Abstract
The second-to-fourth finger length ratio (2D:4D) is an indication of prenatal sex hormone exposure, and has sex-specifically been associated with several lethal illnesses including ischemic heart disease, diverse cancers, and suicide. Our primary aim was to verify that 2D:4D sex-specifically relates to life expectancy and suicide numbers on a national level (23 countries). We also used a hypothesis-free approach to investigate associations with other causes of death [p value adjustment for multiple hypothesis testing using the false discovery rate procedure (FDR)]. All parameters were normalized to the national mean (of males and females) and analyzed across nations. Normalized male 2D:4D correlated positively with normalized male life expectancy (at birth, r = 0.46, p = 0.029; at the age of 60, r = 0.44, p = 0.038) and negatively with normalized male suicide rates (r = − 0.49, p = 0.017). In the exploratory analyses, the normalized male 2D:4D values were negatively associated with the normalized male deaths rates from communicable, maternal, perinatal, and nutritional conditions [r = − 0.65, p(FDR) = 0.011], respiratory infections [r = − 0.69, p(FDR) = 0.008], asthma [r = − 0.65, p(FDR) = 0.011], neurological conditions [r = − 0.56, p(FDR) = 0.046], and Alzheimer’s disease and other dementias [r = − 0.59, p(FDR) = 0.036]. The normalized female parameters showed the same cross-national correlations. In line with the previous individual level findings, the results suggest that prenatal sex hormone effects are sex-specifically involved in suicide and neurological conditions. Moreover, we provide novel national level evidence that prenatal sex hormone priming may sex-specifically influence life expectancy and death risk from respiratory diseases.
Keywords: Digit ratio, Suicide, Causes of death, Life expectancy, Respiratory infections, Asthma
Introduction
Emerging evidence suggests that sex hormone levels during early developmental periods influence the risk of severe diseases with high mortality in later life. Based on preclinical causal and clinical associational studies, the second-to-fourth finger length ratio (2D:4D) is seen as a non-invasive retrospective biomarker for prenatal androgenization and estrogenization (Berenbaum et al. 2009; Breedlove 2010; Zheng and Cohn 2011; Auger et al. 2013) (but see also Wallen 2009 for a critical review). Many hypothesis-driven studies have shown associations between 2D: 4D and severe diseases. For example, lower (prenatally more androgenized) 2D:4D has been reported in individuals with poorer health (Rapoza 2017), prostate cancer (Mendes et al. 2016), primary brain tumors (Bunevicius et al. 2016), alcohol dependence (Kornhuber et al. 2011; Han et al. 2016; Lenz et al. 2017), anorexia nervosa in females (Quinton et al. 2011), Alzheimer’s disease in females (Vladeanu et al. 2014), and aggression-related injuries (Joyce et al. 2013; O’Briain et al. 2017). In addition, we recently provided the initial evidence for lower 2D:4D values in males who had died from suicide in comparison to males with other causes of death (Lenz et al. 2016). Higher (prenatally more estrogenized) 2D:4D has also been associated with lethal diseases such as breast cancer (Muller et al. 2012; Hong et al. 2014), cervical dysplasia (Brabin et al. 2008), oral squamous cell carcinoma in males (Nicolás Hopp and Jorge 2011), gastric cancer (Nicolás Hopp et al. 2013), disordered eating in males (Smith et al. 2010), Alzheimer’s disease in males (Vladeanu et al. 2014), coronary heart disease (Lu et al. 2015), and myocardial infarction (Kyriakidis et al. 2010). These studies, though, are often limited in sample size. Different methods for 2D:4D quantification complicate the comparison of investigations and demand cautious interpretation [e.g., Vernier caliper, photocopies, X-rays; usage of scans and photocopies results in lower 2D:4D in comparison to direct measures (Ribeiro et al. 2016)]. Most findings have not been replicated and thus negative or contradictory results have been reported [e.g., prostate cancer (Muller et al. 2011; García-Cruz et al. 2012)]. The overall effect sizes may be over interpreted due to publication bias of positive findings.
Of the above reported and 2D:4D-related diseases, ischemic heart disease and dementias reached the ranks of 1 (8.8 million deaths) and 7 (1.5 million deaths) for the World Health Organization (WHO) top 10 list of global causes of death in 2015 (WHO 2017). This indicates that prenatal sex hormone exposure may significantly contribute to the global death burden.
Our primary aims were to demonstrate a link between 2D:4D and life expectancy and to provide additional evidence for a role of 2D:4D in suicide. Moreover, we used a hypothesis-free approach to further explore associations with specific causes of death, as defined by WHO. Many studies have shown sex-dependent relationships between 2D:4D and illness with stronger or exclusively significant associations in males, suggesting that prenatal sex hormone priming might be more relevant to lifetime health status in males in comparison to females (Martin et al. 1999; Bailey and Hurd 2005; Martel et al. 2008; Collinson et al. 2010; Kyriakidis et al. 2010; Kornhuber et al. 2011; Nicolás Hopp et al. 2013; Portnoy et al. 2015; Lenz et al. 2016, 2017; Rapoza 2017) (but see also: Smedley et al. 2014; O’Briain et al. 2017). Thus, we analyzed sex-specifically whether normalized 2D:4D correlated with normalized life expectancy, suicide rates, and death rates from other causes across nations.
