Abstract
We review the details of preparation and of the recently elucidated mechanism of biological (regenerative) activity of a collagen scaffold (dermis regeneration template, DRT) that has induced regeneration of skin and peripheral nerves (PN) in a variety of animal models and in the clinic. DRT is a 3D protein network with optimized pore size in the range 20–125 μm, degradation half-life 14 ± 7 d and ligand densities that exceed 200 μ α1β1 or α2β1 ligands. The pore has been optimized to allow migration of contractile cells (myofibroblasts, MFB) into the scaffold and to provide sufficient specific surface for cell–scaffold interaction; the degradation half-life provides the required time window for satisfactory binding interaction of MFB with the scaffold surface; and the ligand density supplies the appropriate ligands for specific binding of MFB on the scaffold surface. A dramatic change in MFB phenotype takes place following MFB-scaffold binding which has been shown to result in blocking of wound contraction. In both skin wounds and PN wounds the evidence has shown clearly that contraction blocking by DRT is followed by induction of regeneration of nearly perfect organs. The biologically active structure of DRT is required for contraction blocking; well-matched collagen scaffold controls of DRT, with structures that varied from that of DRT, have failed to induce regeneration. Careful processing of collagen scaffolds is required for adequate biological activity of the scaffold surface. The newly understood mechanism provides a relatively complete paradigm of regenerative medicine that can be used to prepare scaffolds that may induce regeneration of other organs in future studies.
Keywords: skin regeneration, nerve regeneration, collagen scaffolds, wound contraction, scar formation, surface biology, phenotype change
Introduction
Scaffolds are widely studied in various areas of regenerative medicine and tissue engineering. Yet the biomaterials literature does not show many examples of scaffolds that possess confirmed regenerative activity, i.e. scaffolds that can induce clinically-relevant regeneration by inducing synthesis of nearly physiological organs in vivo. There is also a dearth of theoretical or mechanistic information on the regenerative activity of scaffolds. Without such information it is very difficult to design new biomaterials that regenerate tissues and organs.
In this review article we describe a particular collagen scaffold, referred to as dermis regeneration template (DRT), that has induced regeneration of skin, peripheral nerves and the conjunctiva in various animal models, as well as inducing regeneration of skin and nerves in diverse clinical settings. This review includes both a summary of the processing steps required for its synthesis and the mechanism of its regenerative activity. Detailed description of the structure, including the surface chemistry, and of the regenerative activity of DRT leads to a paradigm in biomaterials science that we believe can be used as a guide in the rational design of new biomaterials.
Materials science of collagen scaffolds with specific structure
Early studies with collagen implants
Implants based on type I collagen have been reported in very early studies. With few exceptions these implants were typically prepared as nonporous solid-like materials, rather than in scaffold-like form. In most cases reported in the early literature implants were described simply as being made of ‘collagen’ with no detailed information provided either on the processing steps used in fabrication of implants nor the structural features of the collagen used. One of these studies showed that the level of crosslink density controlled the degradation rate of implanted collagen sutures (Grillo and Gross 1962, Kline and Hayes 1964). In addition, the immune response of the host to collagen was found to be finite but minimal (Grillo and Gross 1962), a conclusion that was confirmed later for DRT in a clinical study (MacPherson and Michaeli 1990). In another very early study it was shown that the average pore diameter of collagen implants controlled the migration of cells into collagen implants (Chvapil and Holusa 1968, Chvapil et al 1969). Nonporous collagen tubes have been used in peripheral nerve (PN) regeneration (e.g. Kline and Hayes 1964, Mackinnon and Dellon 1990, Archibald et al 1991, 1995, Navarro et al 1996).
Regeneratively active collagen scaffolds
Unlike these early reports of collagen-based implants the studies with DRT reviewed below have marked the first time that a complex biological activity, such as the ability to induce tissue regeneration, could be incorporated in a collagen-based macromolecular network. Lengthy studies eventually showed that the unexpected regenerative activity depended on precisely defined physicochemical structural features, including quantitative descriptions of the pore structure, half-life for degradation and surface chemistry (Yannas et al 1981, 1982a, 1982b, 1984, 1987b, 1989, Chang et al 1990, Chang and Yannas 1992, Chamberlain et al 2000a, 2000b, Harley et al 2004, Tzeranis et al 2010, Soller et al 2012). Most implants, defined as ‘collagen’, that have been described in the literature lacked regenerative activity either in part or entirely, most probably because the structural features of the raw material collagen were not adjusted to the critical levels that appear to be required and are described below. Furthermore, scaffolds based on synthetic polymers intrinsically lack what appear to be essential structural features, most importantly, ligands with which collagen-binding integrins α1β1 and α2β1 of contractile cells can interact specifically (Tzeranis et al 2010), thereby blocking contraction and inducing regeneration, as reported below.
DRT is a 3D protein network
DRT is a highly porous analog of the extracellular matrix (ECM), a graft copolymer of type I collagen and chondroitin 6-sulfate (a glycosaminoglycan, GAG), and has been referred to as a ‘scaffold’. It is synthesized as a cell-free scaffold and is, therefore, ‘nonliving’ unless cells are seeded into it prior to implantation. After about the year 2000, collagen scaffolds have been prepared in the author’s laboratory without GAG (see discussion below).
Collagen scaffolds are 3D protein networks. Being insoluble they cannot be characterized using the panoply of analytical tools that have been employed to study soluble polymers in such detail over the years. Neither the detailed configuration in space nor the detailed structural features of a collagen scaffold are as simply described as even those of a synthetic polymer with very high molecular weight. To our knowledge, the vast majority of 3D collagen scaffolds that have been reported have little or no regenerative activity. In contrast, certain structural features of the most regeneratively active collagen scaffold (DRT), including average pore size, degradation rate and surface chemistry (and of a few other structural features that are discussed below), serve to define this insoluble material to a reasonably clear extent. A few collagen-based scaffolds, including DRT, have been classified as simple analogs of the extracellular matrix (ECM) (Yannas 1990) and have been characterized extensively by physicochemical methods (Dagalakis et al 1980, Yannas et al 1980, 2010, Yannas 1990, Harley et al 2008). Multiyear experience in the laboratory and in industrial practice with DRT have repeatedly shown that, when prepared with certain restrictions in processing parameters, collagen extracted from an animal source is converted to a well-defined protein network that has shown reproducible regenerative activity in several animal models as well as in the clinic.
A 3D protein network is defined here as a macromolecular substance with chemical composition described by a known amino acid sequence and a defined physicochemical structure, including the average molecular weight between crosslinks of the network (related to the crosslink density), as well as the pore structure of the solid (Yannas et al 1980, Yannas 1981, 1990). The structural features of the scaffold DRT serve to define a foam-like solid material sufficiently well to provide for a reproducible surface chemistry and degradation rate. Detailed protocols for synthesizing DRT have been published (Chamberlain and Yannas 1998, Yannas 2015) and updated (Soller et al 2012).
Preparation of active collagen scaffolds
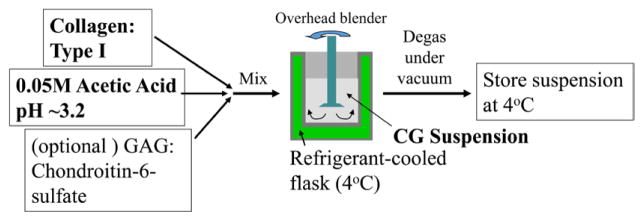
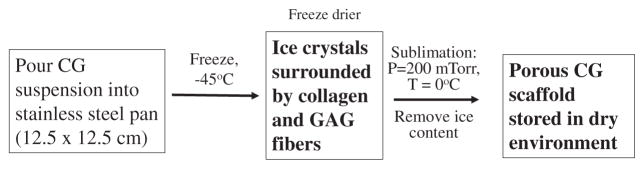
Briefly, to prepare DRT and scaffolds with closely related structure, acid soluble collagen extracted from one of several animal sources is coprecipitated with GAG from dilute aqueous acetic acid at pH 3, the suspension is frozen and the ice is sublimated (freeze-drying) to produce a porous material containing only 0.05% solid material (collagen and GAG); the highly porous solid is then treated at high temperature and vacuum to introduce covalent crosslinks between collagen and GAG. Solubilization of collagen by treatment in aqueous acetic acid (pH < 4.25 ± 0.30) melts out the collagen banding pattern in a fraction of the fibrils but conserves the triple helical structure. The resulting collagen preparation is nonthrombogenic and is associated with a significantly lower concentration of TGFβ1, a factor in downregulation of the myofibroblast density (see below) (Sylvester et al 1989). The GAG component serves mostly to increase the resistance to degradation by collagenases (Yannas et al 1975a, 1975b) and does not affect significantly the contraction-blocking ability or the regenerative activity of collagen scaffolds (Shafritz et al 1994) (see below for a description of the biological/medical activity of collagen scaffolds). Later protocols have omitted use of GAG without apparent loss of regenerative activity (Soller et al 2012) (see also Discussion). DRT was originally synthesized as a graft copolymer of collagen and GAG, in an attempt to simulate the ECM that is present in many tissues (Yannas and Burke 1980, Yannas 1990). The early use of the term ‘ECM analog’ or ‘artificial skin’ for this scaffold was mostly replaced by use of ‘DRT’ after it was confirmed, based on a number of studies with animals and humans (see below), that this scaffold unexpectedly induced regeneration of tissues, such as the dermis, that do not spontaneously regenerate in the adult mammal (Yannas et al 1982a, 1989).
Specific processing steps during preparation of the scaffold have been shown to control certain structural features of DRT that critically determine regenerative activity. The basic processing steps are illustrated in the simplified flowsheet shown in figures 2 and 3. The pore structure is controlled in the freeze dryer primarily by the shelf temperature which largely determines the freezing rate of the aqueous suspension; high freezing rates lead to smaller ice crystals which, following sublimation of ice, lead to smaller pores (Dagalakis et al 1980, Harley et al 2004, 2008, O’Brien et al 2004). Degradation rate in vivo depends primarily on the conditions of the crosslinking reaction that lead to specific levels of the crosslink density (Yannas et al 1975b, Huang and Yannas 1977), corresponding to corresponding values of the average molecular weight between crosslinks (Yannas 1981, 1990). Finally, the identity and density of ligands for specific integrins depend on the presence of particular hexapeptides in the collagen molecule and is, therefore, a property of the basic collagen protein (Tzeranis 2013).
Figure 2.
Flowsheet for preparation of a suspension of collagen or collagen-GAG for use in preparation of sheets (skin grafts). Sterile conditions must apply during manufacture. (Graphic by Tzeranis.)
Figure 3.
Flowsheet for preparation of porous sheets of collagen scaffold for use in skin grafts. Sterile conditions must apply during manufacture.(Graphic by Tzeranis 2012.)
An example of a processing treatment that can practically eliminate the regenerative activity of the scaffold is the conversion of collagen to gelatin (amorphous collagen) during a melting transformation (Yannas 1972), which may inadvertently occur during processing. Because gelatin lacks the triple helical structure of collagen, ligands on its surface bind poorly to collagen-binding integrins (Tuckwell et al 1995, Calderwood et al 1997). α1β1 and α2β1 integrins bind tightly to peptides that contain the GFOGER (glycine-phenylalanine-hydroxyproline-glycine-glutamic acid-arginine) ligand as long as these peptides participate in the triple helical structure of the collagen molecule, which is absent in gelatin (Knight et al 2000, Siljander et al 2004). There is also evidence that the hexapeptide motif GLOGEN (glycine-leucine-hydroxyproline-glycine-glutamic acid-asparagine) is a high affinity binding site for α1β1 (Hamaia et al 2012).
Collagen libraries
A homologous series of collagen scaffolds can be said to comprise a collagen library. These libraries have been prepared using variants of the standard processing steps (figures 1 and 2) in order to obtain quantitative understanding of the regenerative effect of structural features of collagen scaffolds. These collections of collagen scaffolds are closely related in structure and are especially useful as probes of regenerative activity. A few of the scaffolds that have been synthesized are regeneratively active but most are inactive. The basic experimental strategy in using these libraries is to prepare a homologous series of scaffolds, with members that differ from each other only with respect to the level of just one structural variable (reference variable) and test the ability of the individual library members to yield a specific medical/biological outcome that is known to correlate well with the quality of regeneration induced through its use.
