Introduction
Key Teaching Points.
-
•
Comprehensive phenotyping and deep knowledge of the underlying genotype guide successful management in complex long QT syndrome.
-
•
Left cardiac sympathetic denervation may be a successful option for long QT patients who cannot tolerate beta blockade.
-
•
Competing risks in complex patients with long QT syndrome require a personalized, patient-centered approach to medical management.
Long QT syndrome (LQTS) is an inherited cardiac arrhythmia disorder characterized by QT prolongation and/or abnormal T-wave morphology on the electrocardiogram (ECG) and symptoms including syncope, cardiac arrest, and sudden cardiac death.1 Beta-blockers have long been accepted as the first line of treatment, and are highly effective, especially in patients with LQTS type 1. This type of LQTS is related to abnormalities in the KvLQT1, which controls the slow delayed rectifier potassium current of cardiac repolarization, where cardiac events are typically triggered by adrenergic stimulation.2 An alternative antiadrenergic treatment strategy is the use of left cardiac sympathetic denervation (LCSD), initially advocated as an effective treatment in addition to beta-blockade for those with recurrent symptoms and appropriate implantable cardioverter-defibrillator discharges, but more recently used in patients who are intolerant to beta-blockade.3 The effects of LCSD are to both interrupt the main source of myocardial norepinephrine, thereby limiting the catecholaminergic activation of dysfunctional KvLQT1 channels, and to increase myocardial refractoriness and fibrillatory threshold.4 This report details patient-specific treatment strategies used in a child with LQTS type 1 who had complications on beta-blocker therapy. The strategies were based on genetic results and continual assessment of the phenotype.
