Abstract
The development of new drugs from plants is an interesting alternative approach to overcoming microbial resistance. Passiflora cincinnata shows resistance to diseases and pests and a higher concentration of chemical components that may be useful in the pharmaceutical industry. We investigated the potential antimicrobial and antibiotic-modifying activity of hydroalcoholic extracts of leaves, stems, bark, pulp and seeds of P. cincinnata. The extracts were prepared by homogenization of material in 50% ethanol. Minimum inhibitory concentration (MIC) was determined by the broth dilution method, and the bacterial strains tested were Staphylococcus aureus and Escherichia coli. Antibiotic-modifying activity was evaluated against the strains S. aureus 03 and E. coli 08, using a subinhibitory concentration of extract. The antibiotics tested were: amikacin, gentamicin, ampicillin, potassium benzylpenicillin and oxacillin. The extracts did not show antimicrobial activity of clinical relevance, where the MIC was equal to or greater than 1024 μg/mL. S. aureus showed 13 events, while E. coli showed only 4 events. Among these events, 14 involved synergistic activity, potentiating the effect of the antibiotics, and only 3 events demonstrated antagonistic activity toward ampicillin. Hydroalcoholic extracts are potential antimicrobial agents when combined with conventional drugs little utilized in in vivo treatment.
Keywords: Antibiotic-modifying activity, Antimicrobial activity, Medicinal plants, Passiflora, Passiflora cincinnata, Phytotherapeutic, Staphylococcus aureus, Escherichia coli
1. Introduction
The history of humanity, from the medical point of view, can be considered a battle against infectious diseases. In the beginning of the 21st century, the occurrence of bacterial infections resistant to drugs became common and research to develop new drugs with antimicrobial activity has spread to new clinical areas (Harbarth and Samore, 2005, Chin et al., 2012). Accordingly, natural products from plants are interesting alternatives for solving this problem, since many plant extracts and their phytochemical constituents are known to have antimicrobial activities (Coutinho et al., 2009a). In the last years, various studies in different countries have been conducted aimed at demonstrating this efficacy (Gibbons, 2004, Coutinho et al., 2009b).
Clinical microbiologists have two reasons for being interested in plant extracts with antimicrobial activity. In the first place, it is very likely that the phytochemicals found have a role in the arsenal of antimicrobial agents, since scientists know that the useful effective life of any antibiotic is limited. In the second place, the public is increasingly aware of the problems with the overprescription and misuse of conventional antibiotics. A large number of compounds from plants (often of reliable nature) are readily available without the need for a doctor’s prescription, in drug stores and natural food stores, where self-medication with these substances is common. The use of plant extracts as another form of medical treatment has gained popularity since the 1990s (Cowan, 1999).
The family Passifloraceae stands out in the Brazilian flora because of its extensive use in phytotherapy, and the genus Passiflora is the most found in the country. Passion fruit belongs to the genus, and this popular name designates more than 120 species (encountered and native) in Brazil (Barbosa, 2006).
Passiflora cincinnata Mast. is one of the wild species of the genus that has a great potential for raw consumption, the production of juice concentrate and utilization as functional food. Its high commercial yield, as well as content of phytochemical compounds, has stirred great interest in its research, besides diversified utilization. The phytochemical characteristics shown for this species are of interest to the food industry, aimed at developing those with bioactive substances to meet the needs of the consumer, and also to the pharmaceutical industry (Wondracec, 2009), which despite continuous efforts, has had difficulties in finding or developing new effective drugs, with the urgency needed (Vermelho et al., 2007).
Here, were evaluated the antimicrobial potential and antibiotic-modifying activity of hydroalcoholic extracts of leaves, stems, epicarp, pulp and seeds of P. cincinnata Mast., against standard and multiresistant strains of Staphylococcus aureus and Escherichia coli.
2. Materials and methods
2.1. Collection and preparation of plant material
Leaves, stems and fruits (epicarp, pulp and seeds) were collected in the municipality of Crato, Ceara, Brazil. The plant material was identified, and a dried specimen was deposited in the Herbario Caririense Dardano de Andrade-Lima of the Cariri Regional University (URCA), Crato, Ceara, Brazil, under identification No. HCDAL 8097.
