Abstract
Reseda pentagyna is the only endemic species among the seven species of the genera Reseda found in Saudi Arabia. Probably no information is available on regeneration by conventional method of regeneration through seeds or cuttings. Therefore, alternative method of tissue culture was attempted to regenerate and multiply the plant. High shoot regeneration (14.44 shoots/explant) was obtained after four weeks, when shoot cuttings cultured on MS containing BA at 1.0 µM. Other cytokinins e.g., Kn, 2iP and TDZ found to be less effective in bud induction and shoot multiplication. Individual shoots were rooted on MS medium supplemented with various auxins at 0.5–5.0 µM concentrations. The IBA (1.5 µM) supplemented MS media induced maximum (83.3%) rooting. The plantlets were acclimatized and hardened under greenhouse conditions in plastic pots containing soil and farm yard manure with 95.0% success. The protocol developed would help to multiply the plant as well as conserve them in natural habitat. This can also be utilized to obtain active constituents for pharmaceutics and genetic manipulations.
Keywords: Reseda pentagyna, Endemic, Tissue culture, Conservation, Micropropagation
1. Introduction
Seven species of the genus Reseda are found in Saudi Arabia, viz., R. pentagyna Abdallah & A.G. Miller, R. arabica Boiss., R. lutea, R. alba R. aucheri Boiss., R. muricata C. Presl, and R. sphenocleoides Deflers (Chaudhary, 1999). Among the seven species, R. pentagyna has an endemic status in Saudi Arabia and distributed widely in Wadi Sawawin, Northern Hijaz mountains and Tabuk of north western parts of Saudi Arabia (Miller and Nyberg, 1994, Chaudhary, 1999, Llewellyn et al., 2010). The presence of only 3–4 toothed capsules in R. pentagyna differentiate it from R. stenostachya that show the occurrence of 5–6 toothed capsules.
The conservation of endemic, threatened and endangered medicinal species is vital for the future of humankind. A rich in diversity is found in the flora of Saudi Arabia, with numerous rare and endangered plants spreading to different genera of plant kingdom. There is continuous threat to the survival of these plants because of the prevalent environmental conditions. Therefore, the number of threatened plant species are more and increasing yearly resulting from harsh conditions and anthropogenic activities Khan et al., 2012. Over-exploitation, loss of habitat, speedy urbanization, over-grazing, selective species extraction from wilds, and damage is leading to genetic erosion. Climatic conditions in Arabian region is tough that has resulted in declining plant population, fragmented habitats, endangerment, narrowed genetic diversity, rarity, poor regeneration and reproductive inefficiencies, are wide spread in the Kingdom of Saudi Arabia (Al-Farhan et al., 2005).
Hence, it is required to search for the development of alternative propagation method for this important plant. To augment this conventional as well as in vitro approaches can be implemented for mass multiplication and value addition to the plant. In general plants regenerates through seeds, a very few literature is available for this plant for propagation and multiplication. To start the regeneration Plant tissue culture would be an attractive and alternative method of propagation. This method is being applied extensively for the conservation of important, rare, endangered medicinal plants and also commercial purpose (Rout et al., 2000). Plant biotechnology and tissue culture provide contamination free plants as well as explants to exploit in propagation and transformation. In vitro propagation can be utilized for conservation of germplasm and cryopreservation. Probably there is no report on in vitro regeneration and propagation on Reseda pentagyna. The objective of the present investigation was to develop a highly reproducible in vitro propagation protocol from axillary bud of Reseda pentagyna.
Micropropagation of plants is important to maintain the genetic fidelity in regeneration if the explant is chosen from pre-existing meristems. The regeneration and multiplication of shoots improves when PGRs are added to the media. The addition of PGRs attracts the possibility of mutation and genetic instability (Howell et al., 2003, Bairu et al., 2006) and soma clonal variations may occur (Larkin and Scowcroft, 1981). Therefore, it becomes necessary to evaluate the genetic consistency of micropropagated plants by using cytological or molecular markers to ensure the quality of the plants (Mallon et al., 2010). To establish the genetic fidelity, there are various techniques present such as isozymes, cytological, and molecular markers to determine anomalies and confirm genetic homogeneity of the in vitro raised plantlets. However, ISSR and RAPD markers are widely tested to elucidate genetic uniformity and quality of the micropropagated plants and mother plant, thus warranting the quality of clonal plantlets (Chalageri and Babu, 2012, Paul et al., 2010, Sun et al., 2009). The genetic homogeneity has also been established by ISSR marker in micropropagated plants of Saccharum officinarum, Lippia integrifolia (Griseb.) Hier., Pittosporum eriocarpum Royle (Hsie et al., 2015, Iannicelli et al., 2016, Thakur et al., 2016).
