Abstract
Objective
β-Lactamase-negative ampicillin-resistant Haemophilus influenzae is a common opportunistic pathogen of hospital- and community-acquired infections, harboring multiple single nucleotide polymorphisms in the ftsI gene, which codes for penicillin-binding protein-3. The objectives of this study were to perform comprehensive genetic analyses of whole regions of the penicillin-binding proteins in H. influenzae and to identify additional single nucleotide polymorphisms related to antibiotic resistance, especially to ampicillin and other cephalosporins.
Results
In this genome analysis of the ftsI gene in 27 strains of H. influenzae, 10 of 23 (43.5%) specimens of group III genotype β-lactamase-negative ampicillin-resistant H. influenzae were paradoxically classified as ampicillin-sensitive phenotypes. Unfortunately, we could not identify any novel mutations that were significantly associated with ampicillin minimum inhibitory concentrations in other regions of the penicillin-binding proteins, and we reconfirmed that susceptibility to β-lactam antibiotics was mainly defined by previously reported SNPs in the ftsI gene. We should also consider detailed changes in expression that lead to antibiotic resistance in the future because the acquisition of resistance to antimicrobials can be predicted by the expression levels of a small number of genes.
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3169-0) contains supplementary material, which is available to authorized users.
Keywords: Haemophilus influenzae, β-Lactamase-negative ampicillin-resistant (BLNAR), Penicillin binding protein, SNP
Introduction
The main molecular mechanism of non-β-lactamase resistance among β-lactamase-negative ampicillin-resistant (BLNAR) strains is assumed to be a decreased affinity of penicillin-binding protein (PBP)3 to β-lactams [1–3]. Ubukata et al. proved that amino acid substitutions in the SSN motifs (M377I, S385T, and L389F) and KTG motifs (R517H and N526K) in BLNAR strains are major causes of resistance to ampicillin (ABPC), and that these SNPs lead to changes in the three-dimensional structure of the active binding site of PBP3 [4].
To date, along with the widespread global incidence of BLNAR H. influenzae, several isolates have been found to harbor the typical mutation patterns of BLNAR mutants (genotype BLNAR, gBLNAR) with susceptibility to low ABPC minimum inhibitory concentrations (MICs) [5, 6]. Osaki et al. reported elevated cephalosporin MICs in a strain of H. influenzae possessing artificially mutated ftsI genes, which was expected; however, the ABPC MICs were not elevated, contrary to expectations [7]. These data also suggest that the ftsI gene could play a different role in penicillin resistance from its role in resistance to other cephalosporin antibiotics, and that resistance to ABPC is not only determined by the presence or absence of mutations on the ftsI gene.
Few studies have investigated the recent trends of genetic polymorphisms in the ftsI gene, despite the worldwide spread of BLNAR H. influenzae. We hypothesized that novel SNPs on the ftsI gene that affect the susceptibility to β-lactams have emerged and could become a threat to the world in the future. The objectives of this study were to perform comprehensive genetic analyses of whole regions of the PBPs in H. influenzae and to identify new SNPs related to antibiotic resistance, especially to ABPC and other cephalosporins.
Main text
Materials and methods
We collected 39 clinical isolates of H. influenzae from January 2014 to March 2016 at the National Defense Medical College Hospital. These isolates were identified using standard microbiological methods to identify H. influenzae (rabbit blood agar and conventional X and V factor requirements test) (Eiken Chemical Co., Tokyo, Japan, Cat. No. E-DC08) [8]. Isolates were stored at − 30 °C until use and recovered after inoculation onto chocolate agar (Kyokuto Pharmaceuticals, Tokyo, Japan, Cat. No. 251169) at 37 °C in 5% CO2 for 24 h. Genomic DNA was extracted with the EZ Extract for DNA kit (Advanced Microorganism Research, Gifu, Japan, Cat. No. 76815M). MICs of the antibiotics were determined by the broth dilution method (Kyokuto Pharmaceuticals, Cat. Nos. 551-0000-0 and 08930), in accordance with the guidelines of the Clinical Laboratory Standard Institute (document M100-S25) [9]. β-Lactamase production was identified using the nitrocefin method with BBL Cefinase Paper Disc kit (Becton–Dickinson, Franklin Lakes, NJ, Cat. No. 231650).
