer saline (PBS) (25 mM, pH 6.5) pre-chilled in a cold room. The citrinin solution was then added drop by drop to the BSA solution and kept 2 h in a cold room while gently stirred. Rho123 (1 mg) and EDC/NHS (1:1 molar ratio) dissolved in 1 mL double-distilled water and then added drop by drop to the citrinin–BSA mixture and kept 2 h in a cold room while gently stirred. The citrinin–Rho123–BSA mixture was then dialyzed against 3 × 1 L PBS (25 mM, pH 7) in a cold room to remove un-wanted materials. Optical density of the dialyzed mixture at 510 nm was used for confirmation of the labeling of BSA with Rho123. Citrinin binding to BSA and functionality of the citrinin–Rho123–BSA conjugate to bind to the antigen binding site of the anti-citrinin antibody were confirmed by a self-designed heterogeneous assay. Briefly, anti-citrinin antibody was immobilized in a micro-well plate and the diluted citrinin–Rho123–BSA mixture was then added to the micro-well. The plate was incubated at room temperature for 30 min and then washed three times with PBS to remove un-reacted molecules. Optical density of the plate was then recorded on a micro-plate reader at 510 nm (Zekavati et al., 2013).
2.6. Biosensor evaluation
To evaluate the ability and sensitivity of the nanobiosensor in the detection of citrinin, enhancement in the fluorescence intensity of the immobilized QDs was monitored at 530 nm upon excitation at 375 nm. Then the citrinin–Rho123–BSA conjugate solution was added to the micro-wells and kept at room temperature for 30 min. The micro-wells were then washed three times with PBS buffer solution to remove any excess of the citrinin–Rho123–BSA conjugate. Enhancement in the fluorescence intensity of the Rho123 and reduction in the fluorescence intensity of the QDs were monitored at 400–650 nm upon excitation at 375 nm. Free citrinin was then sequentially added to the system in a range of 1–6 pM and incubated in the dark at room temperature for 30 min to complete competitive substitution of the free and the conjugated citrinin. Then the Emission spectra of the system related to each concentration of free citrinin were monitored at 400–650 nm upon excitation at 375 nm. To evaluate the ability of the system to detect citrinin in a real sample, a stock solution of 100 pg mL−1 of citrinin in a healthy human serum was prepared and was then sequentially added to the micro-wells. The emission spectra of the system were recorded at the same condition as mentioned. Un-treated human serum was used as a blank to eliminate non-specific bindings (Zekavati et al., 2013 and Rad et al., 2012).
3. Results and discussion
3.1. Preparation of Cd/Te QDs and immobilization of the prepared QDs on the amino-functionalized micro-well plate
Fig. 1 shows the absorption and emission spectra of the prepared QDs and Rho123. As shown here, the maximum emission peak of the QDs was seen at 535 nm. While, the maximum absorption and emission peaks of Rho123 were observed at 510 nm and 580 nm, respectively. The full width at half maximum (FWHM) of the QDs emission spectrum was about 35 nm. Emission wavelength of the QDs and absorption wavelength of Rho123 showed maximum spectral overlap that is critical to obtain optimum FRET phenomenon. According to the previous studies, TEM image and DLS analysis of the prepared TGA-capped Cd/Te QDs indicated that the core–shell has a good mono-dispersity and spherical morphology with a particle size of 2–3 nm. The obtained QDs dispersed in the phosphate buffer (pH 7.4) showed high optical stability, without considerable loss of fluorescence intensity during three months. Immobilization of the QDs on the micro-wells was assessed by reading the emission spectra of the immobilized QDs in a range of 400–650 nm while excited at 375 nm. As shown in Fig. 2, an emission peak at 530 nm showed that the QDs were effectively immobilized on the micro-wells.
Figure 1.

Normalized absorbance of Rho123 and normalized emission spectra of QDs and Rho123 were illustrated.
Figure 2.
TEM images of magnetic nanoparticles (Left) and magnetic/silica core–shell (Right) Preparation of citrinin–Rho123–BSA conjugate.
