Abstract
This study aimed to estimate the proximate, phenolic and flavonoids contents and phytochemicals present in seeds of twenty four soybeans (Glycine max (L.) Merr) genotypes to explore their nutritional and medicinal values. Crude protein composition ranged between 35.63 and 43.13% in Argentinian and USA (Clark) genotypes, respectively. Total phenolic content varied from 1.15 to 1.77 mg GAE/g, whereas flavonoids varied from 0.68 to 2.13 mg QE/g. The GC–MS analysis resulted identification of 88 compounds categorized into aldehydes (5), ketones (13), alcohols (5), carboxylic acids (7), esters (13), alkanes (2), heterocyclic compounds (19), phenolic compound (9), sugar moiety (7) ether (4) and amide (3), one Alkene and one fatty acid ester. Indonesian genotypes (Ijen and Indo-1) had the highest phenolic compounds than others genotype having antioxidant activities, while the Australian genotype contains the maximum in esters compounds. The major phytocompounds identified in majority of genotypes were Phenol, 2,6-dimethoxy-, 2-Methoxy-4-vinylphenol, 3,5-Dimethoxyacetophenone, 1,2-cyclopentanedione and Hexadecanoic acid, methyl ester. The presence of phytochemicals with strong pharmacological actions like antimicrobial and antioxidants activities could be considered as sources of quality raw materials for food and pharmaceutical industries. This study further set a platform for isolating and understanding the characteristics of each compound for it pharmacological properties.
Keywords: Glycine max, Phytochemicals, GC–MS, Antioxidants
1. Introduction
Soybean (Glycine max (L.) Merr.) is one of the most widely grown leguminous crops in the world grow in tropical, subtropical, and temperate climates and providing abundant protein and oil for human diet and animal feeding. Its seeds contain more than 36% protein, 30% carbohydrates, and appreciable amounts of dietary fiber, vitamins, and minerals. It also contains about 20% oil, which makes soybean the most important crop for edible oil production (Lim, 2012). Seeds of soybean have been used in Asia and other parts of the world to prepare a variety of fresh, fermented and dried foods (Probst and Judd, 1973). Soya-based food products such as tofu, soy milk, soy sauce, miso, etc. have been developed for human consumption while the oil extracted soya meal is used as a nutritious animal feed. Besides its use for domestic purposes, soy oil has multifarious uses in related industries for the production of pharmaceuticals, plastics, papers, inks, paints, varnishes, pesticides and cosmetics (Gupta, 2012). Recently, use of soy oil as biodiesel has opened up another possibility of renewable sources of energy for industrial uses. As a legume crop, soybean is capable of biological nitrogen fixation and is therefore less dependent on synthetic nitrogen fertilizers (Gupta, 2012).
Soybean contains numerous bioactive phytochemicals such as phenolic acids, flavonoids, isoflavones, saponins, phytosterols and sphingolipids (Luthria et al., 2007, Lee et al., 2008, Gutierrez et al., 2004) and possesses excellent immune-active effects in the human body (Sakai and Kogiso, 2008). The reported pharmacological properties of soy and its phytochemicals include antioxidant, estrogenic, antidiabetic, antihypercholesterolemic, antihyperlipidemic, antiobesity, antihypertensive, anticancer, antimutagenic, hepatoprotective, antiosteoporotic, antiviral, bifidogenic, antiinflammatory, immunomodulatory, neuroprotective, wound healing, antimicrobial, goitrogenic anti-skin aging, anti-photoaging activity and the effects of antinutritional factors (Lim, 2012). To a large extent, these pharmacological attributes of soybean are attributable to the presence of isoflavones in soybean (Messina, 2010).
Due to importance of soybean and its products, it is necessary to investigate chemical composition across various genotypes. Therefore, this study was carried out to estimate the active ingredients including total phenolic acid content (TPC), total flavonoid content (TFC), protein content (PC) across 24 genotypes and Phytochemicals in methanolic extracts were evaluated using GC–MS.
2. Materials and methods
2.1. Plant materials
Twenty four soybean genotypes were introduced from nine countries (USA, Egypt, Syria, China, India, Pakistan, Indonesia, Argentina and Australia). Name and geographical origin of these genotypes are presented in Table 1. Seeds of these genotypes were grown in Dirab Agriculture Research Station, King Saud University, Riyadh, Kingdom of Saudi Arabia (24°25′49.2″N 46°22′12.5″E) on August, 2014.
Table 1.
Name and geographical origin of the twenty four soybean genotypes.
| Entry no. | Genotype name | Source/Origin | Entry no. | Genotype name | Origin |
|---|---|---|---|---|---|
| 1 | Admaril | Pakistan | 13 | Giza 111 | Egypt |
| 2 | Romal-1 | Pakistan | 14 | Clark | USA |
| 3 | NARC-2 | Pakistan | 15 | 3803 | Syria |
| 4 | Williams 82 | USA | 15 | A-1 | Australia |
| 5 | X 32 | Egypt | 17 | Ijen | Indonesia |
| 6 | Giza 22 | Egypt | 18 | Indo-black | Indonesia |
| 7 | Giza 21 | Egypt | 29 | Indo-I | Indonesia |
| 8 | X2 L 12 | Egypt | 20 | Indo-II | Indonesia |
| 9 | Giza 83 | Egypt | 21 | USA-1 | USA |
| 10 | Crawford | USA | 22 | Indian | India |
| 11 | Giza 35 | Egypt | 23 | Chinese | China |
| 12 | X 30 | Egypt | 24 | Argentinian | Argentina |
2.2. Chemical analysis
2.2.1. Proximate composition
The proximate analyses of soybean samples for crude proteins, moisture, total ash, fat and carbohydrate were carried out in triplicate using the methods described in AOAC, (AOAC, 1990). All the proximate values were reported in g/100 g seed sample. The Kjeldahl method was used to estimate protein content by titration and percentage nitrogen in the food samples were calculated according to (Markham, 1942) equation.