Extracted DNA specimens subsequently underwent library preparation using TruSeq Nano DNA Sample Preparation Kit (Illumina, San Diego, CA, Cat. Nos. FC-121-4001 and 4002), according to the low sample protocol provided. Single sequencing was performed with an Illumina HiSeq 2500 (Illumina) using 100 bp paired-end runs (Illumina, Cat. Nos. PE-401-3001, FC-401-3001 and FC-121-1003). BWA (v 0.7.15) was used to map raw FASTQ data [10]. The reference sequences used in this study are listed in Additional file 1. Sequences of 16S ribosomal RNA (rRNA), recA, fucK, hpd, and sodC were used to distinguish H. influenzae from H. haemolyticus according to previous reports [11–14]. Capsular formation and serotyping were performed according to the BexDCBA and region II in the cap locus [15]. Consensus FASTQ data were extracted from the mapping data, and bases with a quality score of less than 20 were eliminated. SNP detection, prediction of amino acid substitutions, and phylogenetic analyses of 16S rRNA and recA were performed with MEGA (v 7.0.21) [16]. The presence of fucK, hpd, and sodC was determined from mapping data using Integrative Genomic Viewer (v 2.3.81) [17, 18]. All synonymous SNPs were removed in this study.
Mann–Whitney U tests were used to ascertain the association between the MICs of the antibiotics and the presence or absence of each SNP. Associations were considered statistically significant if the p value for any of the SNPs was less than the Bonferroni-adjusted significance threshold. All statistical analyses were calculated with R (v 3.4.0; R Foundation for Statistical Computing, Vienna, Austria [http://www.R-project.org/]) and package “EnvStats” [19].
Based on the ftsI gene sequence information, each specimen was classified into one of the following major genotypes: β-lactamase-negative ampicillin-susceptible (gBLNAS), strains without amino acid substitutions in the KTG motif (N526K or R517H) and SSN motif (M377I, S385T, or L389F) in ftsI; group I BLNAR, strains with N526K in the KTG motif and no mutations in the SSN motif; group II BLNAR, strains with R517H in the KTG motif and no mutations in the SSN motif; and group III BLNAR, strains with mutations in both the SSN and the KTG motifs [4, 20]. In addition, we defined a strain with the bla gene and without amino acid substitutions in the ftsI gene as a β-lactamase-positive ampicillin-resistant (BLPAR) strain.
Results
Origins of the clinical samples and β-lactamase producibility
Among the 39 isolates, 18 were extracted from sputum and the rest from nasal discharge (15/39), bronchoalveolar lavage fluid (3/39), pharyngeal swabs (2/39), blood (2/39), and biliary drain fluid (1/39). There were 5 (12.8%) BLPAR strains, and the other 34 were non-producing strains.
Antibiotics MICs and genotypes
The MIC profiles of the antibiotics examined in this study are shown in Additional file 2. ABPC MICs were significantly higher in group III BLNAR than in the gBLNAS strains and group I/II gBLNAR strains. However, 10 of 23 specimens in group III BLNAR (43.5%) were paradoxically classified as ABPC-sensitive strains (MIC ≤ 1 µg/mL; Fig. 1).
Fig. 1.
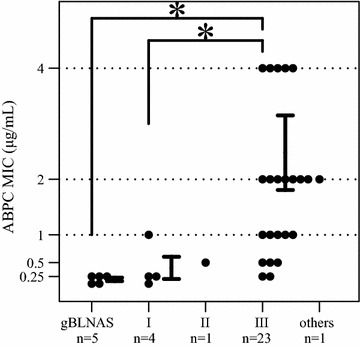
Relationship between non-β-lactamase-producing genotype strains and ABPC MICs. * represents p value < 0.05. I, II, and III represent gBLNAR groups I, II, and III respectively
Genetic confirmation of H. influenzae and serotyping
Genome analyses of 34 non-β-lactamase-producing strains were performed. All strains were identified as H. influenzae and simultaneously distinguished from both H. parainfluenzae and H. haemolyticus based on the phylogenetic analysis of 16S rRNA and recA genes. All 34 strains possessed the hpd gene; nevertheless, 2 strains were finally excluded from further SNP analysis because they lacked the fucK gene and had the sodC gene instead. The reason for their exclusion was that most H. haemolyticus strains possess sodC gene, which is a characteristic of the species, and H. influenzae possesses both fucK and hpd genes. The remaining 32 strains were confirmed as nontypeable serotypes because they did not have BexDCBA and region II in the cap locus.