3.2. Preparation of MSCS and immobilization of the prepared MSCS on the micro-well
As shown in Fig. 3, the TEM analysis of the prepared magnetic nanoparticles and MSCS shows that, the magnetic nanoparticles have a spherical morphology with an average size of 10–15 nm (left) and the MSCS have a spherical morphology with an average particle size of 70 nm and good monodispersity (right). The thickness of the silica shell was estimated as 50–55 nm. The obtained MSCS dispersed in the phosphate buffer (pH 7.4) showed high stability in optical properties, without considerable agglomeration (data not shown). Immobilization of the MSCS was assessed by labeling the immobilized amino-functionalized MSCS by Rho123 using glutaraldehyde as a cross linker. In fact, the emission of the Rho123 was considered as a tool to confirm in one hand the immobilization of the MSCS on the micro-well and the functionalization of the amino-functionalized MSCS with glutaraldehyde on the other hand. In order to exclude the non-specific binding of the Rho123, Tris/HCl buffer (50 mM, pH 6.5) was used to block the negative charges and also to neutralize the remaining EDC/NHS-activated carboxylic groups of the immobilized QDs. The remaining amino groups of the micro-wells were also blocked by the EDC/NHS-activated adipic acid. In fact, in the absence of the MSCS in the micro-well, the emission of Rho123 is negligible.
Figure 3.

Changes in fluorescence intensity of QDs and Rho123 upon addition of free citrinin.
3.3. Preparation of citrinin–Rho123–BSA conjugate
Citrinin and Rho123 could be covalently attached to one molecule of BSA, citrinin by its carboxylic group to the amino groups of BSA and Rho123 by its amino group to the carboxylic groups of the BSA. EDC/NHS was used as a zero-length cross-linker in these reactions. In direct conjugation of citrinin by Rho123 only one molecule of Rho123 binds to one molecule of citrinin while the citrinin–Rho123–BSA conjugate has higher amount of Rho123. This may lead to generate a stronger signal in an optical system. Citrinin and Rho123 binding to BSA and also functionality of the citrinin–Rho123–BSA conjugate to bind to the antigen binding site of the immobilized anti-citrinin antibody were confirmed by a self-designed heterogeneous assay. Briefly, anti-citrinin antibody was immobilized in a micro-well plate and the diluted citrinin–Rho123–BSA conjugate was then added to the micro-well. The plate was kept at room temperature for 30 min and then washed three times with PBS to remove un-reacted molecules. Optical density of the plate was then recorded on a micro-plate reader at maximal wavelength of Rho123 (510 nm). The optical density at 510 nm confirmed conjugation of the BSA with Rho123 and citrinin in one hand and the ability of the citrini–Rho123–BSA conjugate to bind to the antigen binding site of the immobilized anti-citrinin antibody on the other hand.
3.4. Biosensor evaluation
Ability and sensitivity of the designed nanobiosensor for detecting of citrinin were evaluated by the enhancement in the fluorescence intensity of the BSA-conjugated Rho123 with a maximal fluorescence peak at 580 nm upon excitation of the immobilized QDs at 375 nm. The fluorescence intensity of the system was remarkably intensified by the large amount of the immobilized QDs in one hand and the intensifying properties of the immobilized MSCS on the other hand. In the presence of free citrinin, the citrinin–Rho123–BSA conjugate leaves the antigen binding site of the immobilized anti-citrinin antibody that leads to a remarkable reduction in the fluorescence intensity of the Rho123. Reduction in the fluorescence intensity of the Rho123 was linearly correlated with the concentration of the free citrinin. Reduction in the fluorescence intensity of the Rho123 was linearly correlated with enhancement in the fluorescence intensity of the QDs (see Fig. 4). The limit of detection (LOD) was estimated at 0.1 pM, based on equation LOD = 3S0 K−1, where S0 is the standard deviation of blank measurements (n: 7) and K is the slope of calibration curve. Based on our review of the published papers and regarding to the results of the similar studies that was briefly mentioned in Table 1, the sensitivity of the designed nanobiosensor is even higher than the limit of detection of the other nanobiosensors. The ability of the system to detect citrinin in a real sample was evaluated by adding free citrinin in a healthy human serum followed by estimation of the concentration of the added citrinin using the designed nanobiosensor. The difference between the real concentration of the added citrinin and the estimated concentration of citrinin in the real samples was expressed as coefficient variation (C.V.%). In the real sample analysis the C.V. less than 7% was achieved that showed acceptable accuracy in the estimation of citrinin in the real samples. Un-treated human serum was used as a blank to eliminate non-specific bindings. It is also concluded that the substances in the human serum do not really interfere with the immuno-reaction and the emission signal of the detection system. In other words, the designed nanobiosensor could be homogenously detecting the citrinin without any further isolation, purification and concentrating of the analyte of interest. Here, we proposed these effective strategies to enhance the sensitivity of a detection system principally based on the immobilization of the magnetic/silica core–shell.
