Abstract
The present study aimed to investigate the efficacy of traditionally used anti-mastitis plants (Allium sativum, Bunium persicum, Oryza sativa and Triticum aestivum) in northwest Pakistan against bacterial pathogens. Selected plants were phytochemically screened for Alkaloids, Flavonoids, and Saponins and checked for in vitro antibacterial activity at concentration of 50 mg/ml against S. aureus, E. coli and K. pneumoniae by agar well diffusion method. Minimum inhibitory concentration and minimum bactericidal concentration was determined against multidrug resistant bacteria using tube dilution method. All extracts were found to significantly inhibit (p < 0.01, p < 0.05) the activity against bacterial strains examined. Among phytochemicals, alkaloids of all tested antimastitis plants produced significantly higher inhibition zones against bacteria. The minimum inhibitory concentration and minimum bactericidal concentration of phytochemicals and crude methanolic extracts against tested bacterial strains ranged between 12.5–50 mg/ml and 25–50 mg/ml, respectively. Medicinal plants traditionally used against mastitis are therapeutically active against bacterial pathogens. A. sativum and B. persicum were found to be potential candidate species for the development of novel veterinary drugs with low cost and fewer side effects.
Abbreviations: MIC, Minimum Inhibitory Concentration; MBC, Minimum Bactericidal Concentration; MDR, Multidrug resistant; ATCC, American Type Culture Collection
Keywords: Ethnomedicines, Phytochemicals, Dairy animals, Infectious disease, In vitro activities
1. Introduction
Mastitis is an infectious zoonotic disease of dairy animals categorized by pathological changes in the glandular tissues of milk producing organ (udder) and drastic changes in the taste, color and odor of milk (Ratafia, 1987). The main etiological agents for mastitis are Staphylococcus aureus, Escherichia coli and Klebsiella pneumonia (Dharajiya et al., 2012) while many other bacterial species like Streptococcus agalactiae, Corynebacterium pyogenes, Pseudomonas mendocina, Micrococcus pyogenes, Streptococcus dysgalactiae, Streptococcus parauberis and Streptococcus uberis are also reported as mastitogens (Watts, 1989, Sharif et al., 2009). Mastitis is prevalent in all types of animals, while it is commonly believed that every dairy cow and buffalo develops mastitis before death. It is also reported that cattle are at high risk of mastitis infection as compared to buffaloes and prevalence rate of mastitis in dairy cows is about 50% (Awale et al., 2012). In Pakistan the prevalence rate of mastitis in cows and buffaloes is about 93% and 48% respectively (Sharif et al., 2009). Those animals, which suffered from mastitis even once in life, will never return to their natural milk production state resulting in a great economic loss (Hamadani et al., 2014). Mastitis is associated with annual economic losses of about $35 billion worldwide and $2 billion in the United States (Mubarack et al., 2011). Although recent data of economic losses in Pakistan are unavailable however it was estimated that only in its one district the annual economic losses due to mastitis are $3.75 (Bilal et al., 2004). Mastitis is considered to be one of the most economically destructive diseases of dairy industries for both developed and developing nations including Pakistan. According to the field surveys on major animal diseases, mastitis ranked 1st in Pakistan and 2nd in district Dera Ismail Khan (Khan and Khan, 2006, Mussarat et al., 2014a, Mussarat et al., 2014b).
Livestock provides raw material to food industries of Pakistan with milk being most important. It has been estimated 75% of poor and food insecure peoples of Pakistan reside in rural areas and depend directly or indirectly on agriculture especially income comes from the production of milk (Dilshad et al., 2012). Different antibiotics are being used worldwide for the treatment of mastitogens and many bacterial mastitis pathogens are now resistant to various antibiotics. Drug residues in milk have a potential health hazards effects for the consumer and may cause allergic reactions, interference in the intestinal flora and resistant populations of bacteria in the general populations, thereby rendering antibiotic treatment ineffective (Bharti et al., 2012). Bacteria showing resistance to several antibiotics like Escherichia coli resistance to third-generation cephalosporins, Klebsiella pneumoniae resistance to third-generation cephalosporins and to carbapenems, Staphylococcus aureus resistance to beta-lactam antibacterial drugs.
The emerging potential of drug resistance of pathogens coupled with high cost and more side effects of antibiotics have drawn the interest of researchers and general population towards ethnomedicinal plants for the potential discovery of useful compounds (Hassan et al., 2014). A variety of plant species are being traditionally used in northwest Pakistan for the treatment of mastitis including Allium sativum, Bunium persicum, Oryza sativa and Triticum aestivum (Mussarat et al., 2014a, Mussarat et al., 2014b).
Present study was aimed to provide scientific validation to ethnomedicinal anti-mastitis plants against common mastitis pathogens through their phytochemical investigation and in vitro antimicrobial activities against American Type Culture Collection (ATCC) and Multidrug resistant (MDR) bacterial strains. The present research will provide baseline information to chemists and pharmacologists to further investigate these plants both in vitro and in vivo for the search of new bioactive compounds and production of novel veterinary drugs. The study will also provide scientific validation to traditional knowledge and increase the reliability of local farmers on ethnoveterinary practices.
2. Materials and methods
2.1. Study area
The present study was carried out in district Dera Ismail Khan, Khyber Pakhtunkhwa, northwest region of Pakistan. With an area of about 7326 km2, D.I. Khan is situated between 31°.15′ and 32°.32′N latitude and between 70°.11′ and 71°.20′E longitude. Most of the area is rural in nature, people’s lives in villages and depends on livestock for food and income generation. They use various medicinal plants to treat their animals due to expensive allopathic drugs in order to compensate with their income resources and to improve their livelihood (Mussarat et al., 2014a, Mussarat et al., 2014b). Dera Ismail Khan is an agricultural and maximum milk producing district of Khyber Pakhtunkhwa. Each farmer has minimum 4–5 number of livestock and milk yields between 4 and 8 l/day. Dominant plants of the region included Acacia modesta, Acacia nilotica, Morus alba, Calotropis procera, Eucalyptus camaldulensis and Ziziphus jujuba. Wheat, rice, sugarcane, dates and mangoes are the commonly cultivated crops in the area. Among these, “Village Dhakki dates” are the most famous product, which is used in the country as well as exported to the Middle East, Europe, India and United States (Mussarat et al., 2014a, Mussarat et al., 2014b). Due to mastitis majority of the animals got infected that ultimately leads toward low milk production and great economic loss at district as well as country level (Akhtar et al., 2012).