SNP analysis of PBP-coding genes
We targeted 7 distinct genes encoding PBPs (1A, 1B, 2, 3, 4, 5, and 7) according to the NCBI information for H. influenzae Rd KW20 (Accession No. NC_000907). Unfortunately, we could not obtain the complete sequences of the PBP-coding genes in 5 of 32 strains, but we successfully completed SNP sequence analysis in 27. As a result of our analysis, we found SNPs with significant associations with the MICs of ABPC on the SSN motif (D350N, S357N, S385T, and L389F) and near the KTG motif (V562L) in the ftsI gene (coding PBP-3). Figure 2 illustrates the associations between the p-values derived from Mann–Whitney U tests of non-β-lactamase-producing strains for the ftsI gene and the SNPs that show significant associations with the MICs of ABPC. We also found SNPs with low p-values on the SSN motif (M377I) and near the KTG motif (V562L) in the ftsI gene (coding PBP-3). No novel SNPs were found in the other PBP-coding genes (Fig. 2a). The MICs of the other cephalosporin antibiotics were similar to those of ABPC (Fig. 2b–f). Additionally, these SNPs showed strong positive correlations with each other (Table 1).
Fig. 2.
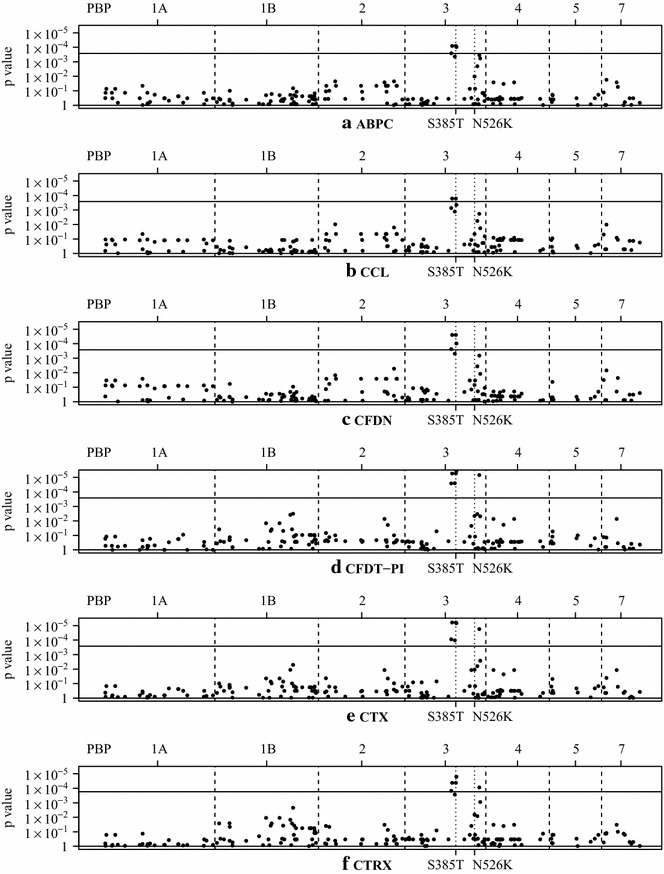
Association between SNPs and MICs. Plot of Mann–Whitney test p values of non-β-lactamase-producing strains. The horizontal axis shows the positions of the SNPs in each PBP coding gene. Dots represent the p values for each SNP. The upper, solid, horizontal lines show the level of significance after adjustment with the Bonferroni method (0.05 divided by 190). The two dotted lines in pbp3 represent the positions of the major SSN and KTG motif mutations: S385T and N526K. a ampicillin (ABPC), b cefaclor (CCL), c cefdinir (CFDN), d cefditoren pivoxil (CFDN-PI), e cefotaxime (CTX), and f ceftriaxone (CTRX)
Table 1.
