Abstract
Diabetes is a major risk factor for cardiovascular disease (CVD) including stroke, coronary heart disease, and peripheral artery disease. It remains a leading cause of mortality throughout the world, affecting both women and men. This investigation was aimed to study gender based differences in cardiovascular risk factors of adult population with type-2 diabetes mellitus (T2DM) and to check the correlation between serum HbA1C, lipid profile and serum vitamin D levels, in T2DM patients of Riyadh, Saudi Arabia. This hospital-based cross-sectional study involving subjects was divided into two gender based groups; normal male (800), diabetic male (800) and normal female (800) and T2DM females (800). Blood samples were analyzed for fasting glucose (FBG), HbA1c, total cholesterol (TC), triglycerides (Tg), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C) and serum levels of 25(OH)-vitamin D in all groups. All the glycemic control parameters and lipid profile parameters were found to be significantly different in diabetic vs non-diabetic group (p < 0.001) in both genders. The results also show that vitamin D concentration decreased significantly (p < 0.001) in diabetic patients than the healthy individuals in both the genders. Vitamin-D and HbA1C were negatively correlated in both males and females in T2DM patients and significant at P < 0.05. Our study reveals that dyslipidemia remains one of the major risk factors of CVD in T2DM. In addition to dyslipidemia, decreased levels of vitamin-D associated with increased HbA1C alarms the early diagnosis of Type 2 Diabetes.
Keywords: Lipoproteins, HbA1c, Vitamin-D, Type 2 diabetes mellitus
1. Introduction
Diabetes mellitus (DM) is a major health problem among both males and females worldwide and a known risk factor for coronary artery disease (CAD). Diabetes is of two types- type 1 diabetes mellitus (T1DM) which leads to insulin deficiency as a result of β cell destruction, and type 2 diabetes mellitus (T2DM) with lack of insulin secretion and action. According to World Health Organization (WHO) by 2030 the global prevalence of T2DM will increase from 171 million people in 2000 to 366 million (Wild et al., 2004). If T2DM is undiagnosed and untreated it can result in retinopathy, neuropathy, nephropathy and cardio vascular disease (CVD). The major contributing risk factors for development of type 2 diabetes mellitus are the poor nutrition, overweight, and physical inactivity (Sadie et al., 2003). In KSA the prevalence of DM was estimated to be 23.7%. (Al-Nozh et al., 2004). In 2013, diabetes was reported as 8.3% among the age group of 20 and 70 years and was predicted to rise by 2035 to 10.1% (WHO, 2013, IDF, 2013). In a recent review on different population of Saudi Arabia by Al-Daghri, 2016, the prevalence of vitamin-D deficiency studied from 2011 to 2016 was found to be 81% (Al-Daghri, 2016).
Diabetic patients apart from suffering with numerous complications of chronic hyperglycaemia, are also susceptible to deadly cardiovascular disease (CVD). CVDs are one of the major reasons that reduces the life expectancy in these groups of patients (Schnell and Stand, 2006). The ability of glycated haemoglobin (HbA1c) to report the glycaemic history of the preceding 2–3 months makes it an important biomaker of glycaemic control. Its enhanced levels are considered as independent risk factor for stroke and coronary heart disease (CHD) in diabetic and non-diabetic patients (Selvin et al., 2005). Direct correlation between CVD and HbA1C in diabetic patients was observed by Ravipati et al. (2006). In pregnant women, poor glycaemic control impact can be so severe that higher HbA1c levels can impair foetal cardiac function; if glycaemic control is improved it can reduce the risk of cardiovascular events in diabetics to a major extent (Selvin et al., 2006, Kawasumi et al., 2006). In type 2 diabetes mellitus, dyslipidemia characterized by raised levels of LDL-C, triglycerides and low HDL-C is a major risk factor of CVD. The observed changes in diabetes mellitus with respect to lipid parameters are due to enhanced free fatty acid flux secondary to insulin resistance (Chahil and Ginsberg, 2006). In non-diabetic cases a positive relationship has been reported between HbA1c and CVD even within normal range of HbA1c (Khaw et al., 2004).