2.2.2. Antioxidants determination
Soybean seeds were ground to a fine powder in a blender. A portion of 1 g of powder was extracted in a capped centrifuge tube with 10 ml 80% methanol. The mixture was shaken at 300 rpm at ambient temperature on an orbital shaker for 3 h. The extracts were then centrifuged by at 3000 rpm for 10 min, and the supernatants were transferred to new tubes. Residues were extracted with 5 ml of the same solvent overnight for the 2nd time. Both extracts were combined and stored at 4 °C in the dark. Extractions were performed in 3 replicates. The amount of total phenolic in extracts was determined with the Folin-Ciocalteu reagent using the calibration curve of gallic acid as standard. The total phenolics were expressed as mg/g gallic acid equivalents (GAE). An aliquot (50 μl) of extract was mixed with 250 μl of Folin-Ciocalteau reagent and 750 μl of 7.5% sodium carbonate. The volume was made up to 5 ml with water and sample was incubated for two hours. The absorbance was measured at 765 nm against distilled water as blank. Aluminum chloride method was used for flavonoid determination using Quercetin equivalent as standard. An aliquot (250 μl) of extract was mixed 1.25 ml of water and 75 μl 5%NaNO2 added to the flask. After 6 min, 150 μl 10% AlCl3 was added to the mixture. At the 5th min, 500 μl 1 M NaOH was added and volume made up to 2.5 ml with distills water. The absorbance was noted at 410 nm using UV–Visible spectrophotometer.
2.2.3. Gas chromatography—mass spectroscopy
The GC–MS analysis of methanolic extract of 24 genotypes were carried out using a TSQ™ 8000 Evo Triple Quadrupole GC–MS/MS (Thermo Fisher Scientific) equipped with a Elite-5 capillary column (30 nm × 0.25 mm ID × 0.25 µmdf) and mass detector was operated in electron impact (EI) mode with full scan (50–550 amu). Helium was the carriers gas at a flow rate of 1 ml/min. and the injector was operated at 290 °C and the oven temperature was programmed as follows; 50 °C at 8 °C/min to 200 °C (5 min) at 7 °C/min to 290 °C (10 min). The peaks in the chromatogram were identified on the basis of their mass spectra. Interpretation of mass spectrum of GC–MS was done using the database of National Institute Standard and Technology (NIST). The mass spectrum of phytochemicals was compared with the spectrum of known compounds stored in the NIST library.
2.3. Data analysis
The data were analyzed for descriptive statistics (mean, standard deviation, coefficient of variability, minimum and maximum values) and principal component analysis using statistical software Past3 program (Hammer et al., 2001).
3. Results and discussion
3.1. Proximate analysis
Descriptive values of 24 soybean genotypes for proximate analysis (crude protein, ash fat, carbohydrate, and moisture contents), total phenolic and flavonoid contents are presented in Table 2, and the detailed proximate analysis estimates are shown in supplementary Table S1. Crude protein content values ranged between 35.63 in Argentinian to 43.13% in Clark genotypes, with a mean value of 39.02%. Genotypes recorded higher than 40% crude protein were Clark, Indo-1, Indo-black, Ijen, Romal-1, X 30 and 3803. The results revealed significant differences among genotypes in crude protein content, which reflect the variations in genetic background and/or origin. The higher protein content in these genotypes is coherent with those described previously which ranged from 43 to 45% (Machado et al., 2008). Zarkadas et al., 1999, Zarkadas et al., 1993 have also reported crude protein values in soybean ranging from 33.67% to 42.11%. Moisture content ranged from 3.08% to 5.88% in Giza 83 and Indo-1 respectively with overall mean 4.90% with no significant difference. Ash content in soybean genotypes ranged from 4.55% and 6.28% with average of 5.44%. Romal-1 genotype had the lowest value of ash (4.88%) and Giza 111 genotype had the highest value 6.28%. The values of moisture and ash obtained were lower than that reported by Monteiro et al. (2004). Total fat also varied from 16.92% to 22.94% with a mean value of 21.16%. The genotype 3803 recorded the highest content while the genotype Indo- black contained the lowest. Soybean is considered to be high in fat content compared to other legumes. It was mentioned that soybean has about 47% of its energy value in fat content (Liu, 1997, Messina, 1999). Our total fat values were more or less close to that of (Guillon and Champ, 2002) who reported total fat ranged between 18 and 22 g/100 g in different soybean genotypes. The carbohydrate content ranged from 26.11% in Clark to 33.18% in Argentinian with an average value of 29.48%.
Table 2.