The plant material collected was transported to the Pharmacology and Molecular Chemistry Laboratory (LFQM), URCA, where it was selected according to the degree of apparent health (the absence of mechanical damage and fungal spots), washed and air-dried until excess moisture was removed. The material was then processed, according to its physical characteristics, pulverizing it to increase the surface in contact with the extraction solvent. The plant mass obtained was extracted with 50% aqueous ethanol for 72 h. The extract was filtered and then concentrated using a rotary evaporator and warm-water bath. Afterward, the extracts were frozen and lyophilized (Matos, 1997).
2.2. Microbiological tests
The clinical isolates used were from the Clinical Mycology Laboratory of Universidade Federal da Paraiba, and were as follows: standard bacterial strains S. aureus ATCC 25923 and E. coli ATCC 11105 and multiresistant bacterial strains S. aureus 03 and E. coli 08. The aminoglycosides amikacin and gentamicin and beta-lactams ampicillin, potassium benzylpenicillin and oxacillin were utilized at a starting concentration of 5000 μg/mL. All drugs were dissolved in sterile water. Antibacterial susceptibility was determined using the broth microdilution method. The highest concentration of plant extract used in the tests was 1024 μg/mL, which was obtained by dissolving 0.010 g of each extract in 1 mL of dimethylsulfoxide (DMSO) and diluting with sterile distilled water to a test concentration of 100 mg/mL. The inoculum was diluted in 10% BHI (brain heart infusion) to give a concentration of 105 CFU/mL. A volume of 100 μL of BHI and inoculum was added to each well of a 96-well plate, followed by 100 μL of serial dilutions of extracts, at concentrations of 512 to 8 μg/mL. The plates were incubated for 24 h at 37 °C (Javadpour et al., 2013). Bacterial growth was assessed using resazurin to determine the minimum inhibitory concentration (MIC). MIC was defined as the lowest concentration at which no growth was observed according to NCCLS guidelines (2005). In the drug-modifying test, the method proposed by Coutinho et al. (2008) was utilized, where the extracts were tested using a subinhibitory concentration (MIC/8). A 100-μL mixture of 10% BHI, inoculum and extract was added to each well in the alphabetic order of the plate. Next, 100 μL of the drug were added to the first well, followed by 2-fold serial dilutions from the next to the last well. The concentrations of aminoglycosides and beta-lactams varied gradually from 5000 to 1.22 μg/mL.
3. Results
The hydroalcoholic extracts of leaves, stems, epicarp, pulp and seeds of P. cincinnata Mast. did not show antimicrobial activity of clinical relevance against the bacterial strains S. aureus ATCC 25923 and E. coli ATCC 11105, where the minimum inhibitory concentration was equal to or greater than 1024 μg/mL.
The extracts were tested at a subinhibitory concentration for antibiotic-modifying activity using the multiresistant strains S. aureus 03 and E. coli 08 treated with antibiotics of the aminoglycoside and beta-lactam classes. They showed significant effects compared to the activity of the antibiotic alone, in accordance with the resistance profile of the strains utilized (Table 1).
Table 1.
Origin of the bacterial strains and antibiotic resistance profile.