Figure 4.
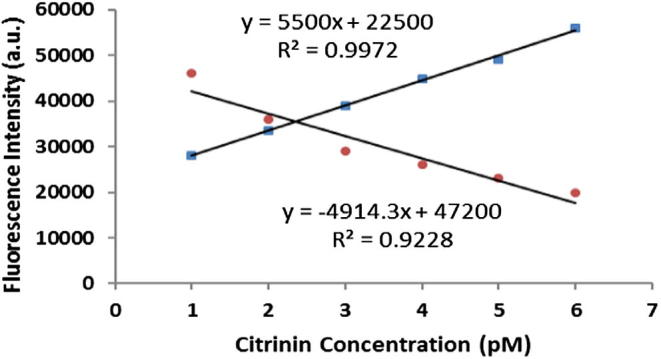
Linear standard curve based on changes in fluorescence intensity of QDs (Blue Dots) and Rho123 (Red Dots).
Table 1.
Different detection methods of Citrinin using nanobiosensors.
| Detection method | Title of the published paper | LD | LWR | Ref. |
|---|---|---|---|---|
| Amperometric biosensor based on Peroxidases | Development of an amperometric biosensor based on peroxidases to quantify citrinin in rice samples | 0.25 nM | 1–11.6 nM | Zachetti et al. (2013) |
| Micro fluidic electrochemical immunosensor | Citrinin (CIT) determination in rice samples using a micro fluidic electrochemical immunosensor | 0.1 ng mL−1 | 0.5 and 50 ng mL−1 | Arévalo et al. (2011) |
| Molecular Imprinted SPR Biosensor | A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice | 0.0017 ng mL−1 | 0.005–1 ng mL−1 | Atar et al. (2015) |
| FRET-based immunosensor | Intensified fluorescence signal of a FRET-based immunosensor for detection of citrinin using quantum dots, MSCS and Rho123 conjugated to bovine serum albumin | 0.1 pM | 1–6 pM | Present Study |
4. Conclusion
Based on the results of this study, the MSCS and the large number of the immobilized QDs on the bed of the micro-plate and the large number of the Rho123 molecules that conjugated to BSA effectively intensified the fluorescence signal of the system. So the proposed mechanism could be used in other optical-based detection systems to make the system more sensitive.
Acknowledgment
The authors would like to acknowledge the financial and technical support from the Nanozino R&D Company.
Footnotes
Peer review under responsibility of King Saud University.