Case report
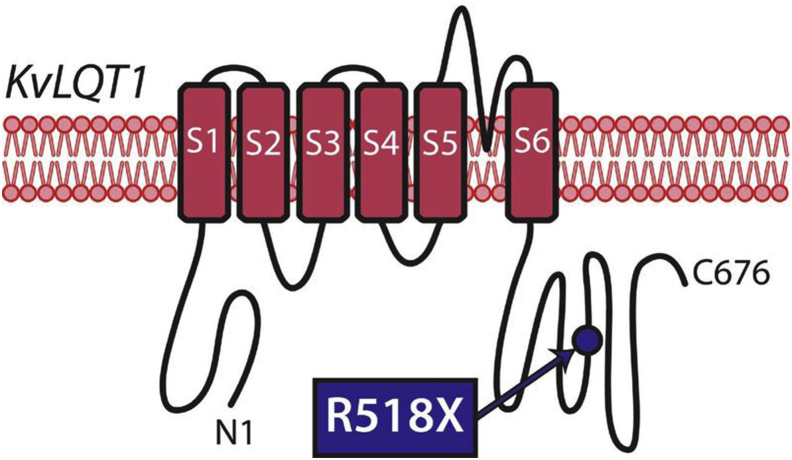
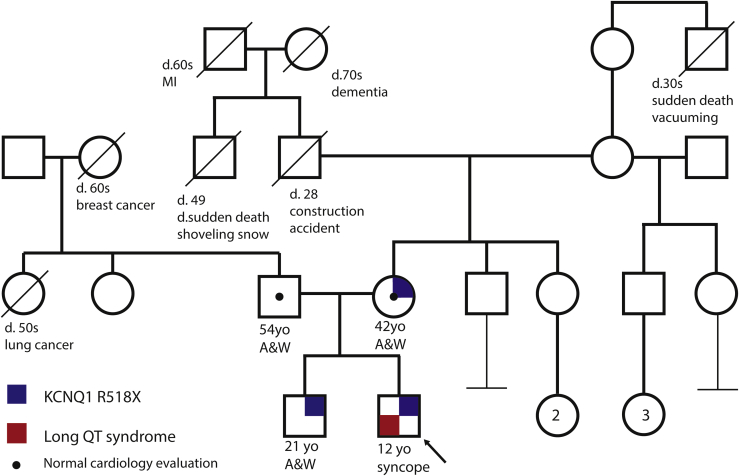
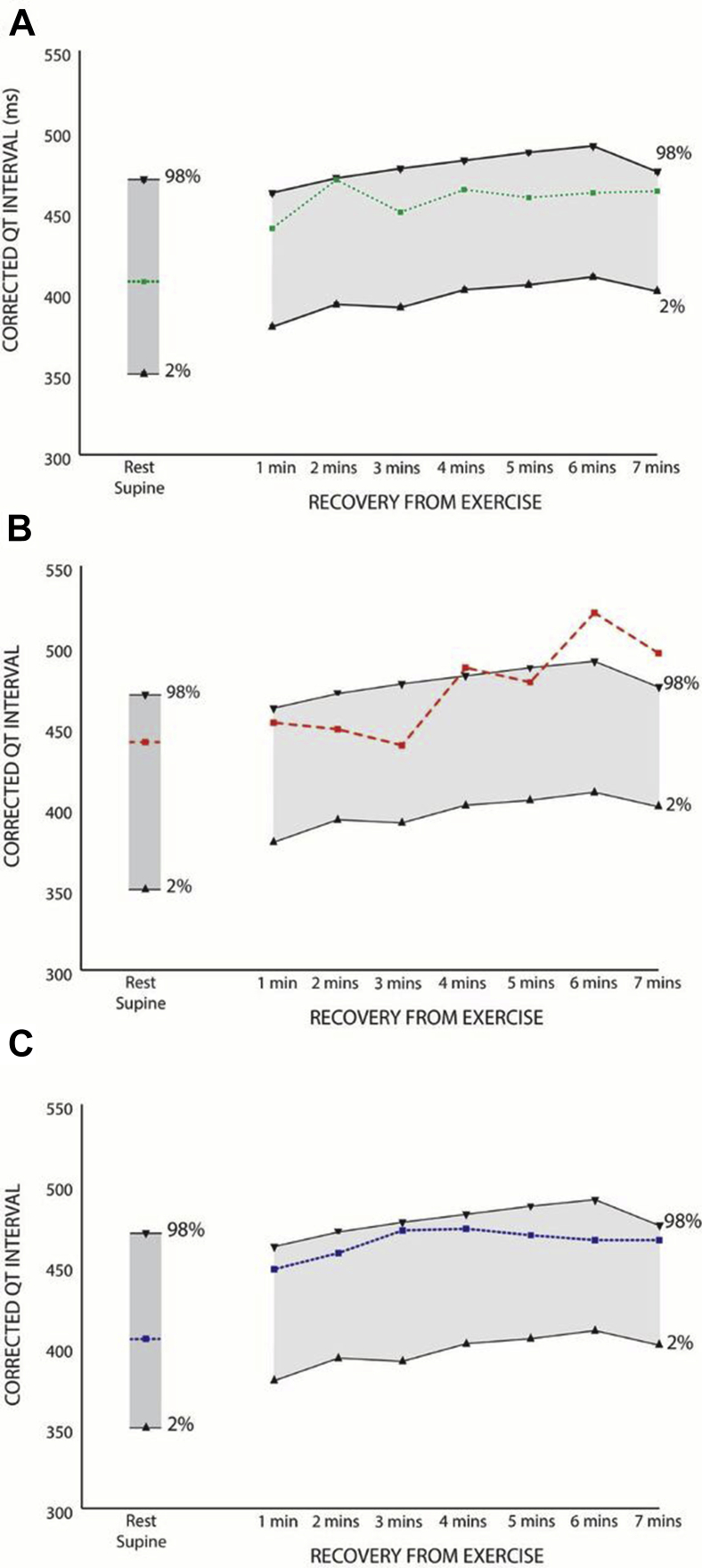
A 4-year-old boy was diagnosed with LQTS after an episode of syncope while climbing out of a swimming pool. His corrected QT interval (QTc) was reportedly measured between 480 and 520 ms at an external institution, although the original ECGs were unavailable for review. He was treated with mexiletine until genetic testing exposed a maternally inherited truncating Swedish founder mutation in the C-terminal of KCNQ1 (c.1552C>T; p.R518X) (Figure 1). Mexiletine was discontinued and he was treated with nadolol.5 He remained asymptomatic for many years, and was reviewed at our institution at the age of 11 when his family relocated. At this time, on a daily dose of 20 mg of nadolol (0.74 mg/kg), the QTc on a resting ECG was 407 ms. In the family, he is the second child of nonconsanguineous parents. The KCNQ1 variant was inherited from his asymptomatic mother with normal QT intervals on repeated ECGs. The proband's 21-year-old asymptomatic brother also carries the same variant. Additionally, the family history is significant for sudden cardiac death in a 49-year-old maternal great uncle who died while shoveling snow and a 28-year-old maternal grandfather who died in a construction accident. The deceased relatives were not genotyped and no further details were available (Figure 2). An exercise test was performed on our patient, which demonstrated a peak heart rate of 173 beats per minute and a QTc measured within the normal limits during the recovery period from exercise (Figure 3A). He was continued on the same dose of nadolol and reviewed annually.