Hence, this study was undertaken to establish an efficient plant propagation protocol through direct organogenesis from apical and axillary buds of Reseda pentagyna. This is probably the first report on tissue culture studies of wild R. pentagyna plant species. The genetic uniformity of clonally raised plants was verified by ISSR to assure the true-to-type of the plantlets.
2. Materials and methods
2.1. Plant material collection and explants
Stem cuttings of mother plants of Reseda pentagyna were collected from a wild population from Tabuk region of Saudi Arabia, in the month of October and authenticated from Department of Botany and Microbiology, College of Science, King Saud University, KSA. The collected cuttings were stored in a refrigerator for further use. Stem cuttings bearing apical and axillary buds of about 2–3 cm were washed under running tap water for 30 min to remove traces foreign material. Now the cuttings were sterilized with Sodium hypochlorite solution (4% available chlorine) for 12 min. The explants (axillary and apical buds) were trimmed at the basal end and cultured on nutrient media under aseptic conditions of a Laminar hood to initiate shoot multiplication.
2.2. Culture media and conditions for shoot multiplication
The explants (about 1.5–2.0 cm) were inoculated on MS media under aseptic conditions (Murashige and Skoog, 1962) containing sucrose (2%), supplied with various concentrations and combinations of Benzyl-6-adenine (BA), Thidiazuron (TDZ), 2-isopentenyladenine (2iP) and Kinetin (Kn) (0.5, 1.0, 2.5 and 5.0 µM) for bud and shoot induction and multiplication. The pH of the medium was adjusted to 5.8 before the addition of 0.8% agar and autoclaved at 121 °C, 15 lbs. pressure for 15 min. The cultures were placed in a growth chamber and the temperature of the room was maintained at 25 ± 2 °C fitted with cool-white fluorescent tubes in culture racks. The duration of light was set to 16 h light and 8 h dark with a light intensity of 3000 lux. Data were recorded after 25–45-day culture cycle on percent shoot multiplication, number of shoots/explants and shoot length per culture. Each treatment, had a total of three explants in triplicate. Cultures were incubated under the same growth conditions as stated above. The explants were routinely subcultured with shoot clusters, produced after more than 4 weeks of incubation on MS medium containing optimum concentration of PGRs (all the chemical and PGRs were from Sigma-Aldrich, St. Louis, USA).
2.3. Rooting and acclimatization
The regenerated shoots (1.5–2.0 cm) bearing at least 1–2 internodes were dissected individually and cultured on a fresh rooting MS medium supplemented with different concentrations (0.5–5.0 µM) of Indole-3-acetic Acid (IAA), Indole-3-butyric acid (IBA), 2,4-Dichlorophenoxyacetic acid (2,4-D) and α-Naphthalene acetic acid (NAA). After 4 weeks of culture, the frequency of root formation, number of roots produced per cultured shoot, and length of the root were recorded. Well rooted plantlets were taken out from the culture and thoroughly washed under running tap water to remove the agar from root system. Then the plants were transplanted to plastic pots containing sterilized soil and farm yard manure (FYM) (3:1). The pots were covered with a polythene bag to maintain maximum humidity (80%) and watered for every 3–4 days. After 20 days, the polythene cover was gradually removed from the pots, and subsequently planted in the field.