Figure 1.
A scaffold with regenerative activity was initially synthesized as a highly porous graft copolymer of type I collagen and chondroitin 6-sulfate (GAG). It was modified later by omitting the GAG (see text). Regenerative activity depends on optimization of pore diameter and half-life for degradation; a minimal density of ligands for integrins α1β1 and α2β1 is also required on the collagen surface. When the structure has been appropriately optimized to yield a so-called dermis regeneration template (DRT), the scaffold induces regeneration of skin (guinea pig, swine, human) peripheral nerves (rat) and of the conjunctiva (rabbit). (Photo by Alexandra Kourgiantaki.)
Examples of such structural variables that can be ‘fine-tuned’ are the average pore size of the scaffold, which can be varied from 10 μm to more than 500 μm, the scaffold half-life for in vivo degradation between 1 week and more than 100 weeks, and the concentration level of ligands for specific cell receptors, such as the collagen-binding integrins α1β1 and α2β1 which bind to contractile cells. These variables can be often adjusted to the desired level by modifying one of the processing conditions during fabrication of scaffolds while keeping other processing variables fixed. The value of such a library derives from the tight internal control of structural properties of its members, a characteristic which allows to pinpoint with clarity whether changes in the reference variable (e.g. pore size, degradation half-life) affect the outcome or not (e.g. contraction inhibition, regenerative activity). Furthermore, discovery of a maximal value in a given outcome along a series identifies the structural property of the library member that maximizes that outcome and serves to eventually define the scaffold structure that possesses optimal activity.
Biological and medical rationale for use of collagen scaffolds with specific structure
Irreversibly injured tissues
Acute injury to any organ generates a wound within a short period of time (seconds, minutes) and is immediately followed by a spontaneous healing process at the end of which (roughly 3–4 weeks) a normal wound has closed. (Chronic wounds are generated incrementally over a long period, often months, and usually do not heal spontaneously.) In the early mammalian fetus and in certain amphibians, healing is a largely reversible process, leading to restoration of the original organ (regeneration). However, in the adult mammal, injury to the stroma (connective tissues) is typically irreversible and heals by wound contraction and scar formation (repair). Every organ in the adult can be irreversibly injured. In certain organs injury is irreversible when it has damaged specific tissues whereas in other organs it becomes irreversible when the injury exceeds a critical size. Irreversible injury disease process (chronic). As examples of severe medical problems directly caused by irreversible injury we mention here paralysis of extremities due to severe trauma in peripheral nerves of hands or legs, heart failure due to formation of extensive scar in the heart muscle and kidney failure, requiring kidney dialysis treatment, due to severe scarring of tissues of the kidney (Yannas 2015). Chronic injuries include extensive focal scar formation in the kidney or liver; or skin wounds in extremities of diabetics, that do not close.
Several approaches have been used in attempts to restore the loss of organ function that results from an extensive acute or chronic repair process in the adult. They include organ transplantation, autografting, implantation of a permanent prosthesis, use of stem cells, in vitro synthesis, and induced regeneration. The topic of this review article, induced regeneration, is a process in which physiological tissue, rather than scar, is deliberately synthesized by the investigator at the anatomical site of the adult host that has been irreversibly injured. This approach is embodied in the collagen scaffold regeneration paradigm, described below.
Antagonistic relation between wound contraction and regeneration
A number of theories have been proposed in the medical literature to explain the inability of the adult to regenerate its organs (Yannas 2015). Among these, scar formation has been frequently cited as the cause for inhibition of regeneration. Nevertheless, we have compiled from independent studies and from our own studies a large body of data in different species strongly suggesting that scar formation is not the primary reason for inability of the adult mammal to regenerate organs. The evidence from these diverse sources supports instead the conclusion that an antagonistic relation exists between wound contraction and regeneration during normal healing of severe injuries both in skin and peripheral nerve injuries; and that wound contraction, rather than scar, is the process likely associated with the failure to obtain spontaneous regeneration in the adult mammal.
Spontaneously healing skin wounds in different species provide the first set of data that support the hypothesis of an antagonistic relation between contraction and regeneration. It is important to cite the detailed evidence in order to support a theoretical position that appears to have been neglected in the literature. The evidence includes the well-known example of skin wounds in the perforated rabbit ear where wound contraction is practically excluded due to tight binding between skin and cartilage, and where regeneration, or scarless healing, of skin is observed (Joseph and Dyson 1966, Goss and Grimes 1972, 1975 Goss 1980, Mustoe et al 1991, Goss 1992), data from a study of the developing tadpole, where contraction and scarless healing appear to be mutually exclusive processes of skin wound closure (Yannas et al 1996), studies of regeneration of the injured oral mucosa in mice showing greatly reduced scar formation or even scarless healing (Schrementi et al 2008, Mak et al 2009, Wong et al 2009, Larjava et al 2011, Glim et al 2013) while studies with swine showed that oral mucosal wounds contracted significantly less than skin wounds (Mak et al 2009) or else showed lower levels of TGFβ1 (cytokine that induces differentiation of fibroblasts to contractile cells) than in control skin wounds (Schrementi et al 2008), studies with full-thickness excisional skin wounds in the axolotl which led to regeneration of the skin (Seifert et al 2012), while study of similar skin wounds in the axolotl showed that alpha smooth muscle actin (αSMA), a protein characteristic of contractile cells, was absent and TGFβ1, which is required for expression of the αSMA phenotype in fibroblasts, was only transiently expressed during wound healing (Lévesque et al 2007, 2010). Each of these studies in various species with skin wounds that heal spontaneously have led us to the conclusion that scarless healing (regeneration) of these wounds was persistently observed in association with inhibition of wound contraction (Yannas 2015).
A second set of data that supports an antagonistic relation between wound contraction and regeneration includes several studies of induced (rather than spontaneous) regeneration using a collagen scaffold (DRT). This scaffold has been shown to block contraction in the guinea pig, rat, rabbit, swine, and (with limited quantitative data) in the human; and in three organs, skin, peripheral nerves and the conjunctiva. For brevity, we focus here on studies of an active porous sheet-like collagen scaffold (DRT), either unseeded or seeded with keratinocytes, which delayed contraction (Yannas 1981) or arrested contraction (Yannas et al 1982, 1989) while inducing regeneration, in skin wounds of the guinea pig. We also include a report of studies with a porous tube, fabricated from a scaffold similar to DRT, which was used to show that the quality of rat sciatic nerve regeneration decreased monotonically with increasing thickness of the contractile cell capsule surrounding healing nerve stumps and with increasing diameter shrinkage of the healing nerve (Soller et al 2012). Finally, we refer to a study of the fully excised stroma in the rabbit conjunctiva, which regenerated when grafted with DRT while contraction of neighboring tissues was simultaneously reduced (Hsu et al 2000).
In a separate series of studies, skin wounds that were characterized by impaired healing, failed to contract but also failed to regenerate (Billingham and Russell 1956, Fiddes et al 1991, Hayward et al 1992). These results showed that a hypothetical relation between contraction blocking and induction of regeneration may be necessary but is not sufficient for regeneration in every type of wound.
Deformation theory of scar formation
Studies of scar formation in guinea pig skin wounds (Ferdman and Yannas 1993) and peripheral nerve wounds in the rat sciatic nerve (Chamberlain et al 2000a) showed that scar was virtually abolished in the presence of scaffolds that were associated with significant reduction of contraction (while also inducing regeneration). This evidence is consistent with the deformation field theory of scar formation which states that scar, rather than physiological stroma, is synthesized by fibroblasts that have become oriented in a tensile stress field generated along the major deformation axis of wound contraction (Ferdman and Yannas 1993, Yannas 2001, 2015). According to the deformation theory, scar formation originates in the contractile mechanical field that closes wounds and is therefore derivative to wound contraction. If so, regeneration in the adult mammal must be principally inhibited by wound contraction rather than by scar formation.
Other evidence shows that skin wounds and peripheral nerve wounds undergo spontaneous contraction along axes that seem to be determined by the symmetry of their respective anatomical site (Yannas 2015). Accordingly, scar formation in these two organs results in collagen fiber orientation that is planar in contracting excisional skin wounds and circumferential around contracting peripheral nerve stumps following transection (Chamberlain et al 2000a, Soller et al 2012).
The simplest explanation of the diverse evidence summarized above is an inverse relation between contraction and regeneration. This relation has been directly observed during peripheral nerve regeneration using tubular implants that were members of a collagen scaffold library (Soller et al 2012). Studies of regeneration of skin wounds using a different collagen scaffold library also showed that contracting wounds failed to regenerate while wounds where contraction was strongly inhibited (using DRT grafts) closed by regeneration (Yannas et al 1989).
Surface biological events that account for contraction blocking by an active scaffold
Cellular origin of wound contraction
It follows from the preceding section that collagen scaffolds that are capable to induce regeneration must block contraction of the injured site; and to do that, collagen scaffolds must possess certain critical structural features which dramatically modify the healing behavior of severe wounds, converting the healing outcome from repair to regeneration. However, it is first necessary to identify the specific biological process that is associated with a regeneratively active scaffold at the cell scale. A description of such activity at the cell scale will help to explain much more informatively how an active scaffold blocks contraction.
Contraction can be measured either as force or as deformation, the result of application of a balanced force. Measurements of contractile forces in wounds have appeared very rarely in the literature, a testament to the inherent difficulty in making such measurements with animal models. The contraction force required to close a skin wound in the rabbit thorax was measured to be about 0.1 N (Higton and James 1964). In contrast to the contractile force, measurement of the resulting tissue deformation is much more readily available and is based on microscopic observation of tissues in contracting wounds.
Contractile fibroblasts, often referred to as myofibroblasts (MFB), have been credited with generating most of the contractile forces and tissue deformations in skin wounds (Gabbiani et al 1971, Rudolph et al 1977, Rudolph et al 1992, Desmoulière and Gabbiani 1996, Gabbiani 1998, Desmoulière et al 2005, Hinz et al 2012, Daimon et al 2013). The α-smooth muscle actin isoform is currently considered to be the most useful marker of the myofibroblastic phenotype (Hinz et al 2012). These cells are highly elongated and display densely bundled actin microfilaments at their perimeter. Perhaps the most widely known phenotype of myofibroblasts are ‘stress fibers’, prominent filaments containing α-smooth muscle actin (Gabbiani et al 1971, Desmoulière et al 2005). The differentiation of the fibroblast to the contractile fibroblast requires transforming growth factor beta1 (TGFβ1), the ED-A splice variant of cellular fibronectin, and the presence of mechanical tension (Desmoulière et al 2005). Following wound closure, myofibroblasts disappear by apoptosis (Desmoulière et al 1995, 1997).
Physiological role of myofibroblasts in wound closure
Myofibroblasts close a full-thickness skin wound by deforming the tissues of the wound in the plane of the epidermis until wound edges from opposite sides move close to each other. Scar tissue also forms between the wound edges during the healing process. At least three structural features appear to be required for wound closure. First, these cells must have been differentiated to the specialized contractile cell phenotype and be available during healing in large enough number. Considering that a macroscopic force of about 0.1 N is required for skin wound closure in the rabbit (Higton and James 1964) and that the force generated by an individual cell is about 1–10 nN (Freyman et al 2001, Harley et al 2008), it appears necessary to apply forces from as many as 108–109 cells during wound contraction to close a skin wound in the rodent. Second, contractile cells need to form assemblies that are known to be held together by stress fibers in neighboring myofibroblasts, joined at sites of cadherin-type intercellular adherens junctions (AJs) (Hinz et al 2004). The required macroscopic force appears to be generated by assemblies of myofibroblasts, organized to apply cooperative forces, and providing thereby conditions for a ‘mechanically coherent’ wound which closes efficiently (Yannas 1998). There is evidence that local contractile events are mechanically coordinated by AJs, via synchronization of periodic intracellular Ca2 + oscillations between physically contacting myofibroblasts (Follonier et al 2010). Third, the long axes of MFB have to be oriented along the major deformation axis, the main direction along which wound tissues deform until the wound closes (Troxel and Yannas 1991).
In the transected peripheral nerve, myofibroblasts, in the form of a thick cylindrical sheet (capsule), spontaneously surround each nerve stump following injury and apparently apply compressive forces along the radial direction of the stump. It has been observed that the diameter of the healing nerve is inversely related to the quality of regeneration (Soller et al 2012), and, in most cases, the wound in the healing nerve stump closes by capping with scar (neuroma) (Chamberlain et al 2000a).
