Abstract
This study was aimed to evaluate the impact of high frequency electromagnetic fields (HF-EMF at 900 and 1800 MHz) on DNA, growth rate and antibiotic susceptibility of S. aureus, S. epidermidis, and P. aeruginosa. In this study, bacteria were exposed to 900 and 1800 MHz for 2 h and then inoculated to new medium when their growth rate and antibiotic susceptibility were evaluated. Results for the study of bacterial DNA unsuccessful to appearance any difference exposed and non-exposed S. aureus and S. epidermidis. Exposure of S. epidermidis and S. aureus to electromagnetic fields mostly produced no statistically significant decrease in bacterial growth, except for S. aureus when exposure to 900 MHz at 12 h. Exposure of P. aeruginosa to electromagnetic fields at 900 MHz however, lead to a significant reduction in growth rate, while 1800 MHz had insignificant effect. With the exception of S. aureus, treated with amoxicillin (30 µg) and exposed to electromagnetic fields, radiation treatment had no significant effect on bacterial sensitivity to antibiotics.
Keywords: Electromagnetic fields, Bacterial DNA, Antibiotic sensitivity, Bacterial growth
1. Introduction
Electromagnetic fields (EMF) have a major impact on biological systems (Panagopoulos et al., 2002, Balcavage et al., 1996, Grassi et al., 2004) including human health (Feychting and Ahlbom, 1993, Berg, 1999, Valberg et al., 1997). Recently, microorganisms are being exposed to radiofrequency and microwaves radiation signals from several sources (Balmori, 2016). Some studies were showed to confirm the effects of electromagnetic radiation (EMR) on functions of cell (Oncul et al. 2016). Studies on the effect of magnetic fields on prokaryotes have recently increased significantly, including studies on the effects of static magnetic fields, short frequency electromagnetic fields and pulsed electromagnetic fields. Fojt et al. (2004) for example, detected a decrease in colony forming units of E. coli, S. aureus and L. adecarboxylata at 50 Hz, 10 mT (particularly short frequency electromagnetic fields), while Strasak et al. (1998) reported that exposure of E. coli to extremely low frequency electromagnetic fields for 0–24 h for 0 – 24 h decreased the viability and growth rate of this bacterium when subjected to 50 Hz. Static magnetic fields have also been shown to inhibit bacterial growth (Chang et al., 2005, El-Sayed et al., 2006, Piatti et al., 2002, Zhang et al., 2003).
Cellini et al. (2008) studied the genetic effect of electromagnetic fields (EMF) on DNA when exposed to 50 Hz, DNA fingerprinting revealed no obvious differences among the DNA patterns at each conditions of study. Segatore et al. (2012) determined the effect of particularly short frequency electromagnetic fields (EL-FEMF) on antibiotic sensitivity and growth rate in P. aeruginosa and E. coli. Electromagnetic field (EMF) exposure significantly affected the growth rate of P. aeruginosa and E. coli when incubated with sub-inhibitory concentrations of kanamycin (1 μg/mL) and amikacin (0.5 μg/mL), individually. The potential of a synergistic and/or antagonistic impact on bacteria of antibiotics and exposure to electromagnetic fields deserves special attention because of the increased incidence of antibiotics resistance (Bush et al., 2011, Levy, 2001).
The aim of the present study was to determine the effect of electromagnetic fields (900 and 1800 MHz), i.e. the frequency produced by mobile phones on the growth and antibiotic sensitivity of a variety of bacteria.
2. Materials and methods
2.1. Exposure to electromagnetic fields
All bacteria were exposed to electromagnetic radiation using a mobile handset (Nokia). A dummy voice call was established between Radio Communication Tester CMU 200 by Rhode and Schwarz and the mobile handset (Fig. 1), with CMU 200 acting as a base station emulator. Bacteria were placed in close proximity to this source of electromagnetic radiation at frequencies of 900 MHz and 1800 MHz for two hours.
Fig. 1.
CMU 200 generator used to generate the frequencies of electromagnetic radiation.
