Abstract
Blue tilapia, Oreochromis aureus, was experimentally infected with Aeromonas hydrophila, a bacterium that damages the gills, liver, and intestine, resulting in histopathological changes in the infected organs. Our histopathological study showed an aggregation of hemocytes with cell necrosis in gills; a massive aggregation of hemocytes and pyknotic nuclei in the hepatopancreas; and a lower rate of hemocyte aggregation in the digestive system of the infected fish.
Keywords: Freshwater fish, Bacterial disease, Hemocyte aggregations
1. Introduction
Tilapia is the second most farmed fish worldwide, and its production has multiplied over the past decade because of its suitability for aquaculture, good marketability and stable prices (Wang and Lu, 2016). Aquaculture is hampered by a wide range of microorganisms causing diseases (Van Hai, 2015). Aeromonas hydrophila is a bacterial pathogen that results in high economic losses causing a disease known as motile septicemia or hemorrhagic septicemia.
A. hydrophila, as a food-borne pathogen, causes zoonotic diseases (Guz and Kozinska, 2004). It can cause local pathology to the host tissue. The host’s reaction may be in the form of tissue proliferation, degeneration and inflammation (Stratev et al., 2015). A. hydrophila can be a pathogenic agent not only for fish, amphibians and reptiles, but also for mammals, including humans (Aoki, 1999, Janda and Abbott, 2010, Lallier and Higgins, 1988, Plumb and Hanson, 2011, Stratev et al., 2015, Trott, 2006). A. hydrophila can be isolated from estuarine waters, marine, brackish and freshwaters (Aoki, 1999, Dias et al., 2016, Janda and Abbott, 2010, Lallier and Higgins, 1988, Trott, 2006). Freshwater environments, especially with a high organic load, are normally considered to be natural habitats of A. hydrophila, but recent evidence from Japan and USA suggest that it may also be a part of the resident gut flora of fish (Sreedharan et al., 2012).
Motile Aeromonas septicemia displays chronic characteristics that persist for weeks, during which time the mortality rate increases gradually and the cumulative mortality can be high (Plumb and Hanson, 2011, Zhang et al., 2016). A. hydrophila pathological findings have recently been studied on other fish species (Abdelhamed et al., 2017, Hamid et al., 2017, Kumar et al., 2016).
Degenerative histopathological changes were commonly observed in organs such as kidney, liver, gills, stomach and spleen. Other Aeromonas spp., such as A. jandaei and A. veronii, have also been studied recently and similar clinical signs and histological manifestation have been observed (Dong et al., 2017).
Aeromonads are considered to be opportunistic pathogens, capable of causing diseases only in weakened fish populations or as secondary invaders in fish suffering from other diseases (Roberts, 2012). The pathogenic A. hydrophila is common in freshwater habitats worldwide, and frequently causes disease in cultured and feral fish (Laith et al., 2014). An outbreak of motile Aeromonas septicemia in channel catfish (Ictalurus punctatus) in USA farms has stepped up Aeromonas studies (Abdelhamed et al., 2017, Baumgartner et al., 2017, Peatman et al., 2017, Zhang et al., 2016) and increased the need to study this possibly highly virulent A. hydrophila also in other important fish species. The aim of this study was to identify histopathological symptoms caused by bacterial A. hydrophila in the organs of blue tilapia Oreochromis aureus.
2. Materials and methods
Skin samples from A. hydrophila infected blue tilapia from ponds of the Marine Center Science, Unversity of Basrah, were transferred to tryptic soy agar (TSA) medium and incubated, resulting in the growth of many bacterial colonies. A. hydrophila was identified according to (Hossain et al., 2013); macroscopic observations included size, shape and pigmentation seen from inside, beside and top; microscopic analysis was done by Gram staining and observing the color and shape of bacteria; biochemical tests were: Motile, indol, O/F and H2S. A specific medium, Rimler-Shotts Agar, was used to confirm the isolated bacteria (Sayed, 2010). Cell densities were photometrically obtained at a wavelength of 590 nm (Saulnier et al., 2000), based on the McFarland standard and serially diluted to reach the target density (1 × 107 CFU). A total of 20 fish was used for the laboratory experiments from October 2015 to February 2016 (total weight 80–90 g). Four ponds, where fish were farmed, were sampled, five blue tilapia from each pond. Fifteen fish chosen randomly were injected with the dose of 1 × 107 CFU per fish (injected intraperitoneally with 0.5 ml); this dose was selected as the challenger dose according to Brooks et al. (2015). Five fish were used as controls. The blue tilapia was monitored for 10 days after being infected to study the histopathological changes in their organs.