Effect of DRT grafting on myofibroblast morphology
Grafting of skin wounds or peripheral nerve wounds with DRT changes the morphology of myofibroblast assemblies to a remarkable extent. All three of the processes that appear to be required for mounting the macroscopic contractile force that suffices to close a wound (see above) are interfered with dramatically. In the presence of DRT there was a significant decrease in the number of myofibroblasts (Murphy et al 1990), dispersion of the MFB state of assembly (Troxel 1994) and loss of the axial orientation of the long axes of MFB. These morphological changes (figures 4–6) appear to explain the observation that wounds treated with DRT in skin, peripheral nerves and the conjunctiva either failed to contract entirely or else contracted poorly prior to showing clear evidence of regeneration of tissues at the injured site.
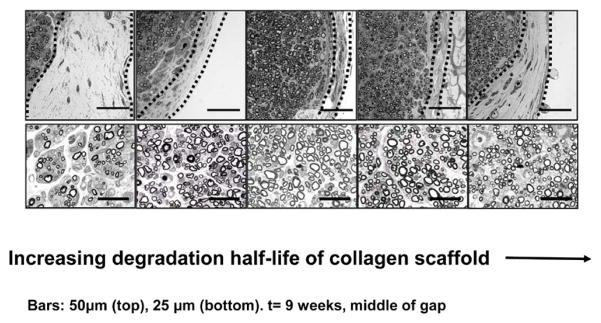
Figure 4.
Relation between the thickness of the contractile cell capsule surrounding a transected peripheral nerve and the quality of nerve regeneration at 9 weeks. The rat sciatic nerve was transected and the stumps were inserted inside collagen tubes with closely matched but nonidentical structures differing in half-life for degradation. The stumps were originally separated by a gap length of 15 ± 1 mm. Representative quarter cross sections of transected nerves tubulated with collagen scaffolds of increasing degradation half-life, stained with osmium tetroxide. Top row: contractile capsule, shown bounded by broken lines, around the regenerate. Data obtained at nerve gap midpoint at 9 weeks following transection of the rat sciatic nerve. Bottom row: newly-formed myelinated nerve tissue in animals that formed the capsule shown in the photos directly above. Broken line: capsule border. Scale bars: 50 μm (top); 25 μm (bottom). (Quantitative data from the same study are shown in figure 6) (Soller et al 2012).
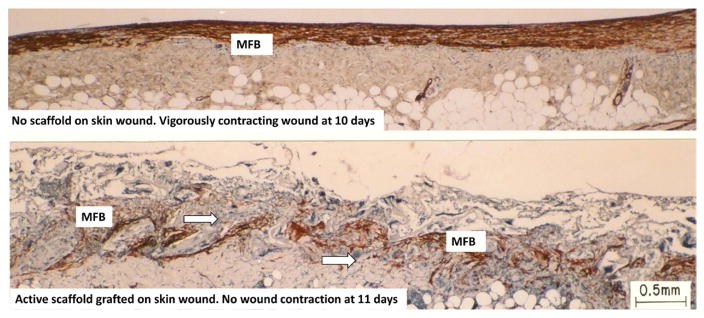
Figure 6.
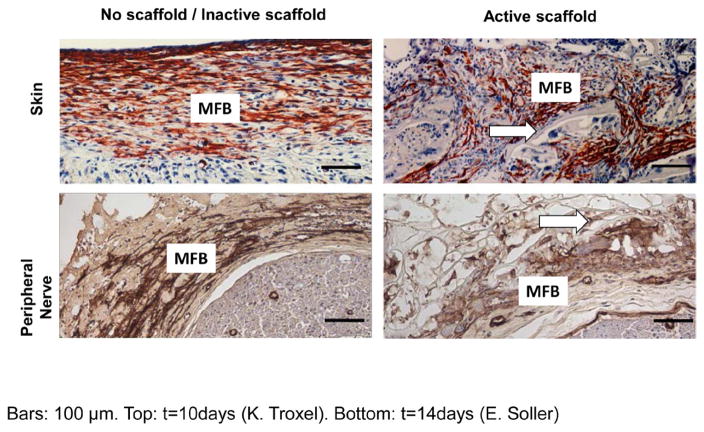
Two full-thickness skin wounds in the guinea pig, showing sharply contrasting behavior during healing. Immunohistochemical localization of αSMA, indicative of myofibroblasts (red brown). Top: ungrafted wound is vigorously contracting on day 10. It shows dense assemblies of contractile cells (myofibroblasts), all across the wound, with long axes oriented in the plane of the wound. Bottom: wound grafted with dermis regeneration template (DRT) is not contracting on day 11. It shows fewer myofibroblsts, with random orientation of long cell axes, and dispersed myofibroblast assemblies. Arrows: Scaffold struts. Scale bar: 0.5 mm. (Troxel 1994.)
The decrease in myofibroblast density in the presence of DRT was measured in guinea pig skin wounds by direct cell count using immmunoelectron microscopy (Murphy et al 1990). By 14 d, in the ungrafted site (no DRT), a fraction greater than 50% of dermal fibroblasts were identified as myofibroblasts, whereas a fibroblast fraction less than 10% exhibited features of myofibroblasts in the DRT-grafted site, a reduction in myofibroblast density down to one-fifth of normal level in the presence of DRT (Murphy et al 1990). A large decrease in density of myofibroblasts is also qualitatively evident in the longitudinal section of a skin wound that was grafted with DRT (noncontracting wound), compared with the ungrafted control (contracting wound) (figures 6 and 7).
Figure 7.
Immunohistochemical localization of αSMA (indicative of myofibroblast phenotype) in the contractile cell capsule in skin wounds (top row, red, 10 d post-injury) and peripheral nerve wounds (bottom row, brown, gap midpoint of transected rat sciatic nerve, 7 d post-injury). Left column: control wounds (top left: ungrafted skin; bottom left: nerve grafted with silicone tube). Right column: wounds grafted with active collagen scaffolds (DRT). Arrows: scaffold struts. Scale bars: 100 μm. MFB, myofibroblasts. (Soller et al 2012.)
In the transected rat sciatic nerve the thickness of the capsule that comprised myofibroblasts (as well as connective tissue) was sharply reduced from 73 ± 18 μm in the presence of an inactive collagen scaffold tube down to 31 ± 5 μm in the presence of a closely matched active DRT tube (Soller et al 2012) (figure 4). The drop in MFB density appears to be associated with the finding that the TGFβ1 concentration drops significantly in nerve wounds, probably following extensive nonspecific binding of TGFβ1 that has been observed to take place on the DRT surface (Thies 2010, Soller et al 2012, Yannas 2015). This finding raises the question whether the surface-bound TGFβ1 is an equally active contributor to differentiation of myofibroblasts as is the free growth factor. We recall that TGFβ1 is required for MFB differentiation; its putative absence in a free (unbound) form in a wound would be expected to lead to reduced MFB differentiation (Desmoulière et al 1993, 2005).
Dispersion of contractile cell assemblies by DRT accounts for further reduction of the macroscopic contractile force. Independent studies of the forces that maintain contractile cells assembled during normal contraction suggest that cell–cell contact and consequent coordination between myofibroblasts are required for generation of mechanical forces between cells in normally contracting wounds (Follonier et al 2008, 2010). These specific cell–cell contacts appear to be cancelled in the presence of DRT (figures 6 and 7).
Finally, disorientation of long axes of contractile cells present inside the pores of the DRT scaffold is clearly evident in photographs of myofibroblasts in skin wounds and nerve wounds (figures 7 and 8). Such disorientation stands in contrast to the high orientation observed in the absence of DRT, where the long axes of cells were oriented in the plane of the epidermis in skin wounds (figure 6) or circumferentially oriented around the stumps of transected nerves (figure 8) (Troxel and Yannas 1991, Chamberlain et al 2000a). The resulting randomization of force vectors contributed by individual cells is expected to lead to extensive mutual cancellation of vectors, leading to reduction in the resultant macroscopic force.
Figure 8.
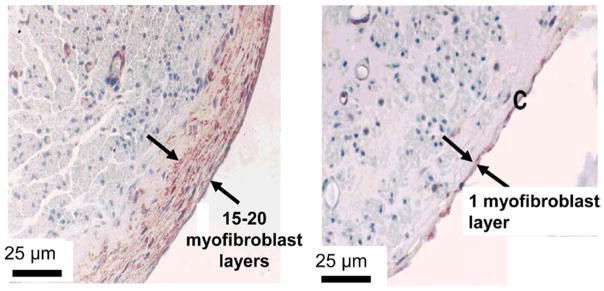
Myofibroblast layers formed around mature regenerated peripheral nerves. The two groups of regenerated rat sciatic nerves were formed following transection, tubulation with different tubes and were examined at 60 weeks following injury. Left: use of silicone tube yielded poorly regenerated nerves that were surrounded by 15–20 layers of myofibroblasts. Right: use of tube based on a porous collagen scaffold with high regenerative activity (DRT) led to nerves surrounded with only 1 layer of myofibroblasts. Bar: 25 μm (Chamberlain et al 2000a).
Donwregulation of all three of these normal morphological features of myofibroblasts in contracting wounds (high cell density, tight cell assembly, high axial orientation) appears likely to be responsible for the cancellation of healing tissue deformation that has been observed in the presence of DRT, both in skin wounds and peripheral nerve wounds.
Adhesive binding of contractile cells on the DRT surface is the simplest explanation for dispersion of cell assemblies and disorientation of long axes of cells in the presence of DRT. These morphological changes in contractile cells are not observed in the absence of DRT or in the presence of very similar collagen scaffolds used as inactive controls, suggesting that DRT possesses molecular features that are required for binding on the scaffold surface. Direct evidence of contact of contractile cells to the scaffold surface, suggestive of adhesive binding of cells on the scaffold surface, has been obtained by transmission electron microscopy (Murphy et al 1990).
What is the molecular evidence that binding between cells and DRT actually takes place? This question will be answered below.
Specific cell adhesion on the surface of collagen scaffolds
Binding of cells on surface of collagen
The most abundant isoform of collagen is type I, organized into supramolecular structures (fibrils, fibers) in a manner that is specific to the tissue type (e.g. tendons, dermis, endoneurium, cornea) and regulated by other components of the extracellular matrix (ECM) (Gelse et al 2003, Canty et al 2005). Collagen adhesion receptors of cells participate in binding various types of mammalian cells on the surface of collagen fibers. The most prominent of adhesion receptors are members of the integrin family; those that bind to collagen are known as collagen-binding integrins (CBI) (Barczyk et al 2010, Leitinger 2011). Integrins consist of two subunits, referred to as α and β, each subunit comprising four parts: head, leg, transmembrane section, and cytoplasmic domain. α subunits of CBI include an additional domain close to their N terminus, usually called ‘I domain’ (occasionally referred to as ‘A domain’). Binding of an integrin on a ligand appears to be mediated entirely by the I domain of the integrin (Hynes 2002, Luo et al 2007). I domains, isolated using protocols that have been published (Tzeranis et al 2010), have been used as markers for adhesion ligands of the parent integrin, providing means for assay of ligands on DRT; see below (Tzeranis 2013).
Ligands on the surface of several collagens have been identified. The I domains of CBI bind to small peptide motifs (‘adhesion ligands’) on the collagen surface. The affinity and specificity of binding depends on the molecular details of the particular I domain-ligand interaction. Molecular details of the interaction have been clarified from study of crystal structures of I domains bound to the triple helical peptide that contains a given ligand, as well as from related studies (Emsley et al 1997, Symersky et al 1997, Emsley et al 2000) (figure 9). The hexapeptide motif GFOGER (glycine-phenylalanine-hydroxyproline-glycine-glutamic acid-arginine), corresponding to residues 502–516 of the alpha(1)(I) chain of collagen, was identified as a high affinity binding site both for α1β1 and α2β1 integrins in collagen I (Knight et al 2000, Siljander et al 2004). The hexapeptide motif GLOGEN (glycine-leucine-hydroxyproline-glycine-glutamic acid-asparagine) was identified as a high affinity binding site for α1β1 (Hamaia et al 2012). The triple helical conformation is required for effective binding; the α1 I and α2 I domains bind collagen with approximately 10 times higher affinity than heat-denatured collagen (gelatin) (Tuckwell et al 1995, Calderwood et al 1997). The affinity of α1 I and α2 I domains for collagen depends on the presence of divalent cations (Tuckwell et al 1995, Calderwood et al 1997). Binding of Mg + 2 or Mn + 2 to the I domain is a prerequisite for I domain binding to its ligand (Rich et al 1999, Knight et al 2000, Zhang et al 2003, Hamaia et al 2012), its presence confirms the bond between cell and scaffold. Although the available evidence appears to implicate integrins α1β1 and α2β1 in myofibroblast-collagen binding events, there are other receptors, less well-studied in wound healing phenomena, which could conceivably be involved in the binding process. In future studies of regenerative phenomena it would be useful to examine the potential role of other integrins, e.g., integrin α11β1 (Helary et al 2012), and well as the role of discoidin domain receptors (Leitinger 2014).