Methods
We normalized the male and female variables of interest (2D:4D, life expectancies, suicide rates, death rates from specific causes) to their national means (= sex-specific value divided by the mean of the male and female values) and calculated cross-national Pearson correlations between normalized 2D:4D and normalized variables of interest for males and females. In comparison to the direct correlation of 2D:4D values with the variables of interest, the correlation of normalized 2D:4D with the other normalized variables of interest may reduce the risk of misleading associations induced by psycho-social confounders (e.g., national differences in health care systems).
We extracted the national 2D:4D values from a follow-up analysis of the BBC Internet study (Reimers 2007) investigating gender inequality between 29 nations (158, 753 participants) (Manning et al. 2014). Due to how ethnicity influences 2D:4D, predominantly Caucasian national samples were analyzed. An online self-measure method for length of the second and fourth fingers has been employed. To reduce the number of statistical tests, we used the means of the national right-hand and left-hand 2D:4D values. Although some studies show stronger associations between 2D:4D and the target traits for the right hand (e.g., Manning et al. 1998; Hönekopp and Watson 2010; Kornhuber et al. 2011; Masuya et al. 2015; Bilgic et al. 2016), others found stronger effects for the left hand (e.g., Muller et al. 2012; Kilduff et al. 2013; Kornhuber et al. 2013; Hong et al. 2014; Lu et al. 2015). To our knowledge, there is no reliable explanation for differing associations of right- and left-hand 2D:4D with prenatal androgen load.
For confirmatory testing, gender-specific national life expectancies (at birth and at the age of 60) and suicide rates (age-standardized suicide rates per 100,000) for the year 2012 were extracted from World Health Statistics (WHO 2014c) and the WHO suicide report (WHO 2014b). For exploratory analyses, point estimates for deaths (adjusted for the population size) by cause, sex, and Member State in 2012 from the Global Health Estimates summary tables were used (WHO 2014a). We included the following 23 countries, as they provided high-quality data for variables of interest [comprehensive vital registration with at least 5 years of data for suicide estimates (WHO 2014b) and direct nationally representative death registration data for death numbers (WHO 2014a)]: Australia, Austria, Belgium, Canada, Croatia, Czech Republic, Denmark, Finland, France, Germany, Hungary, Iceland, Ireland, Italy, Netherlands, New Zealand, Norway, Romania, Spain, Sweden, Switzerland, United Kingdom, and the United States of America.
Variables with a significant deviation from the normal distribution (Shapiro–Wilk test) were transformed into rankit normal scores (Bishara and Hittner 2012). The p values < 0.05 were considered statistically significant, and p values < 0.1 were interpreted as a trend. To manage type 1 error risk in exploratory analyses, we adjusted p values for multiple hypothesis testing with the false discovery rate (FDR) procedure using a macro for Microsoft Excel [Appendix S1 of Pike (2011), graphically sharpened method; (Benjamini and Hochberg 2000)]. Data were analyzed with SPSS for Windows 21.0 (SPSS Inc., Chicago, IL, USA) and Graph Pad Prism 5 (Graph Pad Software Inc., San Diego, CA, USA).
Results
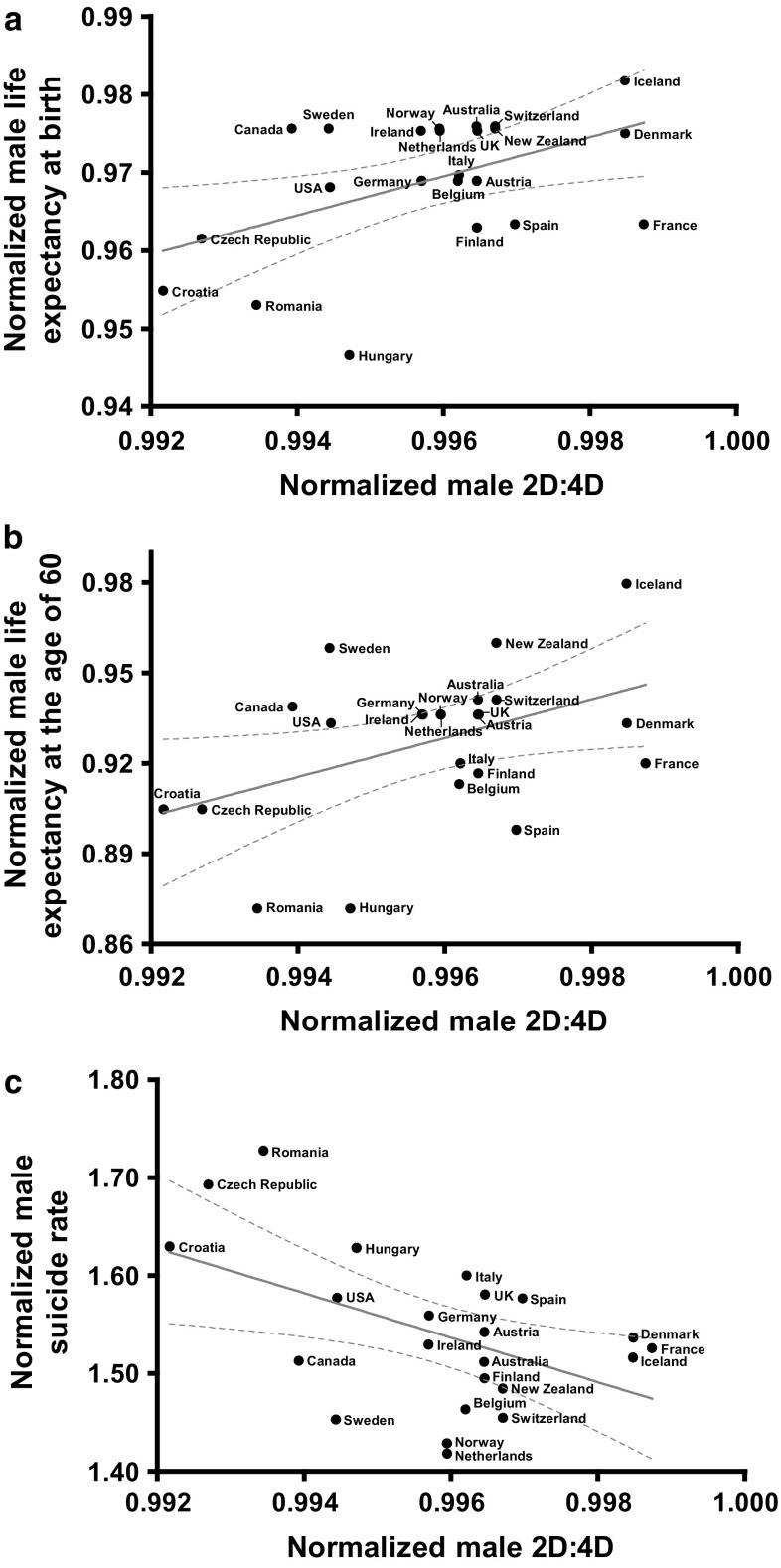
In support of our confirmatory hypotheses, normalized male 2D:4D correlated positively with normalized male life expectancy (at birth, see Fig. 1a, at the age of 60, see Fig. 1b) and negatively with normalized age-standardized male suicide rates (see Fig. 1c) across nations. On an individual level, these findings indicate that lower 2D:4D values might sex-specifically be associated with reduced life expectancy and a higher risk for death by suicide.
Fig. 1.