| Bacteria/origin | Resistance |
|---|---|
| Staphylococcus aureus 03 Eschar | Cefadroxil/Cephalexin/Cephalothin/Oxacillin/Penicillin/Ampicillin/Amoxicillin/Moxifloxacin /Ciprofloxacin/Levofloxacin Ampicillin + Sulbactam/Amoxicillin + Clavulanic Acid/Erythromycin/Clarithromycin Azithromycin/Clindamycin/Mupirocin |
| Escherichia coli 08 Uroculture | Ceftriaxone/Cephalothin/Cephalexin/Cefadroxil/Cefepime/Ciprofloxacin/Levofloxacin/Ampicillin + Sulbactam |
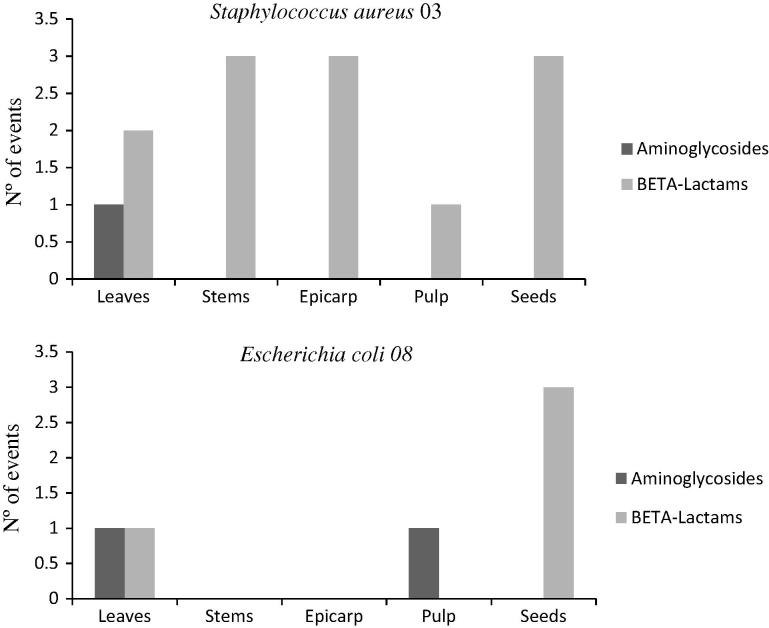
In analyzing the number of events where MIC was modified by the combination of antibiotic and extract, it was seen that S. aureus 03 showed 13 events of MIC modulation, while E. coli 08 showed only 4 events, taking into consideration all extracts combined with the two classes of antimicrobials tested (Fig.1). It is important to point out that among the 17 events observed, 14 were activities synergistic with the expected effect of the antibiotic and only 3 events showed activity antagonistic with the effect of ampicillin, namely with the strain S. aureus 03 and extracts of stems, epicarp and seeds of P. cincinnata (Table 2, Table 3, Table 4, Table 5, Table 6). The comparative analysis between the classes of antibiotics tested shows that the beta-lactams surpassed the aminoglycosides in the number of synergistic events and also in the number of antagonistic events (Table 7).
Figure 1.
Comparison of number of drug modifying events with extracts of P. cincinnata when combined with aminoglycosides and beta-lactams, against the strains S. aureus 03 and E. coli 08.
Table 2.
Antibiotic modifying activity of the hydroalcoholic extract of leaves of Passiflora cincinnata Mast. in combination with aminoglycosides and beta-lactams.
| Antibiotics |
Staphylococcus aureus 03 |
Escherichia coli 08 |
||
|---|---|---|---|---|
| Control | +EHFPC | Control | +EHFPC | |
| Amikacin | 312.5 | 312.5 | 156.25 | 39.06 |
| Gentamicin | 312.5 | 78.125 | 39.06 | 39.06 |
| Ampicillin | 156.25 | 156.25 | 312.5 | 312.5 |
| Benzylpenicillin | 2500 | 625 | 2500 | 2500 |
| Oxacillin | 2500 | 625 | ⩾5000 | 2500 |
∗ HELPC: hydroalcoholic extract of the leaves of Passiflora cincinnata Mast.
Table 3.
Antibiotic modifying activity of the hydroalcoholic extract of seeds of Passiflora cincinnata Mast. in combination with aminoglycosides and beta-lactams.
| Antibiotics |
Staphylococcus aureus 03 |
Escherichia coli 08 |
||
|---|---|---|---|---|
| Control | +EHSPC | Control | +EHSPC | |
| Amikacin | 625 | 625 | 78.125 | 78.125 |
| Gentamicin | 625 | 625 | 312.5 | 312.5 |
| Ampicillin | 78.125 | 312.5 | 312.5 | 312.5 |
| Benzylpenicillin | ⩾5000 | 1250 | ⩾5000 | 2500 |
| Oxacillin | 2500 | 625 | 2500 | 2500 |
∗ HESPC: hydroalcoholic extract of seeds of Passiflora cincinnata Mast.
Table 4.