Unacceptable high rates of vitamin D deficiency (30–90%) have been reported in Middle-East region inspite of abundant supply of sunlight throughout the year (Bassil et al., 2013). Vitamin D is a fat soluble vitamin with well known functions in bone homeostasis and metabolism. Extensive research in the last few decades has established a crucial role of vitamin D deficiency in numerous non-skeletal diseases like CVD, hypertension, diabetes (type-1 and 2), osteoporosis, immune disorders and cancers (Gulseth et al., 2014, Papandreou and Hamid, 2015).
In addition to the pivotal role of vitamin D in calcium/phosphorus homeostasis and bone physiology, several lines of evidence suggest that vitamin D status might have a specific role in Type 2 diabetes and progression of metabolic syndrome and also may have a general role in glucose homeostasis. (Thomas et al., 2012). Studies in animals and humans suggest that vitamin D affects insulin secretion and tyrosine phosphorylation of the insulin receptor. Low levels of vitamin D were associated with surrogate measures of insulin resistance, major adverse cardiovascular events, cancers, and all-cause mortality, at least in subjects with metabolic syndrome (Chiu et al., 2004). In Middle East and North African region, in adults-gender, increasing age, season, obesity related diseases like T2DM, insulin resistance and partial or complete metabolic syndrome are the risk factors which have been consistently linked to vitamin D deficiency (Chiu et al., 2004, Al-Shoumer and Al-Essa, 2015). The aim of our study was to assess the gender related differences in serum levels of vitamin D, HbA1C, lipid profile in T2DM patients and also to study the correlation between serum level of vitamin D with HbA1C, and lipid profile in T2DM male and female patients of Riyadh, Saudi Arabia.
2. Materials and methods
2.1. Study population
In the present study, 4843 volunteers participated, of which 3200 were selected based on levels of HbA1c and gender (35–79 years). The participants were divided into four groups-normal male (800), diabetic male (800), normal female (800) and T2DM females (800). The study was carried out in Prince Sultan Military Medical City in collaboration with Clinical laboratory Sciences department, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia. The study was approved by the Prince Sultan Military Medical City hospital ethics committee. At the beginning of the study, written informed consents from the patients and the subjects were obtained. Participants were asked to fill-in questionnaires containing demographic information, as well as past and present medical history.
Inclusion criteria: Adult male and female patients with vitamin D deficiency suffering with Type 2 Diabetes were included in the study.
Exclusion criteria: Subjects were screened and patients with Type 1 diabetes or recently diagnosed diabetes, cardiac problems, other chronic disorders and on Vitamin D supplementation were excluded from the study.
2.2. Sample collection and laboratory investigations
Blood samples were collected in sterile labeled vacutainers (12 h fasting) and separately 1 ml of blood sample was collected in an EDTA-coated tube for HbA1C analysis. All lipid profile parameters- triglycerides (Tg), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and fasting blood glucose (FBG), HbA1C, were analyzed by fully automatic analyzer, ROCHE, Cobas 8000 (C701 & C702). LDL-C was estimated by formula by Friedewald et al. (1972). Serum 25(OH)D3 or vitamin-D was measured by kits in Roche Elecsys 2010 Modular Analytics E170-Cobase 411 utilizing electro-chemiluminescence immunoassay (Roche Diagnostics, Germany).
2.3. Statistical analysis
SPSS software was used for statistical analysis. Comparison between the groups was done by one way ANOVA followed by Holm-Sidaktest. A value of P < 0.05 was considered to be statistically significant. Vitamin-D correlation with HbA1C, fasting blood glucose (FBG), lipid profile parameters were determined in both genders using Pearsons correlation (r) and multiple regression analysis.
3. Results
3.1. Relationship between vitamin D status, HbA1c and serum lipid profile in relation to gender in T2DM patients
The mean ± SD values of vitamin-D, HbA1C, FBG, total cholesterol (TC), triglycerides (Tg), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) in both genders of T2DM are shown in Table 1. Table 2 depicts the comparison of the biochemical markers between the groups-normal male, diabetic male, normal female and diabetic female.