Figure 9.
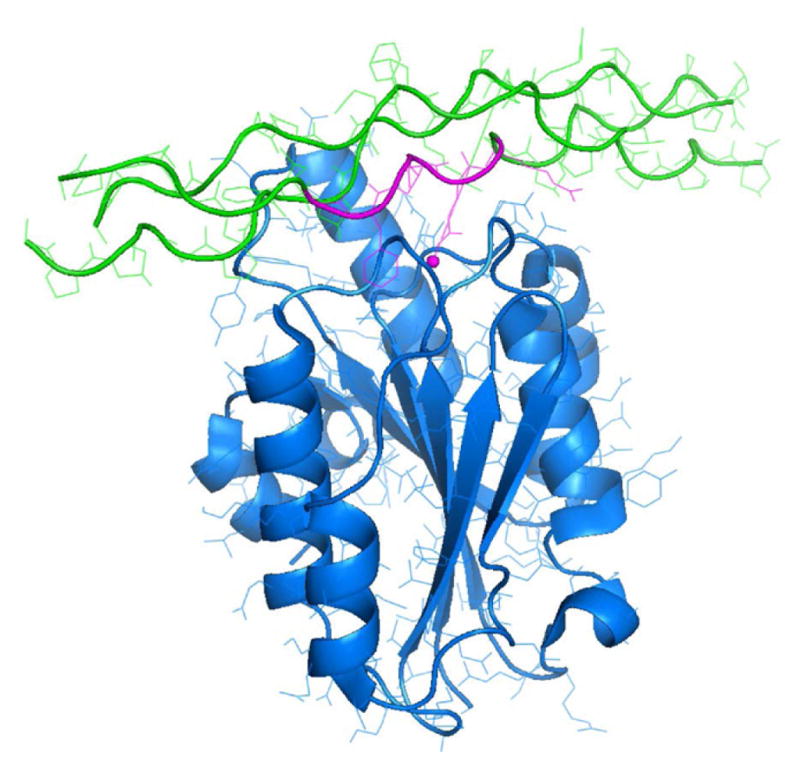
Structure of integrin-ligand complex at the surface of collagen fibers. The cell receptor is the α2β1 integrin (blue). It adheres to the collagen (green) hexapeptide ligand GFOGER (purple). (Emsley et al 2000, Knight et al 2000.)
The contractile cell phenotype is cancelled following binding on the scaffold surface. Changes in cell phenotype are mediated by binding of cells to collagen via their integrins. As examples, α1β1-mediated adhesion to collagen has been reported to promote cell proliferation and to impede collagen synthesis as well as suppress remodeling (Riikonen et al 1995, Heino 2000, Shi et al 2012). α2β1-mediated adhesion was shown to inhibit growth of a number of cell types as well as stimulate ECM synthesis and remodeling (Riikonen et al 1995, Heino 2000, Tulla et al 2001, Jokinen et al 2004, Shi et al 2012). Wound contraction processes mediated by adhesion of α1β1 and α2β1 to collagen have been widely reported. Myofibroblast differentiation, identified by expression of alpha smooth muscle actin (αSMA), was induced by α1β1 (Ng et al 2005, Rodriguez et al 2009). α1β1 (Znoyko et al 2006, Rodriguez et al 2009) and α2β1 were reported to play contrasting roles in myofibroblast differentiation (Znoyko et al 2006).
The surface chemistry of a matrix is defined here as the density of ligands for particular adhesion receptors available to cells. It is a quantity that can be measured meaningfully only with the intact, insoluble matrix. It identifies which adhesion receptors can be utilized by cells, defines the perception of the cell about its insoluble environment, and affects intracellular signaling downstream (Schiller et al 2011). Despite its importance, the vast majority of published studies on cell–matrix interactions have not quantified the surface chemistry of the matrix used in these studies due to lack of appropriate methods. A few studies have reported the mass of adsorbed matrix biomolecules on a flat cell culture dish (Maheshwari et al 2000, Engler et al 2004, Valenick et al 2006). Other methods measure quantities related to the density of RGD (arginine-glycine-aspartic acid) ligands in artificial biomaterials that contain a single ligand type (Barber et al 2005, Harbers et al 2005, Kong et al 2006, Huebsch and Mooney 2007, Hsiong et al 2008). Certain spectroscopic techniques quantify chemical groups on the surface of biomaterials (Ma et al 2007, Kingshott et al 2011). However, data from such measurements cannot be converted straightforwardly to density of ligands recognized by particular adhesion receptors. Although the density of ligands is a very useful metric, a complete description of the surface chemistry of the substrate eludes us at this time. A more advanced description of the surface would likely include measurements leading to the steric arrangement of ligands (clustering) (Upla et al 2004).
Quantitative description of the scaffold surface
A novel methodology has been described for quantifying the density of ligands of a particular adhesion receptor on the surface of a 3D matrix in situ (Tzeranis et al 2010, 2014, Tzeranis 2013). It consists of: (i) developing soluble fluorescently-labeled markers whose binding properties mimic those of particular adhesion receptors, (ii) using a binding assay of the markers, based on 3D microscopy to detect the fluorescent markers bound on the matrix, and (iii) assaying the density of adhesion ligands using a novel model that describes binding of soluble receptors on ligands present on an insoluble surface. Confirmation of cell receptor binding onto the scaffold surface was provided by observing that binding of Mg + 2 to the I domain had taken place.
The methodology has been used to measure the adhesion ligand density for the two major collagen-binding integrins (α1β1, α2β1) in two kinds of porous collagen scaffolds, named here ‘active’ and ‘inactive’ (Tzeranis et al 2010, Tzeranis 2013). The active scaffold had previously shown strong regenerative activity in a peripheral nerve study while the inactive control was minimally active (Soller et al 2012). The two scaffolds had identical pore geometry and chemical composition (having been fabricated by freeze-drying microfibrillar type I collagen using the same protocol) but differed in the cross-linking treatment used to introduce intermolecular bonds. The active scaffold was crosslinked by dehydrothermal treatment (DHT), a physicochemical treatment that forms peptide bonds between chains without use of a crosslinking agent (Yannas and Tobolsky 1967). The inactive scaffold was crosslinked chemically using the reagents EDAC and NHS (Hermanson 2008, Tzeranis 2013). EDAC-NHS crosslinking agents are known to react with carboxylic groups, identified in key acidic residues in all major ligands of α1β1 and α2β1 (Leitinger 2011). This type of crosslinking amounts to masking of ligands. Used as baseline in this study, a scaffold was prepared by the same freeze-drying process but had not been crosslinked; this scaffold had previously shown negligible regenerative activity. The two nearly identically structured, crosslinked scaffolds were selected for comparison after showing remarkably different ability to induce peripheral nerve regeneration in the transected rat sciatic nerve model (Soller et al 2012). The density of adhesion ligands in each of the two scaffolds for each receptor of interest was estimated based on a series of binding experiments described in detail elsewhere (Tzeranis 2013, Tzeranis et al 2014) using quantitative multiphoton microscopy (Buehler et al 2005).
Difference in ligand density between active and inactive scaffold
The results from use of multiphoton microscopy showed that the active scaffold (lightly crosslinked, high regenerative activity) had ligand density levels of 204.9 ± 41 μM α1β1 ligands and 248.3 ± 61 μM α2β1 ligands. The inactive scaffold (heavily crosslinked, poor regenerative activity) had levels of 29.4 ± 7.2 μM α1β1 ligands and 75.6 ± 11.0 μM α2β1 ligands. The baseline control scaffold (uncrosslinked control, poor regenerative activity) showed levels of 148.2 ± 26.3 μM α1β1 ligands and 214.3 ± 50.1 μM α2β1 ligands. It is likely that the baseline control scaffold lacked regenerative activity because it has been shown to degrade with a half-life < 1.5 week, shorter than the half-life associated with optimal regenerative activity (see discussion below). The finding that the inactive scaffold was associated with a reduced ligand density is consistent with the known effect of DHT and EDAC-NHS crosslinking on collagen. Both of these reagents have been shown to react with carboxylic groups present in α1β1 and α2β1 ligands, thereby eliminating their activity as ligands for these integrins. Because of expected differences in steric specificity between the two treatments, it is not clear whether identical amino acid residues were involved in these two crosslinking treatments.
These results are preliminary and cannot be used to conclude that the difference in regenerative activity between the two scaffolds described here is explained entirely by the observed difference in ligand density. Although the crosslinking process does affect the ligand density (by reacting with functional groups that appear in collagen ligands, such as the carboxylic group), it also affects the half-life of degradation (half-life becomes longer with crosslinking) as well as the stiffness of the scaffolds (stiffness increases with crosslinking). The half-life is known to affect regenerative activity (Soller et al 2012), as noted above. The effect of scaffold stiffness on regenerative activity is not known.
In summary, measurements of the density of ligands for integrins α1β1 and α2β1 on the two collagen scaffolds with identical pore structure (and differing also in half life for degradation) but quite different levels of regenerative activity showed that the scaffolds differed sharply in their respective surface chemistry. A scaffold which had been shown to block contraction in peripheral nerve wounds and to induce regeneration of nerves of high quality (active scaffold) (Soller et al 2012) had a much higher ligand density for α1β1, α2β1 integrins than the inactive scaffold which blocked contraction poorly and also resulted in regenerated nerves of poor quality. These results are currently limited to very few scaffolds; they must be extended. There are also questions associated with the half-life and the stiffness, both of which change with crosslinking treatment, as discussed above. The data are nevertheless consistent with a mechanism of scaffold activity that depends strongly on cell adhesion on the surface, mediated by high levels of ligand densities for the two integrins which appear to control much of the molecular biology of wound contraction in injured nerves. Additional evidence is required before a clearer association between ligand density and phenotype changes in contractile cells can be confirmed.
Critical structural features of a collagen scaffold that blocks contraction and induces regeneration
Critical structural features of DRT
The evidence collected in the previous sections can be used to identify specific structural features of the dermis regeneration template (DRT), a collagen scaffold that blocks wound contraction in skin and peripheral nerves and induces regeneration of high, though imperfect, quality. These structural features of a collagen scaffold define an insoluble material in terms of its average pore size, degradation rate and surface chemistry.
Maximum blocking of wound contraction and highest quality of regeneration coincided with DRT but not with a large number of other collagen-based scaffolds with closely matched structure. The optimal pore size for DRT was in the range 20–125 μm (Yannas et al 1989) while the optimal degradation half-life for this scaffold was 14 ± 7 d (Soller et al 2012). Insufficient data are available to identify optimal values for ligand densities for integrins α1β1 and α2β1. The limited evidence suggests a guideline for ligand densities that exceed 200 μM α1β1 or α2β1 ligands (Tzeranis et al 2014). Modification of any of the first two structural features, and possibly of the third as well, deactivates DRT almost completely.
Although it is clear that these structural criteria are necessary there is little evidence that they are also sufficient. For example, there is little information on which to decide the optimal level of pore volume fraction (currently set at 0.995) (Chen 1982) or the optimal fraction of collagen fibers that carry the native D-banding of collagen (quaternary structure) that has been retained following treatment in acetic acid during preparation of DRT (Sylvester et al 1989). Changes in activity from the presence of GAG are discussed below.