Correlation coefficients between 2 SNPs in the ftsI gene
| D350N | S357N | M377I | S385T | L389F | V562L | |
|---|---|---|---|---|---|---|
| D350N | 0.924 | 0.924 | 0.924 | 0.795 | 0.739 | |
| S357N | 0.846 | 1.000 | 0.860 | 0.798 | ||
| M377I | 0.846 | 0.860 | 0.798 | |||
| S385T | 0.860 | 0.798 | ||||
| L389F | 0.928 | |||||
| V562L |
Analysis of acrA, acrB, acrR, and tolC genes
The sequences of acrA, acrB, acrR, and tolC genes were also analyzed in 27 samples. Whereas 16 samples had about 46 bp deletions in the acrB gene according to the mapping data, no significant changes in the MICs of the antibiotics were identified in either the presence or absence of the acrB mutations (Additional file 3).
Discussion
In this study, our data reconfirmed the significant associations between SNPs located in the ftsI gene and the MICs of ABPC or other cephalosporins in β-lactamase-negative H. influenzae; however, no other novel SNPs could be identified. We also found that group III BLNAR strains of H. influenzae with low ABPC MICs could paradoxically harbor typical SNPs in the ftsI gene. It was also suggested that discrepancies between the genotypes and phenotypes among BLNAR strains have already become commonplace in Japan. We identified that about up to 40% of isolates within group III gBLNAR genotype had low ABPC MICs, and these were classed as ABPC-sensitive phenotype.
We considered several reasons for the discrepancies between phenotypes and genotypes. First, MICs from the disk-testing method are considered to be influenced by the amount of bacteria in H. influenzae and our data reaffirmed the difficulty of predicting ABPC MICs based on the mutation pattern of the ftsI gene [21]. Second, ABPC affinity for PBPs was different from that of the other cephalosporins. Cefotaxime and other cephalosporins have the highest affinity for PBP2, whereas ampicillin had higher affinity for PBP1A and PBP4 [4]. Our data showed that cephalosporin agents tended to have a higher association with the MICs than ABPC. These findings confirm that the active sites of PBPs depend on the antibiotic agents. Third, subtle differences among clusters of species might be one of the reasons for the discrepancies between phenotypes and genotypes in clinical practice. Among the 27 isolates of H. influenzae identified via conventional biochemical methods, 2 were lacking the fucK gene and possessed the sodC gene instead, which is a typical profile of H. haemolyticus and not H. influenzae. Conventionally, the identification of H. influenzae is determined based on the results of hemolysis in rabbit blood agar and the conventional X and V factor requirements test. However, H. haemolyticus strains could be misdiagnosed as H. influenzae using these conventional protocols in clinical practice. Furthermore, even 16S rRNA phylogenetic analysis is not enough to distinguish these two species, and some of them can be categorized as “fuzzy species” [13, 14, 22–24]. Although β-lactamase resistance in H. haemolyticus is similar to that in H. influenzae, detailed information about mutations in the ftsI gene and the sensitivity of H. haemolyticus to ABPC and fuzzy species remains limited [25].
Some reports have also suggested that efflux pumps are involved in the development of antibiotic resistance, including to ABPC [26–28]. Kaczmarek et al. indicated that both the ftsI and acrR genes are associated with the antibiotic efflux pump and frameshift mutations, especially in acrR, could promote high ABPC MICs by accelerating AcrAB-mediated efflux [29]. However, our data did not show significant changes in the MICs either in the presence or absence of the acrB mutation [30].
Conclusion
It is possible that the gBLNAR strains could spread more widely in communities than can be estimated from MIC values. Unfortunately, we could not exclude the possibility that other mechanisms independent of the SNPs could contribute to the elevations in the MICs of the antibiotics. Therefore, continuous studies are crucial in order to gain an understanding of the molecular basis of β-lactam antibiotic resistance in BLNAR H. influenzae. Furthermore, we should consider in detail the changes in expression that lead to antibiotic resistance in our future plans because the acquisition of resistance to antimicrobials can also be predicted by the levels of expression of a small number of genes.
Limitations
First, our findings demonstrated that SNPs located in the SSN motif in the ftsI gene correlated highly with each other and we could not conclude which of the three types of SNPs in the SSN motif contributed most to the MICs of the antibiotics. Furthermore, we cannot rule out the possibility that horizontal transfer of a resistance gene could be involved [31]. Second, variations among the mutations were limited due to the small number of samples. Third, β-lactamase-positive amoxicillin/clavulanate resistant strains of H. influenzae have recently emerged in clinical settings [32]. However, we did not include these strains because only a few were found in this study.