Table 1.
Serum biochemistry of male and female in Normal and Type 2 Diabetes patients.
| Males |
Females |
|||
|---|---|---|---|---|
| Normal | Diabetes | Normal | Diabetes | |
| Age (years) | (35–79) | (35–78) | ||
| HbA1C (%) | 5.37 ± 0.23 | 6.82 ± 0.5 | 5.37 ± 0.22 | 9.97 ± 0.77 |
| FBG (mmol/l) | 4.80 ± 0.36 | 10.91 ± 0.64 | 4.76 ± 0.34 | 10.17 ± 0.50 |
| Cholesterol (mmol/l) | 4.66 ± 0.32 | 5.38 ± 0.13 | 4.61 ± 0.31 | 5.62 ± 0.15 |
| Triglycerides (mmol/l) | 1.46 ± 0.06 | 1.99 ± 0.25 | 1.61 ± 0.07 | 2.51 ± 0.74 |
| HDL (mmol/l) | 1.62 ± 0.07 | 0.92 ± 0.12 | 1.96 ± 0.24 | 1.02 ± 0.15 |
| LDL (mmol/l) | 1.90 ± 0.06 | 2.89 ± 0.08 | 2.24 ± 0.11 | 3.69 ± 0.20 |
| Vitamin-D (nmol/l) | 70.12 ± 16.18 | 26.68 ± 2.64 | 66.26 ± 14.54 | 27.12 ± 3.15 |
Table 2.
Comparison of the glycemic indices, lipid profile and vitamin D among the groups.
| Normal male with normal female |
Normal male with Diabetic male |
Normal female with Diabetic female |
Diabetic male with Diabetic female |
|||||
|---|---|---|---|---|---|---|---|---|
| t | p | t | p | t | p | t | p | |
| HbA1C | 0.14 | 0.87 | 58.62 | <0.001* | 187.1 | <0.001* | 128.33 | <0.001* |
| FBG | 1.68 | 0.09 | 254.76 | <0.001* | 225.3 | <0.001* | 31.05 | <0.001* |
| Cholesterol | 3.96 | <0.001* | 57.67 | <0.001* | 81.66 | <0.001* | 19.52 | <0.001* |
| Triglycerides | 7.3 | <0.001* | 26.90 | <0.001* | 45.57 | <0.001* | 25.96 | <0.001* |
| HDL | 42.87 | <0.001* | 86.29 | <0.001* | 117.03 | <0.001* | 12.13 | <0.001* |
| LDL | 53.68 | <0.001* | 155.95 | <0.001* | 227.92 | <0.001* | 125.65 | <0.001* |
| Vitamin-D | 6.96 | <0.001* | 78.45 | <0.001* | 70.68 | <0.001* | 0.80 | 0.42 |
** p < 0.05.
p < 0.001.
Levels of HbA1C increased significantly in diabetic group compared to normal subjects in both males and females (p < 0.001). HbA1C increased significantly in diabetic females compared to normal females, whereas in diabetic males borderline increase in HbA1C was observed. However there was no significant change between normal male and normal female. In addition to HbA1c, FBG was found to increase in diabetic group in both genders but the values of FBG were lower in females than males in both diabetic and non-diabetic groups and significant at p < 0.001 level of significance.
Similar to HbA1C and FBG, the parameters of lipid profile like TC, Tg and LDL-C increased significantly in both genders in T2DM patients compared to normal subjects (p < 0.001). TC, Tg and LDL-C increased significantly in T2DM patients in both genders compared to normal subjects. Serum levels of TC were similar in both genders in non-diabetic group. Tg and LDL were higher in normal females compared to normal males. However the change in levels of TC, Tg and LDL was statistically significant in non-diabetic males and females. T2DM females exhibited marked increase in the levels of these lipids and lipoproteins compared to diabetic males and was significant at P < 0.001 level of significance.
In contrast to these parameters, serum levels of HDL-C decreased significantly in T2DM patients in both genders compared to non-Diabetic group. Females have significantly higher HDL-C levels than males in both normal and T2DM patients. Like HDL-C, Vitamin-D levels decreased significantly in T2DM males and females compared to normal subjects. The levels of Vitamin-D were higher in males than females in both non-Diabetic and T2DM patients.