2.2. Anti-mastitis plants collection, identification and sample preparation
Data collection was done using structured questionnaires. Field visits were made for the documentation and collection of anti-mastitis medicinal plants. Among all the documented plants only four highly used plants (A. sativum, B. persicum, O. sativa and T. aestivum) were selected and for further in vitro screening. Ethnobotanical data on their botanical names, family names, habit, life form, part used, recipe formulation, mode of administration and types of animal treated were collected (Table 1). Selected plants were identified by the taxonomist at the department of Botany, Kohat University of science and Technology, Kohat, Pakistan. Vouchers numbers were given to the plant samples and deposited at the herbarium of department of Botany, Kohat University of science and Technology, Kohat. Collected samples were washed using tape water, dried and then sliced into tiny pieces. Sliced plant pieces were dried under the shade and then mashed into powder with the help of grinder. All powdered samples were preserved in dirt free closed glass container and stored until used for the quantitative determination of major phytochemicals (Alkaloids, Flavonoids and Saponins) and antibacterial activities.
Table 1.
Medicinal plants used to treat Mastitis in D.I. Khan.
| Plant/Family/Local name/Voucher No | Habit | Life form | Part used | Type of animal treated | Recipe | Dosage (g) | Mode of administration | Recovery days |
|---|---|---|---|---|---|---|---|---|
| Allium sativum L./Amaryllidaceae/Lehsan, Thoom/KUH-723 | Herb | Annual | Bulb | Cows, buffaloes, sheep, goats | Plant parts are grinded and mixed with butter | 100 | Oral | 7 |
| Bunium persicum (Boiss.) B. Fedtsch/Apiaceae/Kala zeera/KUH-786 | Herb | Annual | Seeds | Cows, buffaloes | Seeds are grinded in powder form and mixed with wheat flour | 80 | Oral | 7 |
| Oryza sativa L./Poaceae/Chawal/KUH-749 | Herb | Annual | Seeds | Cows, buffaloes | Decoction of seeds is given | 1–2 glass | Oral | 9 |
| Triticum aestivum L./Poaceae/Gandam, Kanak/KUH-760 | Herb | Annual | Seeds/Fruit | Cows, buffaloes | Seeds/Fruits are grinded to powder form and mix with fodder | 500 g | Oral | 8 |
2.3. Crude extract determination
200 ml methanol was taken in 250 ml of beaker and about 100 g of powdered plant samples were soaked in it. Then it was kept in a rotary shaker at about 30 °C for 24 h. After that plant sample was filtered through a Whatman filter paper and filtrate was centrifuged at 2000 rpm for about 10 min. The supernatant was collected and allowed to evaporate until completely dried (Lakshmi et al., 2013).
2.4. Alkaloids determination
A total of 5 grams of plant sample was taken into a 250 ml beaker and subjected to weight balance, after which 200 ml of 20% acetic acid in ethanol was poured into the beaker and was properly covered for four hours. Afterwards, filtration process was carried out and the resultant extract was concentrated with the help of water bath to one-quarter of its original volume. In next step concentrated ammonium hydroxide was taken and added in the extract drop wise until the precipitation was completed. Whole solution was kept for some time to settle down in bottom followed by the collection of precipitate using filtration, which was weighed on electric balance (Obadoni and Ochuko, 2001).
2.5. Flavonoids determination
Ten grams of plant sample was weighed and extracted in 100 ml of 80% aqueous methanol at room temperature. The whole solution was then filtered using Whatman filter paper (125 mm). The filtrate was then transferred into a crucible and evaporated to dryness over a water bath, and weighed (Harborne, 1973).
2.6. Saponins determination
Twenty grams of plant sample was mixed in 200 ml of 20% ethanol. The solution was heated on water bath for 4 h at 55 °C. Filtrate was obtained and the process was again repeated using another 200 ml of 20% ethanol solution. The resultant collective extracts were condensed to an amount of 40 ml over water bath at a temperature of 90 °C. The concentrate was then shifted into a separate funnel of 250 ml and added 20 ml of diethyl ether with constant shaking. Afterwards, ether layer was removed while aqueous layer was collected, in which 60 ml of n-butanol was added. The collective n-butanol extract was washed two times using 10 ml of 5% aqueous sodium chloride and the residual solution was heated on water bath. After evaporation, the remaining sample was dried using electric oven. Saponins concentration was measured in percentage (Boham and Kocipai, 1994).
2.7. In vitro antibacterial assay
Each of crude extract, alkaloids, flavonoids and saponins were dissolved in Dimethyl sulfoxide (DMSO) to obtain 50 mg/ml concentration. This is the initial concentration used for testing the antibacterial activities, however further this concentration was diluted for the determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC). Three multidrug resistant (MDR) bacterial strains (Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus) and American Type Culture Collection (ATCC) bacterial strain (E. coli (25922), K. pneumoniae (13889), S. aureus (29213)) were used and taken from the Department of Microbiology, KUST. A little amount of 0.25 g nutrient broth was dissolved in 15 ml of distilled water, then mixed completely and autoclaved at 121 °C for 15 min for sub-culturing of bacterial strains. Next day, the titre form culture appeared in small test tube and the McFarland Equivalence Standards were used to check the turbidity of culture. Antibacterial activity was carried out using agar well diffusion method (Parekh and Chanda, 2007). An amount of 11.4 g Muller Hinton Agar (MHA) was taken after weighting it on electronic balance and then added in 500 ml flask containing 300 ml distilled water and autoclaved. After autoclaving, the media was poured aseptically into Petri plates in laminar air flow cabinet.. In each Petri plate, 20 ml of the nutrient media was poured and allowed to solidify for about 10 min before wells formation through cork borer. The bacterial strains were spread with sterile swabs on the nutrient agar plates. Each Petri plate was punched in 5 wells with a sterile borer of 6 mm diameter. A little amount of 200 µl stock solutions were added to each respective well except the one well to which DMSO was added as a negative control. As a positive control, Meropenem standard disc (10 µg) was used against each strain. In order to avoid contamination, all the steps were carried out in the laminar flow hood and then plates were incubated for 24 h at 37 °C in incubator. Bacterial zones of inhibitions were measured in mm (Kirby et al., 1957).