Antibiotic modifying activity of the hydroalcoholic extract of stems of Passiflora cincinnata Mast. in combination with aminoglycosides and beta-lactams.
| Antibiotics |
Staphylococcus aureus 03 |
Escherichia coli 08 |
||
|---|---|---|---|---|
| Control | +EHHPC | Control | +EHHPC | |
| Amikacin | 625 | 625 | 78.125 | 78.125 |
| Gentamicin | 625 | 625 | 312.5 | 312.5 |
| Ampicillin | 78.125 | 312.5 | 312.5 | 312.5 |
| Benzylpenicillin | 2500 | 625 | 2500 | 2500 |
| Oxacillin | 2500 | 625 | 2500 | 2500 |
∗ HEStPC: hydroalcoholic extract of stems of Passiflora cincinnata Mast.
Table 5.
Antibiotic modifying activity of the hydroalcoholic extract of epicarp of Passiflora cincinnata Mast. in combination with aminoglycosides and beta-lactams.
| Antibiotics |
Staphylococcus aureus 03 |
Escherichia coli 08 |
||
|---|---|---|---|---|
| Controle | +EHEPC | Controle | +EHEPC | |
| Amicacina | 312.5 | 312.5 | 78.125 | 78.125 |
| Gentamicina | 625 | 625 | 312.5 | 312.5 |
| Ampicilina | 78.125 | 312.5 | 312.5 | 312.5 |
| Benzylpenicilina | 2500 | 625 | 2500 | 2500 |
| Oxacilina | 2500 | 625 | 2500 | 2500 |
∗ EHEPC: extrato hidroalcoólico do epicarpo de Passiflora cincinnata Mast.
Table 6.
Antibiotic modifying activity of the hydroalcoholic extract of pulp of Passiflora cincinnata Mast. in combination with aminoglycosides and beta-lactams.
| Antibiotics |
Staphylococcus aureus 03 |
Escherichia coli 08 |
||
|---|---|---|---|---|
| Control | +EHPpC | Control | +EHPpC | |
| Amikacin | 312.5 | 312.5 | 78.125 | 19.531 |
| Gentamicin | 312.5 | 312.5 | 39.06 | 39.06 |
| Ampicillin | 156.25 | 156.25 | 625 | 625 |
| Benzylpenicillin | 2500 | 625 | 2500 | 2500 |
| Oxacillin | 2500 | 2500 | 2500 | 2500 |
∗ HEPPC: hydroalcoholic extract of pulp of Passiflora cincinnata Mast.
Table 7.
Comparative study of antimicrobial drug activity of the pharmacological classes of aminoglycosides and beta-lactams when combined with hydroalcoholic extracts of aerial parts of P. cincinnata, with regard to their synergistic and antagonistic effects.
| Antimicrobials | S. aureus 03 | E. coli 08 | Total |
|---|---|---|---|
| Aminoglycosides | |||
| Amikacin | 0 | 2 synergism | 2 |
| Gentamicin | 1 synergism | 0 | 1 |
| BETA-Lactams | |||
| Ampicillin | 3 antagonisms | 0 | 3 |
| Benzylpenicillin | 5 synergismo | 1 synergism | 6 |
| Oxacillin | 4 synergisms | 1 synergism | 5 |
| Total | 13 | 04 | 17 |
4. Discussion
Phytochemical assays previously performed with hydroalcoholic extracts of the aerial parts (leaves, stems, epicarp, pulp and seeds) established that P. cincinnata possesses the following classes of secondary metabolites: condensed tannins, phlobaphenes, flavones, flavononols, flavonols, xanthones, chalcones, aurones, flavanones, leucoanthocyanidin, catechins and alkaloids. Since these phytoconstituents originate from the secondary metabolism of plants, they almost always act in plant defense against pathogens and may therefore have interesting biological activities (Simões et al., 2010).