Descriptive statistics of chemical composition in 24 soybean genotypes.
| Crude protein (g/100 g) | Moisture (g/100 g) | Ash (g/100 g) | Total Fat (g/100 g) | Carbohydrate (g/100 g) | Total phenolic content (TPC) | Total flavonoid content (TFC) | |
|---|---|---|---|---|---|---|---|
| N | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| Min | 35.63 | 3.08 | 4.55 | 16.92 | 26.11 | 1.15 | 0.68 |
| Max | 43.13 | 5.88 | 6.28 | 22.94 | 33.18 | 1.77 | 2.13 |
| Mean | 39.02 | 4.90 | 5.44 | 21.16 | 29.48 | 1.45 | 1.24 |
| Stand. dev. | 2.09 | 0.65 | 0.33 | 1.41 | 1.86 | 0.16 | 0.36 |
| Coeff. Var. | 5.35 | 13.26 | 6.11 | 6.68 | 6.30 | 11.58 | 29.32 |
3.2. Total phenolic and flavonoid content
Phenolic and flavonoid compounds, as important phytochemicals, are natural antioxidants found in vegetable, fruits and cereal grains. They have multiple biological functions and can play an essential role in defense against cardiovascular disease, cancer and aging (Karimi et al., 2010). Total phenolic and flavonoids contents of seeds from the 24 genotypes of soybean are presented in supplementary Table S1.Total phenolic and flavonoids content of seeds in all genotypes were significantly different from each other. Among all seed extracts investigated, total phenolic content ranged from 1.15 to 1.77 mg GAE/g with mean of 1.45 GAE/g mg/g (Table 2). Giza 111 genotype contained the lowest phenolic content (1.15 mg/g) while the Romal-1 had the highest value of 1.7 mg/g. The total flavonoid content ranged from 0.68 to 2.13 mg QE/g (Table 2). TPC and TFC of 24 soybean genotype are presented in Table S1. It has been reported that the phenolic content is strongly linked with antioxidant capacity (Zheng and Wang, 2001, Skerget et al., 2005) and can contribute directly to the antioxidative activities (Duh et al., 1999). Applications of phenolic are spreading rapidly in the food industry to improve the nutritional value and quality of food (Aneta et al., 2007).
3.3. GC–MS analysis
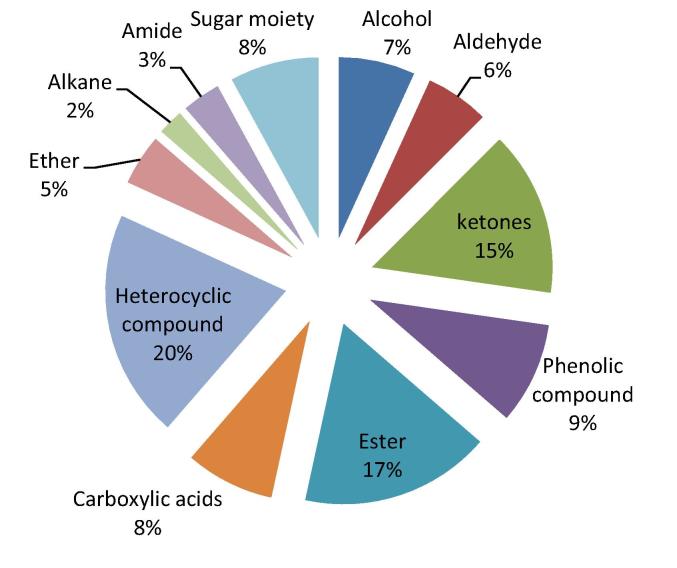
A large number of phytochemical were identified in the methanolic extract of 24 soybean genotypes using GC–MS analysis. A total of 88 compounds were identified based on peak area, retention time and molecular formula. A large number of bioactive phytochemicals including phenolic acids, flavonoids, isoflavones, saponins, phytosterols and sphingolipids were also reported previously for soybean (Luthria et al., 2007, Lee et al., 2008, Gutierrez et al., 2004). The first compound identified at retention time of 3.67 min was carbamide, whereas methyl 10 Trans, 12-cis-octadecadienoate was the last compound which with longest retention time (48.53 min) (Table S2). There were wide variation in the compositions of phytochemicals in 24 soybean genotypes and are shown in supplementary Table S3. Table 3 lists some major phytocompounds and their biological activities in different genotypes of soybean. The phytocompounds identified in 24 genotypes were classified into different groups (Fig. 1). The identified 88 compounds were mainly categorized into aldehydes (5), ketones (13), alcohols (5), carboxylic acids (7), esters (13), alkanes (2), heterocyclic compounds (19), phenolic compound (9), sugar moiety (7), ether (4), amide (3), one Alkene and one fatty acid ester.
Table 3.
List of important phytocompounds identified in the methanolic seed extract of soybean genotypes by GC–MS.