2.2. Bacteria used
The three bacteria used in these studies, Staphylococcus aureus (ATCC 29213), Staphylococcus epidermidis (ATCC 25923), and Pseudomonas aeruginosa (ATCC 27853) were obtained from the Microbiology Laboratory of the College of Medicine, King Khalid University Hospital, Riyadh, Saudi Arabia.
2.3. Molecular method
2.3.1. Isolation and purification of DNA
The chromosomal DNA of both exposed and unexposed bacteria was extracted using QIAamp® DNA mini kit (QIAGEN, Italy) according to the method of Chomczynski et al. (1997). The quality of the isolated bacterial DNA was then quantified by a NanoDrop 8000 a muti-sample micro-volume UV–Vis spectrophotometer (Thermo Fisher Scientific, USA).
2.3.2. Repetitive sequence-based PCR (Rep-PCR)
Repetitive sequence-based PCR (Rep-PCR) was carried out using the primers REP1R (5′-III ICG ICG ICA TCI GGC-3′) and REP2I (5′-ICG ICT TAT CIG GCC TAC-3′) (Busch and Nitschko, 1999). The amplifications were carried out in PCR mixture containing (25 μl): DMSO, 5X Gauthier buffer, Go Taq® Green Master Mix ready-to-use solutions (GoTaq® DNA Polymerase, MgCl2, dNTPs, and reaction buffers at appropriate concentrations for active amplification, Promega, USA), primers of Rep-PCR reaction, and DNA template. GoTaq® contained two dyes (yellow and blue) that allow watching of progress through electrophoresis. Amplifications of PCR were carried out in a ProFlex™ PCR System (ThermoFisher Scientific, USA) with an initial denaturation step (95 °C, 2 min) followed by 30 cycles of denaturation (94 °C for 30 s and 92 °C for 30 s), annealing (40 °C for 1 min) and extension (65 °C for 8 min), and a single final extension step (65 °C for 8 min). The Rep–PCR fragments were divided using 1.5% agarose gels electrophoresis (3 V cm−1), which was carried out in 1xTAE electrophorests buffer. Computer-image analysis of photographs of the ethidium bromide-stained agarose gels was carried out by the gel analysis system (Ingenius Syngene Bio Inaging, Syngene, Cambridge, UK). Photographs were taken by the visual imaging system, and the images were saved. The patterns of DNA were standardized by the 1 Kilo base pair (kbp) marker lanes in gel, and the saved images were scanned in the 2.0–0.1-kbp range.
2.4. Bacterial growth assay
The bacteria were separately inoculated into Nutrient Broth (Oxoid) and incubated at 37 °C overnight according to the method of Schoenknecht et al. (1985). The cultures were then re-inoculated onto fresh Nutrient Broth and incubated for an additional 18 h to produce late logarithmic phase cultures. The bacteria were then sub-cultured in 100 ml fresh Nutrient Broth and the optical density was adjusted to 0.1 (OD) at 600 nm to obtain 1 × 106 colony-forming units (CFU) per ml. One set of flasks was exposed to the magnetic field generator while another, non-exposed set, acted a controls. Bacterial growth rate following exposure to EMF application (CMU 200 Generator) for two hours was determined using optical density measurements (600 nm) (Biowave, CO8000); all measurements were made on three separate aliquots.
2.5. Sensitivity to antibiotics
The sensitivity of EMF exposed bacteria to antibiotics was evaluated using the antibiotics amoxicillin (30 µg), azithromycin (15 µg) chloramphenicol (10 µg) and ciprofloxacin (5 µg) (Oxoid) and employing disc diffusion test.
2.5.1. Disc diffusion test
The disc diffusion method was used for determining the susceptibility of bacteria to antibiotics following exposure to electromagnetic fields using standard methods as described by the Clinical and Laboratory Standards Institute (CLSI, 2005).
2.6. Statistical analysis
Data were presented as mean ± standard deviation of three separate experiments. The data was then exposed to one-way ANOVA and Dunnett' test with Graphpad InStat software in order to determine statistical significance at p < 0.05.