Figure 1.
A depiction of the potassium channel, KvLQT1, encoded by the gene KCNQ1, is shown with 6 transmembrane domains (S1–S6) seen spanning the cardiomyocyte membrane. The site of the pathogenic variant identified in the proband—KCNQ1 c.1552C>T; p.R518X—is depicted by the blue circle in the C-terminus.
Figure 2.
A multigenerational family pedigree with the proband denoted by an arrow. Males are represented by squares and females by circles. Blue quadrants represent the KCNQ1 R518X genotype, and red quadrants represent long QT syndrome. A&W= alive and well; d. = died; MI = myocardial infarct; yo = years old.
Figure 3.
Corrected QT intervals measured in the patient during the recovery period from exercise under the following conditions: A: on 20 mg nadolol therapy, B: on no therapy, C: post left cardiac sympathectomy denervation. The normal range (2nd to 98th percentiles) derived from control subjects is shown in gray.15
At the age of 11 he presented to the Emergency Department after 3 days of anxiety and suicidal ideation, prompted by an encounter with a sexual predator in an online video game chat room, necessitating nadolol discontinuation. Psychiatric evaluation reported an unspecified anxiety disorder with moderately severe suicidal ideation and recommended an inpatient admission with 24-hour surveillance and discontinuation of nadolol. On further questioning regarding his psychological status, his parents reported that he was asymptomatic prior to starting nadolol and during early treatment, but had noted marked personality changes 6–8 months before this acute psychotic reaction, including fatigue, depression, and anxiety.
Given his prior normal QT response to exercise, a repeat test was performed after 3 days without nadolol therapy, which unmasked an LQTS phenotype in the late recovery period from exercise, with a maximal QTc of 520 ms recorded at 6 minutes (Figure 3B). Detailed consideration of future management was multifactorial, including his personal phenotypic expression, namely a male patient with long QT1 under the age of 12,6 who had suffered prior symptoms consistent with LQTS, albeit without documented arrhythmia; his documented genotype and the associated phenotype in many carriers of the same variant7; and the potential need for QT-prolonging agents in the immediate term vs the risk of recurrent psychosis if beta-blockers were reintroduced. Ultimately, given the phenotypic expression in the absence of beta-blockers, age, and prior symptoms, he underwent LCSD, comprising excision of the lower half of the left stellate ganglion and removal of thoracic ganglia (T1–T5) of the left sympathetic chain with concurrent implantation of a Reveal LINQ loop recorder (Medtronic, Minneapolis, MN). He was started on the selective serotonin reuptake inhibitor escitalopram as the optimal management strategy for his acute psychosis, an agent known to prolong the QT interval. Once steady state was achieved, he was re-exercised, which again demonstrated that the QTc was within normal limits during the recovery period (Figure 3C). He remains asymptomatic more than 18 months later, on a decreasing dose of escitalopram. He has improved psychologically, with decreased fatigue, decreased anxiety, and termination of suicidal and intrusive thoughts noted on recurrent evaluation. His resting ECG is within normal limits with a QTc of 430 ms, although subtle nonspecific morphologic T-wave changes consistent with LQT1 are still evident. There have been no arrhythmias documented on his implantable loop recorder.