Normalized male 2D:4D values correlate positively with normalized male life expectancies at birth (n = 23, r = 0.46, p = 0.029; Fig. 1a) and at the age of 60 (n = 23, r = 0.44, p = 0.038; Fig. 1b) and negatively with normalized male age-standardized suicide rates (n = 23, r = − 0.49, p = 0.017; Fig. 1c). The analyzed variables were normalized to their national means (= male value divided by the mean of the male and female values), i.e., in countries with lower (prenatally more androgenized) national male 2D:4D values (divided by the national mean of the male and female values), we found lower male life expectancies at birth and at the age of 60 (divided by the national mean of the male and female values) and higher male age-standardized suicide rates (divided by the national mean of the male and female values). These cross-national associations indicate that lower 2D:4D ratios might sex-specifically be related to reduced life expectancy and increased risk for suicide at individual levels. For national sex-specific right- and left-hand 2D:4D values, see Table 1 in Manning et al. (2014); dotted lines represent the 95% confidence intervals of the best-fit from a linear regression analysis
Subsequently, we explored cross-national correlations between the normalized male 2D:4D and normalized male death rates from other specific causes, as defined by WHO (see Table 1). After correction for multiple hypothesis testing according to the FDR procedure, the analyses revealed exclusively negative Pearson correlations. Across nations, normalized male 2D:4D values were negatively related to the normalized male death rates of the following causes: Communicable, maternal, perinatal, and nutritional conditions; respiratory infections; neurological conditions; Alzheimer’s disease and other dementias; asthma; and other respiratory diseases. The normalized male 2D:4D values were only nominally related to death rates in the following groups: All causes, pancreas cancer, lymphomas and multiple myeloma, ischemic heart disease, digestive diseases, other digestive diseases, intentional injuries, and self-harm. For reasons of clarity and comprehensibility, we have focused on normalized male variables. However, we have repeated all analyses with normalized female variables of interest; the results showed the same correlations as in males (statistical values are not shown).
Table 1.
Cross-national Pearson correlations between normalized male 2D:4D and normalized male death rates from specific causes [Global Health Estimates summary tables (WHO 2014a)]
| r | p | p(FDR) | |
|---|---|---|---|
| All causes | − 0.43 | 0.0391 | 0.1485 |
| I. Communicable, maternal, perinatal, and nutritional conditions | − 0.65 | 0.0008 | 0.0109 |
| A. Infectious and parasitic diseases | − 0.36 | 0.0896 | 0.1929 |
| 1. Tuberculosis | − 0.39 | 0.0666 | 0.1769 |
| 4. Diarrheal diseases | 0.12 | 0.6011 | 0.5271 |
| 6. Meningitis | − 0.09 | 0.6759 | 0.5504 |
| 7. Encephalitis | − 0.20 | 0.3698 | 0.4088 |
| 10. Parasitic and vector diseases | − 0.17 | 0.4319 | 0.4245 |
| 12. Other infectious diseases | − 0.26 | 0.2225 | 0.3381 |
| B. Respiratory infections | − 0.69 | 0.0003 | 0.0078 |
| 1. Lower respiratory infections | − 0.69 | 0.0003 | 0.0078 |
| D. Neonatal conditions | 0.15 | 0.4960 | 0.4635 |
| 1. Preterm birth complications | 0.31 | 0.1525 | 0.2484 |
| 4. Other neonatal conditions | − 0.25 | 0.2510 | 0.3460 |
| E. Nutritional deficiencies | − 0.31 | 0.1485 | 0.2484 |
| II. Non-communicable diseases | − 0.36 | 0.0948 | 0.1929 |
| A. Malignant neoplasms | − 0.24 | 0.2608 | 0.3460 |
| 1. Mouth and oropharynx cancers | − 0.37 | 0.0782 | 0.1853 |
| 2. Esophagus cancer | − 0.39 | 0.0683 | 0.1769 |
| 3. Stomach cancer | 0.19 | 0.3846 | 0.4136 |
| 4. Colon and rectum cancers | − 0.07 | 0.7574 | 0.5680 |
| 5. Liver cancer | − 0.07 | 0.7485 | 0.5680 |
| 6. Pancreas cancer | − 0.48 | 0.0211 | 0.1095 |
| 7. Trachea, bronchus, lung cancers | − 0.26 | 0.2333 | 0.3409 |
| 8. Melanoma and other skin cancers | − 0.02 | 0.9288 | 0.6618 |
| 14. Bladder cancer | − 0.03 | 0.8777 | 0.6333 |
| 15. Lymphomas, multiple myeloma | 0.45 | 0.0331 | 0.1485 |
| 16. Leukemia | − 0.15 | 0.4851 | 0.4609 |
| Other malignant neoplasms | − 0.18 | 0.4130 | 0.4240 |
| B. Other neoplasms | − 0.36 | 0.0926 | 0.1929 |
| C. Diabetes mellitus | − 0.05 | 0.8081 | 0.5905 |
| D. Endocrine, blood, immune disorders | − 0.24 | 0.2640 | 0.3460 |
| E. Mental and behavioral disorders | − 0.17 | 0.4289 | 0.4245 |
| 4. Alcohol use disorders | − 0.18 | 0.4166 | 0.4240 |