| Compound | Other names | Nature | Activity | RT | MW | |
|---|---|---|---|---|---|---|
| 22 | 2H-1-Benzopyran, 3,5,6,8a-tetrahydro-2,5,5,8a-tetramethyl-,(2S-cis)- | Edulan II | Heterocyclic compound | 7.58 | 192 | |
| 27 | 1,2-cyclopentanedione | Ketone | Antioxidant | 7.98 | 98 | |
| 28 | Pyran-4-Carboxylic acid, 4-(4-methoxyphenyl)-tetrahydro- | Heterocyclic compound | 8.02 | 236 | ||
| 34 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | Ketone | 9.74 | 144 | ||
| 36 | 2H-Pyran-2,6(3H)-dione | Glutaconic anhydride | Heterocyclic compound | 10.75 | 112 | |
| 39 | 2-Pyrrolidinone, 1-methyl | M-Pyrol | Ketone | 11.86 | 99 | |
| 42 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | Ketone | 12.28 | 128 | ||
| 44 | Phenol, 2-methoxy- | Phenolic compound | Antimicrobial, Antioxidant, Anti inflammatory, Analgesic | 13.83 | 124 | |
| 49 | 4H-Pyran-4-one,3-hydroxy-2-methyl- | Maltol | Heterocyclic compound | Flavor enhancer | 14.78 | 126 |
| 50 | 5-hepten-3-one, 5-methyl- | Ketone compound | 15.09 | 126 | ||
| 52 | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one | Heterocyclic compound | Antimicrobial, anti-inflammatory | 16.61 | 144 | |
| 57 | Phenol, 4-ethenyl-, acetate | 4-Vinylphenyl acetate | Phenolic compound | Antimicrobial, Antioxidant, Anti inflammatory, | 19.29 | 162 |
| 60 | Benzofuran, 2,3-dihydro | Coumaran | Heterocyclic compound | Antihelminthic, Anti-inflammatory, Anti-diarrhoeal | 20.16 | 120 |
| 62 | Benzeneacetaldehyde, 3-methyl | m-tolualdehyde | Aldehyde | Antimicrobial | 20.34 | 120 |
| 61 | 1,2-Benzenediol,3-methoxy- | Pyrocatechol, 3-methoxy | Phenolic compound | Antioxidant | 21.01 | 140 |
| 64 | 2-Methoxy-4-vinylphenol | Phenol, 4-ethenyl-2-methoxy- | Phenolic compound | Antimicrobial, Antioxidant, Anti inflammatory, | 23.35 | 150 |
| 68 | Phenol, 2,6-dimethoxy- | Pyrogallol 1,3-dimethyl ether | Phenolic compound | Antimicrobial, Antioxidant, Anti inflammatory, | 24.99 | 154 |
| 70 | Phenol,2,6-bis(1,1-dimethylethyl)-4-methyl- | Butylated Hydroxytoluene | Phenolic compound | Antimicrobial,Antioxidant, Anti inflammatory, Analgesic | 30.99 | 220 |
| 71 | Phenol, 2,4-bis(1,1-dimethylethyl)- | Phenol, 2,4-di-tert-butyl- | Phenolic compound | Antimicrobial,Antioxidant, Anti inflammatory, | 31.19 | 206 |
| 72 | 5-tert-Butyl-1,2,3-benzenetriol | 5-tert-Butylpyrogallol | Phenolic compound | Antioxidant,antiseptic antibacterial, antidermatitic fungicide, pesticide | 31.91 | 182 |
| 76 | 3,5-Dimethoxyacetophenone | Ketone | Antioxidant | 33.65 | 180 | |
| 85 | Hexadecanoic acid, methyl ester | Palmitic acid, methyl ester | Ester | Antioxidant, Flavor, Hypocholesterolemic, Nematicide | 46.13 | 270 |
Fig. 1.
Pie Diagram showing the percentage of phytochemical groups identified in 24 soybean genotypes.
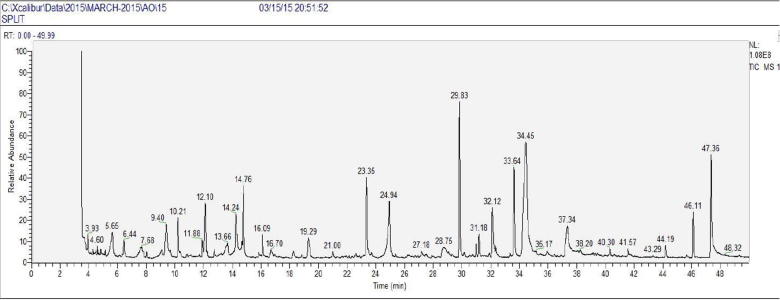
The total ion chromatogram (TIC) of methanol extract of 24 soybean genotypes showing the GC–MS profile of the compounds identified is given (Figs. S1–S24). A typical chromatogram of one soybean genotype is shown in Fig. 2. The GC–MS analyses revealed that the methanolic extract is predominantly composed of heterocyclic compound, ester and phenolic compound. Phenol, 2,6-dimethoxy-, 2-Methoxy-4-vinylphenol, 3,5-Dimethoxyacetophenone, 1,2-cyclopentanedione and Hexadecanoic acid, methyl ester were detected in most of the genotypes. These phytochemicals are responsible for various pharmacological actions like antimicrobial and antioxidants activities (Tapiero et al., 2002). The phytochemicals identified through GC–MS analysis proved to be active in many biological activities as listed in (Table S2). Phytochemicals have been reported to possess potent antioxidant and anti-cancer, anti-carcinogenic, anti-bacterial, anti-viral or anti-inflammatory activities to a greater or lesser extent and play a vital role in plant metabolism (Tapiero et al., 2002, Wei et al., 1999).
Fig. 2.
A typical GC–MS profile of seeds of soybean genotype.