2.8. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
MIC was determined as the lowest concentration of extracts that inhibited visible growth (turbidity) of the test organisms after 24 h (Davidson and Parish, 1989). MIC was investigated for the MDR bacterial strains, being sensitive to the plant extracts in the disc diffusion assay. MIC was carried out using tube dilution method as described by the Clinical and Laboratory Standards Institute (NCCLS, 2000). Three test tubes contained 3 ml of 12.5 mg/ml, 25 mg/ml and 50 mg/ml of extract in nutrient broth. One drop (0.025 ml) of the standardized bacteria was then suspended in each of the test tubes. Further, they were incubated at 37 °C for 15 to 24 h. The experiment was carried out in triplicates.
MBC is the lowest concentration of an antimicrobial agent needed to kill 99.9% of the initial inoculums. MBC of the plant extract was determined following the method of Spencer and Spencer (Spencer and Spencer, 2004). One milliliter of sample from the test tubes used in MIC, which did not show any visible growth, was streaked out on nutrient agar plates to determine the minimum concentration required to kill pathogens. The concentration of extract indicating a bactericidal effect after 24 h of incubation at 37 °C was regarded as MBC.
2.9. Data organization and statistical analysis
Data was organized and tabulated using Microsoft Word 2007 and Microsoft Excel 2007. Experiments were carried out in replicates and average zone of inhibition and standard deviations were calculated. Data was analyzed by using ANNOVA for the determination of statistical significance (p value) among the tested phytochemical classes of a single plant producing zones of inhibitions in a single bacterial strain. Moreover, t-test was applied to determine the significant difference among tested ATCC and MDR bacterial strains. ANNOVA and t-test were performed in SPSS, 2007.
3. Results
3.1. Ethnomedicinal plants used to treat mastitis
A. sativum, B. persicum, O. sativa and T. aestivum belong to three families namely Amaryllidaceae, Apiaceae and Poaceae. Different plant parts like seeds, rhizome, bulb, fruit and stem were used for traditional recipe formulation against mastitis that mostly administered orally to the livestock. Additives like water, sugar, milk and butter were used in ethnomedicinal recipe preparations for reducing astringent taste of the remedy while ensuring the intake of complete dosage of medication. All the four plants used to treat mastitis are herbs with annual life form. Cows and buffaloes were treated the most by using traditional medicines because mastitis is most commonly present in lactating animals (Table 1).
3.2. Quantitative evaluation of phytochemicals extracted from anti-mastitis plants
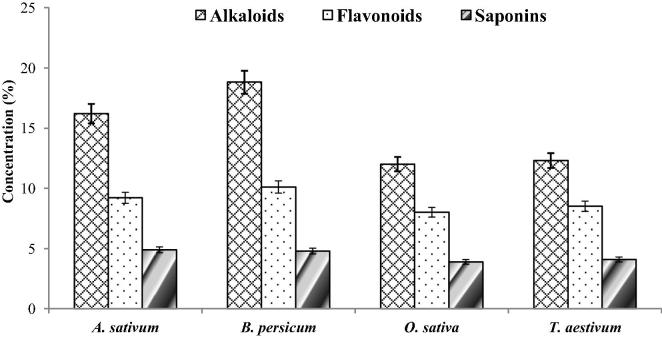
Alkaloids are present in large quantity in the selected anti-mastitis plants as compared to other secondary metabolites. Across the plant species, B. persicum contains higher concentration of alkaloids (18.8%) followed by A. sativum with 16.2%, T. aestivum with 12.3% and O. sativa with 12%. Flavonoids were observed second largest in quantity among the studied plant species after alkaloids. B. persicum was observed with the highest amount of flavonoids (10.1%) followed by A. sativum (9.2%). O. sativa and T. aestivum were observed with 8% and 8.5% flavonoids, respectively. Saponins were present in a very small quantity as compared to alkaloids and flavonoids among the selected plants. Highest saponins contents were found in A. sativum (4.9%) followed by B. persicum (4.8%), T. aestivum (4.1%) and O. sativa (3.9%) (Fig. 1).
Fig. 1.
Quantitative phytochemical evaluation of tested anti-mastitis plants. Concentration (%) represents the total amount of alkaloids, flavonoids and saponin in selected anti-mastitis plants.
3.3. Antibacterial activities
Alkaloids of A. sativum showed strong bacterial inhibition zones as compared to crude extract, flavonoids and saponins. Among the tested phytochemical classes, alkaloids produced significantly higher inhibition zones against K. pneumoniae ATCC (22 mm; p < 0.01), K. pneumoniae MDR (19 mm; p < 0.05), S. aureus ATCC (18.3 mm; p < 0.01) and S. aureus MDR (17.7 mm; p < 0.01) across the bacterial strains. Crude methanolic extract of A. sativum showed significantly higher inhibition zones against E. coli ATCC (21.3 mm; p < 0.01) and E. coli MDR (19 mm; p < 0.01) (Table 2; Fig. 3).
Table 2.