| 5. Drug use disorders | − 0.09 | 0.6986 | 0.5608 |
| 7. Eating disorders | − 0.21 | 0.3371 | 0.4003 |
| 11. Other mental and behavioral disorders | − 0.18 | 0.3993 | 0.4214 |
| F. Neurological conditions | − 0.56 | 0.0056 | 0.0456 |
| 1. Alzheimer’s disease and other dementias | − 0.59 | 0.0032 | 0.0361 |
| 2. Parkinson’s disease | − 0.01 | 0.9768 | 0.6874 |
| 3. Epilepsy | − 0.29 | 0.1875 | 0.2968 |
| 4. Multiple sclerosis | − 0.25 | 0.2505 | 0.3460 |
| 7. Other neurological conditions | − 0.37 | 0.0787 | 0.1853 |
| H. Cardiovascular diseases | − 0.11 | 0.6266 | 0.5412 |
| 1. Rheumatic heart disease | − 0.35 | 0.1019 | 0.2003 |
| 2. Hypertensive heart disease | − 0.40 | 0.0585 | 0.1769 |
| 3. Ischemic heart disease | 0.44 | 0.0361 | 0.1485 |
| 4. Stroke | − 0.37 | 0.0813 | 0.1853 |
| 5. Cardiomyopathy, myocarditis, endocarditis | − 0.33 | 0.1183 | 0.2248 |
| 6. Other circulatory diseases | 0.13 | 0.5481 | 0.5039 |
| I. Respiratory diseases | − 0.40 | 0.0570 | 0.1769 |
| 1. Chronic obstructive pulmonary disease | − 0.10 | 0.6566 | 0.5485 |
| 2. Asthma | − 0.65 | 0.0007 | 0.0109 |
| 3. Other respiratory diseases | − 0.57 | 0.0043 | 0.0413 |
| J. Digestive diseases | − 0.50 | 0.0141 | 0.0805 |
| 1. Peptic ulcer disease | − 0.41 | 0.0522 | 0.1769 |
| 2. Cirrhosis of the liver | 0.08 | 0.7153 | 0.5663 |
| 3. Appendicitis | 0.26 | 0.2254 | 0.3381 |
| Other digestive diseases | − 0.44 | 0.0367 | 0.1485 |
| K. Genitourinary diseases | − 0.10 | 0.6640 | 0.5485 |
| 1. Kidney diseases | 0.12 | 0.5954 | 0.5271 |
| 3. Urolithiasis | − 0.40 | 0.0601 | 0.1769 |
| 4. Other genitourinary diseases | − 0.23 | 0.2846 | 0.3528 |
| L. Skin diseases | − 0.22 | 0.3191 | 0.3871 |
| M. Musculoskeletal diseases | 0.10 | 0.6406 | 0.5450 |
| Other musculoskeletal disorders | − 0.08 | 0.7271 | 0.5677 |
| N. Congenital anomalies | − 0.19 | 0.3729 | 0.4088 |
| 4. Congenital heart anomalies | − 0.20 | 0.3560 | 0.4088 |
| 5. Other chromosomal anomalies | − 0.31 | 0.1483 | 0.2484 |
| 6. Other congenital anomalies | − 0.17 | 0.4425 | 0.4275 |
| III. Injuries | − 0.39 | 0.0641 | 0.1769 |
| A. Unintentional injuries | − 0.32 | 0.1316 | 0.2345 |
| 1. Road injury | − 0.07 | 0.7561 | 0.5680 |
| 2. Poisonings | − 0.33 | 0.1282 | 0.2345 |
| 3. Falls | − 0.23 | 0.2847 | 0.3528 |
| 4. Fire, heat, and hot substances | − 0.24 | 0.2671 | 0.3460 |
| 5. Drowning | 0.12 | 0.5813 | 0.5259 |
| 7. Other unintentional injuries | − 0.20 | 0.3655 | 0.4088 |
| B. Intentional injuries | − 0.53 | 0.0090 | 0.0639 |
| 1. Self-harm | − 0.52 | 0.0109 | 0.0690 |
| 2. Interpersonal violence | − 0.05 | 0.8049 | 0.5905 |
The analyzed variables were normalized to their national means (= male value divided by the mean of the male and female values). A negative cross-national correlation between normalized male 2D:4D and normalized male death rates from specific causes means that lower (prenatally more androgenized) national male 2D:4D values (divided by the national mean of the male and female values) correlate with a higher national risk for cause-specific death in males (divided by the national mean of the male and female values). The normalized female parameters showed the same correlations. We calculated only correlations for which male and female causes of death estimates according to the Global Health Estimates summary tables (WHO 2014a) in all 23 countries included in this study were available. The following categories have been excluded due to missing national values: I: A2-3, A5, A8-9, A10a-k, A11, B2-3, C, D2-3, E1-5; II: A9-13, E1-3, E6, E8-10, F5-6, G, K2, K5-6, M1-4, N1-3, O; III: A6, B3. For national sex-specific right- and left-hand 2D:4D values, see Table 1 in Manning et al. (2014). Significant results (p < 0.05) are illustrated in bold letters. FDR False discovery rate
Discussion
This investigation shows that, across nations, normalized male 2D:4D values correlate significantly with normalized male life expectancies (at birth and at the age of 60), suicide rates, and other cause-specific death rates (using a hypothesis-free approach). The reported associations are also true for females. However, for reasons of clarity and comprehensibility, we focus on males when elaborating the relationships in the following discussion.