For the histopathological study, tissue specimens of the skin, gills, liver, and intestine were excised, rinsed in normal saline, and fixed in a 10% formalin buffer for 24 h. After fixation, the tissues were dehydrated in an alcohol series of ascending concentration (70%, 80%, 90%, and 100%, respectively), embedded in paraffin and sectioned at 5 µm and the tissue sections were stained with hematoxylin-eosin (H&E).
3. Results and discussion
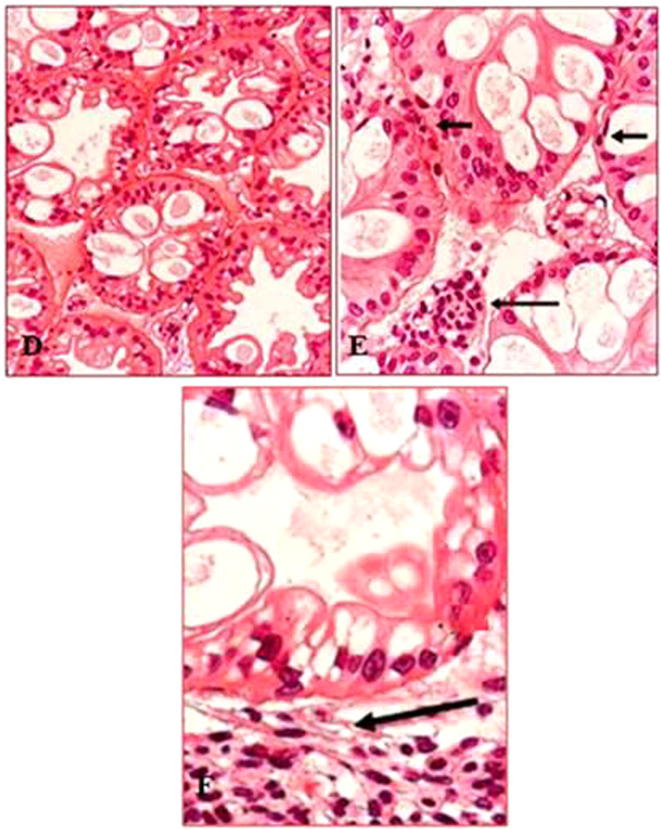
The histopathological study showed degraded hemocytes with cell necrosis and aggregation in gill tissue (Fig. 1A –C); a massive aggregation of hemocytes and pyknotic nuclei in the hepatopancreas (Fig. 2D –F); and a hemocyte aggregation in the digestive system (Fig. 3G, H) of the infected fish.
Fig. 1.
Pathological changes in lamellae tissues of blue tilapia. A: Normal gill lamella in control fishes, (H&E, X 1000). B: Infected gill lamella, hemocyte aggregation (arrow) (H&E, X1000), C: Necrosis and hemocyte aggregation (H&E, X 1000).
Fig. 2.
Pathological changes in the haepatopancreas of blue tilapia. D: Normal in cells of the haepatopancreas in control fishes (H&E, X 1000). E: Infected haepatopancreas, hemocyte aggregation (long arrow) and pyknotic nuclei (small arrow) (H&E, X 1000). F: Massive hemocytes aggregations are infiltrated (arrow) (H&E, X 1000).
Fig. 3.
Pathological changes in the digestive tract of blue tilapia. G: Normal digestive tract tissue in control fishes (H&E, X 400). H: Infected fish, Hemocyte infiltration in digestive system (arrow) (H&E, X 1000).
The histopathological results of A. hydrophila infection in blue tilapia showed massive hemocyte aggregations in the hepatopancreas and fewer aggregations in the gills and digestive system, which may be caused by stress. Abundant hemocyte aggregations in the hepatopancreas indicated that the hepatopancreas is possibly the target organ for hemocyte aggregation and bacterial pathogenesis.