Discussion
This case demonstrates the myriad complexities that can arise while managing patients with LQTS, where both the condition itself and treatment strategies present variable risks to the patient. In this instance management was dictated by an ongoing arrhythmia risk in a previously symptomatic individual, who had been rendered entirely asymptomatic from the cardiac perspective by beta-blockade, although this may have significantly contributed to an acute psychotic reaction to an external stressor.
Syncope associated with swimming in a boy under the age of 12 is a classical presentation of LQTS type 1, and although no ECG monitoring was performed at the time this would appear likely to be an arrhythmic event. The genetic variant was inherited from his mother and was also identified in his older brother, both of whom were asymptomatic with normal QTc and both of whom declined treatment. This demonstrates the significantly reduced penetrance seen in LQT1 generally, and particularly in heterozygote carriers of this specific variant, where the vast majority of carriers are asymptomatic.7 A study of 86 Swedish patients heterozygous for the same variant reported that the average QTc was 462 ± 34 ms, with 25% of individuals with a QTc <440 ms and 17% with a QTc >500 ms. The mechanism behind clinical disease expression in specific individuals with KCNQ1 variants is uncertain, although it may relate to a number of factors, including sex, age, activity, and potentially the specific expression ratio of the wild-type and mutant proteins.
Although truncating variants in KCNQ1 are a well-recognized mechanism of pathogenicity in LQTS, the gene is notoriously tolerant of loss of function (probability of loss-of-function intolerance = 0.0),8 and many patients with such variants remain asymptomatic. KCNQ1 encodes the pore-forming subunit of the Kv7.1 voltage-gated potassium channel required for repolarization of the cardiac action potential. The variant identified in our patient is well described in the literature, and has been seen in the heterozygous state in autosomal dominant LQTS and in both the homozygous and compound heterozygous states in autosomal recessive Jervell and Lange-Nielsen syndrome. Heterozygote carriers typically display a mild or absent long QT phenotype,7 with 46% displaying abnormal QT intervals and 17% experiencing syncope off therapy, and a much lower cardiac arrest rate than seen with other KCNQ1 variants and associated LQTS (0% vs 3%).7, 9 Functional studies of KCNQ1 R518X have shown the mutant protein cannot co-assemble with the wild-type subunit and therefore cannot cause a dominant-negative effect. One study demonstrated simple loss of a functional allele in a 1:1 wild-type–to–mutant ratio, whereas a dominant-negative effect is only present at a 1:3 ratio.10 Additionally, the mutant protein is not trafficked from the endoplasmic reticulum to its functional position at the cardiomyocyte surface, although it does not interfere with the wild-type channel.11
Beta-blockers are highly effective in LQTS type 1, and although anecdotal concern has long existed regarding side effects including depression and, rarely, psychosis, these have not been validated in larger clinical trials comparing beta-blockers with placebo.12 Though we cannot definitively attribute our patient's psychotic episode to beta-blockers, consideration of his overall wellbeing necessitated the discontinuation of a medication that could have potentially contributed to his pronounced psychological symptoms, and his suicidal risk was considered sufficient to warrant immediate hospital admission and 24-hour continual observation. Beta-blocker intolerance or, more commonly, nonadherence are indications to consider other treatment options.13 Although side effects such as dry skin, harlequin-type facial flushing, ptosis, and hyperhidrosis following LCSD are common, the vast majority of patients typically feel safer after the procedure and would recommend it to others.14
Despite the typically low penetrance of KCNQ1 R518X, detailed phenotyping demonstrated normal QTc intervals in the recovery period from exercise in the presence of nadolol, but disease expression became evident once beta-blockers were discontinued. Given the combination of disease expression and prior symptoms, it was felt that continued antiadrenergic therapy was warranted and an LCSD performed. Based on the subsequent exercise test, this strategy appeared to confer adequate protection based on normalization of his QT intervals. This decision was further enhanced by the potential need for QT-prolonging antidepressant medication. Therefore, in this complex situation of competing risks, comprehensive phenotyping and detailed knowledge of the underlying genetic variant guided successful management.