Additional files
Additional file 1. Reference sequences used in this study.
Additional file 2. Antibiotic minimum inhibitory concentrations (MICs) for β-lactamase non-producing H. influenzae examined in this study.
Additional file 3. AcrB mutations and their relationship with ABPC MICs for each genotype.
Authors’ contributions
KM, YS, and TM1 conceived and designed the study; KM, NT, ST, MO, TH, AY, YK, KI, RLR, and TM1 gathered and analyzed the data and drafted and edited the manuscript; KM, TY, TM2, SM, AK, and TM1 supervised the study and revised the manuscript; YS and TM1 performed project administration (TM1: Takuya Maeda, TM2: Takashi Murakami.). All authors read and approved the final manuscript
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the National Defense Medical College Research Ethics Committee (reference 2521). Informed written consent was not applicable since this study was not directly involved with patients.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ABPC
ampicillin
- BLNAR
β-lactamase-negative ampicillin-resistant
- BLNAS
β-lactamase-negative ampicillin-susceptible
- BLPAR
β-lactamase-producing ampicillin-resistant
- MICs
minimum inhibitory concentrations
- PBP
penicillin-binding protein
- rRNA
ribosomal RNA
- SNP
single nucleotide polymorphisms
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13104-018-3169-0) contains supplementary material, which is available to authorized users.
Contributor Information
Kazuhisa Misawa, Email: k_misawa28@ndmc.ac.jp.
Norihito Tarumoto, Email: tarumoto@saitama-med.ac.jp.
Shinsuke Tamura, Email: tamshin007@gmail.com.
Morichika Osa, Email: nagumomoritika@yahoo.co.jp.
Takaaki Hamamoto, Email: hamamoto@ndmc.ac.jp.
Atsushi Yuki, Email: ayuki@ndmc.ac.jp.
Yuji Kouzaki, Email: yukouzaki@gmail.com.
Kazuo Imai, Email: k_imai@saitama-med.ac.jp.
Runtuwene Lucky Ronald, Email: luckyruntuwene@edu.k.u-tokyo.ac.jp.
Toshiyuki Yamaguchi, Email: desfier@gmail.com.
Takashi Murakami, Email: takmu@saitama-med.ac.jp.
Shigefumi Maesaki, Email: maesaki@saitama-med.ac.jp.
Yutaka Suzuki, Email: ysuzuki@k.u-tokyo.ac.jp.
Akihiko Kawana, Email: kawana59@ndmc.ac.jp.
Takuya Maeda, Phone: +81-49-276-1166, Email: t_maeda@saitama-med.ac.jp.
References
- 1.Mendelman PM, Chaffin DO, Stull TL, Rubens CE, Mack KD, Smith AL. Characterization of non-β-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984;26:235–244. doi: 10.1128/AAC.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007;20:368–389. doi: 10.1128/CMR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skaare D, Anthonisen IL, Caugant DA, Jenkins A, Steinbakk M, Strand L, et al. Multilocus sequence typing and ftsI sequencing: a powerful tool for surveillance of penicillin-binding protein 3-mediated beta-lactam resistance in nontypeable Haemophilus influenzae. BMC Microbiol. 2014;14:131. doi: 10.1186/1471-2180-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ubukata K, Shibasaki Y, Yamamoto K, Chiba N, Hasegawa K, Takeuchi Y, et al. Association of amino acid substitutions in penicillin-binding protein 3 with β-lactam resistance in β-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 2001;45:1693–1699. doi: 10.1128/AAC.45.6.1693-1699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lâm TT, Claus H, Elias J, Frosch M, Vogel U. Ampicillin resistance of invasive Haemophilus influenzae isolates in Germany 2009–2012. Int J Med Microbiol. 2015;305:748–755. doi: 10.1016/j.ijmm.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Dabernat H, Delmas C, Seguy M, Pelissier R, Faucon G, Bennamani S, et al. Diversity of β-lactam resistance-conferring amino acid substitutions in penicillin-binding protein 3 of Haemophilus influenzae. Antimicrob Agents Chemother. 2002;46:2208–2218. doi: 10.1128/AAC.46.7.2208-2218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osaki Y, Sanbongi Y, Ishikawa M, Kataoka H, Suzuki T, Maeda K, et al. Genetic approach to study the relationship between penicillin-binding protein 3 mutations and Haemophilus influenzae β-lactam resistance by using site-directed mutagenesis and gene recombinants. Antimicrob Agents Chemother. 2005;49:2834–2889. doi: 10.1128/AAC.49.7.2834-2839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia LS. Clinical microbiology procedures handbook. 3. Washington, D.C.: American Society for Microbiology; 2010. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing: twenty-fifth informational supplement M100-S25. Wayne: NCCLS; 2015. [Google Scholar]