2.4. Molecular characterization of regenerated plants
2.4.1. Genomic DNA isolation and PCR amplification
High quality and quantity of whole genomic DNA was isolated from the mother plant of Reseda pentagyna and its tissue culture originated plantlets using the modified CTAB method (Khan et al., 2007). The fresh leaf material was powdered with motar and pestle in liquid nitrogen. The 200 mg powder was taken into 2 ml microcentrifuge tube with 600 μl extraction bufeer (100 mmol/L Tris buffer pH 8.0, 25 mmol/L Na2 EDTA, 2.0 mol/L NaCl, 3% CTAB, 3% polyvinyl pyrrolidone). This was mixed slowly and incubated at 60 C for 20 min. After cooling equal volume of chloroform: isoamyl alcohol (24:1) was added and centrifuged at 12000 rpm for 10 min. The supernatant was taken in another tube and 2/3 volume mixed with cold isopropanol and kept at −20 C for 30 min. The nucleic acid was precipitated by centrifugating at 10,000 rpm for 10 min. The pellet obtained was washed twice with 80% ethanol and air dried to remove the ethanol. The resulting nucleic acid was dissolved in TE (10 mmol/L Tris buffer pH 8.0, 1 mmol/L Na2 EDTA) buffer and stored at 4 C in a fridge. The extract was treated with RNase A (10 mg/ml) for 30 min at 37 °C) to get pure DNA. The purity and concentration of DNA was measure on a spectrophotometer at wavelength at 260 and 280 nm. The quality of DNA was observed on agrose gel electrophoresis stained by ethidium bromide. Master mixture (GE health care) was used for PCR amplification. PCR reaction was performed in 25 µL volume in which primer and template DNA was added in the last after adding the doubled distilled water (see Table 1). The PCR amplification program used as follows: Initial denaturation for 5 min at 95 °C, followed by 40 cycles for 1 min at 94 °C denaturation, annealing at 43 °C for 1 min, extension at 72 °C for 2 min and final extension at 72 °C for 5 min was used for amplification performed in the Techne thermal cycler. The amplified products were electrophoretically separated on a 1.3% agarose gel using 1X TBE buffer, and stained with ethidium bromide. The stained gels were scanned and photographed under UVI gel documentation system.
Table 1.
List of ISSR primers used for testing genetic fidelity among the clones of Reseda pentagyna.
| S.N | Primer sequence | |
|---|---|---|
| 1. | ISSR-25 | 5′-GAGAGAGAGAGAGAGAGAT-3′ |
| 2. | ISSR-60 | 5′-GGGTGGGGTGGGGTG-3′ |
| 3. | ISSR-65 | 5′-ACACACACACACACACCTT-3′ |
| 4. | ISSR-69 | 5′-CTCTCTCTCTCTCTCTRG-3′ |
| 5. | ISSR-87 | 5′-CACACACACACACACARG-3′ |
| 6. | ISSR-89 | 5′-CACACACACACACACAGT-3′ |
| 7. | ISSR-90 | 5′-GATAGATAGATAGAT-3′ |
| 8. | ISSR-91 | 5′-GACAGACAGACAGAC-3′ |
| 9. | ISSR-92 | 5′-CAACAACAACAACAA-3′ |
| 10. | ISSR-93 | 5′-CAGCAGCAGCAGCAG-3′ |
| 11. | ISSR-94 | 5′-GTGTGTGTGTGTGG-3′ |
| 12. | ISSR-95 | 5′-GAGAGAGAGAGAGG-3′ |
| 13. | ISSR-96 | 5′-CACACACACACAGT-3′ |
| 14. | ISSR-97 | 5′-CACACACACACAAG-3′ |
| 15. | ISSR-98 | 5′-CTCTCTCTCTCTCTCTGC-3′ |
2.5. Statistical analysis
The experiments were set up in completely randomized design. The different treatments were in three replicates. Experimental data was recorded after 30 days of culture. The significance of differences among means was carried by Duncan’s multiple range tests (DMRT) at P > 5%. The results expressed are mean ± SE of three independent experiments and subjected to one-way analysis of variance (ANOVA) using SPSS v.17 (SPSS, Chicago, USA).
3. Results and discussion
3.1. Shoot multiplication
The morphogenetic response (bud induction and shoot multiplication) of explant to the treatment of various cytokinins and concentrations were evaluated after 30 days of culture on MS medium (Table 2). Direct organogenesis was exhibited by explants on MS medium supplemented with different concentrations of BA, KN, TDZ and 2iP (0.5, 1.0, 2.5 and 5.0 µM) separately. In general, cytokinin concentrations was inversely related to number of shoots and bud proliferation on MS medium. The medium containing 0.5 µM BA was best for bud break and resulted in an optimum number of shoots/explant (14.4 ± 0.44, Table 2). Among different concentrations of cytokinins applied, the explants cultured on BA-supplemented media resulted to a greater response when compared to explants cultured on KN, TDZ and 2iP fortified media. The PGR BA is known to stimulate multiple shoots in Passiflora mollussima (Johnson et al., 2007), Mentha viridis (Raja and Arokiasamy, 2008), and Rubia cardifolia (Swaroopa et al., 2011) Trichosanthes dioica (Kumar et al., 2016) Strobilanthes tonkinensis (Srikun, 2017).
Table 2.