Bacterial zone of inhibition (mm) by phytochemicals of selected anti-mastitis plants at 50 mg/ml.
| Plant name | Phytoconstituents | E. coli (ATCC) | E. coli (MDR) | K. pneumoniae (ATCC) | K. pneumoniae (MDR) | S. aureus (ATCC) | S. aureus (MDR) |
|---|---|---|---|---|---|---|---|
| A. sativum | Alkaloids | 21 ± 1 | 18 ± 1 | 22 ± 2 | 19 ± 2 | 18.3 ± 1.5 | 17.7 ± 1.5 |
| Flavonoids | 16 ± 2.6 | 13 ± 1 | 13 ± 1 | 14 ± 1 | 11.7 ± 1.5 | 10.7 ± 2.1 | |
| Saponin | 14.3 ± 1.5 | 13.3 ± 2.3 | 14 ± 1 | 13.3 ± 2.1 | 11.7 ± 0.6 | 12 ± 1 | |
| Crude | 21.3 ± 1.5 | 19 ± 1 | 16.3 ± 1.5 | 16.3 ± 0.6 | 16.3 ± 1.5 | 17 ± 2 | |
| P value | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.05 | P < 0.01 | P < 0.01 | |
| B. persicum | Alkaloids | 19 ± 1 | 17 ± 1 | 21.3 ± 1.5 | 18.3 ± 2.5 | 18 ± 2 | 17.3 ± 1.5 |
| Flavonoids | 15.7 ± 2.1 | 16.7 ± 1.2 | 14.3 ± 1.5 | 12.3 ± 1.5 | 15 ± 1 | 10.3 ± 1.5 | |
| Saponin | 15.3 ± 0.6 | 19 ± 1 | 13.7 ± 1.5 | 13.7 ± 0.6 | 16.3 ± 1.5 | 12 ± 1 | |
| Crude | 20 ± 2 | 20.3 ± 2.1 | 18.7 ± 1.5 | 19.3 ± 1.5 | 19.3 ± 1.2 | 17 ± 1 | |
| P value | P < 0.05 | P < 0.05 | P < 0.01 | P < 0.01 | P < 0.05 | P < 0.01 | |
| O. sativa | Alkaloids | 14.3 ± 1.5 | 9.3 ± 0.6 | 12.3 ± 1.5 | 10 ± 1 | 11.7 ± 1.5 | 10.3 ± 1.5 |
| Flavonoids | 12.3 ± 1.5 | 8.7 ± 2.1 | 11.3 ± 1.5 | 6.8 ± 0.8 | 10 ± 1 | 8.7 ± 0.6 | |
| Saponin | 11 ± 1 | 12 ± 1 | 10 ± 1 | 7.8 ± 1.3 | 10 ± 2 | 9.7 ± 1.2 | |
| Crude | 12.3 ± 1.5 | 13 ± 1 | 11 ± 1 | 10.3 ± 1 | 13 ± 2 | 13 ± 1 | |
| P value | Ns | P < 0.01 | Ns | P < 0.05 | Ns | P < 0.01 | |
| T. aestivum | Alkaloids | 13.3 ± 1.5 | 12 ± 1 | 14 ± 2 | 11.7 ± 1.2 | 12.3 ± 2.1 | 9.3 ± 1.5 |
| Flavonoids | 11 ± 1 | 11.3 ± 1.5 | 11.3 ± 1.5 | 13 ± 1 | 11 ± 1 | 6.8 ± 0.8 | |
| Saponin | 12 ± 1 | 14.7 ± 0.6 | 10.3 ± 1.5 | 14 ± 2 | 12 ± 2 | 7.8 ± 1.3 | |
| Crude | 12.3 ± 1.5 | 9.7 ± 2.1 | 11.3 ± 1.5 | 13.7 ± 2.1 | 10.7 ± 1.5 | 10.3 ± 1.5 | |
| P value | Ns | P < 0.05 | Ns | Ns | Ns | P < 0.05 | |
| Antibiotic | 25 | 21 | 23 | 20 | 20 | 18 | |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | |
P value corresponds to ANOVA.
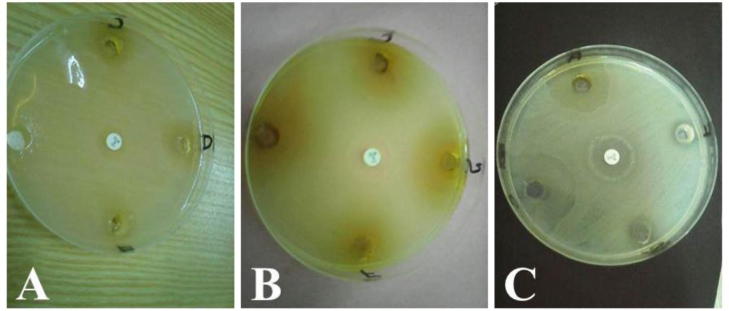
Fig. 3.
A–C. Antimicrobial activities of selected medicinal plants.
Alkaloids and crude extract B. persicum showed significantly higher zones of inhibitions across the phytochemical classes against the tested bacteria (Fig. 3). Significantly highest bacterial zone of inhibition for alkaloid extract was observed in K. pneumonia ATCC (21.3 mm; p < 0.01) followed by S. aureus MDR (17.3 mm; p < 0.01). Crude extract have shown significantly higher inhibition against E. coli MDR (20.3 mm; p < 0.05) and K. pneumonia MDR (19.3 mm; p < 0.01) (Table 2). Similar trends were also observed for the bacterial zone of inhibitions produced by the different extracts of O. sativa. Crude methanolic extract of O. sativa showed excellent antibacterial activity against the tested pathogens particularly E. coli MDR (13 mm; p < 0.01), K. pneumonia MDR (10.3 mm; p < 0.05) and S. aureus MDR (13 mm; p < 0.01). Among the tested phytochemical classes in T. aestivum, saponins has shown highest inhibitory activities against E. coli MDR (14.7 mm; p < 0.05) while crude extract has higher inhibitory activities against S. aureus MDR (10.3 mm; p < 0.05).
Across all the extracts of studied plant species, the values corresponding to bacterial inhibition zones produced by the alkaloids of A. sativum and B. persicum were close to the inhibition zones of standard antibiotics (Meropenem) indicating the efficacy of natural products. DMSO was used as negative control and did not show any activity against the bacterial strains (Table 2).