Biological assays using combinations alone reveal that flavonoids have a strong impact on biological systems, demonstrating antimicrobial, antiviral, antiulcerogenic, cytotoxic, antineoplastic, antioxidant, antihepatotoxic, antihypertensive, hypolipidemic, antiinflammatory and antiplatelet effects (Machado et al., 2008). The activity demonstrated in our in vitro assays carried out with the hydroalcoholic extracts of P. cincinnata is likely due to the action of phenolic compounds present in all plant parts, confirming the antimicrobial activity attributed to this class of secondary metabolites. The combination of natural products with conventional antibiotics can exert a direct activity against many bacterial species, modulating or even increasing the activity of specific antibiotic, reversing the natural resistance of the bacteria. The potentiation of the activity or reversal of the resistance to antimicrobials, allows the classification of these compounds as modifiers of antibiotic activity (Coutinho et al., 2009a). The use of extracts is interesting because they show a low possibility of the microorganisms acquiring resistance to their action, since they are complex mixtures, making it difficult for microbial adaptation (Daferera et al., 2003).
S. aureus is one of the principal causes of infectious diseases in humans, associated with community and hospital origins, varying from minor infections of the skin to severe infections such as pneumonia and septicemia (Archer and Climo, 2013, Sugimoto et al., 2013). In the last years, the emergence of virulent strains resistant to various antibiotics, such as methicillin-resistant S. aureus (MRSA) and methicillin resistant staphylococcus (MRS), represents a major problem worldwide (Sugimoto et al., 2013, Zetola et al., 2005). Historically, the resistance to penicillinase-stable penicillins has been called “resistance to methicillin.” In the case of S. aureus resistant to oxacillin and coagulase-negative staphylococci (MRS), other β-lactams/β-lactamase inhibitors, cephems and carbapenems, appear to be active in vitro, but are clinically ineffective. The results for these drugs should be reported as resistant, or should not be reported. This is due to the fact that the majority of the reported cases of MRS infections have not responded adequately to therapy with β-lactams, or because relevant clinical data have not yet been presented documenting the clinical efficacy of these agents under these conditions (CLSI/NCCLS, 2005).
The bacterial strain S. aureus 03, utilized in the antibiotic-modifying experiments with the combination of aminoglycosides or beta-lactams and hydroalcoholic extracts of P. cincinnata is classified as a MRSA, showing a resistance profile for the antimicrobials utilized. The modulatory effect shown by the extracts, especially on the antimicrobials oxacillin and benzylpenicillin, was not capable of altering the phenotype of this strain from resistant to sensitive, but there was a decrease in MIC enough to impede the growth of the pathogen. The number of events was relevant, especially because at least 10 synergistic activities were repeated, in all the combination of extracts and antimicrobials in this test.
Considering that Gram-positive bacteria have a cell wall that is chemically less complex and has a lower lipid level than do Gram-negative bacteria (Loguercio et al., 2005), it is suggested that the structure of S. aureus (Gram-positive bacterium) can be a facilitating factor in the synergistic activity of the hydroalcoholic extracts of P. cincinnata with aminoglycosides and beta-lactams. Studies have shown that the inhibition of the growth of S. aureus can be associated with the ability of flavonoids to complex with the bacterial cell wall, causing rupture of the cell membrane (Cowan, 1999). Therefore, the combination of natural products with antibiotics favors the disintegration of bacterial cell membranes through complexation by agents associated with this structure.
The antagonistic effect observed with the extracts of stems, epicarp and seeds, when combined with ampicillin, can be explained by the mixture of compounds present in crude extracts, which contain higher amounts of metabolites capable of decreasing the effects of active substances (Peitz et al., 2003).
The emergence of MRS strains as serious human pathogens and their growing prevalence in nosocomial infections increase the necessity to identify and limit their spread. The detection of methicillin-resistant Staphylococcus strains is complex; there are difficulties in identifying them with precision, especially because resistance is frequently heterogeneous and its expression is affected by different factors (Chambers, 1997). Continuous efforts are needed to understand the epidemiological changes in S. aureus in humans and animals, not only for adequate and effective antimicrobial treatment to control the infection but also to follow the evolution of the species (Stefani et al., 2012).
E. coli is one of the best characterized model organisms. Some of its variants have been the key to advances in genetics, molecular biology, physiology and biochemistry, where they have the interesting characteristic of being commensal in the intestine of vertebrates and at the same time pathogens capable of killing more than 2 million people per year, through intestinal and extra-intestinal diseases (Kosek et al., 2003, Russo and Johnson, 2003). Therefore, it is the perfect candidate for the study of the transition between commensalism and pathogenicity (Pinheiro et al., 2007) or, more broadly, how the strict connection between bacteria and their host can fluctuate between mutualism, commensalism and opportunistic pathogen (Tenaillon et al., 2010).