3. Results and discussion
3.1. Bacterial DNA analysis
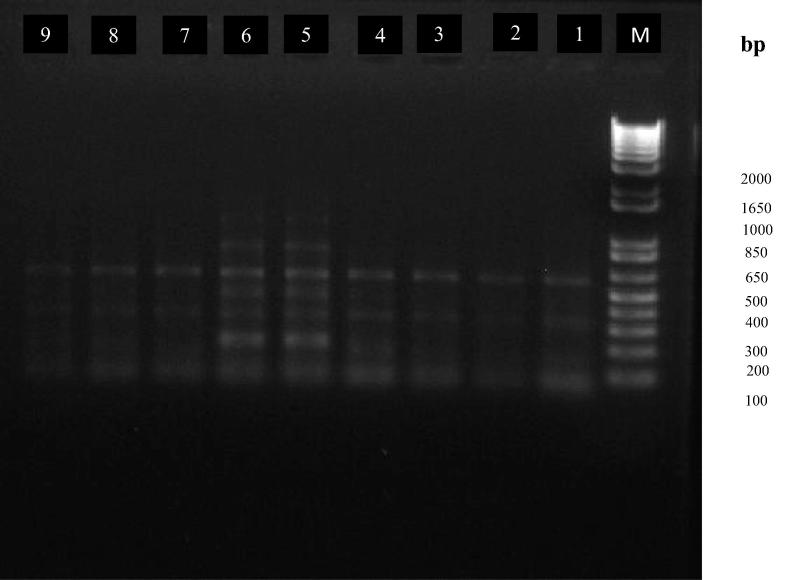
The effect of exposure to HF-EMF at radio frequencies of 900 MHz and 1800 MHz on chromosomal DNA of S. aureus, S. epidermidis and P. aeruginosa was determined by Rep-PCR analysis to estimate the presence of a noticeable variation induced in DNA structure. Bacterial DNA patterns obtained after agarose gel electrophoresis did not show any differentiation between those S. aureus and S. epidermidis exposed to 900 and 1800 MHz and the controls (Fig. 2). Three DNA band patterns were observed on gel electrophoreses for S. aureus and S. epidermidis with amplicon sizes ranging from 100 to1650 bp. Results obtained for P. aeruginosa on the other hand showed differences between the control and following exposure to 900 MHz and 1800 MHz radiation with more band appearing following exposure to 900 and 1800 MHz in comparison with control. Seven patterns were noted on gel electrophoreses for those bacteria exposed to 900 MHz and 1800 MHz with amplicon sizes ranging from 100 to 1000 bp, while three patterns with amplicon sizes ranging from 100 to 1650 bp were recorded for the controls. Similar results of unvaried electrophoretic patterns between E. coli XL-1 control cells and those which were exposed to high static magnetic field (HSMF) in in vivo experiments using random amplified polymorphic DNA (RAPD-PCR) were reported by Potenza et al. (2004), who, when recording DNA changes during studies on the stability of the genome in in vitro assays, found that exposure to magnetic fields radiation can induce DNA alterations normalized in organisms with cellular protective responses. Cellini et al. (2008) also, studied the effects of exposure to (LF-EMF) at 50 Hz on E. coli DNA using amplified fragment length polymorphism (AFLP) technique. However, their results did not reveal any remarkable differences between the DNA patterns for each studied condition.
Fig. 2.
Typical Rep-PCR fingerprints of bacteria exposed to electromagnetic field, lane (1) S. aureus unexposed-control, lane (2) S. aureus exposed to 900 MHz, lane (3) S. aureus exposed to 1800 MHz, lane (4) S. epidermidis unexposed-control, lane (5) S. epidermidis exposed to 900 MHz, lane (6) S. epidermidis exposed to 1800 MHz, lane (7) P. aeruginosa unexposed control, lane (8) P. aeruginosa exposed to 900 MHz, lane (9) P. aeruginosa exposed to 1800 MHz and lane (M) contains a 1-Kb molecular weight DNA ladder (Promega).
The results obtained in this study using S. aureus and S. epidermidis are in agreement with those results of the abive mentioned earlier studies, although the results for DNA patterns recorded for P. aeruginosa differed from the reported studies. This difference may be attributed to the techniques used in the present study compared to different techniques and bacteria employed in previous studies.