In conclusion, although LQT1 is typically treated very successfully with beta-blockade, other treatment options should be considered if the patient cannot tolerate this medication. Clinical management in LQTS can be guided by comprehensive knowledge of the genotype and phenotype, in addition to a risk-benefit ratio of invasive and noninvasive treatment strategies.
Footnotes
The Inherited Cardiac Arrhythmia Program is generously supported by the Mannion and Roberts families.
References
- 1.Schwartz P.J., Crotti L., Insolia R. Long-QT Syndrome. Circ Arrhythm Electrophysiol. 2012;5(4) doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz P.J., Priori S.G., Spazzolini C. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103(1):89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz P.J., Priori S.G., Cerrone M. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 4.Wilde A.A.M., Bhuiyan Z.A., Crotti L. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–2049. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 5.Vincent G.M., Schwartz P.J., Denjoy I. High efficiency of beta-blockers in long QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of B-blocker treatment “failures.”. Circulation. 2009;119:215–221. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
- 6.Zareba W., Moss A.J., Locati E.H. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–109. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 7.Winbo A., Stattin E.-L., Nordin C., Diamant U.B., Persson J., Jensen S.M., Rydberg A. Phenotype, origin and estimated prevalence of a common long QT syndrome mutation: a clinical, genealogical and molecular genetics study including Swedish R518X/KCNQ1 families. BMC Cardiovasc Disord. 2014;14(1):22. doi: 10.1186/1471-2261-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gene: KCNQ1. http://exac.broadinstitute.org/gene/ENSG00000053918 Available at:
- 9.Moss A.J., Shimizu W., Wilde A.A. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–2489. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L., Bitner-Glindzicz M., Tranebjaerg L., Tinker A. A spectrum of functional effects for disease causing mutations in the Jervell and Lange-Nielsen syndrome. Cardiovasc Res. 2001;51:670–680. doi: 10.1016/s0008-6363(01)00350-9. [DOI] [PubMed] [Google Scholar]
- 11.Wilson A.J., Quinn K.V., Graves F.M., Bitner-Glindzicz M., Tinker A. Abnormal KCNQ1 trafficking influences disease pathogenesis in hereditary long QT syndromes (LQT1) Cardiovasc Res. 2005;67:476–486. doi: 10.1016/j.cardiores.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 12.Barron A.J., Zaman N., Cole G.D., Wensel R., Okonko D.O., Francis D.P. Systematic review of genuine versus spurious side-effects of beta-blockers in heart failure using placebo control: recommendations for patient information. Int J Cardiol. 2013;168:3572–3579. doi: 10.1016/j.ijcard.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waddell-Smith K.E., Li J., Smith W., Crawford J., Skinner J.R. Cardiac Inherited Disease Group New Zealand. β-blocker adherence in familial long QT syndrome. Circ Arrhythm Electrophysiol. 2016;9(8) doi: 10.1161/CIRCEP.115.003591. [DOI] [PubMed] [Google Scholar]
- 14.Waddell-Smith K.E., Ertresvaag K.N., Li J., Chaudhuri K., Crawford J.R., Hamill J.K., Haydock D., Skinner J.R. Physical and psychological consequences of left cardiac sympathetic denervation in long-QT syndrome and catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2015;8:1151–1158. doi: 10.1161/CIRCEP.115.003159. [DOI] [PubMed] [Google Scholar]
- 15.Berger W.R., Gow R.M., Kamberi S., Cheung M., Smith K.R., Davis A.M. The QT and corrected QT interval in recovery after exercise in children. Circ Arrhythm Electrophysiol. 2011;4:448–455. doi: 10.1161/CIRCEP.110.961094. [DOI] [PubMed] [Google Scholar]