Effect of different cytokinins on shoot and bud induction in Reseda pentagyna on MS medium.
| SN | Treatment concentration | Average no. of shoots/explant ± SE |
Average no. of buds/explant ± SE |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Kn | TDZ | BA | 2iP | Kn | TDZ | BA | 2iP | ||
| 1 | 0.5 | 11.77 ± 0.66a | 6.44 ± 0.37a | 10.00 ± 0.57b | 5.66 ± 0.44a | 2.40 ± 0.19a | 2.73 ± 0.22a | 3.06 ± 0.18ab | 2.20 ± 0.22a |
| 2 | 1.0 | 9.00 ± 0.68ab | 5.33 ± 0.40ab | 14.44 ± 0.44a | 4.55 ± 0.37ab | 2.20 ± 0.22ab | 2.46 ± 0.13ab | 3.73 ± 0.18a | 1.86 ± 0.16a |
| 3 | 2.5 | 8.11 ± 0.48ab | 3.66 ± 0.37bc | 7.33 ± 0.52c | 3.88 ± 0.42ab | 1.80 ± 0.17bc | 1.93 ± 0.18bc | 3.00 ± 0.19bc | 1.60 ± 1.6a |
| 4 | 5.0 | 7.11 ± 0.63c | 3.44 ± 0.37c | 5.88 ± 0.42d | 2.88 ± 0.42bc | 1.93 ± 0.20c | 1.80 ± 0.20c | 2.40 ± 0.23c | 1.46 ± 0.16a |
Data denotes mean ± SE of three replicates per treatment. ANOVA tested by the Duncans range test at significance level (p ≤ 0.05).
The concentration of cytokinin used in the culture medium plays a significant role in shoot organogenesis that increased concentrations of cytokinins exhibited a decreased number of shoot buds coupled with callus proliferation. This may be caused by fast cell division resulting in profuse callus proliferation and inhibiting the morphogenesis. Similar observations have also been made by Chaudhari et al. (2004) in Tylophora indica, Ahmad et al. (2008) in Vitex negundo, Nikam et al. (2009) in Leptadenia reticulata, Paul et al. (2010) in Momordica cymbalaria, Sudipta et al. (2011) and Swamy et al. (2014) in Pogostemon cablin. Although Kn and other cytoinin proved to be poorer for shoot production in, its usefulness either alone or with BA induced direct shoot organogenesis in Asparagus maritimus (Stajner et al., 2002) and Bixa orellana (De Paiva et al., 2003). The maximum shoot multiplication was observed on MS media containing BA alone, which gave the highest regeneration rate (Fig. 1B). The maximum number of shoots/explants (14.44 ± 1.35) and length of shoot (2.8 ± 1.15) was also evidenced on the same treatment. Jose and Satheesh (2004) accomplished a maximum mean of 10.4 shoots per shoot explants of O. mungos inoculated on MS media containing 2.22 μM BA.
Fig. 1.
In vitro multiplication and acclimatization of Reseda pentagyna A: Shoot bud induction on MS medium containing 1.0 μM of BA, B: Shoot multiplication on MS medium supplemented with BA, C: Regeneration of shoots on preexisting shoots on MS medium and BA, D: Bud proliferation on MS medium supplied with Kn, E: Individual shoots cultured for rooting on MS medium with IBA, F: Hardened and acclimatized plant of Reseda pentagyna.
3.2. In vitro rooting of plantlets and acclimatization
The effect of various types and concentration of auxin on rhizogenesis of R. pentagyna were evaluated (Table 3). Among the treatments tried However, a high percent frequency of rooting (83.33%) was achieved within 25 days of shoot implantation on mean number of roots/shoot (3.2 ± 0.32) and root length (2.57 ± 0.10 cm) was found to be better the same treatment of auxins (Fig. 1D). Among hormones, IBA was the most effective auxin as compared to NAA and IAA in root induction. The highest frequency of root formation (83.3%) 1.5 µM IBA while maximum number of roots (3.9 ± 0.37) and root length (2.57 ± 0.10 cm) were achieved on MS medium supplied with 0.5 µM IBA (Table 3). Though auxins (IAA and NAA) were potent to induce rooting formation with varying degrees, small amount of callusing was also observed in a few number of shoots. This suggests that the addition of auxins is beneficial for rooting. Arikat et al. (2004) and Shekhawat et al. (2014) described that auxins (especially IBA) play an important role in the induction of roots from the cut ends of the in vitro raised shoots of Salvia fruticosa and Turnera ulmifolia, respectively. Similarly IBA induced rooting in in vitro raised shoots of Trichosanthes dioica (Kumar et al., 2016) and Strobilanthes tonkinensis (Srikun, 2017). Forty well-developed plantlets having four to six fully expanded leaves and roots were acclimatized successfully in pots containing soil and FYM (3:1) within 4 weeks. The plant growth started after two weeks of transfer to pot and considerable development observed up to four weeks. The leaves expanded and stem looked hardened compared to the plant at the time of transfer. The plantlets were soon after established in a nursery with a survival rate of 95%. The established plants were phenotypically identical with mother plant without any visible variation (Fig. 1).