The results obtained showed five compounds belongs to aldehyde group namely Methoxy-propanal, Benzeneacetaldehyde, 3,4-dimethylbenzaldehyde, p-Hydroxyphenyl)glyoxal and Propanal, 2-(benzoyloxy)-, Benzeneacetaldehyde, were detected in 10 genotypes (Table S2). 3-Methoxy-propanal was found in two genotypes (Admaril and Williams 82). 3,4-dimethylbenzaldehyde was detected in three genotypes (Indo- black, Indo- I and Indo -II) whereas p-Hydroxyphenyl)glyoxal and Propanal, 2-benzoyloxy, were present only in one genotype (Giza 35 and X30 respectively). The genotype Williams 82 recorded the highest number of aldehyde compounds (2). Aldehydes are reported to possess powerful antimicrobial activity because of their highly electronegative arrangement of an aldehyde group conjugated to a carbon to carbon double bond (Moleyar and Narasimham, 1986), suggesting an increase in electronegativity increases the antibacterial activities in those genotypes (Kurita et al., 1979, Kurita et al., 1981). Such electronegative compounds react with vital nitrogen components, e.g. proteins and nucleic acids and consequently inhibit microorganisms.
A total of 13 compounds related to ketone were identified. They included 1,2-propanone, 1-(dimethylamino)-, 1,2-cyclopentanedione, 6-Oxa-bicyclo[3.1.0]hexan-3-one, Dihydroxyacetone, 2-Pyrrolidinone, 1-methyl, 2,4,6,-Cycloheptatrien-1-one,4-methyl-, 3,5-Dimethoxyacetophenone, 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one,2-acetyl-2,3,5,6-tetrahydro-1,4-thiazine, Butyrolactone, 2,5-Dimethyl-4-hydroxy-3(2H)-furanone, 5-hepten-3-one, 5-methyl-, were detected. The genotype 3803 and Indo-11 recorded the highest number of ketonic compounds which is eight (8) in number followed by Giza 35 and USA-1 genotypes having six (6) ketonic compounds each. Ketones might be formed by beta-oxidation of fatty acids, which generated a few important flavor compounds (Yu et al., 2008).
Volatile compounds are also formed through fatty acid metabolism, producing alcohols, acids, and esters. Most alcohols are derived from bioremediation of unsaturated fatty acids, and are also a prerequisite for the formation of long-chain esters. Many alcoholic compounds like 4-methyl-2-haptanol, 1-undecanol alcohol, 1,2,3-Propanetriol, Isosorbide (D-Glucitol, 1,4,3,6-dianhydro) and 1,3-Dioxolane-4-methanol (Glycerol formal) were detected among the tested genotypes (Table S3). 4-methyl-2-haptanol was detected in one genotype (Giza 35) while 1,2,3-Propanetriol was present in nine genotype and Isosorbide was appeared in three genotype (Table S3). The genotype clark recorded the three (3) alcoholic compounds which is highest than others genotypes. Alcohols are known to possess antibacterial against vegetative cells. Glycerol and its derivatives are reported to have bacterial inhibiting effect (Saegeman et al., 2008).
The following seven carboxylic acids namely acetic acid, Acetic acid, 2,2-[oxybis(2,1-ethanediyloxy)]bis, 2-Pyridinecarboxylic acid (also called Picolinic acid), Butanoic acid, 4-hydroxy-, Propanedioic acid, Propyl-(also called 2-Propylmalonic acid), Benzoic Acid, Butanoic acid, 4,4-dithiobis[2-amino-,[S-(R∗,R∗)] were detected (Table S3). Acetic acid was recorded in seven genotypes. 2-Pyridinecarboxylic acid was recorded in five genotypes. Butanoic acid, 4-hydroxy- was recorded in three genotypes while Benzoic Acid was present in one genotype (Table S3). The maximum numbers of carboxylic acids compounds were found in genotypes X30, Giza 35, Chinese and Argentinian. Each one of these contained two (2) carboxylic compounds.
A total of 13 esters were identified. The Butyrolactone, Acetic acid, 2-(dimethylamino)ethyl ester, Formic acid, 3-methylbut-2-yl ester, pentanoic acid, 2-isopropoxyphenyl ester, Phthalic acid, hex-3-yl-isobutyl ester, Hexadecanoic acid, methyl ester, 5,8,11-heptadecatriynoic acid methyl ester, Phthalic acid, butyl undecyl ester, 1,2-benzenedicarboxylic acid, dibutyl ester, methyl 10-trans, 12-cis-octadecadienoate, 9,12-Octadecadienoic acid(Z,Z)-methyl ester, Benzoic acid, 4-ethoxy-, ethyl ester, and pentanoic acid, 2,2-4-trimethyl-3-carboxyisopropyl, isobutyl, ester were detected. The genotype A-1 contained maximum 6 esters compounds followed by others genotypes (Romal-1, Giza 83, Clark, 3803 and Argentinian) having five (5) esters compounds. Hexadecanoic acid ethyl ester shows antioxidant, hypocholesterolemic, and nematicidal activities (Priya and Vijaylakshmi, 2011).