Status of Vitamin D and other biochemical variables were compared by Pearson correlation and are shown in Table 3 and regression graphs showing correlation between Vitamin-D and other variables like HbA1C, FBG, TC, Tg, LDL-C and HDL-C in males and females of T2DM patients are depicted in Fig. 1, Fig. 2, Fig. 3, Fig. 4. It was found that Vitamin-D and HbA1C were negatively correlated in both males and females in T2DM patients and significant at P < 0.05. FBG and TC were positively correlated with Vitamin-D in both genders but the correlation was not statistically significant. Vitamin-D and TC were positively correlated in both genders and were significant only in males (p < 0.001). Pearsons correlation exhibited differential statistically non-significant correlation in males compared to female T2DM patients.
Table 3.
Correlation between Vitamin D, HbA1C, lipid profile and FBG in type-2 Diabetic male and females by Pearsons Correlation.
| Parameters | Diabetic male |
Diabetic female |
||
|---|---|---|---|---|
| Vitamin-D | Pearson correlation r | p | Pearson correlation r | p |
| HbA1C | −0.06 | 0.04** | −0.13 | <0.001* |
| FBG | 0.026 | 0.4 | 0.06 | 0.07 |
| Cholesterol | 0.16 | <0.001* | 0.05 | 0.11 |
| Triglycerides | 0.032 | 0.3 | −0.03 | 0.3 |
| HDL | −0.03 | 0.35 | −0.01 | 0.7 |
| LDL | 0.01 | 0.67 | −0.01 | 0.60 |
p < 0.001.
p < 0.05.
Fig. 1.

Regression graphs showing correlation between vitamin-D, HbA1C, and FBG in Diabetic males.
Fig. 2.
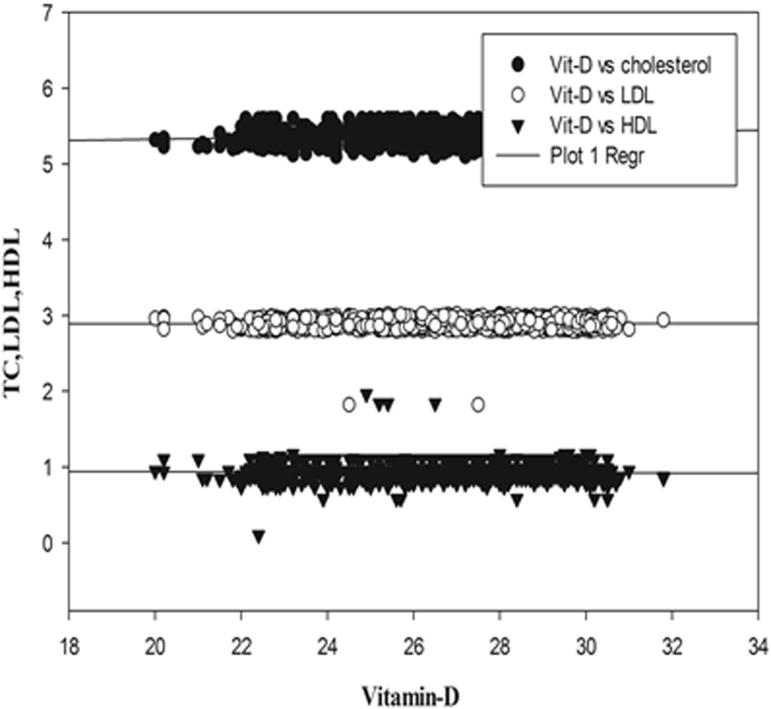
Regression graphs showing correlation between vitamin-D, TC, LDL, HDL in Diabetic males.
Fig. 3.

Regression graphs showing correlation between vitamin-D, HbA1C, lipid profile and FBG in Diabetic females.
Fig. 4.