3.2. Effect of EMF on bacterial growth
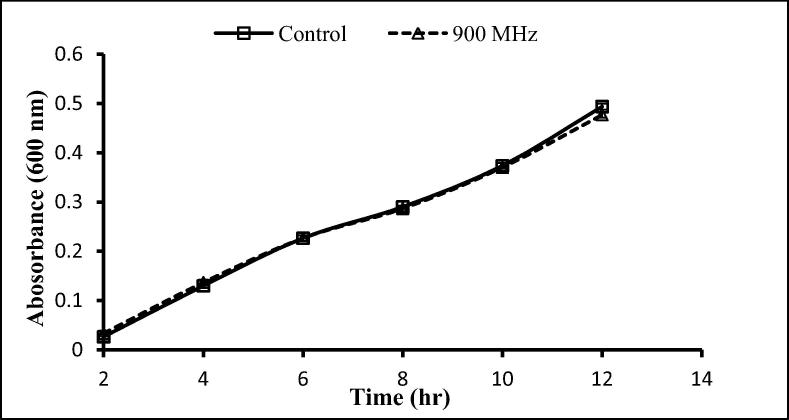
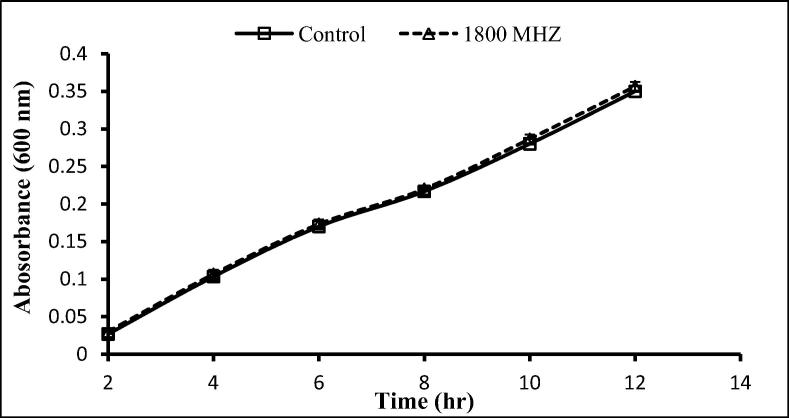
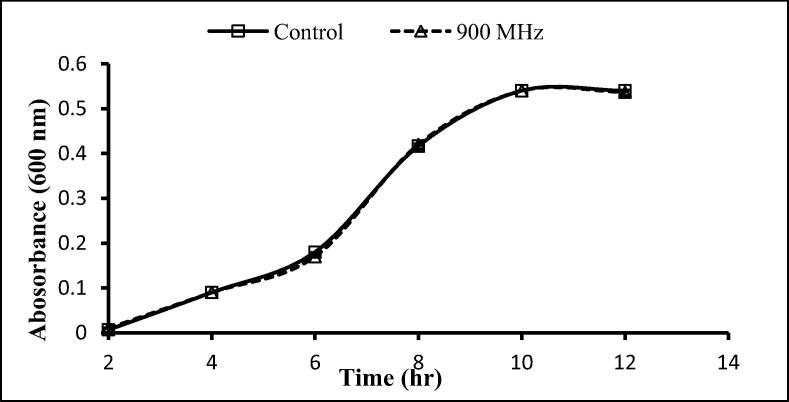
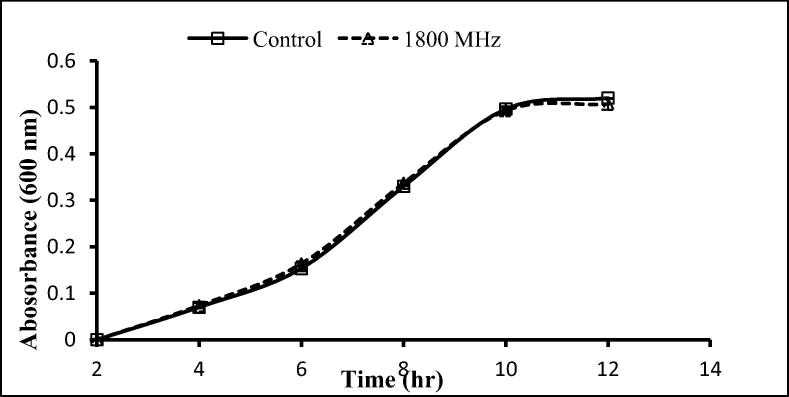
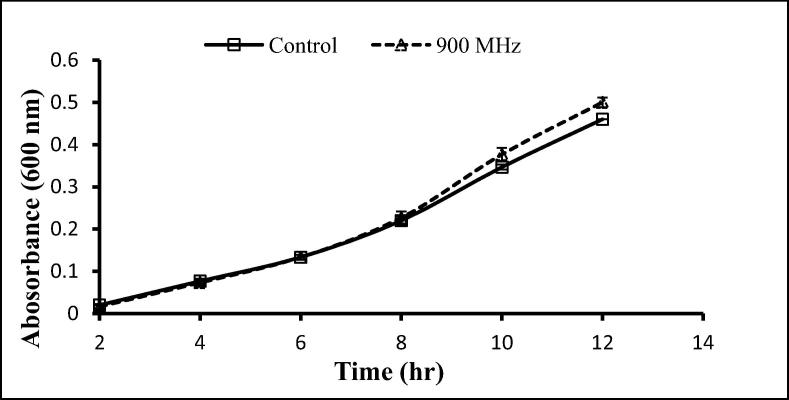
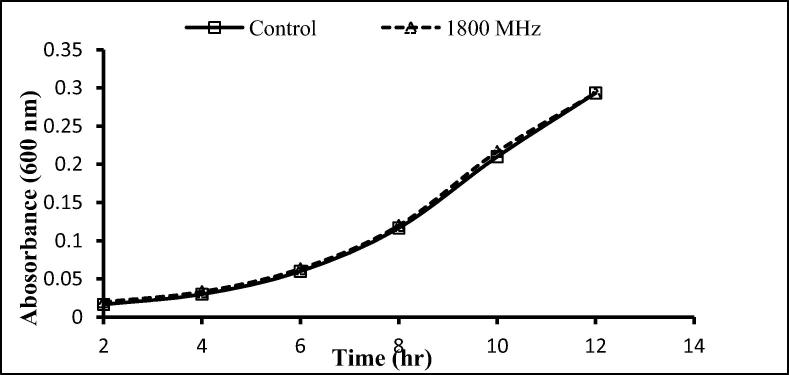
The two gram-positive and one-gram negative bacteria used here were exposed to 900 and 1800 MHz, with growth rates being determined following exposure for 2 h. With the exception of a significant decrease after 12 h exposure to 900 MHz, no significant effects on the growth of S. aureus were seen following exposure to 900 and 1800 MHz (Fig. 3, Fig. 4), similarly, no significant differences in the growth of S. epidermidis was seen following such exposure (Fig. 5, Fig. 6). In contrast, the growth of P. aeruginosa was significantly reduced following exposure for 10 and 12 h to 900 MHz while, no significant reduction in growth followed exposure to 1800 MHz (Fig. 7, Fig. 8).
Fig. 3.
Effect of EMF (900 MHz) on the growth rate of Staphylococcus aureus. Means ± SD from 3 different experiments.
Fig. 4.
Effect of EMF (1800 MHz) on the growth rate of Staphylococcus aureus. Means ± SD from 3 different experiments.
Fig. 5.
Effect of EMF (900 MHz) on the growth rate of Staphylococcus epidermidis Means ± SD from 3 different experiments.
Fig. 6.
Effect of EMF (1800 MHz) on the growth rate of Staphylococcus epidermidis Means ± SD from 3 different experiments.
Fig. 7.
Effect of EMF (900 MHz) on the growth rate of Pseudomonas aeruginosa. Means ± SD from 3 different experiments.
Fig. 8.
Effect of EMF (1800 MHz) on the growth rate of Pseudomonas aeruginosa. Means ± SD from 3 different experiments.