Table 3.
Rooting in Reseda pentagyna after the treatment of various auxins supplied to MS medium.
| Treatment concentration | Rooting% ± SE | No of roots ± SE | Root length ± SE | Shoot length ± SE |
|---|---|---|---|---|
| IBA | ||||
| 0.5 | 60.00 | 3.90 ± 0.37a | 2.56 ± 0.13a | 2.08 ± 0.09ab |
| 1.5 | 83.33 | 3.20 ± 0.32ab | 2.30 ± 0.13a | 2.57 ± 0.10a |
| 2.5 | 50.0 | 2.80 ± 0.29b | 1.92 ± 0.05ab | 1.77 ± 0.07bc |
| 5.0 | 43.33 | 2.30 ± 0.30b | 1.88 ± 0.06ab | 1.59 ± 0.07c |
| NAA | ||||
| 0.5 | 80.00 | 2.60 ± 0.30a | 2.70 ± 0.10a | 1.86 ± 0.06bc |
| 1.5 | 76.66 | 2.40 ± 0.26ab | 2.27 ± 0.11ab | 2.13 ± 0.07ab |
| 2.5 | 63.33 | 1.80 ± 0.20ab | 2.09 ± 0.06bc | 2.37 ± 0.08a |
| 5.0 | 53.33 | 1.30 ± 0.30b | 1.93 ± 0.08c | 1.84 ± 0.11c |
| IAA | ||||
| 0.5 | 63.33 | 3.40 ± 0.30a | 2.49 ± 0.21ab | 1.93 ± 0.05a |
| 1.5 | 53.33 | 3.00 ± 0.42ab | 3.11 ± 0.21a | 2.66 ± 0.15a |
| 2.5 | 50 | 2.60 ± 0.30ab | 2.07 ± 0.06bc | 1.60 ± 0.09ab |
| 5.0 | 43.3 | 2.00 ± 0.29b | 2.09 ± 0.14c | 1.47 ± 0.08b |
Data denotes mean ± SE of three replicates per treatment. ANOVA tested by the Duncans range test at significance level (p ≤ 0.05).
3.3. ISSR analysis of in vitro raised plants
Micropropagation is used to obtain uniform planting material. However, it is necessary to authenticate the clonal uniformity of in vitro-raised plants to confirm the reliability of the protocol for mass propagation. DNA based Molecular markers are more reproducible as compared to the cytological, morphological and protein markers as they have been long practiced for the identification of variations in tissue-cultured raised plantlets, genetic diversity study, plant identification and beyond. Moreover, these markers are highly reproducible, heritable, reliable, detectible in all tissues and easy to perform. The genetic fidelity was assessed among the clones of R. pentagyna and compared with their mother plant using the ISSR molecular markers. We used 15 primers for genetic fidelity testing and out of these, 14 primers amplified the genomic fragments and gave reproducible bands. All primers produced the monomorphic bands among the all regenerated plantlets as results of two primers shown in (Fig. 2, Fig. 3). Thus, regenerated plantlets of R. pentagyna maintained the genetic stability even after sub-culturing of calli for long term duration on the same media. Our result was congruence in line with other researchers as they did not find any genetic variations on tissue cultured raised plantlets such as Dendrocalamus strictus (Roxb.) (Goyal et al., 2015), Banana cv Robusta (Chaudhary, 2015), Tylophora indica (Sharma et al., 2014) etc. ISSR markers have been used for the assessment of genetic fidelity in the tissue cultured raised plants including Bambusa balcoa (Negi and Saxena, 2010). Simmondsia chinensis (Link) Schneider (Kumar et al., 2011); olive species (Brito et al., 2010); Capparis spinosa L. (Carra et al., 2012); etc. The application of ISSR marker is very easy and it uses single primer in PCR reaction like RAPD marker.
Fig. 2.