E. coli is a facultative anaerobic, non-sporulating, Gram-negative bacterium, found in the intestinal microbiota, where it is the predominant microorganism in the gastrointestinal tract. Gram-negative bacteria have many resources of resistance, such as the production of beta-lactamases, which inactivate antibiotics that are resistant to the action of the majority of bacterial enzymes (Koneman et al., 2008). There is even a large variety of mechanisms of resistance to β-lactams, one of the most important being the production of beta-lactamases, which are enzymes capable of hydrolyzing the β-lactam ring of penicillins, cephalosporins and other related antimicrobials, resulting in their inactivation. Of special interest is extended-spectrum beta-lactamase (ESBL), mainly produced by some species of Gram-negative bacteria (Francisco and Jea, 2011). The first report of ESBL-producing strains occurred in Frankfurt, Germany, in 1983, where enzymes of the SHV type were isolated from Klebsiella pneumoniae and E. coli. The analysis of these strains demonstrated that resistance was due to a transferable beta-lactamase plasmid, derived from SHV-1, which was called SHV-2 (Sousa-Júnior et al., 2004).
ESBL-producing strains are generally multiresistant, and ESBL-producing enterobacteria have been isolated more often in samples from hospitalized patients, but they can also be found in samples of community origin (Sousa-Júnior et al., 2004). Despite the apparent in vitro sensitivity to various antimicrobials, patients with infections with ESBL strains may not respond to therapy with beta-lactams. Generally, these strains exhibit co-resistance to aminoglycosides, due to the use of these agents being based on the antibiogram (Sousa-Júnior et al., 2004).
A comparison of the results for E. coli versus S. aureus revealed a difference in drug susceptibility, explained by the difference in the composition of their membranes. The outer membrane of Gram-negative bacteria has a barrier resistant to the penetration of various antimicrobial agents, where it also harbors a periplasmic space containing enzymes capable of inactivating some antibiotics (Vermelho et al., 2007). This could account for the difference in vulnerability exhibited by the microorganisms, since drug-modifying activity with S. aureus was seen in 13 events of MIC reduction between synergism and antagonism, against aminoglycosides and beta-lactams. On the other hand, the combination of the same antimicrobials with P. cincinnata extracts against the ESBL-producing multiresistant strain E. coli 08, showed efficacy in only 4 events and only for the extracts of leaves, pulp and seeds, all with synergistic activity. Extracts of stems and bark did not produce any effect compared to controls.
Multiresistance in Gram-negative bacteria occurs through the accumulation of resistance plasmids or by horizontal gene transfer, associated with an outer membrane barrier of low permeability, with an efficient complex of efflux pumps (capable of pumping out of the bacteria more than one type of drug), combined with various specific mechanisms of resistance (Nikaido, 2009). In addition, the susceptibility of the bacterial cells to antibiotics can be affected by their physiological state. An important consequence of this phenomenon is the discovery of “persistent” cells, revealing that even high concentrations of antibiotics do not kill the whole bacterial population, leaving a resistant population that is genetically identical to the susceptible cells (Tikhonova et al., 2007).
We therefore conclude that the hydroalcoholic extract of P. cincinnata has potential as an antibacterial agent when combined with drugs that are little effective in the treatment of various human diseases but are often utilized by the public in general. Its profile of action is thereby altered by decreasing the MIC of conventional antibiotics, where this plant extract can be developed as a new therapeutic weapon, potentiating aminoglycosides and beta-lactams, which are not very effective in the treatment of infections caused by MRSA and ESBL strains.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ana Luiza A. Siebra, Email: analu_farm@yahoo.com.br.
Larissa R. Oliveira, Email: larissarolim.ufcg@hotmail.com.
Anita O.B.P.B. Martins, Email: anitaoliveira24@yahoo.com.br.
David C. Siebra, Email: david.siebra@outlook.com.
Rosimeire S. Albuquerque, Email: rosi_sabino87@hotmail.com.
Izabel Cristina Santiago Lemos, Email: izabel_santiago@hotmail.com.