To confirm the specificity of structural features that define the regenerative activity of DRT we provide below a brief list of collagen scaffolds that, even though very similar in structure to DRT, were shown incapable of blocking contraction or inducing regeneration. The requirement for porosity in a scaffold was confirmed in this early study by comparing a highly porous implant with pore size 50 ± 20 μm and a scaffold that had been prepared identically except that, following all preparation steps, the porous structure was virtually eliminated by simple evaporative drying at atmospheric pressure, a process that yielded a nonporous collagen film. When implanted subcutaneously, the nonporous film became surrounded with scar rather than a small mass of dermis, as observed with the porous film (Yannas 1981). A later study made use of a library of collagen scaffolds with variable pore size and otherwise identical structure and showed that scaffolds fabricated with pore sizes of 5 ± 2.5 μm, 450 ± 100 μm, or 850 ± 200 μm, failed to block contraction and lacked regenerative activity (Yannas et al 1989). In a related study (Troxel 1994), a scaffold with a pore size of 400 μm was compared by microscopy with a DRT-like scaffold having a pore size of 40 μm. This comparison showed fibroblasts clustered very tightly inside the large pores of the first scaffold (which was inactive) while fibroblasts in the second (active) scaffold were present instead as isolated cells inside individual pores (Yannas 2015). In a later study with a different collagen library comprising members that differed in degradation rate (Harley et al 2004, Soller et al 2012), the baseline uncrosslinked control (half-life <1.5 week) and the inactive scaffold (highly crosslinked; half life >100 weeks) did not inhibit contraction nor did they show any significant regenerative activity. These examples indicate the high degree of structural specificity of the DRT scaffold.
Optimization of scaffold structure
Optimal values of structural parameters of DRT are required for maximum blocking of wound contraction. A mechanistic analysis of these optimal values provides an explanation for their existence.
The average pore size is required to have a lower limit of approximately 20 μm, as shown in measurements of contraction delay (Yannas et al 1989), in order to allow cells to migrate inside the scaffold and bind on the surface (figure 10). In the literature, cells have not been generally observed to migrate in any significant number inside scaffolds with much smaller average pore size. Migration inside the scaffold is a requirement for activity: once inside the scaffold, cell binding occurs via integrins for specific ligands on the collagen surface, leading to loss of the contractile phenotype. A sufficient number of cells from the healing wound must be bound on the scaffold surface in order to remove themselves from the pool of actively contracting cells and reduce contraction sufficiently. This requires a sufficiently large specific surface, σ (measured in mm2 scaffold surface per mm3 scaffold volume) for adhesion of a large enough number of cells (Yannas 1997). The specific surface of porous materials is known to decrease monotonously with increasing pore diameter. Estimated values of σ range from about 40 000 at an average pore diameter of 20 μm down to 4,000 mm2 mm−3 at a pore diameter of 200 μm (Chang 1988). Contraction was inhibited poorly above a pore size level of about 120 μm (Yannas et al 1989), corresponding to a minimum specific surface of about 6,000 mm2 mm−, The data can be simply explained by assuming that above this limiting value of the pore size there was insufficient specific surface to bind most of the contractile cells in the wound.
Figure 10.
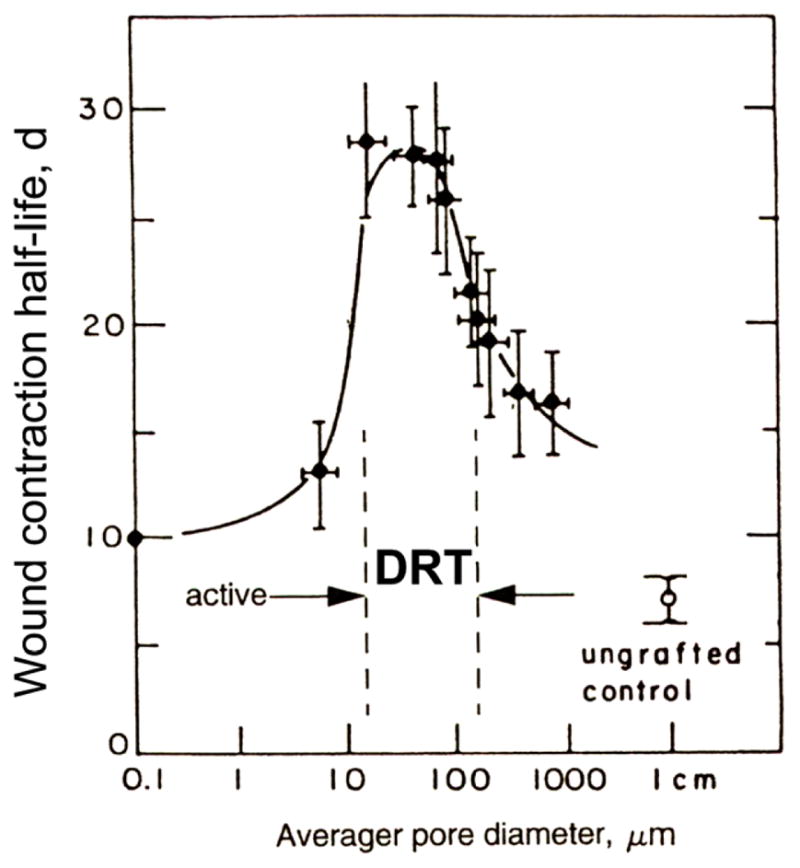
Partial identification of the dermis regeneration template (DRT). The average pore diameter of a collagen scaffold profoundly affects its ability to block wound contraction and its regenerative activity. Full-thickness excisional skin defects in the guinea pig were treated with several scaffolds, differing in pore diameter but otherwise identical in structure, that were members of a collagen library. The delay in onset of wound contraction is shown on the vertical axis. The horizontal axis is logarithmic to accommodate the wide range in pore sizes in this study. DRT is defined within the range 20 μm to 125 μm where contraction delay is maximized and regeneration occurs. (Yannas et al 1989.)
The degradation rate is optimized on a different basis. As mentioned above, the proposed mechanism of contraction blocking requires adhesive contact between integrins of contractile cells and ligands on the scaffold surface. Contractile cells have been observed to bind on the surface of the randomly oriented scaffold struts, thereby losing their axial orientation and cell–cells contacts (Murphy et al 1990), and becoming mechanically incompetent to apply an axially directed contraction force. The details of cell-scaffold binding are not clear at this time. However, to achieve specific binding on the scaffold surface, it appears necessary for a large enough surface to be present in an undissolved state at the same time that contractile cells are available for binding on it. Following binding on such a solid-like surface it is expected that the cells are temporarily ‘fixed’ in a randomized spatial orientation. It is hypothesized that a dissolved (highly degraded) scaffold would be diffusible and would be unsuitable for stable fixation of cells on the surface. Myofibroblasts appear in a full-thickness skin wound at about 1 week following injury and disappear in about 3–4 weeks (probably due to apoptosis; see above). A scaffold that degrades with a half-life less than about 7 d would therefore be excessively degraded by the time it makes contact with the relatively few contractile cells available in the wound at that time; very little blocking of wound contraction would take place under these conditions. At the other extreme, a scaffold with a half-life much longer than 3–4 weeks would likely prompt prolonged recruitment of macrophages, required to remain in situ in order to complete dismantling of the intractable implant; such an outcome would be expected to lead to a lengthy inflammatory response that might negate the myofibroblast-inhibiting activity observed with DRT. In contrast, when grafted with DRT (half-life, 14 ± 7 d) (Soller et al 2012), macrophages have been observed to engulf scaffold fragments by day 14 and to solubilize these fragments entirely by day 21 (Murphy et al 1990). An alternative explanation for the upper limit in scaffold half-life is suggested by the observed paucity of ligands on the surface of the inactive scaffold that has been extensively crosslinked with agents (EDAC and NHS; see Soller et al 2012, Tzeranis 2013). These crosslinking agents are known to react with carboxylic groups present in the ligands GFOGER and GLOGEN (see above), thereby masking these functional groups, leading to loss of most available ligands on the surface and eventually inhibiting cell-scaffold adhesion. Possibly both mechanisms may contribute to setting up the upper limit of scaffold half-life. Whatever the detailed mechanism, the evidence shows that the adhesive cell-scaffold contact that contributes to contraction blockade appears to require a critical window of opportunity between 1 and 3–4 weeks. Additional studies, especially with slowly degrading scaffolds, are needed to understand the diminished regenerative activity of such scaffolds. Another open question focuses on the minimal size of a partly degraded scaffold fragment that is required to ensure firm adhesive contact of contractile cells with the scaffold surface.
A requirement for finite density of ligands for integrins α1β1 and α2β1 is consistent with the requirement for adhesive contact of contractile cells with the scaffold, itself a prerequisite for contraction blockade. Although it seems plausible to hypothesize that the active scaffold which blocks contraction efficiently (see above) owes its activity to its superior ligand density, the data are limited to very few scaffolds and do not provide basis for such a conclusion. The available data are also limited and preclude a quantitative optimization of the ligand density. It is possible to make the qualitative case for existence of a minimal ligand density, below which binding would practically disappear; and of a maximal ligand density, limited by availability on the collagens structure. The incomplete evidence supports a requirement for a finite ligand density on the scaffold surface but provides no more information that could be used to set an optimal value. In the absence of such information it is suggested that a temporary guideline for ligand densities of active scaffolds that exceeds approximately 200 μM α1β1 or α2β1 ligands could be used until further information becomes available.
Discussion
Missing information on specificity of DRT structure
The structure of the active scaffold (DRT) is not as narrowly defined as many synthetic macromolecular substances (e.g. polylactic acid) or as a protein crystallized from solution (e.g. serum albumin). Nevertheless, its structure is not mutable to an unlimited extent since regenerative activity is lost following small, finite modifications to it. In general terms the physicochemical structure of the active scaffold is relatively well-defined and can be reproduced by following the standardized processing steps outlined above. When pursued carefully, the processing steps (figures 2 and 3) have led to reproducible levels of regenerative activity in the laboratory and the clinic.
The critical features of an active collagen scaffold that have been described above appear to have a significance similar to the structural determinants of biological activity for enzymes: they are required for regenerative activity. Although it appears clear that these structural criteria are necessary, they are most probably not sufficient. For example, there is little information on which to decide the optimal level of pore volume fraction (currently set at 0.995) (Chen 1982), or the optimal fraction of collagen fibers that carry the native D-banding, retained following standardized treatment in acetic acid during scaffold preparation which partly melts the quaternary (but not the tertiary) structure (Sylvester et al 1989). Although, for several years, reports of DRT activity described a chemical composition based on the original graft copolymer of collagen and chondroitin 6-sulfate (glycosaminoglycan, GAG), the GAG component was omitted in experimental scaffold preparations in our MIT laboratory approximately after year 2000 (however, GAG was not omitted from the industrial version of the scaffold, Integra™). Omission of GAG in our laboratory was implemented in deference to reported inhibition of peripheral nerve regeneration in the presence of certain glycosaminoglycans (Carbonetto 1991, Carbonetto et al 1983, Carbonetto and Cochard 1987). It has been reported that reaction of collagen with GAG increases the half-life for degradation of collagen (Yannas et al 1975a) and has a moderate delaying effect on wound contraction (Shafritz et al 1994) but it does not appear to affect the overall regenerative outcome. Quantitative limits for optimal values of ligand densities for integrins α1β1 and α2β1 in DRT are not available at this time. Since the crosslinking treatment used (Tzeranis 2013) affects not only the ligand density but also the half-life for degradation, as well as the stiffness, future studies are required in order to separate these effects on the regenerative activity of DRT clearly from each other.
A biologically active surface
The combined data from skin and nerve studies support the somewhat unusual view of biological activity that resides not in a soluble substance, e.g. an enzyme, but on a (temporarily) insoluble surface. A paradigm of surface biology explains the available evidence which shows that cell binding on the insoluble collagen surface dramatically modifies the phenotype of cells that control the incidence of repair versus regeneration. In the presence of the active surface, to which they adhere, contractile cells lose their normal states of assembly and high orientation along a deformation axis, resulting thereby in almost complete cancellation of the macroscopic contractile force that normally closes wounds in skin and peripheral nerves of the adult mammal. A premature onset of scaffold degradation destroys this biological activity, as evidenced by inactive scaffolds that degrade earlier than the optimal level of 2–3 weeks (Soller et al 2012).
A structural feature on the scaffold surface that might be responsible for the observed significant reduction in TGFβ1 concentration in DRT-treated nerve wounds (presumably by binding of the cytokine on the DRT surface, as described above) is not apparent at this time.