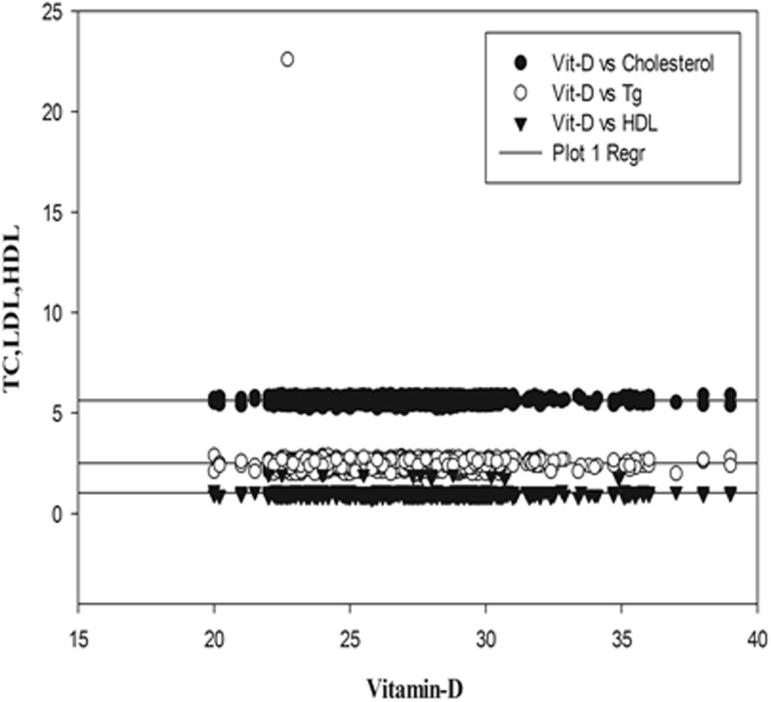
Regression graphs showing correlation between vitamin-D, TC, LDL, HDL in Diabetic females.
4. Discussion
Cardio vascular disease (CVD) leads to a high risk of mortality in diabetes characterized by dyslipidemia, the key features of which are raised triglycerides, low high density lipoprotein and high small density lipoprotein particles. According to American Diabetes Association, one of the standard measures for care of diabetic patients is regular monitoring of serum lipid levels as they are likely to contribute towards risk of coronary artery disease (The American Diabetes Association, 1999). Aberrant lipid peroxidation and altered lipid homeostasis are frequently observed in diabetes and become worse with poor glycaemic control. Regular monitoring of lipid profile in diabetic patients is essential in order to study how their lipid metabolism is affected by diabetes.
In the present investigation, lipid profile and glycemic indices (HbA1c and FBG) were measured and analyzed to test the correlation of these parameters with serum levels of vitamin-D in both genders of type 2 diabetic patients. The results revealed that T2DM patients exhibits hypertriglyceridemia, with high cholesterol levels, high LDL-C and low HDL-C. It was observed that lipid profile and the lipoproteins were higher in diabetics than non-diabetic group. The results of the present study are in line with previous reports (Idogun et al., 2007, Albrki et al., 2007). HbA1C differed significantly between male and female subjects. HbA1C, Tg, HDL-C and LDL-C were found to be higher in females than male patients. Additionally, the gender effect may account for raised levels of HDL-C in females compared to males, particularly estrogen effect and reflect better adherence to diabetic management by females. This study demonstrated gender difference with respect to lipid metabolism between males and females of diabetic and non-diabetic groups. The findings of our study are in accordance with the observation of Gustafsson et al. (2004). In contrast to our results, Khan et al. (2007) reported no significant differences between males and females for the levels of HbA1C, FBG, LDL. Female diabetic patients are at high risk and may suffer with more adverse changes than male patients with respect to changes in coagulation, vascular function and cardiovascular risk factor. The results obtained for lipid profile of female diabetic patients revealed higher levels of LDL, total cholesterol and HDL which is in agreement with previous studies (Walden et al., 1984). In females, hyperlipidemia is due to effect of sex hormones and body fat distribution which results in altered lipoproteins.