Gyllyandeson A. Delmondes, Email: gyllyandesondelmondes@hotmail.com.
Saulo R. Tintino, Email: saulorelison@gmail.com.
Fernando G. Figueredo, Email: fgfigueredo@gmail.com.
Jose Galberto M. da Costa, Email: galberto@urca.br.
Henrique D.M. Coutinho, Email: hdmcoutinho@gmail.com.
Irwin R.A. Menezes, Email: irwinalencar@yahoo.com.br.
Cicero F.B. Felipe, Email: cicerof@hotmail.com.
Marta R. Kerntopf, Email: martareginakerntopfm@outlook.com.
References
- Archer G.L., Climo M.W. Staphylococcus aureus bacteremia − consider the source. N. Engl. J. Med. 2013;344:55–56. doi: 10.1056/NEJM200101043440110. In: Sugimoto, S., Iwamoto, T., Takada, K., Okuda, K., Tajima, A., Iwase, T., Mizunoe, Y., 2013. Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J. Bacteriol., vol. 155, no. 10, pp. 1–46. [DOI] [PubMed] [Google Scholar]
- Barbosa, P.R., 2006. Estudo da Ação Psicofarmacológica de Extratos de Passiflora alata Dryander e Passiflora edulis Sims. Dissertação (Mestrado). Universidade do Extremo Sul Catarinense, Criciúma – Santa Catarina, 79p.
- Chambers H.F. Methicilin resistance in Staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Y.P., Tsui K.C., Chen M.C., Wang C.Y., Yang C.Y., Lin Y.L. Bactericidal activity of soymilk fermentation broth by in vitro and animal models. J. Med. Food. 2012;15(6):520–526. doi: 10.1089/jmf.2011.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI/NCCLS, 2005. Clinical and Laboratory Standards Institute/National Comitee for Clinical Laboratory Standards document M100-S15. National Committee for Clinical Laboratory Standards, Wayne.
- Coutinho H.D.M., Costa J.G.M., Lima E.O., Falcão-Silva V.S., Siqueira-Júnior Enhancement on the antibiotic activity against a Multiresistant Escherichia coli by Mentha arvensis L. and Chlorpromazine. Chemotherapy. 2008;54:328–330. doi: 10.1159/000151267. [DOI] [PubMed] [Google Scholar]
- Coutinho H.D.M., Costa J.G.M., Lima O.E., Falcão-Silva V.S., Junior-Siqueira J.P. In vitro interference of Momordica charantia in the resistance to aminoglycosides. Pharm. Biol. 2009;47:1056–1059. [Google Scholar]
- Coutinho H.D., Costa J.G., Lima E.O., Falcão-Silva V.S., Siqueira-Júnior J.P. Potentiating effect of Mentha avensis and chlorpromazine in the resistance to aminoglycosides of methicillin-resistant Staphylococcus aureus. In Vivo. 2009;23:287–290. [PubMed] [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12(4):564–581. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daferera D.J., Ziogas B.N., Polissiou M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop. Prot. 2003;22:39–44. [Google Scholar]
- Francisco, W., Jea, A.H.Y., 2011. Resistência a b-lactamases por presença de ESBL. Disponível em: <www.fleury.com.br/mednews/0301/mdcontfcb0302.htm>. Acesso em: 15 Nov. 2009. In: Martins, A.C., Picoli, S.U., Métodos alternativos para detecção de betalactamase de espectro estendido em Escherichia coli e Klebsiella pneumoniae. J. Bras. Patol. Med. Lab., vol. 47, no. 4, pp. 421–426.