Synthesis of new tissue: Limitations of DRT
A contraction blockade, and the secondary process of scar formation, do not suffice to induce synthesis of new stroma. Organ regeneration also requires the synthesis of normal organ tissue, comprising stroma and tissues formed by differentiated cells. Since the evidence shows that an active scaffold, with optional epithelial cells, suffices for both steps in the regenerative process, it appears that the scaffold further provides the required information for stroma synthesis. An active scaffold hypothetically facilitates the formation of physiologic new stroma by acting as a topographic template for its synthesis. We have provided evidence above that the active scaffold cancels the tensile field in the wound. We now suggest further that the synthesis of stroma, instead of scar, makes use of the scaffold architecture as a topographic template (Yannas 2015).
The most serious regenerative limitation of cell-free DRT is inability to induce synthesis of an epidermis. To account for this limitation in the clinical setting, following synthesis of the dermis a thin autoepidermal graft is usually harvested from a donor site and was applied on the neodermis (Burke et al 1981, Heimbach et al 1988). However, this limitation is lifted when keratinocytes are seeded into DRT prior to grafting on the wound, as shown with animal models; the result is simultaneous regeneration both of a dermis and an epidermis. (Yannas et al 1989). Small-area wounds can also be treated comprehensively by DRT alone, since epidermal cells migrate from the wound perimeter within an acceptable time period to cover the entire wound area.
Skin synthesized using DRT seeded with autologous keratinocytes, as practiced in early studies (Yannas et al 1982a, 1982b, 1989), has also lacked skin adenexa (hair follicles, sweat glands, etc). Such deficiencies can become limiting for patients who have been treated with DRT over a very large fraction of their body surface area. Sweat glands contribute strongly to the thermoregulatory function of normal skin. It follows that lack of normal thermoregulation over a large area of the skin, e.g. 70% total skin area free of sweat glands in the case of a severely burned patient, can have serious consequences. The risk of morbidity or even death may become overwhelming when the treated person works many hours daily in the sun, as documented in the story of such a patient who was a farmer (McCarthy 2007). However, these limitations have been lifted in principle following the discovery by Boyce and coworkers that DRT seeded with keratinocytes from the dermal papillae can produce hair follicles and sweat glands (Sriwiriyanont et al 2012, 2013).
When used in clinical settings, DRT (commercial name Integra™, manufactured by Integra Life-Sciences) has shown considerable versatility. It has been successfully used to treat patients with extensive skin loss, such as those with massive burns, those requiring resurfacing of large scars and in the treatment of chronic skin wounds, among others. Abstracts of over 300 diverse clinical studies of Integra™ can be accessed on the following website: (www.ncbi.nlm.nih.gov/pubmed/?term=Integra+substitute++skin).
Empirical rules for induced organ regeneration in adults
The approach outlined above is based on use of a collagen scaffold with highly specific structure but is rooted in five empirical rules that have been described in detail (Yannas 2013). The rules appear to govern induced regeneration of skin and peripheral nerves in animal models and in the clinic. Extension of the rules to organs other than skin and peripheral nerves is a likely contingency but is not presumed; instead the term organ is assumed to apply to skin and peripheral nerves. Detailed references that justify these rules can be found in a monograph (Yannas 2015). These rules crystallize guidelines on: (1) the identification of spontaneously regenerative and nonregenerative tissues, guiding the investigators to focus on the minimal objectives of their regeneration experiments; (2) the simplest set of reactants (a scaffold and, optionally, autologous epithelial cells), added exogenously at the injured site, that can induce regeneration, allowing investigators to exclude use of exogenous reactants that complicate experiments without being useful; (3) the requirement for a scaffold that inhibits wound contraction and scar formation at the injured site, providing a theoretical foundation for the recommended protocols as well as a methodology for assaying experimental biomaterials for regenerative activity; (4) three quantitatively defined structural features that are required in a scaffold with confirmed regenerative activity, pointing at processing changes that can be made in other types of scaffolds in order to optimize their structure; (5) identification of the scaffold as a topographic template for synthesis of the new stroma. The first three rules appear to be necessary conditions for inducing regeneration and are independent of the specific reactant used to achieve regeneration. The last two rules are specific to the collagen scaffold that has been successfully used in experimental animal models and in the clinic. Other scaffolds or pharmacological agents that satisfy these rules could be used to repeat or exceed the quality of regeneration achieved.
Decellularized matrices
During the past 20 years there has been increasing experimental and clinical use of decellularized (acellular) matrices by several investigators as treatments of organ failure. To overcome the problem of immunogenicity of transplanted organs, decellularized matrices were prepared using tissues that had been processed to remove cells and their antigenic epitopes (decellularization, including perfusion decellularization) (Ott et al 2008, Guyette et al 2014). The extracellular matrix, mostly collagen, that forms most of the DCM implant is of much less concern with respect to antigenicity since the constituent molecules are conserved across species and are therefore tolerated immunologically. In these studies the tissues used were frequently derived either from the small intestinal submucosa or the bladder submucosa. An early approach was the use of decellularized human skin to graft burned patients (Wainright 1995, Wainwright et al 1996). Significant regenerative results were reported since then with organs as diverse as the urethra (Chen et al 1999), large defects in the abdominal wall (Badylak et al 2002), the bladder (Atala et al 2006), Achilles tendon (Gilbert et al 2007), the lung (Ott et al 2010), the larynx (Birchall et al 2012), and other organs.
Decellularized matrices (DCM) mostly consist of extracellular matrix, and the latter consists primarily of collagen. At least one analytical study determined over 90% wt. collagen, mostly type I, in small intestinal submucosa, the most commonly used acellular matrix (Badylak et al 2009). Collagens of other types were also detected in this study, together with a variety of GAGs, such as chondroitin sulfate, heparin sulfate and hyaluronic acid. Although collagen is the most prominent insoluble component in acellular matrices, there appears to be no report from studies with decellularized matrices that collagen may be involved directly in the regeneration processes. We suggest that decellularized matrices possess regenerative activity by virtue of their consisting primarily of tissues that are largely collagen-based and that their activity is largely explained by the structural features described above for DRT.
Conclusions
The theme of this review article is the intimate connection between the materials science aspects of collagen scaffolds and the incidence or absence of induced regeneration. Slight variations in key structural features of these materials have powerful consequences on regenerative activity, and on a clinical outcome that affects the lives of individuals who have sustained severe organ losses. Requiring in most cases no externally supplied cells or growth factors, an active scaffold can induce almost perfect regeneration of skin and peripheral nerves, clinical outcomes of major importance. Occasionally, the regenerative task requires seeding of the scaffold with autologous epithelial cells. But synthesis of the stroma, the key step in induction of organ regeneration, is accomplished by the scaffold without any external assistance, as has been described previously (Yannas 2015, ch. 7)
An active collagen scaffold, such as DRT, is a material that, in spite of being a complex 3D protein network, can be described in great detail by well-known physicochemical methods. These methods suffice to determine its key structural features, that are essentially similar to the structural determinants of enzymes.
A singularly important feature is the surface of the scaffold which, acting as if it were an enzyme, modifies the phenotype of contractile fibroblasts in a definitive manner that has dramatic consequences in the clinic. The cell-surface binding interaction opens up considerations that have been labelled by the authors as surface biology. Surfaces have traditionally been studied by physicists, chemists and materials scientists, not by biologists. In the study of regeneration phenomena it appears essential to recruit the cooperative services both of biologists and materials scientists.
Figure 5.
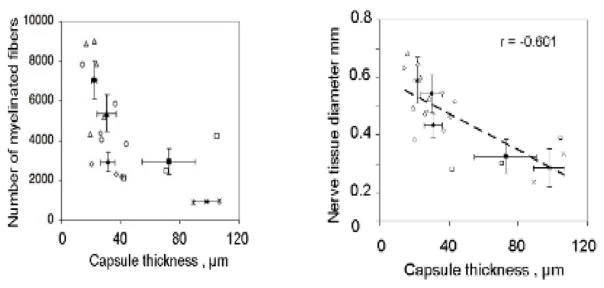
Quantitative relation between the thickness of the contractile cell capsule and two markers of quality of peripheral nerve regeneration at 9 weeks (Soller et al 2012). (The data in figure 4 were obtained in the same study.) The rat sciatic nerve was transected and the stumps were inserted inside collagen tubes with closely matched but nonidentical structures differing in half-life for degradation. The stumps were originally separated by a gap length of 15 ± 1 mm. Data obtained on the regenerated nerve formed at the midpoint of original stump separation, 9 weeks post-injury. Left: the number of myelinated axons decreased sharply with increase in thickness of the contractile cell capsule (between broken lines) surrounding the rat sciatic nerve regenerating nerve. Scatter plot of myelinated fibers number (r = −0.601, N = 18, p = 0.0084) against capsule thickness. Right: an inverse relationship was observed between the thickness of the contractile cell capsule and the diameter of the regenerated rat sciatic nerve. Scatter plot of nerve tissue diameter (square root of total myelinated area, r = −0.669, N = 18, p = 0.0024) as a function of capsule thickness. The resulting linear regression y = 0.6022–0.0032x (R2 = 0.552) is superposed (Soller et al 2012).