Across nations, normalized male 2D:4D values were positively associated with normalized male life expectancies at birth and at the age of 60. This means that lower (prenatally more androgenized) male 2D:4D values (in relation to the mean of male and female 2D:4D) were found in countries with lower male life expectancies (in relation to the male and female mean). This observation supports the assumption that prenatal sex hormone priming entails long-lasting, possibly lifelong effects on sex-specific death risk. The exploratory analyses revealed significant associations between normalized male 2D:4D and normalized male death rates in seven specific causes of death categories which persisted after adjustment for multiple hypothesis testing. Interestingly, each of these seven significant correlations was negative; i.e., lower (prenatally more androgenized) normalized male 2D:4D values were cross-nationally related to higher normalized male disease-specific death rates. This agrees with the observed positive association of normalized male 2D:4D with normalized male life expectancy and underlines the reliability of the finding. Although conclusions of these national level findings for the individual level should be drawn very cautiously, the results suggest that lower (prenatally more androgenized) 2D:4D might be sex-specifically associated with lower life expectancy.
In support of our previous individual level observation of lower 2D:4D in males but not in females who have died from suicide in comparison to sex-specific controls with other causes of death (Lenz et al. 2016), we found, in this study, that the normalized male 2D:4D correlated negatively with the normalized male suicide rates (confirmatory hypothesis) and deaths from intentional injuries and self-harm (statistical trend in the exploratory analysis). These findings are also consistent with individual level studies, showing that 2D:4D is involved in aggression-related injuries (Joyce et al. 2013). They indicate that lower (prenatally more androgenized) 2D:4D might be sex-specifically linked to increased risk for suicide, intentional injuries, and self-harm. This finding is relevant, because suicides represent the second leading cause of death among adolescents and young adults 15–29 years of age (WHO 2014b). It might be hypothesized that depression mediates the association between lower 2D:4D and death from suicide. However, the relevance of this mechanism needs further investigation because of inconsistent literature pointing to an increased risk for depression in individuals with both higher 2D:4D (Bailey and Hurd 2005; Smedley et al. 2014) and lower 2D:4D (Martin et al. 1999; Rapoza 2017).
It must be noted that life expectancy and suicidal behaviors are also influenced by a lot of other biological and non-biological factors. Thus, it is unlikely that 2D:4D alone might serve as an accurate predictor of life expectancy and death from suicide in the future.
To our knowledge, this is the first hypothesis-free investigation which shows after adjustment for multiple hypothesis testing significant cross-national correlations between normalized male 2D:4D and normalized male death rates from respiratory infections, asthma, and other respiratory diseases. On an individual level, these findings indicate that lower (prenatally more androgenized) 2D:4D might be sex-specifically related to deaths caused by respiratory infections and asthma. The importance of this observation is underlined by the fact that lower respiratory infections take rank 3 (3.2 million global deaths) on the 2015 WHO top 10 list of global causes of death (WHO 2017). It is tempting to speculate that prenatal sex hormone effects on adolescent and adult smoking behavior may mediate the association of 2D:4D with respiratory infections and asthma. However, this mechanism is improbable, because higher 2D:4D has been found in smokers in comparison to non-smokers (Manning and Fink 2011; Borkowska and Pawlowski 2013) and because 2D:4D has been positively correlated with the severity of nicotine dependence in male alcohol-dependent smokers and ex-smokers (Lenz et al. 2017). Consistent with the concept of dysanapsis, i.e., an incongruent growth of the lungs and airways, it is more likely that early in life-induced direct pulmonary androgenization entails a higher risk for asthma and respiratory infections in later life; e.g., the female fetal lungs start to produce surfactant earlier and female newborns have lower specific airway resistance. Accordingly, boys are two to four times more prone to asthma than girls; however, after puberty, females are at higher risk for asthma, thereby suggesting that pubertal and postpubertal effects are also very important (Townsend et al. 2012).