- Gibbons S. Anti-staphylococcal plant natural products. Nat. Prod. Rep. 2004;21:263–277. doi: 10.1039/b212695h. [DOI] [PubMed] [Google Scholar]
- Harbarth S., Samore M.H. Antimicrobial resistance determinants and future control. Emerging Infect. Dis. 2005;11:794–801. doi: 10.3201/eid1106.050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadpour M.M., Juban M.M., Lo W.C., Bishop S.M., Alberty J.B., Cowell S.M., Becker C.L., Mclaughlin M.L. Antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 2013;39:107–3113. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- Koneman E.W., Allen S.D., Janda W.M., Schreckenberger P.C., Winn W.C. sixth ed. Editora Guanabara Koogan; 2008. Koneman Diagnóstico Microbiológico. Texto e Atlas Colorido. [Google Scholar]
- Kosek M., Bern C., Guerrant R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- Loguercio A.P., Battistin A., Vargas A.C.D.E., Henzel A., Witt N.M. Atividade antibacteriana do extrato hidroalcoólico de folhas de jambolão (Syzygium cumini L. Skells) Ciênci Rural. 2005;35:371–376. [Google Scholar]
- Machado H., Nagem T.J., Peters V.M., Fonseca C.S., Oliveira T.T. Flavonóides e seu potencial terapêutico. Bol Cent Biol Reprod. 2008;27:33–39. [Google Scholar]
- Matos F.J.A. second ed. Editora UFC; Fortaleza-CE: 1997. Introdução à Fitoquímica Experimental. [Google Scholar]
- NCCLS. National Comitee for Clinical Laboratory Standards . sixth ed. National Committee for Clinical Laboratory Standards; Wayne: 2005. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. [Google Scholar]
- Nikaido H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitz C., Cúnico M.M., Miguel O.G., MigueL M.D., Kerber V.A. Avaliação da atividade antibacteriana e triagem fitoquímica das folhas de Acacia longifólia (Andr.) Willd. (Leguminosae) Rev. Bras. Farmacogn. 2003;13:61–65. [Google Scholar]
- Pinheiro S.F. CD16 promotes Escherichia coli sepsis through an FcRγ inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat. Med. 2007;13:1368–1374. doi: 10.1038/nm1665. [DOI] [PubMed] [Google Scholar]
- Russo T.A., Johnson J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Simões C.M.O., Schenkel E.P., Gosmann G., Mello J.C.P., Mentz L.A., Petrovick P.R. sixth ed. Editora da UFRGS/Editora da UFSC; Porto Alegre/Florianópolis: 2010. Farmacognosia: da planta ao medicamento. [Google Scholar]
- Sousa-Júnior M.A., Ferreira E.S., Conceição G.C. Betalactamase de espectro ampliado (ESBL): um importante mecanismo de resistência bacteriana e sua detecção no laboratório clínico. NewsLab. 2004;63:152–174. [Google Scholar]
- Stefani S., Chung D.R., Lindsay J.A., Friedrich A.W., Kearns A.M., Westh H., MacKenzie F.M. Meticilinresistant Staphylococcus aureus (MRSA): global epidemiology and harmonization of typing methods. Int. J. Antimicrob. Agents. 2012;39:273–282. doi: 10.1016/j.ijantimicag.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Sugimoto S., Iwamoto T., Takada K., Okuda K., Tajima A., Iwase T., Mizunoe Y. Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J. Bacteriol. 2013;155(10):1–46. doi: 10.1128/JB.01672-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenaillon O., Skurnik D., Picard B., Denamur E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010;8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- Tikhonova E.B., Devroy V.K., Lau S.Y., Zgurskaya H.I. Reconstitution of the Escherichia coli macrolide transporter: the periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB. Mol. Microbiol. 2007;63:895–910. doi: 10.1111/j.1365-2958.2006.05549.x. [DOI] [PubMed] [Google Scholar]
- Vermelho A.B., Bastos M.C.F., Branquinha M. Guanabara Koogan; Rio de Janeiro: 2007. Bacteriologia Geral. [Google Scholar]
- Wondracec, D.C., 2009. Caracterização e Diversidade Genética de Acessos de Maracujá do Cerrado com Base no Perfil de Carotenoides. Dissertação (Mestrado). Universidade de Brasília. Faculdade de Agronomia e Medicina Veterinária. Brasília – Distrito Federal: 101.
- Zetola N., Francis J.S., Nuermberger E.L., Bishai W.R. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. In: Sugimoto, S., Iwamoto, T., Takada, K., Okuda, K., Tajima, A., Iwase, T., Mizunoe, Y., 2013. Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J. Bacteriol. vol. 155, no. 10, pp. 1–46. [DOI] [PubMed] [Google Scholar]