3.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MICs of A. sativum (alkaloids, flavonoids and saponins) against all MDR bacterial strains lies between 25 and 50 mg/ml while MICs of crude methanolic extract lies between 12.5 and 25 mg/ml. The MICs of B. persicum (alkaloids, flavonoids, saponins, and crude methanolic extract) against the studied MDR bacterial strains lies between 12.5 and 50 mg/ml. The MICs of O. sativa and T. aestivum (alkaloids, flavonoids, saponins and crude methanolic extract) against the MDR bacterial strains lies between 25 and 50 mg/ml (Table 3). On the other side, MBCs range of studied phytochemicals and crude methanolic extracts of A. sativum, B. persicum, O. sativa and T. aestivum lies between 50 and >50 mg/ml (Table 3).
Table 3.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of plant’s extracts against MDR isolates
| Plant name | Bacteria | Plant extracts |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Alkaloids (mg/ml) |
Flavonoids (mg/ml) |
Saponin (mg/ml) |
Crude (mg/ml) |
||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| A. sativum | E. coli | 25 | 50 | 50 | >50 | 50 | >50 | 12.5–25 | ≤50 |
| K. pneumoniae | 25 | 50 | 25 | 50 | 50 | >50 | 25 | 50 | |
| S. aureus | <25 | >50 | 50 | >50 | 50 | >50 | 25 | 50 | |
| A. persicum | E. coli | 25 | 50 | 50 | >50 | 50 | >50 | 12.5–25 | ≤50 |
| K. pneumoniae | 25 | 50 | 25 | 50 | 50 | >50 | 12.5–25 | ≤50 | |
| S. aureus | 50 | >50 | 50 | >50 | 50 | >50 | 25 | 50 | |
| O. sativa | E. coli | 50 | >50 | 50 | >50 | 25 | 50 | 25 | 50 |
| K. pneumoniae | 25 | 50 | 50 | >50 | 50 | >50 | 50 | >50 | |
| S. aureus | 50 | >50 | 50 | >50 | 50 | >50 | 25 | 50 | |
| T. aestivum | E. coli | 50 | >50 | 50 | >50 | 25 | 50 | 50 | >50 |
| K. pneumoniae | 50 | >50 | 25 | 50 | 25 | 50 | 25 | 50 | |
| S. aureus | 50 | >50 | 50 | >50 | 50 | >50 | 50 | >50 | |
3.5. Comparative analysis of ATCC and MDR strains in response to phytochemicals of anti-mastitis plants
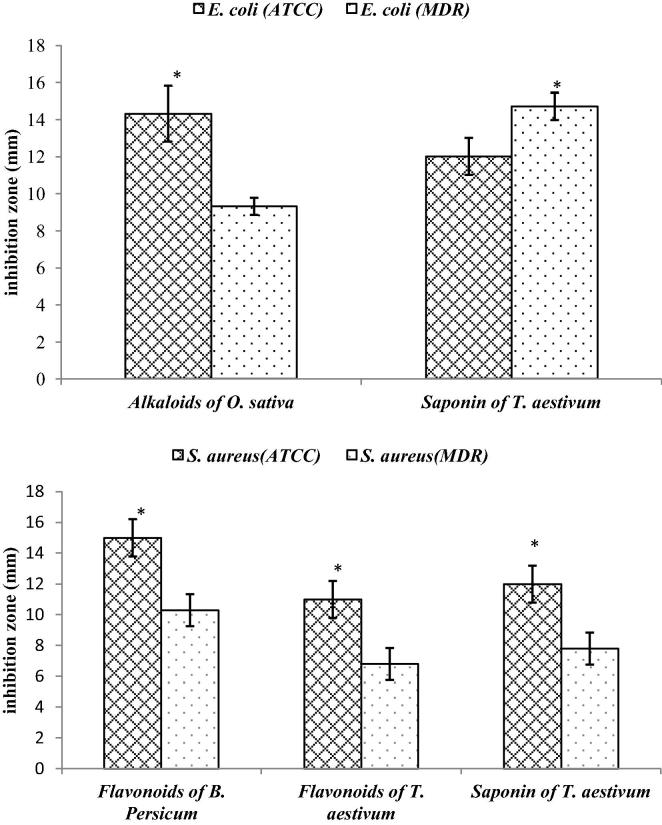
Alkaloids of O. sativa showed significantly higher inhibitory activity (P < 0.05) against E. coli ATCC (14.3 mm) than E. coli MDR (9.3 mm). In contrast, saponins of T. aestivum have shown significantly higher inhibitory activities (P < 0.05) against E. coli MDR (14.7 mm) than E. coli ATCC (12 mm). On the other hand side, flavonoids of B. persicum, flavonoids and saponins of T. aestivum showed significantly higher inhibitory activities against S. aureus ATCC than S. aureus MDR (Fig. 2).
Fig. 2.
Comparative analysis of ATCC and MDR strains in response to phytochemicals of anti-mastitis plants. Inhibition zones of extract at 50 mg/ml concentration. Each bar represents mean value of three independent replicates and the error bars shows standard error. * Represents significant difference at p < 0.05 (t-test).
4. Discussion
4.1. Mastitis and ethnomedicines
Ethnoveterinary practices are very common in Pakistan due to the poor socioeconomic status of the rural farmers (Dilshad et al., 2012). In D.I. Khan, local farmers used variety of medicinal plants especially A. sativum, B. persicum, O. sativa and T. aestivum for the treatment of mastitis in cows and buffaloes. The reason for high dependency on medicinal plants might be due to high cost of allopathic drugs, fewer resources for treating large population of animals, poor socioeconomic conditions of the farmer and their inaccessibility to the modern veterinary practices that are usually in city areas (Mussarat et al., 2014a, Mussarat et al., 2014b). Published literature shows that these medicinal plants are not only used for the treatment of mastitis in D.I. Khan but also in other regions of Pakistan (Dilshad et al., 2012). The high utilization of these plants in ethnoveterinary studies have been reported in various countries worldwide (Tamiru et al., 2013) that might be due to their higher abundance in different regions, their strong efficacy or due to strong traditional belief of local peoples. Poaceae has been reported as highly abundant family in ethnoveterinary practices in the study area. Documented literature shows that family Poaceae is the most diversified family in the world and ranks at fourth position in terms of species richness (Moshi et al., 2009) so that high utilization of its plants in the study area is not surprising. Different plant parts were used for recipe formulation but among four selected anti-mastitis plants seeds were most commonly used. A good reason for using seeds for the treatment of various ailments might be associated to their highest bioactivity because different secondary metabolites are present in ripened seeds. From ecological point of view, herbal formulation that involves stem, whole plant, bulb, roots, etc. have effect on plant life or survival of the mother plant (Yinegar et al., 2007). Seeds are the renewable parts of plant and their collection does not result in the fatality of the mother plants. Furthermore seeds can be stored and used in future. Protection harms due to plant collection may be less important when the collected plant parts are renewable. Traditional farmers have tremendous expertise in formulation of herbal remedies against mastitis. High utilization of herbaceous plants might be associated with their greater abundance, easy availability near households, and century’s old traditional knowledge of the local peoples. Majority of the investigators have also found herbaceous plants dominancy for ethnoveterinary purposes in Pakistan and other parts of the world (Mussarat et al., 2014a, Mussarat et al., 2014b, Haq, 2012).