The exploratory analyses show FDR-adjusted negative cross-national correlations of normalized male 2D:4D with normalized male death rates from neurological conditions and dementias. These results agree with a previously reported sex-specific role of 2D:4D in Alzheimer’s disease based on individual data (Vladeanu et al. 2014). The fact that Alzheimer’s disease and other dementias take rank 7 (1.5 million deaths) on the 2015 WHO top 10 list of global causes of death (WHO 2017) highlights the finding’s relevance. Furthermore, the exploratory analyses revealed that the normalized male 2D:4D values are related to the normalized male death rates of ischemic heart diseases (nominally significant), tuberculosis, and stroke (trends of significance). On the 2015 WHO top 10 list of global causes of death, these illnesses rank at 1, 2, and 9. The observed positive cross-national association between normalized male 2D:4D and normalized male death rates in ischemic heart disease fits with the previous reports of higher 2D:4D values in males with coronary heart disease (Lu et al. 2015) and in males (but not females) with myocardial infarction (Kyriakidis et al. 2010).
The major strength of this investigation is that in addition to verifying the confirmatory hypotheses, we applied a hypothesis-free approach and found significant results after statistical adjustment for multiple hypothesis testing. Moreover, it is important to note that the national 2D:4D values analyzed here represent a large study cohort with a broad age range [> 99% between 10 and 70 years of age (Reimers 2007)].
There are several important limitations. The number of 23 countries investigated is relatively small. The self-measure method applied to quantify 2D:4D reduces the precision. Self-measured 2D:4D show an estimated reliability of 46% of that of expert-measured 2D:4D (Hönekopp and Watson 2010). These factors may be reasons why we here failed to statistically verify associations with lower effect sizes. They may also explain why we were not able to provide further empirical support of previously described significant associations of 2D:4D with alcohol dependence (Kornhuber et al. 2011; Han et al. 2016; Lenz et al. 2017) or eating disorders (Quinton et al. 2011). The analytical approach is limited in that it is based on national gender variations and, thus, depends on a sex-specific relationship between 2D:4D and variable of interest. Moreover, it is important to mention that we explicitly focused on deaths as the endpoint of disease. It may be that 2D:4D is associated with disease prevalence (not in the scope of this study) although not with related mortality. Another important limitation is that our findings cannot easily be generalized to non-Caucasian ethnicities or middle or low income economies, due to the sample of countries that have been included in this analysis. The methodological approach to correlate normalized sex-specific 2D:4D with normalized sex-specific death rates across nations did not allow for the investigation of the role of 2D:4D in sex-specific illnesses such as prostate cancer. Although the reported cross-national correlations of normalized sex-specific 2D:4D with normalized sex-specific death risk from suicide, intentional injuries, and ischemic heart disease agree with the previous reports based on data from individual participants, it remains to be seen to what extent findings such as the national association between 2D:4D and respiratory diseases are relevant to individuals. It is very important to mention that our conclusions drawn from the national level might not correctly reflect true associations on the individual level. The limitations claim a very cautious interpretation of the results reported; an independent replication and support on the individual level, in addition to what is demonstrated here on the national level, is certainly needed. Finally, the limitations related to the use of 2D:4D as a proxy should not be forgotten (e.g., other influencing factors aside from prenatal sex hormone exposure).
Conclusions
We provide novel national level evidence for a sex-specific role of prenatal sex hormone priming in life expectancy and possible causes of death, such as suicide and neurological, pulmonary, and cardiovascular diseases. The results may yield new insight into the mechanisms underlying lethal disease and build a basis to establish predictive and preventive strategies in the future.
Acknowledgements
This work has been presented at the World Psychiatric Association XVII World Congress of Psychiatry, Berlin 2017.
List of abbreviations
- 2D:4D
Second-to-fourth finger length ratio
- FDR
False discovery rate
- WHO
World Health Organization
Author contributions
BL and JK designed the study. BL analyzed the data and wrote the first draft of the manuscript. JK critically revised it for important intellectual content.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable. This investigation analyzed data previously published; no additional human participants have been involved.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This work was supported by intramural grants from the University Hospital of the Friedrich-Alexander University Erlangen-Nürnberg (FAU). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
References