The experiments conducted here differ from others reported in the literature in that a 2 h exposure was used compared to previous studies where radiation exposure was conducted over the entire incubation period. Strasak et al., 1998, Fojt et al., 2004, and El-Sayed et al. 2006 concluded that the inhibitory effects on bacterial growth increased with length of exposure. El-Sayed et al. (2006) applied extremely low frequency-electromagnetic field (ELF-EMF) for 6 and 16 h to E. coli and obtained findings which differ from ours, suggesting that the field result is maximal in the first hours and then decreases, thereby indicating an adaptive response of the showing cells to magnetic field stress. Such an adaptive response can be due to an increase of heat shock proteins; Del Re et al. (2003) for example, showed an increase in DnaK and GroEL in E. coli when exposed 50 Hz, 1 mT. The decrease in growth rate can also be associated with morphological changes, or by metabolic alterations triggered by exposure to magnetic fields. Mega-Tiber et al. (2008) also observed an increased level of reactive oxygen species prompted by ELF25-EMF, which may have affected macromolecular synthesis and caused protein injury (Cabiscol et al., 2000) and thereby lead to a decrease in bacterial growth rate.
3.3. Effect of EMF on the sensitivity of bacteria to antibiotics
The results generally show that exposure to 900 MHz and 1800 MHz radiation did not produce any significant effect on the size of the inhibition zone produced when using the various antibiotics studied. (Table 1, Table 2, Table 3). An exception was provided in the case of amoxicillin (30 µg) against Staphylococcus aureus, where inhibition-zone values following exposure to 900 MHz and 1800 MHz) were significantly different form the control (Table 1). The results are in agreement with those of Segatore et al. (2012) who tested P. aeruginosa and E. coli for their sensitivity to antibiotics in the presence of extremely low frequency (ELF-EMF). They similarly found no significant changes in values of minimum inhibitory concentration (MIC) for treated cells and untreated with ELF-EMF and they concluded that such exposure had no significant effect on the sensitivity of P. aeruginosa and E. coli to antibiotics.
Table 1.
Inhibition zone values (mm) for Staphylococcus aureus when not exposed (control) and following exposure to EMF (900 and 1800 MHz).
| Antibiotics | Control | Expose 900 MHz | Expose 1800 MHz |
|---|---|---|---|
| Chloramphenicol (10 µg) | 18.66a* ± 1.15 | 18.33a ± 0.57 | 19.33a ± 0.57 |
| Ciprofloxacin (5 µg) | 28.33a ± 0.57 | 28a ± 0.0 | 28.33a ± 0.57 |
| Amoxicillin (30 µg) | 31.33b ± 1.15 | 33.66a ± 0.57 | 32.33a ± 0.57 |
| Azithromycin (15 µg) | 24.33a ± 1.15 | 24.33a ± 0.57 | 24.33a ± 0.57 |
Each value is the mean ± SD. Means with different letters in each row are significantly different (P < 0.05).
Table 2.
Inhibition zone values (mm) for Staphylococcus epidermidis when not exposed (control) and following exposure to EMF (900 and 1800 MHZ).
| Antibiotics | Control | Expose 900 MHz | Expose 1800 MHz |
|---|---|---|---|
| Chloramphenicol (10 µg) | 25a* ± 0.0 | 24.33a ± 0.57 | 24.66a ± 0.57 |
| Ciprofloxacin (5 µg) | 14.33a ± 0.57 | 14.66a ± 0.57 | 14a ± 0.0 |
| Amoxicillin (30 µg) | 14.66a ± 0.57 | 15.66a ± 0.57 | 15.33a ± 0.57 |
| Azithromycin (15 µg) | – | – | – |
Each value is the mean ± SD. Means with different letters in each row are significantly different (P < 0.05).
Table 3.
Inhibition zone values (mm) for Pseudomonas aeruginosa when not exposed (control) and following exposure to EMF (900 and 1800 MHZ).
| Antibiotics | Control | Expose 900 MHz | Expose 1800 MHz |
|---|---|---|---|
| Chloramphenicol (10 µg) | – | – | – |
| Ciprofloxacin (5 µg) | 30a*±1.0 | 29.33a ± 0.57 | 30a ± 0.0 |
| Amoxicillin (30 µg) | – | – | – |
| Azithromycin (15 µg) | 20.66a ± 1.15 | 22a ± 0.0 | 20.33a ± 0.57 |
Each value is the mean ± SD. Means with different letters in each row are significantly different (P < 0.05).