Our findings were much similar to the symptoms found in other fish species (Abdelhamed et al., 2017, Alsaphar and Al-Faragi, 2012, Cipriano et al., 1984, Khamees et al., 2013, Kumar et al., 2016, Stratev et al., 2015). Many studies showed that the chronic infections of A. hydrophila have led to dermal ulceration lesions with focal hemorrhages and inflammation. The arrangement of hepatocytes in the liver has shown some cells with vacuolation and severe necrosis. Our results were also similar to those of Afifi et al. (2000), where toxins and extracellular products produced by A. hydrophila, such as hemolysin, protease, and elastase, caused severe necrosis in the liver. Histopathological studies on Nile tilapia (O. niloticus) with bacterial septicemia indicated perivascular cuffing of haepatopancreatic hemolymph vessels and granulocytic hemocyte aggregation in the heart, gills, haepatopancreas, antennal gland, abdominal muscle, and connective tissue (Afifi et al., 2000, Yardimci and Aydin, 2011).
Stratev et al. (2015) noted that the most histopathological damage was seen in the functional epithelium of the liver and kidneys, followed by the intestines and heart. In addition, they found various hemorrhages in the interstitial tissues of visceral organs and some skin hemorrhages on the ventral surface of the body and anal regions. Generally, pathological damage included degenerative changes such as cloudy swelling, granular and/or hyaline droplet degeneration, or vacuolation in the functional epithelium of the respective internal organs.
4. Conclusions
This study suggests that freshwater fishes could always be under the threat of Aeromonas infection, since the species are common in freshwater environments. Aeromonas spp. might also pose a threat to public health, especially to people who come into contact with diseased fish. The study clearly shows that typical histopathological alterations seen in the functional epithelium of the gill, liver, and intestinal tissues are good biomarkers for the field assessment of Aeromonas bacterial disease in blue tilapia.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group NO (RGP-1438-029).
Acknowledgments
Authors contributions
The entire manuscript was prepared by the Authors.
Conflict of interest
Authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelhamed H., Ibrahim I., Baumgartner W., Lawrence M.L., Karsi A. Characterization of histopathological and ultrastructural changes in channel catfish experimentally infected with virulent Aeromonas hydrophila. Front. Microbiol. 2017;8:1519. doi: 10.3389/fmicb.2017.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifi S.H., Al-Thobiati S., Hazaa M.S. Bacteriological and histopathological studies on Aeromonas hydrophila infection of Nile tilapia (Oreochromis niloticus) from fish farms in Saudi Arabia. Assiut. Vet. Med. J. 2000;42:195–205. [Google Scholar]
- Alsaphar S.A.A., Al-Faragi J.K.H. Detection and study of the experimental infection of Aeromonas strain in the common carp (Cyprinus carpio L.) Iraqi J. Vet. Med. 2012;36:222–230. [Google Scholar]
- Aoki, T., 1999. Motile aeromonads (Aeromonas hydrophila).
- Baumgartner W.A., Ford L., Hanson L. Lesions caused by virulent Aeromonas hydrophila in farmed catfish (Ictalurus punctatus and I. punctatus × I. furcatus) in Mississippi. J. Vet. Diagnostic Investig. 2017 doi: 10.1177/1040638717708584. [DOI] [PubMed] [Google Scholar]
- Brooks G., Carroll K., Butel J., Morse S., Mietzner T. McGraw Hill Medical; New York: 2015. Jawetz, Melnick, Adelberg Medical Microbiology. [Google Scholar]
- Cipriano, R.C., Bullock, G.L., Pyle, S.W., 1984. Aeromonas Hydrophila and Motile Aeromonad Septicemias of Fish. US Fish & Wildlife Publications, 134.
- Dias M.K.R., Sampaio L.S., Proietti-Junior A.A., Yoshioka E.T.O., Rodrigues D.P., Rodriguez A.F.R., Ribeiro R.A., Faria F.S., Ozório R.O.A., Tavares-Dias M. Lethal dose and clinical signs of Aeromonas hydrophila in Arapaima gigas (Arapaimidae), the giant fish from Amazon. Vet. Microbiol. 2016;188:12–15. doi: 10.1016/j.vetmic.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Dong H.T., Techatanakitarnan C., Jindakittikul P., Thaiprayoon A., Taengphu S., Charoensapsri W., Khunrae P., Rattanarojpong T., Senapin S. Aeromonas jandaei and Aeromonas veronii caused disease and mortality in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2017 doi: 10.1111/jfd.12617. [DOI] [PubMed] [Google Scholar]
- Guz L., Kozinska A. Antibiotic susceptibility of Aeromonas hydrophila and A. sobria isolated from farmed carp (Cyprinus carpio L.) Bull. Vet. Inst. Pulawy. 2004;48:391–395. [Google Scholar]
- Hamid, N.H., Hassan, M.D., Sabri, M.Y.M., Hasliza, A.H., Hamdan, R.H., Afifah, M.N.F., Raina, M.S., Nadia, A.B.S., Fuad, M.M., 2017. Studies on Pathogenicity effect of Aeromonas hydrophila infection in juvenile red hybrid tilapia Oreochromis sp. In: Proceedings of International Seminar on Livestock Production and Veterinary Technology, pp. 532–539.