- Auger J, Le Denmat D, Berges R, Doridot L, Salmon B, Canivenc-Lavier MC, Eustache F. Environmental levels of oestrogenic and antiandrogenic compounds feminize digit ratios in male rats and their unexposed male progeny. Proc Biol Sci. 2013;280(1768):20131532. doi: 10.1098/rspb.2013.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AA, Hurd PL. Depression in men is associated with more feminine finger length ratios. Personal Indiv Dif. 2005;39(4):829–836. doi: 10.1016/j.paid.2004.12.017. [DOI] [Google Scholar]
- Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25(1):60–83. doi: 10.3102/10769986025001060. [DOI] [Google Scholar]
- Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009;150(11):5119–5124. doi: 10.1210/en.2009-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgic Ö, Altınyazar HC, Eryılmaz D, Tuğrul ZA. Are 2D:4D finger-length ratios an indicator of androgenetic alopecia in males? An Bras Dermatol. 2016;91(2):156–159. doi: 10.1590/abd1806-4841.20164622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishara AJ, Hittner JB. Testing the significance of a correlation with nonnormal data: comparison of Pearson, Spearman, transformation, and resampling approaches. Psychol Methods. 2012;17(3):399–417. doi: 10.1037/a0028087. [DOI] [PubMed] [Google Scholar]
- Borkowska B, Pawlowski B. Alcohol and nicotine intake and prenatal level of androgens measured by digit ratio. Pers Indiv Dif. 2013;55(6):685–687. doi: 10.1016/j.paid.2013.05.020. [DOI] [Google Scholar]
- Brabin L, Roberts SA, Farzaneh F, Fairbrother E, Kitchener HC. The second to fourth digit ratio (2D:4D) in women with and without human papillomavirus and cervical dysplasia. Am J Hum Biol. 2008;20(3):337–341. doi: 10.1002/ajhb.20731. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Minireview: organizational hypothesis: instances of the fingerpost. Endocrinology. 2010;151(9):4116–4122. doi: 10.1210/en.2010-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunevicius A, Tamasauskas S, Deltuva VP, Tamasauskas A, Sliauzys A, Bunevicius R. Digit ratio (2D:4D) in primary brain tumor patients: a case-control study. Early Hum Dev. 2016;103:205–208. doi: 10.1016/j.earlhumdev.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Collinson SL, Lim M, Chaw JH, Verma S, Sim K, Rapisarda A, Chong SA. Increased ratio of 2nd to 4th digit (2D:4D) in schizophrenia. Psychiatry Res. 2010;176(1):8–12. doi: 10.1016/j.psychres.2009.08.023. [DOI] [PubMed] [Google Scholar]
- García-Cruz E, Piqueras M, Huguet J, Ribal MJ, Vilaseca A, Gosálbez D, Castañeda-Argáiz R, Carrión A, Alcover J, Alcaraz A. Higher second fourth digit ratio predicts higher incidence of prostate cancer in prostate biopsy. Arch Esp Urol. 2012;65(9):816–821. [PubMed] [Google Scholar]
- Han C, Bae H, Lee Y-S, Won S-D, Kim DJ. The ratio of 2nd to 4th digit length in Korean alcohol-dependent patients. Clin Psychopharmacol Neurosci. 2016;14(2):148–152. doi: 10.9758/cpn.2016.14.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönekopp J, Watson S. Meta-analysis of digit ratio 2D:4D shows greater sex difference in the right hand. Am J Hum Biol. 2010;22(5):619–630. doi: 10.1002/ajhb.21054. [DOI] [PubMed] [Google Scholar]
- Hong L, Zhan-Bing M, Zhi-Yun S, Xiao-Xia S, Jun-Li Z, Zheng-Hao H. Digit ratio (2D:4D) in Chinese women with breast cancer. Am J Hum Biol. 2014;26(4):562–564. doi: 10.1002/ajhb.22546. [DOI] [PubMed] [Google Scholar]
- Joyce CW, Kelly JC, Chan JC, Colgan G, O’Briain D, Mc Cabe JP, Curtin W. Second to fourth digit ratio confirms aggressive tendencies in patients with boxers fractures. Injury. 2013;44(11):1636–1639. doi: 10.1016/j.injury.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Kilduff LP, Hopp RN, Cook CJ, Crewther BT, Manning JT. Digit ratio (2D:4D), aggression, and testosterone in men exposed to an aggressive video stimulus. Evol Psychol. 2013;11(5):953–964. doi: 10.1177/147470491301100502. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Erhard G, Lenz B, Kraus T, Sperling W, Bayerlein K, Biermann T, Stoessel C. Low digit ratio 2D:4D in alcohol dependent patients. PLoS One. 2011;6(4):e19332. doi: 10.1371/journal.pone.0019332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Zenses EM, Lenz B, Stoessel C, Bouna-Pyrrou P, Rehbein F, Kliem S, Mößle T. Low 2D:4D values are associated with video game addiction. PLoS One. 2013;8(11):e79539. doi: 10.1371/journal.pone.0079539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakidis I, Papaioannidou P, Pantelidou V, Kalles V, Gemitzis K. Digit ratios and relation to myocardial infarction in Greek men and women. Gend Med. 2010;7(6):628–636. doi: 10.1016/j.genm.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Lenz B, Thiem D, Bouna-Pyrrou P, Mühle C, Stoessel C, Betz P, Kornhuber J. Low digit ratio (2D:4D) in male suicide victims. J Neural Transm (Vienna) 2016;123(12):1499–1503. doi: 10.1007/s00702-016-1608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz B, Mühle C, Braun B, Weinland C, Bouna-Pyrrou P, Behrens J, Kubis S, Mikolaiczik K, Muschler M-R, Saigali S, Sibach M, Tanovska P, Huber SE, Hoppe U, Eichler A, Heinrich H, Moll GH, Engel A, Goecke TW, Beckmann MW, Fasching PA, Müller CP, Kornhuber J. Prenatal and adult androgen activities in alcohol dependence. Acta Psychiatr Scand. 2017;136(1):96–107. doi: 10.1111/acps.12725. [DOI] [PubMed] [Google Scholar]
- Lu H, Ma Z, Zhao J, Huo Z. Second to fourth digit ratio (2D:4D) and coronary heart disease. Early Hum Dev. 2015;91(7):417–420. doi: 10.1016/j.earlhumdev.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Manning JT, Fink B. Digit ratio, nicotine and alcohol intake and national rates of smoking and alcohol consumption. Pers Indiv Dif. 2011;50(3):344–348. doi: 10.1016/j.paid.2010.10.016. [DOI] [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13(11):3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- Manning JT, Fink B, Trivers R (2014) Digit ratio (2D:4D) and gender inequalities across nations. Evol Psychol 12(4):757–768. http://nrl.northumbria.ac.uk/17733/ [PubMed]