Different techniques were used for the recipe formation and their common mode of administration was oral. The most preferred techniques for recipe preparation are decoction and powder formation in study area that might be due to easily administration of plants or efficacy of dissolved bioactive compounds in extract. These techniques are also used in other areas of Pakistan and elsewhere throughout the world (Yigezu et al., 2014, Murad et al., 2013). In traditionally used medicine individually plants are rarely used in their pure form, in most of studies there are some ingredients used with plants mixtures (Patel et al., 2011). Different additives like water and butter are used in ethnomedicinal preparations. These are mostly used to minimize the astringent taste of plants recipe, serve as vehicle to transport the remedies, for easy swallowing, to avoid toxic side effects, and ensure to intake complete dosage of medication. These ingredients are also taken in measured quantity and its quantity also depends on plants quantity similarly (Akintobi et al., 2013).
4.2. Activities of phytochemicals and crude extracts from anti-mastitis plants
Quantitative phytochemical analysis shows that A. sativum contains high concentration of alkaloids, flavonoids and saponins as compared to other tested anti-mastitis plants. Alkaloids and crude methanolic extracts of A. sativum showed excellent antibacterial activity against all selected bacterial pathogens comparable to other phytochemicals. Alkaloids showed higher inhibition zone (22 mm) against K. pneumoniae ATCC while crude methanolic extract showed higher inhibition zone (21.3 mm) against E. coli ATCC. Significant antibacterial activity of alkaloids might be due to presence in large quantity and its penetrating power in outer cell membranes of bacterial pathogens (Lewis and Ausubel, 2006). Most studies indicate that alkaloids are bactericidal and reducing the viability of gram positive and gram negative bacteria by penetrates reconstituted lipopolysaccharide monolayers, causes depolarisation of the cytoplasmic membrane, increases bacterial staining with the cell impermeable nucleic acid dye propidium iodide and causes leakage of cytoplasmic contents (Cushnie et al., 2014). The possible reason for highest antibacterial activity of crude methanolic extract is due to the presence of all phytochemicals and their synergistic effect against most common bacterial pathogens (Oroojalia et al., 2010). Methanol extraction of plant parts is highly followed throughout the world (Ncube et al., 2008) due to the polar nature of the solvent that result in easy degradation of cell wall and release of polyphenols from the plant cell (Shinwari et al., 2013). Other extracts like aqueous are usually less stable they get affected by micro organisms, acetone is highly fire sensitive and liver toxic, chloroform also shows liver toxicity and the mode of action seems to be that alcohol acts to keep the active components in solution after ingestion, thus facilitating their absorption into the bloodstream.
Crude methanolic extract and active phytochemicals of B. persicum were showed strong inhibition zone against E. coli, K. pneumoniae and S. aureus (ATCC and MDR). Results indicate the highest inhibitory activity of this plant is due to presence of secondary metabolites in large quantity. Crude extract of B. persicum showed sharp inhibition zone against all mastitis pathogens (E. coli, S. aureus, K. pneumoniae) as compared to alkaloids, flavonoids and saponins alone due to the presence of all phytochemicals. The synergistic effect of phytochemicals of a single plant or more than one plant against bacterial pathogens is evident from published literature (Aires et al., 2016). Studies conducted on phytochemical screening of essential oil from B. persicum shows the presence of various bioactive compounds with potent antibacterial activity. Essential oil of B. persicum was reported for their appreciable antimicrobial activity against several food borne pathogens namely E. coli and S. aureus (Manghani et al., 2011).
Alkaloids, flavonoids, saponins and crude extract of O. sativa seeds showed good antibacterial activity against most common mastitis pathogens. Various studies on the qualitative phytochemical screening of O. sativa shows the presence of various bioactive phytochemicals like terpenoids, alkaloids, steroids, flavonoids, sugar and phenolic compounds which are responsible for their antimicrobial activity (Firdous and Bharathi, 2014). Alkaloids of T. aestivum showed strong inhibition zone against both ATCC and MDR strains of K. pneumoniae, E. coli and S. aureus. As documented literature shows that alkaloids are most active phytoconstituents against various bacterial strains and act differently due to the difference in chemical composition of cell membrane (Lewis and Ausubel, 2006). The crude extract of T. aestivum showed good inhibition zone against all mastitis pathogens and showed similar results to the Jain and Argal (2014). Various studies reported the presence of bioactive phytochemicals like alkaloids; proteins, carbohydrates, phenolics, glycosides, tannins, fibers and saponins in this plant are responsible for their antimicrobial potential (Jain and Argal, 2014). These results provide scientific proof not only for the nutritional value of wheat but also for its medicinal value and various pharmacological activities. According to documented literature it is reported that the antimicrobial potential of wheat is due to the presence of high chlorophyll content in it (Balder et al., 2006).