Regarding phenolic compound, a total of 9 compounds were detected. 1,2-Benzenediol,3-methoxy-, 5-tert-Butyl-1,2,3-benzenetriol, Phenol, 4-ethenyl-, acetate, 2-Methoxy-4-vinylphenol, Phenol, 2,6-dimethoxy-, Phenol,2,6-bis(1,1-dimethylethyl)-4-methyl-, Phenol, 2,4-bis(1,1-dimethylethyl)-, and phenol, 2-methoxy. The distribution of these compounds among genotypes indicated that the genotypes Ijen and Indo-1 contained the highest number of phenolic compounds which is five while the genotypes NARC-2, Giza 35, Clark, Indo-11 and USA-1 contained the four (4) phenolic compounds each. The plant phenolics compounds have great interest to human due to their antioxidative and possible anticarcinogenic activities. The dietary phenolics are considered anti-carcinogens as they are antioxidants, but there is no clear evidence supporting this supposition (Yang et al., 2001). Phenolic may inhibit carcinogenesis by influencing the molecular events in initiation, promotion, and progression stages. Isoflavones and lignans from soybean may affect tumor formation by mediating estrogen-related activities. They also modulate the growth of benign and malignant prostatic epithelial cells in vitro (Hedlund et al., 2003).
The following sugar moiety, l-Galactose, 6-deoxy-, 3,4-0-Isopropylidene-d-galactose, 3-O-methyl-d-glucose,a-Methyl-D-mannopyranoside, a-D-Galactopyranoside, methyl were detected among soybean studied genotypes. The relatively remarkable amounts of heterocyclic compounds were identified in 24 genotypes including the important compound like 2,6-diisopropylnapthalene, 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one,4H-Pyran-4-one,3-hydroxy-2-methyl-, Pyrazine, ethyl-, Oxirane, 2-ethyl-2-methyl, 1H-indazole, 4,5, 6, 7-tetrahydro, N-aminomorpholine, and Benzofuran, 2,3-dihydro. The distribution of these compounds in 24 genotypes is shown in Table S3. The genotype X30 have four sugars compounds while genotypes Indo-1, Indo-11 and USA-1 have three sugars compounds each. Benzofurans are known to possess anti-oxidant, anti-inflammatory and antimicrobial effect (Kamal et al., 2011).
Taken together, some of the detected compounds are reported to be potential therapeutic agents. Moreover, antimicrobial, antioxidant and anti-inflammatory activities were demonstrated by most compounds in this study indicating that different compounds can display similar activity and this could be due to presence of similar functional groups (Table S2). The presence of various antioxidant and anti-inflammatory compounds may be the reason for the presence of antioxidant properties of soybean methanolic seed extract.
3.4. Principal component analysis
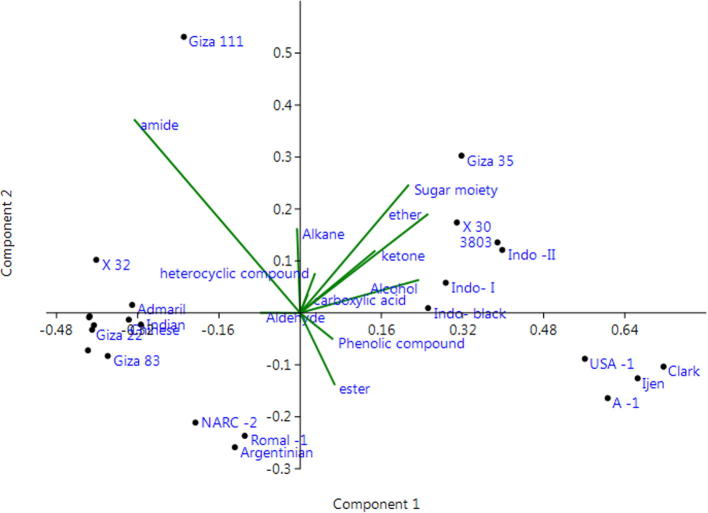
The principal component analysis identified three important components. These components analysis explained 78.64% of total variations among genotypes (Table 4 and Fig. 3). For each main component, greater positive coefficients were considered as a significant factor. In the first component which described 59.65% of total variation, phytochemical classes of ether, alcohol, sugar moiety ketone and phenolic compounds compound had high coefficients. These classes have a positive significant loading for the first component and the genotypes showed most variability based on this component can be selected for these classes. Genotypes Ijen, Clark, A-1, USA-1, Indo–II, 3803, X 30, Giza 35, Indo-black and Indo-I showed the most variability according to these components. The second component illustrated 10.63% of the total variance and classes with higher scores were amide, sugar moiety, ether, alkane, ketone and carboxylic acid. The genotypes showed most variability based on this component were Giza 111, Giza 35, X 30, X 32, Indo-II and 3803. The third component includes Alkane, Aldehyde, Carboxylic acid and Phenolic compound. The genotypes showed most variability based on this component were Giza 35, X 32. According to these results we can concluded that the most divergent genotypes were Giza 35, X 30, Indo -II and genotype 3803 these genotypes showed positive loading in at least two out of the three components. Utilizing PCA effectively reduces the number of variables needed to classify cultivars and permitted soybean researchers to more easily develop significant relationships between important soybean characteristics. Shin et al. (2012) have classified soybean cultivars using (PCA) of the fatty acid data. The four significant principal components yielded together 81.49% of the total variance, where PC1 mainly contributed to by oleic, linoleic, and gondoic acids, PC2 by stearic, linolenic and arachidic acids, PC3 by behenic and lignoceric acids, and PC4 by palmitic acid. The plots generated between PC1-PC2 and PC3-PC4 segregated soybean cultivars based on fatty acid composition. Moreover PCA has been used effectively to define relationships that exist in fatty acid characterization studies of food lipids due to its ability to manage and interpret large data sets (Brodnjak-Voncina et al., 2005). Although soybean oil has been included in some chemometric studies comparing vegetable oils, soybean cultivars have yet to be extensively classified using multivariate techniques (Zagonel et al., 2004, Brandao et al., 2012).