References
- Arévalo F.J., Granero A.M., Fernández H., Raba J., Zón M.A. Citrinin (CIT) determination in rice samples using a micro fluidic electrochemical immunosensor. Talanta. 2011;83:966–973. doi: 10.1016/j.talanta.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Atar N., Eren T., Yola M.L. A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice. Food Chem. 2015;184:7–11. doi: 10.1016/j.foodchem.2015.03.065. [DOI] [PubMed] [Google Scholar]
- Bragulat M.R., Abarca M.L., Cabañes F.J. Low occurrence of patulin- and citrinin-producing species isolated from grapes. Lett. Appl. Microbiol. 2008;47:286–289. doi: 10.1111/j.1472-765x.2008.02422.x. [DOI] [PubMed] [Google Scholar]
- Eslami M., Mashak Z., Heshmati A., Shokrzadeh M., Mozaffari Nejad A.S. Determination of aflatoxin B1 levels in Iranian rice by ELISA method. Toxin Rev. 2015;34:125–128. [Google Scholar]
- Heshmati A., Mozaffari Nejad A.S. Ochratoxin A in dried grapes in Hamadan province. Iran. Food Addit. Contam. Part B Surveill. 2015;8:255–259. doi: 10.1080/19393210.2015.1074945. [DOI] [PubMed] [Google Scholar]
- Kamelipour N., Mohsenifar A., Tabatabaei M., Rahmani-Cherati T., Khoshnevisan K., Allameh A., Milani M.M., Najavand S., Etemadikia B. Fluorometric determination of paraoxon in human serum using a gold nanoparticle-immobilized organophosphorus hydrolase and coumarin 1 as a competitive inhibitor. Microchim. Acta. 2014;181:239–248. [Google Scholar]
- Tavakoli H.R., Kamkar A., Riazipour M., Mozaffari Nejad A.S., Rafati H. Assessment of aflatoxin M1 levels by enzyme-linked immunosorbent Assay in yoghurt consumed in Tehran. Iran. Asian J. Chem. 2013;25:2836–2838. [Google Scholar]
- Khaksarinejad R., Mohsenifar A., Rahmani-Cherati T., Karami R., Tabatabaei M. An organophosphorus hydrolase-based biosensor for direct detection of paraoxon using silica-coated magnetic nanoparticles. Appl. Biochem. Biotechnol. 2015;176:359–371. doi: 10.1007/s12010-015-1579-1. [DOI] [PubMed] [Google Scholar]
- Mozaffari Nejad A.S., Bayat M., Ahmadi A.A. Investigation of aflatoxin B1 in spices marketed in Hyderabad, India by ELISA method. J. Pure Appl. Microbiol. 2013;7:3219–3223. [Google Scholar]
- Mozaffari Nejad A.S., Ghannad M.S., Kamkar A. Determination of aflatoxin B1 levels in Iranian and Indian spices by ELISA method. Toxin Rev. 2014;33:151–154. [Google Scholar]
- Rad F., Mohsenifar A., Tabatabaei M., Safarnejad M.R., Sahryari F., Safarpour H., Foroutan A., Mardi M., Davoudi D., Fotokian M. Detection of candidatus phytoplasma aurantifolia with a quantum dots FRET-based biosensor. J. Plant Pathol. 2012;94:525–534. [Google Scholar]
- Shanehsaz M., Mohsenifar A., Hasannia S., Pirooznia N., Samaei Y., Shamsipur M. Detection of Helicobacter pylori with a nanobiosensor based on fluorescence resonance energy transfer using CdTe quantum dots. Microchim. Acta. 2013;180:195–202. [Google Scholar]
- Shamsipur M., Shanehasz M., Khajeh K., Mollania N., Kazemi S.H. A novel quantum dot-laccase hybrid nanobiosensor for low level determination of dopamine. Analyst. 2012;137:5553–5559. doi: 10.1039/c2an36035g. [DOI] [PubMed] [Google Scholar]
- Zachetti V.G., Granero A.M., Robledo S.N., Zon M.A., Fernández H. Development of an amperometric biosensor based on peroxidases to quantify citrinin in rice samples. Bioelectrochemistry. 2013;91:37–43. doi: 10.1016/j.bioelechem.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Zekavati R., Safi S., Hashemi S.J., Rahmani-Cherati T., Tabatabaei M., Mohsenifar A., Bayat M. Highly sensitive FRET-based fluorescence immunoassay for aflatoxin B1 using cadmium telluride quantum dots. Microchim. Acta. 2013;180:13–14. [Google Scholar]