Comparative analysis of ATCC and MDR strains in response to phytochemicals of anti-mastitis plants showed that alkaloids of O. sativa showed significantly higher inhibitory activity against E. coli ATCC (14.3 mm) than E. coli MDR (9.3 mm). This might be due to resistance potential of MDR isolates against antibiotics. Saponins of T. aestivum have shown significantly higher inhibitory activities against E. coli MDR (14.7 mm) than E. coli ATCC (12 mm). On the other hand side, flavonoids of B. persicum, flavonoids and saponins of T. aestivum showed significantly higher inhibitory activities against S. aureus ATCC than S. aureus MDR. Flavonoids and saponins showed statistically significant higher antibacterial activity as compared to other phytochemicals due to presence of various bioactive compounds in it. Crude methanolic extract of all selected antimastitis plants showed significant antibacterial activity at concentration of 50 mg/ml in comparison with active phytochemicals alone. Crude extract of A. sativum showed strong inhibition zone against all mastitis pathogens followed by B. persicum, T. aestivum and O. sativa. The sharp inhibition zone was measured for A. sativum crude extracts 21.3 mm against E. coli (ATCC). The MICs of A. sativum and B. persicum crude methanolic extract against all MDR bacterial strains lies between 12.5 and 25 mg/ml while MICs of alkaloids, flavonoids and saponins against the studied MDR bacterial strains lies between 25 and 50 mg/ml or 50 to >50 mg/ml concentrations as well there is good inhibition zone of crude extracts as compared to other phytoconstituents so there in crude extracts having active compounds with synergistic effects.
5. Conclusions
Medicinal plants which are being traditionally used as ethnoveterinary treatments are therapeutically active against most common bacterial pathogens causing mastitis. Quantitative phytochemical screening of selected anti-mastitis plants shows that they contain high concentration of phytochemicals especially alkaloids are present in large quantity. A. sativum and B. persicum contains highest concentration of phytochemicals as compared to other selected anti-mastitis plants. Among all selected plants alkaloids of A. sativum and B. persicum have shown significant antibacterial activity. Moreover, in comparison to MDR bacterial strains, the ATCC strains are showing more inhibition to the phytochemicals of tested plant species. A. sativum and B. persicum were found to be potential candidates for the development of novel veterinary drugs with low cost and fewer side effects.
6. Future recommendations
Literature is very scarce on phytocompounds directly checked against most common multidrug resistant bacteria causing mastitis. There is need to identify active phytocompounds present in the alkaloids, flavonoids and saponins of A. sativum and B. persicum and should be in vitro validated. Studies on in vivo trials should also be carried out to validate the efficacy of traditionally used medicinal plants. In addition, toxicology and mechanism of action of these plants extracts against mastitis pathogens need to be studied in detail. New antibiotics and therapeutics should be made from natural products and replaced with those showing resistant to bacterial pathogens. It is the time to increase scientific studies on unexplored medicinal plants traditionally used against mastitis, detailed phytochemical screening and in vivo activities of anti-mastitis plants that could lead toward development of novel veterinary drugs with low cost and fewer side effects. This will be a great contribution in improving animal’s health and agriculture economy of a country.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research group NO (RGP-271). The authors are indebted to the technicians at the laboratory of Zoology Department and Microbiology Department, KUST for providing necessary guidance and support during lab activities. The authors are also indebted to Kohat University of Science and Technology for financial support in carrying out this research.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aires A., Marrinhas E., Carvalho R., Dias C., Saavedra M.J. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus isolates. BioMed Res. Inter. 2016:1–11. doi: 10.1155/2016/5201879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar A., Habibullah, Ameer M., Habibullah, Hidayatullah M., Aeshad M. Prevalence of sub clinical mastitis in buffaloes in district D.I. Khan. Pak. J. Sci. 2012;64:159–160. [Google Scholar]
- Akintobi O.A., Nwanze J.C., Ogele J.O., Idowu A.A. Antimicrobial Activity of Allium sativum (Garlic) extract against some selected pathogenic bacteria. Nat. Sci. 2013;11(1) [Google Scholar]
- Awale M.M., Dudhatra G.B., Kumar A., Chauhan B.N., Kamani D.R., Modi C.M., Patel H.B., Mody S.K. Bovine mastitis: a threat to economy. Open Acc. Scienti. Report. I. 2012;1:295. [Google Scholar]
- Balder H.F., Vogel J., Weijenberg M.P., de-Brandt V. Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol. Biomark. Prev. 2006;15:717–725. doi: 10.1158/1055-9965.EPI-05-0772. [DOI] [PubMed] [Google Scholar]
- Bharti S.K., Sharma N.K., Gupta A.K., Murari K., Kumar A. Pharmacological actions and potential uses of diverse Galactogogues in Cattle. Inter. J. Pharmac. Therap. 2012;2 [Google Scholar]
- Bilal M.Q., Iqbal M.U., Muhammad G., Avais M., Sajid M.S. Factors affecting the prevalence of clinical mastitis in buffaloes around Faisalabad district (Pakistan) Int. J. Agric. Biol. 2004;6:185–187. [Google Scholar]
- Boham A.B., Kocipai A.C. Flavonoid and condensed tannins from leaves of Hawaiian vaccininumvaticulum and vicalycinium. Paci. Scient. J. 1994;48:458–463. [Google Scholar]
- Cushnie T.P.T., Cushnie B., Lamb A.J. Review Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agent. 2014;44:377–386. doi: 10.1016/j.ijantimicag.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Davidson P.M., Parish M.E. Methods for testing the efficacy of food antimicrobials. Food Technol. 1989;43:148–155. [Google Scholar]