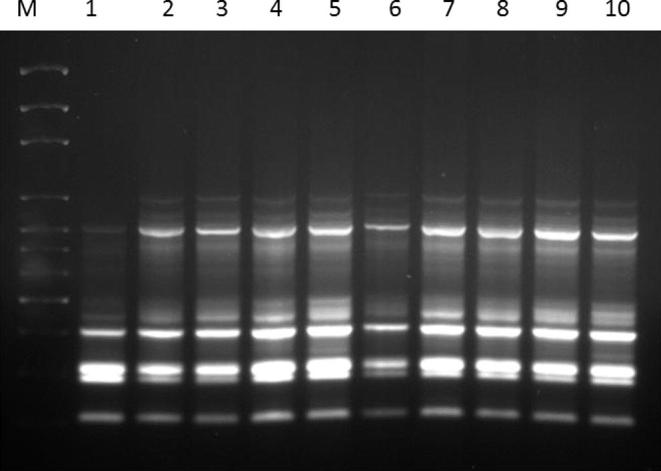
ISSR fingerprint generated with ISSR-25 using the genomic DNA of Reseda pentagyna; M: DNA ladder 100 bp; Lane-1-8 (Tissue cultured raised plantlets and randomly selected); Lane-9-10 (Mother plant).
Fig. 3.
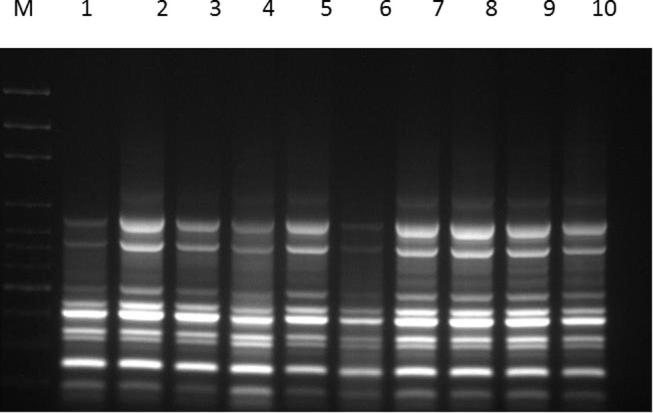
ISSR fingerprint generated with ISSR-87 using the genomic DNA of Reseda pentagyna; M: DNA ladder 100 bp; Lane-1-8 (Tissue cultured raised plantlets and randomly selected); Lane-9-10 (Mother plant).
4. Conclusion
The present study describes an efficient protocol for direct shoot regeneration of R. pentagyna. The genetic uniformity of micropropagated plants were analyzed by using ISSR confirms no genetic variations in the plants regenerated through an in vitro multiplication system. The in vitro-raised cultures could be used as a source for obtaining bioactive compounds on a large scale. Thus the developed protocol for regeneration is a reliable and commercial multiplication but also for conservation of elite clones of R. pentagyna and other genetic manipulation.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group Project no. RGP-014.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Fahad Al-Qurainy, Email: fahad_alqurainy@yahoo.com.
Mohammad Nadeem, Email: mohammadnadeem911@hotmail.com.
Salim Khan, Email: salimkhan17@yahoo.co.in.
Saleh Alansi, Email: alansi1975@yahoo.com.
Mohamed Tarroum, Email: med_taroum@yahoo.fr.
Abdulhafed A. Al-Ameri, Email: alameri73@yahoo.com.
Abdel-Rhman Z. Gaafar, Email: abdou.gaafar@yahoo.com.
Aref Alshameri, Email: arefshamiry@yahoo.com.
References
- Ahmad N., Wali S.A., Anis M. In vitro production of true-to-type plants of Vitex negundo L. from nodal explants. J. Hort. Sci. Biotech. 2008;83(3):313–317. [Google Scholar]
- Al-Farhan, A., Al-Turki, T.A., Basahy, R.A., 2005. Flora of Jizan Region, Final Report of project AR-17-7. King Abdulaziz City for Science and Technology (KACST) KSA 1:1–545.