References
- Archibald SJ, Krarup C, Sheffner J, Li ST, Madison RD. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurology. 1991;306:685–96. doi: 10.1002/cne.903060410. [DOI] [PubMed] [Google Scholar]
- Archibald SJ, Sheffner J, Krarup C, Madison RD. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci. 1995;15:4109–23. doi: 10.1523/JNEUROSCI.15-05-04109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–46. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Badylak S, Kokini K, Tullius B, Simmons-Byrd A, Morff R. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103:190–202. doi: 10.1006/jsre.2001.6349. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Barber TA, Harbers GM, Park S, Gilbert M, Healy KE. Ligand density characterization of peptide-modified biomaterials. Biomaterials. 2005;26:6897–905. doi: 10.1016/j.biomaterials.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–80. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham RE, Russell PS. Studies on wound healing, with special reference to the phenomenon of contracture in experimental wounds in rabbits’ skin. Ann Surg. 1956;144:961–81. doi: 10.1097/00000658-195612000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchall MA, et al. Laryngeal transplantation in minipigs: early immunological outcomes. Clin Exp Immunol. 2012;167:556–64. doi: 10.1111/j.1365-2249.2011.04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler C, Kim KH, Greuter U, Schlumpf N, So PT. Single-photon counting multicolor multiphoton fluorescence microscope. J Fluoresc. 2005;15:41–51. doi: 10.1007/s10895-005-0212-z. [DOI] [PubMed] [Google Scholar]
- Burke JF, Yannas IV, Quinby WC, Jr, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413–28. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA, Tuckwell DS, Eble J, Kühn K, Humphries MJ. The integrin alpha1 A-domain is a ligand binding site for collagens and laminin. J Biol Chem. 1997;272:12311–7. doi: 10.1074/jbc.272.19.12311. [DOI] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–53. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Carbonetto S. Facilitatory and inhibitory effects of glial cells and extracellular matrix in axonal regeneration. Curr Opin Neurobiol. 1991:1407–13. doi: 10.1016/0959-4388(91)90062-c. [DOI] [PubMed] [Google Scholar]
- Carbonetto S, Cochard P. In vitro studies on the control of nerve fiber growth by the extracellular matrix of the nervous system. J Physiol (Paris) 1987;82:258–70. [PubMed] [Google Scholar]
- Carbonetto S, Gruver MM, Turner DC. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J Neurosci. 1983:32324–35. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LJ, Yannas IV. Preparation of collagen-glycosaminoglycan copolymers for tissue regeneration. In: Morgan JR, Yarmush ML, editors. Methods of Molecular Medicine. Tolowa, NJ: Humana Press; 1998. [DOI] [PubMed] [Google Scholar]
- Chamberlain LJ, Yannas IV, Hsu HP, Spector M. Connective tissue response to tubular implants for peripheral nerve regeneration: the role of myofibroblasts. J Comp Neurology. 2000a;417:415–30. doi: 10.1002/(sici)1096-9861(20000221)417:4<415::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chamberlain LJ, Yannas IV, Hsu H-P, Strichartz GR, Spector M. Near terminus axonal structure and function following rat sciatic nerve regeneration through a collagen-GAG matrix in a 10 mm gap. J Neurosci Res. 2000b;60:666–77. doi: 10.1002/(SICI)1097-4547(20000601)60:5<666::AID-JNR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain LJ, Yannas IV, Hsu HP, Strichartz G, Spector M. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp Neurology. 1998;154:315–29. doi: 10.1006/exnr.1998.6955. [DOI] [PubMed] [Google Scholar]
- Chang AS. MSc Thesis. Massachusetts Institute of Technology; 1988. Electrophysiological recovery of peripheral nerves regenerated by biodegradable polymer matrix. [Google Scholar]
- Chang AS, Yannas IV. Peripheral nerve regeneration. In: Smith B, Adelman G, editors. Neuroscience Year. Boston: Birkhauser; 1992. [Google Scholar]
- Chang AS, Yannas IV, Perutz S, Loree H, Sethi RR, Krarup C, Norregaard TV, Zervas NT, Silver J. Electrophysiological study of recovery of peripheral nerves regenerated by a collagen-glycosaminoglycan copolymer matrix. In: Gebelein CG, editor. Progress in Biomedical Polymers. New York: Plenum; 1990. [Google Scholar]
- Chen EHY. M S Thesis. Massachusetts Institute of Technology; Cambridge, MA: 1982. The effects of porosity and crosslinking of a collagen based artificial skin on wound healing. [Google Scholar]
- Chen F, Yoo JJ, Atala A. Acellular collagen matrix as a possible ‘off the shelf’ biomaterial for urethral repair. Urology. 1999;54:407–10. doi: 10.1016/s0090-4295(99)00179-x. [DOI] [PubMed] [Google Scholar]
- Chvapil M, Holusa R. Experimental experiences with the collagen sponge as hemostaticum and tampon. J Biomed Mater Res. 1968;2:245–64. doi: 10.1002/jbm.820020208. [DOI] [PubMed] [Google Scholar]
- Chvapil M, Holusa R, Kliment K, Stoll M. Some chemical and biological characteristics of a new collagen-polymer compound material. J Biomed Mater Res. 1969;3:315–32. doi: 10.1002/jbm.820030211. [DOI] [PubMed] [Google Scholar]
- Dagalakis N, Flink J, Stasikelis P, Burke JF, Yannas IV. Design of an artificial skin. Part III Control of pore structure. J Biomed Mater Res. 1980;14:511–28. doi: 10.1002/jbm.820140417. [DOI] [PubMed] [Google Scholar]
- Daimon E, Shibukawa Y, Wada Y. Calponin 3 regulates stress fiber formation in dermal fibroblasts during wound healing. Arch Dermatol Res. 2013;305:571–84. doi: 10.1007/s00403-013-1343-8. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Gabbiani G. The role of the myofibroblast in wound healing and fibrocontractive diseases. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. [Google Scholar]
- Desmoulière A, Badid C, Bochaton-Piallat M-L, Gabbiani G. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol. 1997;29:19–30. doi: 10.1016/s1357-2725(96)00117-3. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Genioz A, Gabbiani F, Gabbiani G. Transforming growth factor-b1 induces a-smooth muscle actin expression in granulation tissue, myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Emsley J, King SL, Bergelson JM, Liddington RC. Crystal structure of the I domain from integrin alpha2beta1. J Biol Chem. 1997;272:28512–7. doi: 10.1074/jbc.272.45.28512. [DOI] [PubMed] [Google Scholar]
- Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin alpha2beta1. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–28. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdman AG, Yannas IV. Scattering of light from histologic sections: a new method for the analysis of connective tissue. J Invest Dermatol. 1993;100:710–6. doi: 10.1111/1523-1747.ep12472364. [DOI] [PubMed] [Google Scholar]
- Fiddes JC, Hebda PA, Hayward P, Robson MC, Abraham JA, Klingbeil CK. Preclinical wound-healing studies with recombinant human basic fibroblast growth factor. Ann New York Acad Sci. 1991;638:316–28. doi: 10.1111/j.1749-6632.1991.tb49042.x. [DOI] [PubMed] [Google Scholar]
- Freyman TM, Yannas IV, Yokoo R, Gibson LJ. Fibroblast contraction of a collagen-GAG matrix. Biomaterials. 2001;22:2883–91. doi: 10.1016/s0142-9612(01)00034-5. [DOI] [PubMed] [Google Scholar]
- Follonier L, Schaub S, Meister JJ, Hinz B. Myofibroblast communication is controlled by intercellular mechanical coupling. J Cell Sci. 2008;121:3305–16. doi: 10.1242/jcs.024521. [DOI] [PubMed] [Google Scholar]
- Follonier L, Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010;316:2390–401. doi: 10.1016/j.yexcr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. Evolution and clinical implications of the myofibroblast concept. Cardiovasc Res. 1998;38:545–8. doi: 10.1016/s0008-6363(98)00065-0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–46. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621–30. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- Glim JE1, van Egmond M, Niessen FB, Everts V, Beelen RH. Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen. 2013;21:648–60. doi: 10.1111/wrr.12072. [DOI] [PubMed] [Google Scholar]
- Goss RJ. Prospects for regeneration in man. Clin Orth. 1980;151:270–82. [PubMed] [Google Scholar]
- Goss RJ. Regeneration versus repair. In: Cohen IK, et al., editors. Wound Healing. Philadelphia: W. B. Saunders; 1992. [Google Scholar]
- Goss RJ, Grimes LN. Tissue interactions in the regeneration of rabbit ear holes. Am Zool. 1972;12:151–7. [Google Scholar]
- Goss RJ, Grimes LN. Epidermal downgrowths in regenerating rabbit ear holes. J Morphol. 1975;146:533–42. doi: 10.1002/jmor.1051460408. [DOI] [PubMed] [Google Scholar]
- Grillo HC, Gross J. Thermal reconstitution of collagen from solution, and the response to its heterologous implantation. J Surg Res. 1962;2:69–82. doi: 10.1016/s0022-4804(62)80035-3. [DOI] [PubMed] [Google Scholar]
- Guyette JP, Gilpin SE, Charest JM, Tapias LF, Ren X, Ott HC. Perfusion decellularization of whole organs. Nat Protocols. 2014;9:1451–68. doi: 10.1038/nprot.2014.097. [DOI] [PubMed] [Google Scholar]
- Hamaia SW, Pugh N, Raynal N, Némoz B, Stone R, Gullberg D, Bihan D, Farndale RW. Mapping of potent and specific binding motifs, GLOGEN and GVOGEA, for integrin α1β1 using collagen toolkits II and III. J Biol Chem. 2012;287:26019–28. doi: 10.1074/jbc.M112.353144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbers GM, Gamble LJ, Irwin EF, Castner DG, Healy KE. Development and characterization of a high-throughput system for assessing cell-surface receptor-ligand engagement. Langmuir. 2005;21:8374–84. doi: 10.1021/la050396y. [DOI] [PubMed] [Google Scholar]
- Harley BA, Spilker MH, Wu JW, Asano K, Hsu HP, Spector M, Yannas IV. Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues Organs. 2004;176:153–65. doi: 10.1159/000075035. [DOI] [PubMed] [Google Scholar]
- Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Microarchitecture of 3D scaffolds influences cell migration behavior via junction interactions. Biophys J. 2008;95:4013–24. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward P, Hokanson J, Heggers J, Fiddes J, Klingbeil C. Fibroblast growth factor reverses the bacterial retardation of wound contraction. Am J Surg. 1992;163:288–93. doi: 10.1016/0002-9610(92)90004-b. [DOI] [PubMed] [Google Scholar]
- Heimbach D, et al. Artificial dermis for major burns. Ann Surg. 1988;208:313–20. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol. 2000;19:319–23. doi: 10.1016/s0945-053x(00)00076-7. [DOI] [PubMed] [Google Scholar]
- Helary C, Rodriguez-Sanchez B, Vigier S, Giraud Guilli MM. Dense fibrillar collagen matrices to analyse extracellular matrix receptor function. Pathol Biol (Paris) 2012;60:7–14. doi: 10.1016/j.patbio.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Hermanson GT. Bioconjugate Techniques. 2. Academic; 2008. [Google Scholar]
- Higton DLR, James DW. The force of contraction of full-thickness wounds of rabbit skin. Br J Surg. 1964;51:462–6. doi: 10.1002/bjs.1800510616. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell–cell adherens junctions. Mol Biol Cell. 2004;15:4310–20. doi: 10.1091/mbc.E04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiong SX, Huebsch N, Fischbach C, Kong HJ, Mooney DJ. Integrin-adhesion ligand bond formation of preosteoblasts and stem cells in 3D RGD presenting matrices. Biomacromolecules. 2008;9:1843–51. doi: 10.1021/bm8000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WC, Spilker MH, Yannas IV, Rubin PAD. Inhibition of conjunctival scarring and contraction by a porous collagen-GAG implant. Invest Ophthalmol Vis Sci. 2000;41:2404–11. [PubMed] [Google Scholar]
- Huang C, Yannas IV. Mechanochemical studies of enzymatic degradation of insoluble collagen fibers. J Biomed Mater Res. 1977;11:137–54. doi: 10.1002/jbm.820110113. [DOI] [PubMed] [Google Scholar]
- Huebsch ND, Mooney DJ. Fluorescent resonance energy transfer: a tool for probing molecular cell-biomaterial interactions in three dimensions. Biomaterials. 2007;28:2424–37. doi: 10.1016/j.biomaterials.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jokinen J, et al. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279:31956–63. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- Joseph J, Dyson M. Tissue replacement in the rabbit’s ear. Br J Surg. 1966;53:372–80. doi: 10.1002/bjs.1800530415. [DOI] [PubMed] [Google Scholar]
- Kingshott P, Andersson G, McArthur SL, Griesser HJ. Surface modification and chemical surface analysis of biomaterials. Curr Opin Chem Biol. 2011;15:667–76. doi: 10.1016/j.cbpa.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Kline DG, Hayes GJ. The use of resorbable wrapper for peripheral nerve repair. J Neurosurg. 1964;21:737–50. doi: 10.3171/jns.1964.21.9.0737. [DOI] [PubMed] [Google Scholar]
- Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem. 2000;275:35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- Kong HJ, Boontheekul T, Mooney DJ. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proc Natl Acad Sci USA. 2006;103:18534–9. doi: 10.1073/pnas.0605960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H, Wiebe C, Gallant-Behm C, Hart DA, Heino J, Häkkinen L. Exploring scarless healing of oral soft tissues. J Can Dent Assoc. 2011;77:b18. [PubMed] [Google Scholar]
- Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–90. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 2014;310:39–87. doi: 10.1016/B978-0-12-800180-6.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Gatien S, Finnson K, Desmeules S, Villiard E, Pilote M, Philip A, Roy S. Transforming growth factor: beta signaling is essential for limb regeneration in axolotls. PLoS One. 2007;28:e1227. doi: 10.1371/journal.pone.0001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Villiard E, Roy S. Skin wound healing in axolotls: a scarless process. J Exp Zool B Mol Dev Evol. 2010;314:684–97. doi: 10.1002/jez.b.21371. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Mao Z, Gao C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf B Biointerfaces. 2007;60:137–57. doi: 10.1016/j.colsurfb.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL. A study of nerve regeneration across synthetic (Maxon) and biologic (collagen) nerve conduits for nerve gaps up to 5 cm in the primate. J Reconstr Microsurg. 1990:6117–21. doi: 10.1055/s-2007-1006810. [DOI] [PubMed] [Google Scholar]
- Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113:1677–86. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- Mak K, Manji A, Gallant-Behm C, Wiebe C, Hart DA, Larjava H, Häkkinen L. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci. 2009;56:168–80. doi: 10.1016/j.jdermsci.2009.09.005. [DOI] [PubMed] [Google Scholar]
- McCarthy M. The Sun Farmer. Chicago: Ivan R. Dee; 2007. [Google Scholar]
- Michaeli D, McPherson M. Immunologic study of artificial skin used in the treatment of thermal injuries. J Burn Care Rehab. 1990;11:21–6. doi: 10.1097/00004630-199001000-00005. [DOI] [PubMed] [Google Scholar]
- Murphy GF, Orgill DP, Yannas IV. Partial dermal regeneration is induced by biodegradable collagen-glycosaminoglycan grafts. Lab Invest. 1990;62:305–13. [PubMed] [Google Scholar]
- Mustoe TA, Pierce GF, Morishima C, Deuel TF. Growth-factor induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991;87:694–703. doi: 10.1172/JCI115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Rodríguez FJ, Labrador RO, Butí M, Ceballos D, Gómez N, Cuadra J, Perego G. Peripheral nerve regeneration through bioresorbable and durable nerve guides. J Peripher Nerv Syst. 1996:153–64. [PubMed] [Google Scholar]
- Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment. in vitro J Cell Sci. 2005;118:4731–9. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- O’Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25:1077–86. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Rich RL, Deivanayagam CC, Owens RT, Carson M, Höök A, Moore D, Symersky J, Yang VW, Narayana SV, Höök M. Trench-shaped binding sites promote multiple classes of interactions between collagen and the adherence receptors, alpha(1)beta(1) integrin and Staphylococcus aureus cna MSCRAMM. J Biol Chem. 1999;274:24906–13. doi: 10.1074/jbc.274.35.24906. [DOI] [PubMed] [Google Scholar]
- Riikonen T, Westermarck J, Koivisto L, Broberg A, Kähäri VM, Heino J. Integrin α2β1 is a positive regulator of collagenase (MMP-1) and collagen α1(I) gene expression. J Biol Chem. 1995;270:13548–52. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Karen J, Gardner H, Gerdin B, Rubin K, Sundberg C. Integrin alpha1beta1 is involved in the differentiation into myofibroblasts in adult reactive tissues in vivo. J Cell Mol Med. 2009;13:3449–62. doi: 10.1111/j.1582-4934.2008.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph R, Gruber S, Suzuki M, Woodward M. The life cycle of the myofibroblast. Surg Gyn Obst. 1977;145:389–94. [PubMed] [Google Scholar]
- Rudolph R, Van de Berg J, Ehrlich P. Wound contraction and scar contracture. In: Cohen IK, et al., editors. Wound Healing. Philadelphia: W.B. Saunders; 1992. [Google Scholar]
- Schiller HB, Friedel CC, Boulegue C, Faessler R. EMBO reports. EMBO Press. 2011;12:259–66. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrementi ME, Ferreira AM, Zender C, DiPietro LA. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen. 2008;16:80–6. doi: 10.1111/j.1524-475X.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS One. 2012;7:e32875. doi: 10.1371/journal.pone.0032875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz TA, Rosenberg LC, Yannas IV. Specific effects of glycosaminoglycans in an analog of extracellular matrix that delays wound contraction and induces regeneration. Wound Rep Reg. 1994;2:270–6. doi: 10.1046/j.1524-475X.1994.20407.x. [DOI] [PubMed] [Google Scholar]
- Shi M, Pedchenko V, Greer BH, Van Horn WD, Santoro SA, Sanders CR, Hudson BG, Eichman BF, Zent R, Pozzi A. Enhancing integrin α1 inserted (I) domain affinity to ligand potentiates integrin α1β1-mediated down-regulation of collagen synthesis. J Biol Chem. 2012;287:35139–52. doi: 10.1074/jbc.M112.358648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siljander PR, Hamaia S, Peachey AR, Slatter DA, Smethurst PA, Ouwehand WH, Knight CG, Farndale RW. Integrin activation state determines selectivity for novel recognition sites in fibrillar collagens. J Biol Chem. 2004;279:47763–72. doi: 10.1074/jbc.M404685200. [DOI] [PubMed] [Google Scholar]
- Soller EC, Tzeranis DS, Miu K, So PT, Yannas IV. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials. 2012;33:4783–91. doi: 10.1016/j.biomaterials.2012.03.068. [DOI] [PubMed] [Google Scholar]
- Sriwiriyanont P, Lynch KA, Maier EA, Hahn JM, Supp DM, Boyce ST. Morphogenesis of chimeric hair follicles in engineered skin substitutes with human keratinocytes and murine dermal papilla cells. Exp Dermatol. 2012;21:783–5. doi: 10.1111/exd.12003. [DOI] [PubMed] [Google Scholar]
- Sriwiriyanont P, Lynch KA, McFarland KL, Supp DM, Boyce ST. Characterization of hair follicle development in engineered skin substitutes. PLoS One. 2013;8:65664. doi: 10.1371/journal.pone.0065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester MF, Yannas IV, Salzman EW, Forbes MJ. Collagen banded fibril structure and the collagen-platelet reaction. Thromb Res. 1989;55:135–48. doi: 10.1016/0049-3848(89)90463-5. [DOI] [PubMed] [Google Scholar]
- Symersky J, et al. Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat Struct Biol. 1997;4:833–8. doi: 10.1038/nsb1097-833. [DOI] [PubMed] [Google Scholar]
- Thies S. Unpublished data on the nonspecific binding of TGFb1 on the DRT scaffold 2010 [Google Scholar]
- Troxel K. PhD Thesis. Massachusetts Institute of Technology; Cambridge, MA: 1994. Delay of skin wound contraction by porous collagen-GAG matrices. [Google Scholar]
- Troxel K, Yannas IV. Myofibroblast axis orientation (alignment) is required for wound contraction. J Cell Biol Abstracts. 1991;115:114. [Google Scholar]
- Tuckwell D, Calderwood DA, Green LJ, Humphries MJ. Integrin alpha 2 I-domain is a binding site for collagens. J Cell Sci. 1995;108:1629–37. doi: 10.1242/jcs.108.4.1629. [DOI] [PubMed] [Google Scholar]
- Tulla M, Pentikäinen OT, Viitasalo T, Käpylä J, Impola U, Nykvist P, Nissinen L, Johnson MS, Heino J. Selective binding of collagen subtypes by integrin α1I, α2I, and α10I domains. J Biol Chem. 2001;276:48206–12. doi: 10.1074/jbc.M104058200. [DOI] [PubMed] [Google Scholar]
- Tzeranis DS. PhD Thesis. Departments of Mechanical Engineering and Biological Engineering, Massachusetts Institute of Technology; Cambridge, MA: 2013. Imaging studies of peripheral nerve regeneration induced by porous collagen biomaterials. [Google Scholar]
- Tzeranis DS, Roy A, So PT, Yannas IV. An optical method to quantify the density of ligands for cell adhesion receptors in 3D matrices. J R Soc Interface. 2010:7649–61. doi: 10.1098/rsif.2010.0321.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeranis DS, Soller EC, Buydash MC, So PT, Yannas IV. In situ quantification of the surface chemistry in porous collagen biomaterials. Ann Biomed Eng. 2015 doi: 10.1007/s10439-015-1445-x. at press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upla P, Marjomäki V, Kankaanpää P, Ivaska J, Hyypiä T, Van Der Goot FG, Heino J. Clustering induces a lateral redistribution of alpha 2 beta 1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization. Mol Biol Cell. 2004;15:625–36. doi: 10.1091/mbc.E03-08-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenick LV, Schwarzbauer JE. Ligand density and integrin repertoire regulate cellular response to LPA. Matrix Biol. 2006;25:223–31. doi: 10.1016/j.matbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–8. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- Wainwright D, Madden M, Luterman A, Hunt J, Monafo W, Heimbach D, Kagan R, Sittig K, Dimick A, Herndon D. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil. 1996;17:124–36. doi: 10.1097/00004630-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Wong JW, Gallant-Behm C, Wiebe C, Mak K, Hart DA, Larjava H, Häkkinen L. Wound healing in oral mucosa results in reduced scar formation as compared with skin: evidence from the red Duroc pig model and humans. Wound Repair Regen. 2009;17:717–29. doi: 10.1111/j.1524-475X.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- Yannas IV. Collagen and gelatin in the solid state. Rev Macromol Chem. 1972;7:49–104. [Google Scholar]
- Yannas IV. Use of artificial skin in wound management. In: Dineen P, editor. The Surgical Wound. Philadelphia: Lea & Febiger; 1981. [Google Scholar]
- Yannas IV. Biologically active analogues of the extracellular matrix: artificial skin and nerves. Angew Chem Int Ed Engl. 1990;29:20–35. [Google Scholar]
- Yannas IV. Models of organ regeneration processes induced by templates. Ann N Y Acad Sci. 1997;831:280–93. doi: 10.1111/j.1749-6632.1997.tb52203.x. [DOI] [PubMed] [Google Scholar]
- Yannas IV. Studies on the biological activity of the dermal regeneration template. Wound Repair Regen. 1998;6:518–24. doi: 10.1046/j.1524-475x.1998.60604.x. [DOI] [PubMed] [Google Scholar]
- Yannas IV. Tissue and Organ Regeneration in Adults. 1. New York: Springer; 2001. pp. 225–34. [Google Scholar]
- Yannas IV. Emerging rules for inducing organ regeneration. Biomaterials. 2013;34:321–30. doi: 10.1016/j.biomaterials.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Yannas IV. Tissue and Organ Regeneration in Adults. 2. New York: Springer; 2015. pp. 223–7.pp. 233–4. [Google Scholar]
- Yannas IV, Burke JF, Gordon PL, Huang C, Rubinstein RH. Design of an artificial skin II: control of chemical composition. J Biomed Mater Res. 1980;14:107–31. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]
- Yannas IV, Burke JF, Huang C, Gordon PL. Suppression of in vivo degradability and of immunogenicity of collagen by reaction with glycosaminoglycans. Polym Preprare Am Chem Soc. 1975a;16:209–14. [Google Scholar]
- Yannas IV, Burke JF, Huang C, Gordon PL. Correlation of in vivo collagen degradation rate with in vitro measurements. J Biomed Mater Res. 1975b:9623–8. doi: 10.1002/jbm.820090608. [DOI] [PubMed] [Google Scholar]
- Yannas IV, Burke JF, Orgill DP, Skrabut EM. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982a;215:174–6. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- Yannas IV, Burke JF, Orgill DP, Skrabut EM. Regeneration of skin following closure of deep wounds with a biodegradable template. Trans Soc Biomater. 1982b:524–27. [Google Scholar]
- Yannas IV, Burke JF, Warpehoski M, Stasikelis P, Skrabut EM, Orgill D, Giard DJ. Prompt, long-term functional replacement of skin. Trans Am Soc Artif Intern Organs. 1981;27:19–22. [PubMed] [Google Scholar]
- Yannas IV, Colt J, Wai YC. Wound contraction and scar synthesis during development of the amphibian Rana catesbeiana. Wound Rep Reg. 1996:431–41. doi: 10.1046/j.1524-475X.1996.40107.x. [DOI] [PubMed] [Google Scholar]
- Yannas IV, Orgill DP, Silver J, Norregaard TV, Zervas NT, Schoene WC. Polymeric template facilitates regeneration of sciatic nerve across 15 mm gap. Trans Soc Biomater. 1985;8:146. [Google Scholar]
- Yannas IV, Orgill DP, Silver J, Norregaard T, Zervas NT, Schoene WC. Regeneration of sciatic nerve across 15 mm gap by use of a polymeric template. In: Gebelein CG, editor. Advances in Biomedical Materials. Washington, DC: American Chemical Society; 1987a. [Google Scholar]
- Yannas IV, Lee E, Orgill DP, Ferdman A, Skrabut EM, Murphy GF. De novo synthesis of skin. Proc Am Chem Soc Div Polym Mater. 1987b;57:28–32. [Google Scholar]
- Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix which induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933–7. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannas IV, Tobolsky AV. Crosslinking of gelatine by dehydration. Nature. 1967;215:509–10. doi: 10.1038/215509b0. [DOI] [PubMed] [Google Scholar]
- Yannas IV, Tzeranis DS, Harley BA, So PT. Biologically active collagen-based scaffolds: advances in processing and characterization. Phil Trans A Math Phys Eng Sci. 2010;368:2123–39. doi: 10.1098/rsta.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WM, et al. α11β1 Integrin recognizes the GFOGER sequence in interstitial collagens. J Biol Chem. 2003;278:7270–7. doi: 10.1074/jbc.M210313200. [DOI] [PubMed] [Google Scholar]
- Znoyko I, Trojanowska M, Reuben A. Collagen binding alpha2beta1 and alpha1beta1 integrins play contrasting roles in regulation of Ets-1 expression in human liver myofibroblasts. Mol Cell Biochem. 2006;282:89–99. doi: 10.1007/s11010-006-1400-0. [DOI] [PubMed] [Google Scholar]