4. Conclusion
In this study, pathogenic bacteria were exposed to EMF at 900 MHz and 1800 MHz and the effects on DNA, growth rate, and antibiotic sensitivity of bacteria was determined. The DNA of S. aureus and S. epidermidis did not differ from the control when exposed to 900 and 1800 MHz, while P. aeruginosa showed differences in this criterion following such exposure. Fionally, radiation exposure at a frequency of 900 MHz and 1800 MHz generally had no significant effect on bacterial growth rates, except in the case of S. aureus, treated with amoxicillin.
Acknowledgment
This project was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Footnotes
Peer review under responsibility of King Saud University.
References
- Balcavage X., Alvager T., Swez J., Goff C., Fox M., Abdullyava S., King M. A mechanism for action of extremely low frequency electromagnetic fields on biological systems. Biochem. Biophys. Res. Commun. 1996;222:374–378. doi: 10.1006/bbrc.1996.0751. [DOI] [PubMed] [Google Scholar]
- Berg H. Problems of weak electromagnetic field effects in cell biology. Bioelectrochem. Bioenergy. 1999;48:355–360. doi: 10.1016/s0302-4598(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Busch U., Nitschko H. Methods for the differentiation of microorganisms. J. Chromatogr. Bio. Sci. Appl. 1999;722:263–278. doi: 10.1016/s0378-4347(98)00369-7. [DOI] [PubMed] [Google Scholar]
- Bush K.1., Courvalin P., Dantas G., Davies J., Eisenstein B., Huovinen P., Jacoby G.A., Kishony R., Kreiswirth B.N., Kutter E., Lerner S.A., Levy S., Lewis K., Lomovskaya O., Miller J.H., Mobashery S., Piddock L.J., Projan S., Thomas C.M., Tomasz A., Tulkens P.M., Walsh T.R., Watson J.D., Witkowski J., Witte W., Wright G., Yeh P., Zgurskaya H.I. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011;9:894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmori, A., 2016. Radiotelemetry and wildlife: highlighting a gap in the knowledge on radiofrequency radiation effects. Sci. Total Environ. 543 (pt A), 662–669. [DOI] [PubMed]
- Cabiscol E., Tamarit J., Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Intern. Microbiol. 2000;3:3–8. [PubMed] [Google Scholar]
- Cellini L., Grande R., Di Campli E. Bacterial response to the exposure of 50 Hz electromagnetic fields. Bioelectromagnetics. 2008;29:302–311. doi: 10.1002/bem.20391. [DOI] [PubMed] [Google Scholar]
- Chang S.K., Choi J.S., Gil H.W., Yang J.O., Lee E.Y., Jeon Y.S., Lee Z.W., Lee M., Hong M.Y., Ho Son T., Hong S.Y. Genotoxicity evaluation of electromagnetic fields generated by 835-MHz mobile phone frequency band. Eur. J. Cancer. Prev. 2005;14:175–179. doi: 10.1097/00008469-200504000-00014. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Mackey K., Drews R., Wilfinger W. DNAzol: a reagent for the rapid isolation of genomic DNA. Bio-Techniques. 1997;22:550–553. doi: 10.2144/97223pf01. [DOI] [PubMed] [Google Scholar]
- CLSI, 2005. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement, CLSI, Villanova, PA, Publication No. M100-S15.