- Arikat N.A., Jawad F.M., Karam N.S., Shibli R.A. Micropropagation and accumulation of essential oils in wild sage (Salvia fruticosa Mill.) Sci. Hort. 2004;100:193–202. [Google Scholar]
- Bairu M.W., Fennell C.W., Van Staden J. The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA cv. ‘Zelig’) Sci. Hort. 2006;108:347–351. [Google Scholar]
- Brito G., Lopes T., Loureiro J., Rodriguez E., Santos C. Assessment of genetic stability of two micropropagated wild olive species using flow cytometry and microsatellite markers. Tree Struct. Funct. 2010;24:723–773. [Google Scholar]
- Carra A., Sajeva M., Abbate L., Siragusa M., Sottile F., Carimi F. In vitro plant regeneration of caper (Capparis spinosa L.) from floral explants and genetic stability of regenerants. Plant Cell Tissue Organ Cult. 2012;109:373–381. [Google Scholar]
- Chalageri G., Babu U.V. In vitro plant regeneration via petiole callus of Viola patrinii and genetic fidelity assessment using RAPD markers. Turk. J. Bot. 2012;36:358–368. [Google Scholar]
- Chaudhari K.N., Ghosh S., Jha S. The root: A potential source of competent cells for high frequency regeneration in Tylophora indica. Plant Cell Rep. 2004;22:731–740. doi: 10.1007/s00299-003-0753-z. [DOI] [PubMed] [Google Scholar]
- Chaudhary, S., 1999. Resedaceae. In: Chaudhary, S. (Ed.), Flora of the Kingdom of Saudi Arabia. Ministry of Agriculture and Water, National Herbarium, National Agriculture and Water Research Center, Riyadh,Saudi Arabia, pp. 536–543.
- Choudhary D., Kajla S., Poonia A.K., Brar B., Surekha, Duhan J.S. Molecular assessment of genetic stability using ISSR and RAPD markers in in vitro multiplied copies of commercial banana cv. Robusta. Indian J. Biotechnol. 2015;14:420–424. [Google Scholar]
- De Paiva N., Vespasiano B., DaMota T.R., Otoni W.C. Direct organogenesis from hypocotyl-derived explants of annatto (Bixa orellana) Plant Cell Tiss. Org. Cult. 2003;75(2):159–167. [Google Scholar]
- Goyal A.K., Pradhan S., Basistha B.C., Sen A. Micropropagation and assessment of genetic fidelity of Dendrocalamus strictus (Roxb.) nees using RAPD and ISSR markers. Biotech. 2015;5(4):473–482. doi: 10.1007/s13205-014-0244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S.H., Lall S., Che P. Cytokinins and shoot development. Trends Plant Sci. 2003;8:453–459. doi: 10.1016/S1360-1385(03)00191-2. [DOI] [PubMed] [Google Scholar]
- Hsie B.S., 1, Brito J.Z., Vila Nova M.X., Borges-Paluch L.R., Silva M.V., Donato V.M.S.T. Determining the genetic stability of micropropagated sugarcane using inter simple sequence repeat markers. Genet. Mol. Res. 2015;14(4):17651–17659. doi: 10.4238/2015.December.21.38. [DOI] [PubMed] [Google Scholar]
- Iannicelli J., Pérez de la Torre M., Coviella A., Del Valle Aguirre E., Elechosa M.A., van Baren C.M., Pacheco M.G., Escandón A.S. In vitro propagation of Lippia integrifolia (Griseb.) Hier. and detection of genetic instability through ISSR markers of in vitro-cultured plants. Rev. Fac. Agron. 2016;115(1):67–76. [Google Scholar]
- Johnson M., Yasmin N., Sonali D., Rajasekarapandian M. The role of cytokinin and auxin in organogenesis of Passiflora mollissima and evaluation of biochemical changes using isozyme. Eth. J. Sci. Technol. 2007;4:27–36. [Google Scholar]
- Jose B., Satheesh K.K. In vitro mass multiplication of Ophiorrhiza mungos Linn. Indian J. Exp. Biol. 2004;42:639–642. [PubMed] [Google Scholar]
- Khan S., Al-Qurainy F., Nadeem M. Biotechnological approaches for conservation and improvement of rare and endangered plants of Saudi Arabia. Saudi J. Biol. Sci. 2012;19:1–11. doi: 10.1016/j.sjbs.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Qureshi M.I., Kamaluddin T.A., Abdin M.Z. Protocol for isolation of genomic DNA from dry and fresh roots of medicinal plants suitable for RAPD and restriction digestion. Afr. J. Biotechnol. 2007;6(3):175–178. [Google Scholar]
- Kumar S., Mangal M., Dhawan A.K., Singh N. Assessment of genetic fidelity of micropropagated plants of Simmondsia chinensis (Link) Schneider using RAPD and ISSR markers. Acta Physiol. Plant. 2011;33:2541–2545. [Google Scholar]