- Dharajiya D., Moitra N., Patel B., Patel R.K. Preliminary phytochemical analysis of the Indian medicinal plants for antibacterial activity against bovine mastitis pathogens. Wayamba. J. Ani. Sci. 2012:332–342. [Google Scholar]
- Dilshad S.M.R., Rehman N.U., Ahmad N., Iqbal A. Documentation of ethnoveterinary practices for mastitis in dairy animals in Pakistan. Pak. Veter. J. 2012;30(3):167–171. [Google Scholar]
- Firdous S.J., Bharathi V. Phytochemical and antibacterial studies of Oryza Sativa. World J. Pharm. Sci. 2014;3(7):1136–1139. [Google Scholar]
- Hamadani H., Khan A.A., Banday M.T., Ashraf I. Bovine Mastitis: a disease of serious concern for dairy farmers. Int. J. Livestock Res. 2014;3:19. [Google Scholar]
- Haq F.U. The ethnobotanical uses of medicinal plants of Allai Valley Western Himalaya. Int. J. Plant Res. 2012;2:21–34. [Google Scholar]
- Harborne J.B. Chapman and Hall; London: 1973. Phytochemical Methods; p. 113. [Google Scholar]
- Hassan H.U., Murad W., Tariq A., Ahmad A. Ethnoveterinary study of medicinal plants in Malakand Valley, District Dir (Lower), Khyber Pakhtunkhwa, Pakistan. Irish Veter. J. 2014;67 doi: 10.1186/2046-0481-67-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain G., Argal A. Pharmacognostic and phytochemical investigation of young leaves of TriticumaestivumLinn. Int. Curr. Pharm. J. 2014;3(6):280–285. [Google Scholar]
- Khan M.Z., Khan A. Basic facts of mastitis in dairy animals: a review. Pak. Veter. J. 2006;26(4):204–208. [Google Scholar]
- Kirby W.M., Yoshihara G.M., Sundsted K.S., Warren J.H.J. Clinical usefulness of a single disc method for antibiotic sensitivity testing. Antibiotic. Ann. J. 1957:892–897. [PubMed] [Google Scholar]
- Lakshmi M.S., Kumar V.S., Deepika J., Begum F.I. Evaluation of antibacterial activity of methanol extract of leaves of Adhatodavasica on mastitis pathogens. Hygeia. J. D. Med. 2013;5:1–4. [Google Scholar]
- Lewis K., Ausubel F.M. Prospects of plant derived antibacterials. Nat. Biotechnol. 2006;24:1504–1507. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- Manghani E., Pareek A., Neggi R.S., Ojha C.K. Search for antimicrobial potentials from certain Indian medicinal plants. J. Med. Plant Res. 2011;5:295–301. [Google Scholar]
- Moshi M.J., Otieno D.F., Mbabazi P.K., Weisheit A. The ethnomedicine of the Haya people of Bugabo ward, Kagera Region, North Western Tanzania. J. Ethnobiol. Ethnomed. 2009;5:1–10. doi: 10.1186/1746-4269-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarack H.M., Doss A., Dhanabalan R., Venkataswamy R. In-vitro antimicrobial effects of some selected plants against bovine mastitis pathogens. Hygeia. J. D. Med. 2011;3:71–75. [Google Scholar]
- Murad W., Azizullah A., Adnan M., Tariq A. Ethnobotanical assessment of plant resources of Banda Daud Shah, District Karak, Pakistan. J. Ethnobiol. Ethnomed. 2013;9(77):1–10. doi: 10.1186/1746-4269-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussarat S., AbdEl-Salam N.M., Tariq A., Wazir S.M. Use of ethnomedicinal plants by the people living around Indus River. Evid. Based Compl. Alter. Med. 2014:1–14. doi: 10.1155/2014/212634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussarat S., Amber R., Tariq A., Adnan M. Ethnopharmacological assessment of medicinal plants used against livestock infections by the people living around Indus River. BioMed. Res. Int. 2014:1–14. doi: 10.1155/2014/616858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS), 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, M7–A5. Pennsylvania.
- Ncube N.S., Afolayan A.J., Okoh A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr. J. Biotechnol. 2008;7(12):1797–1806. [Google Scholar]
- Obadoni B.O., Ochuko P.O. Phytochemical studies and comparative efficacy of the crude extracts of some homeostatic plants in Edo and Delta States of Nigeria. Global, J. Pure, Appl. Sci. 2001;8:203–208. [Google Scholar]
- Oroojalia F., Kermanshshi R.K., Azizi M., Bassami M.R. Phytochemical composition of the essential oils is assessed on several food-borne pathogens. Food Chem. 2010;120:765–770. [Google Scholar]
- Parekh J., Chanda S.V. In-vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 2007;31:53–58. [Google Scholar]
- Patel H., Shah V., Upadhya U. New pharmaceutical excipients in solid dosage forms – a review. Int. J. Pharm. Life Sci. 2011;2(8):1006–1019. [Google Scholar]
- Ratafia M. Worldwide opportunities in genetically engineered vaccines. Biotechnol. J. 1987;5:1154–1158. [Google Scholar]
- Sharif A., Umer M., Muhammad G. Mastitis control in dairy production. J. Agri. Soc. Sci. 2009;5:102–105. [Google Scholar]
- Shinwari Z.K., Salima M., Rizwan F.R., Huda S., Asrar M. Biological screening of indigenous knowledge based plants used in diarrheal treatment. Pak. J. Bot. 2013;45(4):1375–1382. [Google Scholar]
- Spencer A.L.R., Spencer J.F.T. Human Press Inc.; New Jersey: 2004. Public Health Microbiology: Methods and Protocols; pp. 325–327. [Google Scholar]
- SPSS Inc . SPSS; Chicago, IL: 2007. SPSS Version 160 for Windows. [Google Scholar]
- Tamiru F., Terfa W., Kebede E., Dabessa G. Ethnoknowledge of plants used in veterinary practices in Dabo Hana District, West Ethiopia. J. Med. Plant. Res. 2013;7:2960–2971. [Google Scholar]
- Watts J.L. Evaluation of the Minitek gram-positive set for identification of Streptococci isolated from bovine mammary glands. J. Clin. Microbiol. 1989;27(5):1008–1010. doi: 10.1128/jcm.27.5.1008-1010.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigezu Y., Haile D.B., Ayen W.Y. Ethnoveterinary medicines in four districts of Jimma zone, Ethiopia: cross sectional survey for plant species and mode of use. BMC Veter. Res. 2014;10:76. doi: 10.1186/1746-6148-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinegar H., Kelbessa E., Bekele T., Lulekal E. Ethnoveterinary medicinal plants in Bale Mountains National Park, Ethiopia. J. Ethnopharmacol. 2007;112:55–70. doi: 10.1016/j.jep.2007.02.001. [DOI] [PubMed] [Google Scholar]