- Del Re B., Garoia F., Mesirca P., Agostini C., Bersani F., Giorgi G. Extremely low frequency magnetic fields affect transposition activity in Escherichia coli. Rad. Environ. Biophys. 2003;42:113–118. doi: 10.1007/s00411-003-0192-9. [DOI] [PubMed] [Google Scholar]
- El-Sayed A.G., Magda H.S., Eman Y.T., Mona H.I. Stimulation and control of E.coli by using an extremely low frequency magnetic field. Rom. J. Biophys. 2006;16:283–296. [Google Scholar]
- Feychting M., Ahlbom A. Magnetic fields and cancer in children residing near Swedish high-voltage power lines. Am. J. Epidemiol. 1993;138:467–481. doi: 10.1093/oxfordjournals.aje.a116881. [DOI] [PubMed] [Google Scholar]
- Fojt L., Strasak L., Vetterl V., Smarda J. Comparison of the lowfrequency magnetic field effects on bacteria Escherichia coli, Leclercia adecarboxylata and Staphylococcus aureus. Bioelectrochemistry. 2004;63:337–341. doi: 10.1016/j.bioelechem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Grassi C., D’Ascenzo M., Torsello A., Martinotti G., Wolf F., Cittadini A., Azzena B. Effects of 50 Hz electromagnetic fields on voltage-gated Ca2 channels and their role in modulation of neuroendocrine cell proliferation and death. Cell Calcium. 2004;35:307–315. doi: 10.1016/j.ceca.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Levy B. Antibiotic resistance: consequences of inaction. Clin. Infect. Dis. 2001;33:124–129. doi: 10.1086/321837. [DOI] [PubMed] [Google Scholar]
- Mega-Tiber, P., Aksu, B., Inhan-Garip, A., 2008. The effect of ELF-EMF on bacterial growth; correlation with ROS. In: 2nd International Congress on Cell Membrane and Oxidative Stress. 2008 June 25–28. Isparta, Turkey. Cell Memb. Free Radi. Res. 1:28.
- Oncul S., Cuce E.M., Aksu B., Inhan Garip A. Effect of extremely low frequency electromagnetic fields on bacterial membrane. Int. J. Radiat Biol. 2016;92(1):42–49. doi: 10.3109/09553002.2015.1101500. [DOI] [PubMed] [Google Scholar]
- Panagopoulos J., Karabarbounis A., Margaritis H. Mechanism for action of electromagnetic fields on cells. Biochem. Biophys. Res. Commun. 2002;298:95–102. doi: 10.1016/s0006-291x(02)02393-8. [DOI] [PubMed] [Google Scholar]
- Piatti E., Albertini M.C., Baff W., Fraternale D., Citterio B., Piacentini M.P., Dacha M., Vetrano F., Accorsi A. Antibacterial effect of a magnetic field on Serratia marcescens and related virulence to Hordeum vulgare and Rubus fruticosus callus cells. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2002;132:359–365. doi: 10.1016/s1096-4959(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Potenza L., Cucchiarini L., Piatti E., Angelini U., Dacha M. Effects of high static magnetic field exposure on different DNAs. Bioelectromagnetics. 2004;25:352–355. doi: 10.1002/bem.10206. [DOI] [PubMed] [Google Scholar]
- Schoenknecht F.D., Sabath L.D., Thornsberry C., Jr. Susceptibility tests: special test. In: Lennette E.H., Balows A., Hansler W.J. Jr, Shadomy H.J., editors. Manual of Clinical Microbiology. America Society for Microbiology; Washington, DC, USA: 1985. [Google Scholar]
- Segatore, B., Setacci, D., Bennato, F., Cardigno, R., Amicosante, G, Iorio, R., 2012. Evaluations of the effects of extremely low-frequency electromagnetic fields on growth and antibiotic susceptibility of Escherichia coli and Pseudomonas aeruginosa. Inter. J. Microb. ID:587293 (7). [DOI] [PMC free article] [PubMed]
- Strasak L., Vetter V., Smarda J. The effect of low-frequency electromagnetic fields on living organisms. Sb. Lek. 1998;99:455–464. [PubMed] [Google Scholar]
- Valberg A., Kavet R., Rafferty N. Can low-level 50/60 Hz electric and magnetic fields cause biological effects? Radiat. Res. 1997;148:2–21. [PubMed] [Google Scholar]
- Zhang Q.M., Tokiwa M., Doi T., Nakahara T., Chang P.W., Nakamura N., Hori M., Miyakoshi I., Yonei S. Strong static magnetic field and the induction of mutations through elevated production of reactive oxygen species in Escherichia coli soxR. Intern. J. Rad. Biol. 2003;79:281–286. doi: 10.1080/0955300031000096289. [DOI] [PubMed] [Google Scholar]