There is a scarcity of data with respect to link between serum levels of vitamin D, HbA1C and lipid profiles as relatively fewer studies have been carried out on this aspect. In T2DM patients the levels of serum vitamin-D are comparatively low than healthy control subjects with slight decrease in females than males. The results obtained for correlation revealed negative association between serum levels of vitamin-D with HbA1C and TC which was statistically significant, whereas a non-significant correlation was observed with lipid profile in both the genders. The inverse relationship of vitamin-D with type 2DM is explained by certain mechanisms. Vitamin-D has a direct and indirect effect through genomic and non-genomic pathways on β–cell function, insulin secretion and resistance. On contrary the most frequent cause of mortality of type 2 DM patients is cardiovascular disease. Another proposed mechanism of vitamin-D is an indirect role in endothelial function, calcification of coronary vasculature, control of blood pressure and prevention of CVD.
The correlation studies showed a significant negative correlation (r = −0.06, p < 0.05 in males; r = −0.13, p < 0.001 in females) between vitamin-D and HbA1C, whereas non-significant positive correlations were observed between vitamin-D and FBG, Tg, LDL and HDL-C. The inverse correlation observed in the present study for vitamin-D and HbA1C in T2DM patients is in accordance to that of Shenoy et al. (2014). This study reports dyslipidemia in diabetic group, but Vitamin-D levels did not correlated with the altered lipid abnormalities in T2DM patients in both genders. Similar to our findings, a negative association between vitamin-D and Tg in hypertriglyceridemia patients was reported by another study (Ford et al., 2005). Some of the previous reports have showed a positive association between vitamin-D and Tg whereas as other studies have reported an inverse relation (Gustafsson et al., 2004). Wang et al., 2009 suggested a direct and indirect effect of vitamin-D on lipid profile. According to their study vitamin-D via regulatory action increases the lipoprotein lipase activity resulting in decreasing the levels of Tg in serum (Wang et al., 2009). The present investigation reports a statistically non-significant association between vitamin-D, total cholesterol and LDL. Some of the possible explanations for this observation are – (a) occurrence of vitamin-D deficiency is very common in Saudi Arabia. This is due to limited sunlight exposure for cultural reasons and inadequate consumption of vitamin-D rich foodstuffs. (b) In our country the main source of dietary vitamin-D is through consumption of animal-based foodstuffs, but these food-stuffs in addition to vitamin-D also contain other lipids.
A crucial role of vitamin-D is in calcium metabolism, its deficiency may lead to development of serious cardiovascular diseases, cancer, type-1 and type-2 diabetes (Holick, 2007). In spite of poorly understood biological mechanisms for association between low levels of serum vitamin-D and T2DM, a possible explanation is through effects of vitamin-D on glucose homeostasis, on β-cell function and insulin secretion. Numerous studies have proposed that low levels of vitamin-D leads to insulin resistance (Holick, 2007). Low levels of vitamin-D also lead to glycosylated haemoglobin which is a marker for impaired glucose metabolism. The link between vitamin-D and diabetes is also explained by certain important mechanisms – (a) vitamin-D stimulates expression of insulin receptor thereby improves insulin action, (b) it enhances insulin responsiveness for glucose transport, (c) it has a direct effect on cytokines by improving systemic inflammation and (d) it indirectly effects insulin secretion through calcium effect. (Pittas et al., 2007).
Based on our findings we suggest routine measurements of glycated haemoglobin in diabetic patients as it can be used to monitor the glycemic status and is an indicator of disease progression. Regardless of dyslipidemia, high HbA1C is considered as an independent risk factor for CVD. As increased serum levels of HbA1C are well correlated with decreased vitamin-D, status of vitamin-D together with Hb A1C could be used in early diagnosis of T2DM. Non diabetic individuals with deficient status of vitamin-D levels might have greater risk of developing type 2 diabetes mellitus in near future, than individuals with normal vitamin D levels.
5. Conclusion
Based on the results obtained in the present study it can be concluded that in Type 2 diabetic patients, low levels of vitamin-D is associated with prevalence of hypercholesterolemia, hypertriglyceridemia, high LDL-C and low HDL-C levels and exhibited significant change with respect to gender. Dyslipidemia remains one of the major risk factors of CVD in type 2 diabetic mellitus. In addition, decreased levels of vitamin-D with increased HbA1C as glycemic control alarms the early diagnosis of Type 2 Diabetes and treatment. Low vitamin D levels may be predictive of future development of type 2 diabetes in both sexes.