Table 4.
Eigen values and proportion of the variance explained for the three principal components of the 24 soybean genotypes based on phytochemical components.
| PC 1 | PC 2 | PC 3 | |
|---|---|---|---|
| Eigen values | 0.17 | 0.03 | 0.02 |
| Percent of variance | 59.65 | 10.63 | 8.36 |
| Cumulative percentage | 59.65 | 70.28 | 78.64 |
| Alcohol | 0.42 | 0.11 | −0.12 |
| Aldehyde | −0.14 | 0.00 | 0.24 |
| Alkane | −0.01 | 0.29 | 0.75 |
| Amide | −0.59 | 0.67 | −0.28 |
| Sugar moiety | 0.39 | 0.44 | −0.06 |
| Carboxylic acid | 0.03 | 0.06 | 0.31 |
| Ester | 0.12 | −0.25 | −0.26 |
| Ether | 0.45 | 0.34 | 0.02 |
| Heterocyclic compound | 0.05 | 0.14 | −0.16 |
| Ketone | 0.27 | 0.21 | −0.18 |
| Phenolic compound | 0.11 | −0.09 | 0.24 |
Fig. 3.
Two-dimensional biplot ordination of 24 soybean genotypes on principal component axes according to 11 phytochemical classes.
4. Conclusion
The results demonstrate that soybean genotypes contain variable patterns of total proteins flavonoids, phenolic and various bioactive volatile compounds. On the basis of the mass spectrometry analysis, the studied soybean genotypes are a rich source of bioactive compounds and moreover the most of the obtained compounds have noticeable antioxidant properties. Phenol, 2,6-dimethoxy-, 2-Methoxy-4-vinylphenol, 3,5-Dimethoxyacetophenone, 1,2-cyclopentanedione, Hexadecanoic acid methyl ester and 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one were found in majority of genotypes. The research indicated that majority of soybean genotype are a source of bioactive compounds with antioxidant, anti-inflammatory, antimicrobial and other functions. However, the genotypes Ijen and Indo-1 contributed more phenolic compound than others genotype. Genotype A-1 was contained the maximum compound in esters compounds. From ketone compounds, the genotypes 3803 and Indo-11 contribute maximum compounds. In heterocyclic compound, Giza 111 contribute maximum compounds. Some genotypes may have therapeutic potential and may serve as a potential source in drug development as a health supplement. This study also further set a platform for isolating and understanding the characteristics of each compound for it pharmacological properties.
Acknowledgments
The authors extend their appreciation to the International Scientific Partnership Program (ISPP) at King Saud University for funding this research work through ISPP# 0085.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.sjbs.2017.10.014.
Appendix A. Supplementary materials
References
- Aneta W., Jan O., Renate C. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. [Google Scholar]
- AOAC . 15th ed. AOAC International; Washington DC, USA: 1990. Official Methods of Analysis of the AOAC. [Google Scholar]
- Brandao L.F., Braga J.W., Suarez P.A. Determination of vegetable oils and fats adulterants in diesel oil by high performance liquid chromatography and multivariate methods. J Chromatogr. 2012;1225:150–157. doi: 10.1016/j.chroma.2011.12.076. [DOI] [PubMed] [Google Scholar]
- Brodnjak-Voncina D., Kodba Z.C., Novic M. Multivariate data analysis in classification of vegetable oils characterized by the content of fatty acids. Chemometr. Intell. Lab Syst. 2005;75:31–43. [Google Scholar]
- Duh P.D., Tu Y.Y., Yen G.C. Antioxidative activity of water extracts of Harng Jyur (Chrysanthemum morifolium) Food Sci. Technol. 1999;32:269–277. [Google Scholar]
- Guillon F., Champ M.M.J. Carbohydrate fractions of legumes, uses in human nutrition and potential for health. Br. J. Nutr. 2002;8:293–306. doi: 10.1079/BJN2002720. [DOI] [PubMed] [Google Scholar]
- Gupta S.K. Springer; New York, USA: 2012. Technological Innovations in Major World Oil Crops. [Google Scholar]
- Gutierrez E., Wang T., Fehr W.R. Quantification of sphingolipids in soybeans. J. Am. Oil Chem. Soc. 2004;81:737–742. [Google Scholar]
- Hammer O., Harper D.A.T., Ryan P.D. PAST, Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:1–9. [Google Scholar]
- Hedlund T.E., Johannes W.U., Miller G.J. Soy isoflavonoid equol modulates the growth of benign and malignant prostatic epithelial cells in vitro. Prostate. 2003;54:68–78. doi: 10.1002/pros.10137. [DOI] [PubMed] [Google Scholar]
- Kamal M., Shakya A.K., Jawid T. Benzofurans: A new profile of biological activities. IJMPS. 2011;1:1–15. [Google Scholar]
- Karimi E., Oskoueian E., Hendra R., Jaafar H.Z.E. Evaluation of Crocus sativus L. Stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010;15:6244–6256. doi: 10.3390/molecules15096244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita N., Miyaji M., Kurane R., Takahara Y. Antifungal activity of components of essential oils. Agric. Biol. Chem. 1981;45:945–952. [Google Scholar]
- Kurita N., Miyaji M., Kurane R. Antifungal activity and molecular orbital energies of aldehyde compounds from oils of higher plants. Agric. Biol. Chem. 1979;43:2365–2371. [Google Scholar]
- Lee S.J., Kim J.J., Moon H.I., Ahn J.K., Chun S.C., Jung W.S., Lee O.K., Chung I.M. Analysis of isoflavones and phenolic compounds in Korean soybean Glycine max (L.) seeds of different seed weights. J. Agric. Food Chem. 2008;56:2751–2758. doi: 10.1021/jf073153f. [DOI] [PubMed] [Google Scholar]
- Lim, T.K., 2012. Edible Medicinal and Non-Medicinal Plants, vol 2, Fruits, Springer Science, Business Media B.V.