- Hossain M.F., Rahman M.M., Sayed M.A. Experimental infection of indigenous climbing perch Anabas testudineus with Aeromonas hydrophila bacteria. Progress. Agric. 2013;22:105–114. [Google Scholar]
- Janda J.M., Abbott S.L. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010;23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamees E.S., Al-Rudainy A.J., Faleh E.B. Study of histopathological changes in the common carp (Cyprinus carpio) experimentally infected by bacteria Aeromonas hydrophila. Basrah J. Agric. Sci. 2013;26:193–204. [Google Scholar]
- Kumar R., Pande V., Singh L., Sharma L., Saxena N. Pathological findings of experimental Aeromonas hydrophila infection in golden mahseer (Tor putitora) Fish Aquacult. J. 2016;7:160. [Google Scholar]
- Laith A.R., Najiah M. Aeromonas hydrophila: antimicrobial susceptibility and histopathology of isolates from diseased catfish, Clarias gariepinus (Burchell) J. Aquac. Res. Dev. 2014:5. [Google Scholar]
- Lallier R., Higgins R. Biochemical and toxigenic characteristics of Aeromonas spp. isolated from diseased mammals, moribund and healthy fish. Vet. Microbiol. 1988;18:63–71. doi: 10.1016/0378-1135(88)90116-2. [DOI] [PubMed] [Google Scholar]
- Peatman E., Mohammed H., Kirby A., Shoemaker C.A., Yildirim-Aksoy M., Beck B.H. Mechanisms of pathogen virulence and host susceptibility in virulent Aeromonas hydrophila infections of channel catfish (Ictalurus punctatus) Aquaculture. 2017 [Google Scholar]
- Plumb J.A., Hanson L.A. John Wiley & Sons; 2011. Health Maintenance and Principal Microbial Diseases of Cultured Fishes. [Google Scholar]
- Roberts R.J. John Wiley & Sons; 2012. Fish Pathology. [Google Scholar]
- Saulnier D., Haffner P., Goarant C., Levy P., Ansquer D. Experimental infection models for shrimp vibriosis studies: a review. Aquaculture. 2000;191:133–144. [Google Scholar]
- Sayed, M.A., 2010. Isolation and identification of Aeromonas hydrophila from climbing pearch Anabas testudineus. M.S. Thesis, Department of Aquaculture, Bangladesh Agricultural University.
- Sreedharan K., Philip R., Singh I.S.B. Virulence potential and antibiotic susceptibility pattern of motile aeromonads associated with freshwater ornamental fish culture systems: a possible threat to public health. Brazilian J. Microbiol. 2012;43:754–765. doi: 10.1590/S1517-83822012000200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratev D., Stoev S., Vashin I., Daskalov H. Some varieties of pathological changes in eximentalper infection of carps (Cyprinus carpio) with Aeromonas hydrophila. J. Aquacult. Eng. Fish. Res. 2015;1:191–202. [Google Scholar]
- Trott D.J. Veterinary microbiology: bacterial and fungal agents of animal disease-by J. Glenn Songer, KW Post. Aust. Vet. J. 2006;84:438. [Google Scholar]
- Van Hai N. Research findings from the use of probiotics in tilapia aquaculture: a review. Fish Shellfish Immunol. 2015;45:592–597. doi: 10.1016/j.fsi.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Wang M., Lu M. Tilapia polyculture: a global review. Aquac. Res. 2016;47:2363–2374. [Google Scholar]
- Yardimci B., Aydin Y. Pathological findings of experimental Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) Ankara Univ. Vet. Fak. Derg. 2011;58:47–54. [Google Scholar]
- Zhang D., Moreira G.S.A., Shoemaker C., Newton J.C., Xu D.-H. Detection and quantification of virulent Aeromonas hydrophila in channel catfish tissues following waterborne challenge. FEMS Microbiol. Lett. 2016:363. doi: 10.1093/femsle/fnw080. [DOI] [PubMed] [Google Scholar]