Authors’ contribution
A. Aljohi, M. Abudawood and S. Ansar involved in designing and writing of the proposal; H. Tabassum, K. Almosa, S. Sobki in collection of samples and analyzing biochemical characteristics. M.N. Ali has performed statistical analysis; A. Aljohi and H. Tabassum have been involved in compilation of data and drafting the manuscript in a final version.
Disclosure
All the authors of this study declare the absence of any potential conflicts of interest.
Acknowledgements
This work was supported by the grant from “Research Center, ‘Center for Female Scientific and Medical Colleges’, Deanship of Scientific Research, King Saud University”. Authors are thankful to the Management, Prince Sultan Military Medical City, Riyadh, Saudi Arabia for the samples and facilities for completion of the work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Albrki W.M., Elzouki A.N.Y., EL-Mansoury Z.M., Tashani O.A. Lipid profiles in Libian type 2 diabetes. J. Sci. Appl. 2007;1:18–23. [Google Scholar]
- Al-Daghri N.M. Vitamin D in Saudi Arabia: prevalence, distribution and disease associations. J. Steroid Biochem. Mol. Biol. 2016 doi: 10.1016/j.jsbmb.2016.12.017. (in press) [DOI] [PubMed] [Google Scholar]
- Al-Nozh M.M., Al-Maatouq M.A., Al-Mazrou Y.Y., Al-Harthi S.S., Arafah M.R., Khalil M.Z., Khan N.B., Al-Khadra A., Al-Marzouki K., Nouh M.S., Abdullah M., Attas O., Al-Shahid M.S., Al-Mobeireek A. Diabetes mellitus in Saudi Arabia. Saudi Med. J. 2004;25(11):1603–1610. [PubMed] [Google Scholar]
- Al-Shoumer, Al-Essa T.M. Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J. Diabetes. 2015;25:1057–1064. doi: 10.4239/wjd.v6.i8.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil D., Rahme M., Hoteit M., Fuleihan Gel H. Hypovitaminosis D in the Middle East and North Africa: prevalence, risk factors and impact on outcomes. Dermato. Endocrinol. 2013;5:274–298. doi: 10.4161/derm.25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahil T.J., Ginsberg H.N. Diabetic dyslipidemia. Endocrinol. Metab. Clin. 2006;35:491–510. doi: 10.1016/j.ecl.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Chiu K.C., Chu A., Go V.L.W., Saad M.F. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am. J. Clin. Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- Ford E.S., Ajani U.A., McGuire L.C., Liu S. Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care. 2005;28(5):1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gulseth H.L., Gjelstad I.M., Birkeland K.I., Drveon C.A. Vitamin D and the metabolic syndrome. Curr. Vasc. Pharmacol. 2014;11:968–984. doi: 10.2174/15701611113119990169. [DOI] [PubMed] [Google Scholar]
- Gustafsson I., Brendorp B., Seibaek M., Burchardt H., Hildebrandt P. The influence of diabetes and the diabetes – gender interaction on the risk of death in patients who were hospitalized with congestic heart failure. J. Am. Coll. Cardiol. 2004;43(5):771–777. doi: 10.1016/j.jacc.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation . sixth ed. International Diabetes Federation; Brussels, Belgium: 2013. The IDF Diabetes Atlas. [Google Scholar]
- Idogun E.S., Unuigbe E.I., Ogunro P.S., Akinola O.T., Famodu A.A. Assessment of the serum lipids in Nigerians with type 2 diabetes mellitus complications. Pak. J. Med. Sci. 2007;23(5):708–712. [Google Scholar]
- Kawasumi M., Tanaka Y., Uchino H., Shimizu T., Tamura Y., Sato F., Mita T., Watada H., Sakai K., Hirose T., Kawamori R. Strict glycemic control ameliorates the increase of carotid IMT in patients with type 2 diabetes. Endocr. J. 2006;53:45–50. doi: 10.1507/endocrj.53.45. [DOI] [PubMed] [Google Scholar]