- Liu, K., 1997. Chemistry and nutritional value of soybean components. In: Liu, K., (Ed.), Soybeans, Chemistry, Technology and Utilization. Chapman & Hall, New York, USA, pp. 25–113.
- Luthria D.L., Biswas R., Natarajan S. Comparison of extraction solvents and techniques used for the assay of isoflavones from soybean. Food Chem. 2007;105:325–333. [Google Scholar]
- Machado F.P.P., Queróz J.H., Oliveira M.G.A., Piovesan N.D., Peluzio M.C.G., Costa N.M.B. Effects of heating on protein quality of soybean flour devoid of Kunitz inhibitor and lectin. Food Chem. 2008;107:649–655. [Google Scholar]
- Markham R. Distillation apparatus suitable for microkjeldahl analysis. Biochem. J. 1942;36:970–1791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M. Soybean isoflavone exposure does not have feminizing effects on men, a critical examination of the clinical evidence. Fertil Steril. 2010;93:2095–2104. doi: 10.1016/j.fertnstert.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Messina M. Legumes and soybeans, overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999;70:439–450. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- Moleyar V., Narasimham P. Antifungal activity of some essential oil components. Food Microbiol. 1986;3:331–336. doi: 10.1016/0168-1605(92)90035-2. [DOI] [PubMed] [Google Scholar]
- Monteiro M.R.P., Costa N.M.B., Oliveira M.G.A., Pires C.V., Moreira M.A. Qualidade protéica de linhagens de soja com ausência do inibidor de tripsina kunitz e das isoenzimas lipoxigenases. Rev Nutr. 2004;17:195–205. [Google Scholar]
- Priya K., Vijaylakshmi V.K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. New York Sci J. 2011;4:16–20. [Google Scholar]
- Probst, H., Judd, R.W., 1973. Origin, US history and development, and world distribution. In: Caldwell, B.E. (Ed.), Soybean, Improvement, Production, and Uses. Amer. Soc. Agronomy, Madison, USA, pp. 1–15.
- Saegeman V.S.M., Ectors N.L., Lismont D., Verduykt B., Verhaegen J. Short and long term bacterial inhibiting effect of high concentration of glycerol used in preservation of skin allografts. Burns. 2008;34:205–211. doi: 10.1016/j.burns.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Sakai T., Kogiso M. Soy isoflavones and immuneity. J. Med. Invest. 2008;55:167–173. doi: 10.2152/jmi.55.167. [DOI] [PubMed] [Google Scholar]
- Shin E., Hwang C.E., Lee B.W., Kim H.T., Ko J.M., Baek I.Y., Lee Y., Choi J., Cho E.J., Seo W.T., Cho K.M. Chemometric approach to fatty acid profiles in soybean cultivars by principal component analysis (PCA) Prev. Nutr. Food Sci. 2012;17:184–191. doi: 10.3746/pnf.2012.17.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerget M., Kotnik P., Hadolin M., Hras A.R., Simonic M., Knez Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- Tapiero H., Tew K.D., Nguyen B.G., Mathé G. Polyphenols, do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002;56:200–207. doi: 10.1016/s0753-3322(02)00178-6. [DOI] [PubMed] [Google Scholar]
- Wei Y., Liu Z.Q., Liu Z.L. Antioxidant effect of coumarin derivatives on free radical initiated and photosensitized peroxidation of linoleic acid in micelles. J. Chem. Soc. Perkin Trans. 1999;2:969–974. [Google Scholar]
- Yang C.S., Landau J.M., Huang M.T., Newmark H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann. Rev. Nutr. 2001:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- Yu A.N., Sun B.G., Tian D.T., Qu W.Y. Analysis of volatile compounds in traditional smoke-cured bacon (CSCB) with different fibber coatings using SPME. Food Chem. 2008;110:233–238. doi: 10.1016/j.foodchem.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Zagonel G.F., Peralta-Zamora P., Ramos L.P. Multivariate monitoring of soybean oil ethanolysis by FTIR. Talanta. 2004;63:1021–1025. doi: 10.1016/j.talanta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Zarkadas C.G., Voldeng H.D., Yu Z.R., Choi V. Assessment of the protein quality of nine northern adapted yellow and brown seed coated soybean cultivars by amino acid analysis. J. Agric. Food Chem. 1999;47:5009–5018. doi: 10.1021/jf981381r. [DOI] [PubMed] [Google Scholar]
- Zarkadas C.G., Yu Z.R., Voldeng H.D. Minero-Amador, A. Assessment of the protein quality of a new high-protein soybean cultivar by amino acid analysis. J. Agric. Food Chem. 1993;41:616–623. doi: 10.1021/jf981381r. [DOI] [PubMed] [Google Scholar]
- Zheng W., Wang S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.