- Kumar S., Singh H., Pandey V., Singh B.D. In vitro multiplication of pointed gourd (Trichosanthes dioica) through nodal explant culture, and testing the genetic fidelity of micropropagated plants using RAPD markers. Indian J. Biotechnol. 2016;15:581–588. [Google Scholar]
- Larkin P.J., Scowcroft W.R. Somaclonal variation–a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- Llewellyn O.A., Hall M., Miller A.G., Al-Abbasi T.M., Al-Wetaid A.H., Al-Harbi R.J., Al-Shammari K.F., Al-Farhan A. Important plant areas in the Arabian Peninsula: 1. Jabal Qaraqir. Edinb. J. Bot. 2010;67:37–56. [Google Scholar]
- Mallon R., Oubina J.R., Gonzalez M.L. In vitro propagation of the endangered plant Centaurea ultreiae: assessment of genetic stability by cytological studies, flow cytometry and RAPD analysis. Plant Cell Tiss. Org. Cult. 2010;101:31–39. [Google Scholar]
- Miller A.G., Nyberg J.A. Studies in the flora of Arabia: XXVII some new taxa from the Arabian Peninsula. Edinb. J. Bot. 1994;51(1):33–47. [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- Negi D., Saxena S. Ascertaining clonal fidelity of tissue culture raised plants Bambusa balcooa Roxb. using inter simple sequence repeat markers. New Forest. 2010;40:1–8. [Google Scholar]
- Nikam T.D., Ghane S.G., Nehul J.N., Barmukh R.B. Induction of morphogenic callus and multiple shoot regeneration in Momordica cymbalaria Fenzl. Indian J. Biotechnol. 2009;8:442–447. [Google Scholar]
- Paul A., Thapa G., Basu A., Mazumdar P., Kalita M.C., Sahoo L. Rapid plant regeneration, analysis of genetic fidelity and essential aromatic oil content of micropropagated plants of Patchouli, Pogostemon cablin (Blanco) Benth.–An industrially important aromatic plant. Ind. Crops Prod. 2010;32(3):366–374. [Google Scholar]
- Raja D.H., Arokiasamy D.I. In vitro propagation of Mentha viridis L. from nodal and shoot tip explants. Plant Tissue Cult. Biotech. 2008;18:1–6. [Google Scholar]
- Rout G.R., Samantaray S., Das P. Biotechnology of the banana: a review of recent progress. Plant Biol. 2000;2:512–524. [Google Scholar]
- Sharma M.M., Verma R.N., Singh A., Batra A. Assessment of clonal fidelity of Tylophora indica (Burm. f.) Merrill “in vitro” plantlets by ISSR molecular markers. Springer Plus. 2014;3:400. doi: 10.1186/2193-1801-3-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat M.S., Kannan N., Manokari M., Ramanujam M.P. An efficient micropropagation protocol for high-frequency plantlet regeneration from liquid culture of nodal tissues in a medicinal plant, Turnera ulmifolia L. J. Sustain. For. 2014;33:327–336. [Google Scholar]
- Srikun N. In vitro propagation of the aromatic herb Strobilanthes tonkinensis Lindau. Agri. Nat. Resour. 2017;51:15–19. [Google Scholar]
- Stajner N., Bohanec B., Jake M. In vitro propagation of Asparagus maritimus – a rare Mediterranean salt resistant species. Plant Cell Tiss. Org. Cult. 2002;70(3):269–274. [Google Scholar]
- Sudipta K.M., Kumara, Swamy M., Balasubramanya S., Anuradha M. Cost effective approach for in vitro propagation of (Leptadenia reticulata Wight & Arn.) – a threatened plant of medicinal importance. J. Phytol. 2011;3:72–79. [Google Scholar]
- Sun Y., Zhao Y., Wang X., Qiao G., Chen G., Yang Y., Zhou J., Jin L., Zhuo R. Adventitious bud regeneration from leaf explants of Platanus occidentalis L. and genetic stability assessment. Acta Physiol. Plant. 2009;31:33–41. [Google Scholar]
- Swamy M.K., Mohanty S.K., Anuradha M. The effect of plant growth regulators and natural supplements on in vitro propagation of Pogostemon cablin Benth. J. Crop Sci. Biotech. 2014;17(2):71–78. [Google Scholar]
- Swaroopa G., Subhash K., Ghansham D. An improved plant regeneration system for high frequency multiplication of Rubia cordifolia L.: a rare medicinal plant. Asian J. Biotechnol. 2011;3(4):397–405. [Google Scholar]
- Thakur J., Dwivedi M.D., Sourabh P., Uniyal P.L., Pandey A.K. Genetic homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle an endemic and endangered medicinal plant. PLoS ONE. 2016;11(7):e0159050. doi: 10.1371/journal.pone.0159050. [DOI] [PMC free article] [PubMed] [Google Scholar]