- Khan H.A., Sobki S.H., Khan S.A. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin. Exp. Med. 2007;7:24–29. doi: 10.1007/s10238-007-0121-3. [DOI] [PubMed] [Google Scholar]
- Khaw K.T., Wareham N., Bingham S., Luben R., Welch A., Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European Prospective Investigation in to Cancer in Norfolk. Ann. Intern. Med. 2004;141:413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- Papandreou D., Hamid Z.T.N. The role of Vitamin D in diabetes and cardiovascular disease: an updated review of the literature. Dis. Markers. 2015:1–15. doi: 10.1155/2015/580474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas A.G., Lau J., Hu F.B., Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes: a systematic review and metaanalysis. J. Clin. Endo. Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravipati G., Aronow W.S., Ahn C., Sujata K., Saulle L.N., Weiss M.B. Association of hemoglobin A1c level with the severity of coronary artery disease in patients with diabetes mellitus. Am. J. Cardiol. 2006;97:968–969. doi: 10.1016/j.amjcard.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Sadie H., Styger G., Hapgood J. Expression of the mouse gonadotropinreleasing hormone receptor gene in alpha T3–1 gonadotrope cells is stimulated by cyclic 3′,5′-adenosine monophosphate and protein kinase A, and is modulated by Steroidogenic factor-1 and Nur77. Endocrinology. 2003;144(5):1958–1971. doi: 10.1210/en.2002-220874. [DOI] [PubMed] [Google Scholar]
- Schnell O., Stand I.E. Impaired glucose tolerance, diabetes and cardiovascular disease. Endocr. Pract. 2006;12(1):16–19. doi: 10.4158/EP.12.S1.16. [DOI] [PubMed] [Google Scholar]
- Selvin E., Coresh J., Shahar E., Zhang L., Steffes M., Sharrett A.R. Glycemia (haemoglobin A1c) and incident of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet. Neurol. 2005;4:821–826. doi: 10.1016/S1474-4422(05)70227-1. [DOI] [PubMed] [Google Scholar]
- Selvin E., Wattanakit K., Steffens M.W., Coresh J., Sharrett A.R. HbA1c and peripheral arterial disease in diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2006;29:877–882. doi: 10.2337/diacare.29.04.06.dc05-2018. [DOI] [PubMed] [Google Scholar]
- Shenoy, V., Datta, P., Prabhu, K., Singh, K., 2014. Association between Vitamin D, FastingBlood glucose, HbA1c and fasting lipid profile in euglycemic individuals. J. Res. Diabetes 1–8.
- The American Diabetes Association The management of dyslipidemia in adults with diabetes. Diabetes Care. 1999;22:56–59. doi: 10.2337/diacare.21.1.179. [DOI] [PubMed] [Google Scholar]
- Thomas G.N., Hartaigh B., Bosch J.A., Pilz S., Loerbroks A., Kleber M.E., Fischer J.E., Grammer T.B., Böhm B.O., März W. Vitamin D levels predict all-cause and card Risk and Cardiovascular Health (LURIC) Study. Diabetes Care. 2012;35:1158–1164. doi: 10.2337/dc11-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden C.E., Knopp R.H., Wahl P.W., Beach K.W., Strandness E., Jr. Sex differences in the effect of diabetes mellitus on lipoprotein triglyceride and cholesterol concentrations. N. Engl. J. Med. 1984;311:953–959. doi: 10.1056/NEJM198410113111505. [DOI] [PubMed] [Google Scholar]
- Wang J.H., Keisala T., Solakivi T., Minasyan A., Kalueff A.V., Tuohimaa P. Serum cholesterol and expression of ApoAI, LXR [beta] and SREBP2 in vitamin D receptor knock-out mice. J. Steroid Biochem. 2009;113:222–226. doi: 10.1016/j.jsbmb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2013. Diabetes, Fact Sheet 312. <http://www.who.int/mediacentre/factsheets/fs312/en/>.
- Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]