- Martel MM, Gobrogge KL, Breedlove SM, Nigg JT. Masculinized finger-length ratios of boys, but not girls, are associated with attention-deficit/hyperactivity disorder. Behav Neurosci. 2008;122(2):273–281. doi: 10.1037/0735-7044.122.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SM, Manning JT, Dowrick CF. Fluctuating asymmetry, relative digit length, and depression in men. Evol Hum Behav. 1999;20(3):203–214. doi: 10.1016/S1090-5138(99)00006-9. [DOI] [Google Scholar]
- Masuya Y, Okamoto Y, Inohara K, Matsumura Y, Fujioka T, Wada Y, Kosaka H. Sex-different abnormalities in the right second to fourth digit ratio in Japanese individuals with autism spectrum disorders. Mol Autism. 2015;6:34. doi: 10.1186/s13229-015-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes PHC, Martelli DRB, de Melo Costa S, Gonçalves E, Macedo CP, Silveira MF, Martelli Júnior H. Comparison of digit ratio (2D:4D) between Brazilian men with and without prostate cancer. Prostate Cancer Prostatic Dis. 2016;19(1):107–110. doi: 10.1038/pcan.2015.62. [DOI] [PubMed] [Google Scholar]
- Muller DC, Giles GG, Manning JT, Hopper JL, English DR, Severi G. Second to fourth digit ratio (2D:4D) and prostate cancer risk in the Melbourne Collaborative Cohort Study. Br J Cancer. 2011;105(3):438–440. doi: 10.1038/bjc.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DC, Baglietto L, Manning JT, McLean C, Hopper JL, English DR, Giles GG, Severi G. Second to fourth digit ratio (2D:4D), breast cancer risk factors, and breast cancer risk: a prospective cohort study. Br J Cancer. 2012;107(9):1631–1636. doi: 10.1038/bjc.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolás Hopp R, Jorge J. Right hand digit ratio (2D:4D) is associated with oral cancer. Am J Hum Biol. 2011;23(3):423–425. doi: 10.1002/ajhb.21144. [DOI] [PubMed] [Google Scholar]
- Nicolás Hopp R, de Souza Lima NC, Filho JL, Filho MS, Lima CSP, Jorge J. Digit ratio (2D:4D) is associated with gastric cancer. Early Hum Dev. 2013;89(5):327–329. doi: 10.1016/j.earlhumdev.2012.11.002. [DOI] [PubMed] [Google Scholar]
- O’Briain DE, Dawson PH, Kelly JC, Connolly P. Assessment of the 2D:4D ratio in aggression-related injuries in children attending a paediatric emergency department. Ir J Med Sci. 2017;186(2):441–445. doi: 10.1007/s11845-016-1524-5. [DOI] [PubMed] [Google Scholar]
- Pike N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol. 2011;2(3):278–282. doi: 10.1111/j.2041-210X.2010.00061.x. [DOI] [Google Scholar]
- Portnoy J, Raine A, Glenn AL, Chen FR, Choy O, Granger DA. Digit ratio (2D:4D) moderates the relationship between cortisol reactivity and self-reported externalizing behavior in young adolescent males. Biol Psychol. 2015;112:94–106. doi: 10.1016/j.biopsycho.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Quinton SJ, Smith AR, Joiner T. The 2 to 4 digit ratio (2D:4D) and eating disorder diagnosis in women. Pers Individ Dif. 2011;51(4):402–405. doi: 10.1016/j.paid.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoza KA. Does life stress moderate/mediate the relationship between finger length ratio (2D4D), depression and physical health? Personal Individ Dif. 2017;113:74–80. doi: 10.1016/j.paid.2017.03.009. [DOI] [Google Scholar]
- Reimers S. The BBC internet study: general methodology. Arch Sex Behav. 2007;36(2):147–161. doi: 10.1007/s10508-006-9143-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro E, Neave N, Morais RN, Manning JT. Direct versus indirect measurement of digit ratio (2D:4D): a critical review of the literature and new data. Evol Psychol. 2016;14(1):1–8. doi: 10.1177/1474704916632536. [DOI] [Google Scholar]
- Smedley KD, McKain KJ, McKain DN. 2D:4D digit ratio predicts depression severity for females but not for males. Pers Indiv Dif. 2014;70:136–139. doi: 10.1016/j.paid.2014.06.039. [DOI] [Google Scholar]
- Smith AR, Hawkeswood SE, Joiner TE. The measure of a man: associations between digit ratio and disordered eating in males. Int J Eat Disord. 2010;43(6):543–548. doi: 10.1002/eat.20736. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012;33(1):1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladeanu M, Giuffrida O, Bourne VJ. Prenatal sex hormone exposure and risk of Alzheimer disease: a pilot study using the 2D:4D digit length ratio. Cogn Behav Neurol. 2014;27(2):102–106. doi: 10.1097/WNN.0000000000000024. [DOI] [PubMed] [Google Scholar]
- Wallen K. Does finger fat produce sex differences in second to fourth digit ratios? Endocrinology. 2009;150(11):4819–4822. doi: 10.1210/en.2009-0986. [DOI] [PubMed] [Google Scholar]
- WHO (2014a) Global Health Estimates. http://www.who.int/healthinfo/global_burden_disease/estimates_country_2000_2012/en/. Accessed 21 Jan 2017
- WHO (2014b) Preventing suicide: a global imperative. http://www.who.int/mental_health/suicide-prevention/world_report_2014/en/. Accessed 18 Sept 2015
- WHO (2014c) World Health Statistics 2014. http://www.who.int/gho/publications/world_health_statistics/2014/en/. Accessed 9 March 2017
- WHO (2017) The top 10 causes of death: fact sheet. http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed 26 Feb 2017
- Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci USA. 2011;108(39):16289–16294. doi: 10.1073/pnas.1108312108. [DOI] [PMC free article] [PubMed] [Google Scholar]