- 10.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theodor MJ, Anderson RD, Wang X, Katz LS, Vuong JT, Bell ME, et al. Evaluation of new biomarker genes for differentiating Haemophilus influenzae from Haemophilus haemolyticus. J Clin Microbiol. 2012;50:1422–1424. doi: 10.1128/JCM.06702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nørskov-Lauritsen N, Overballe MD, Kilian M. Delineation of the species Haemophilus influenzae by phenotype, multilocus sequence phylogeny, and detection of marker genes. J Bacteriol. 2009;191:822–831. doi: 10.1128/JB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrea KW, Xie J, LaCross N, Patel M, Mukundan D, Murphy TF, et al. Relationships of nontypeable Haemophilus influenzae strains to hemolytic and nonhemolytic Haemophilus haemolyticus strains. J Clin Microbiol. 2008;46:406–416. doi: 10.1128/JCM.01832-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Binks MJ, Temple B, Kirkham LA, Wiertsema SP, Dunne EM, Richmond PC, et al. Molecular surveillance of true nontypeable Haemophilus influenzae: an evaluation of PCR screening assays. PLoS ONE. 2012;7(3):e34083. doi: 10.1371/journal.pone.0034083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maaroufi Y, Bruyne JMD, Heymans C, Crokaert F. Real-time PCR for determining capsular serotypes of Haemophilus influenzae. J Clin Microbiol. 2007;45:2305–2308. doi: 10.1128/JCM.00102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson James T, Thorvaldsdóttir Helga, Winckler Wendy, Guttman Mitchell, Lander Eric S, Getz Gad, Mesirov Jill P. Integrative Genomics Viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorvaldsdóttir Helga, Robinson James T, Mesirov Jill P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millard SP. EnvStats: an R package for environmental statistics. New York: Springer; 2013. [Google Scholar]
- 20.Hotomi M, Fujihara K, Billal DS, Suzuki K, Nishimura T, Baba S, et al. Genetic characteristics and clonal dissemination of β-lactamase-negative ampicillin-resistant Haemophilus influenzae strains isolated from the upper respiratory tract of patients in Japan. Antimicrob Agents Chemother. 2007;51:3969–3976. doi: 10.1128/AAC.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ubukata K, Chiba N, Hasegawa K, Shibasaki Y, Sunakawa K, Nonoyama M, et al. Differentiation of beta-lactamase-negative ampicillin-resistant Haemophilus influenzae from other H. influenzae strains by a disc method. J Infect Chemother. 2002;8:50–58. doi: 10.1007/s101560200006. [DOI] [PubMed] [Google Scholar]
- 22.Anderson R, Wang X, Briere EC, Katz LS, Cohn AC, Clark TA, et al. Haemophilus haemolyticus isolates causing clinical disease. J Clin Microbiol. 2012;50:2462–2465. doi: 10.1128/JCM.06575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price EP, Sarovich DS, Nosworthy E, Beissbarth J, Marsh RL, Pickering J, et al. Haemophilus influenzae: using comparative genomics to accurately identify a highly recombinogenic human pathogen. BMC Genom. 2015;16:641–650. doi: 10.1186/s12864-015-1857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Gier C, Kirkham LA, Nørskov-Lauritsen N. Complete deletion of the fucose operon in Haemophilus influenzae is associated with a cluster in multilocus sequence analysis-based phylogenetic group II related to Haemophilus haemolyticus: implications for identification and typing. J Clin Microbiol. 2015;53:3773–3778. doi: 10.1128/JCM.01969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witherden EA, Tristram SG. Prevalence and mechanisms of β-lactam resistance in Haemophilus haemolyticus. J Antimicrob Chemother. 2013;68:1049–1053. doi: 10.1093/jac/dks532. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez L, Pan W, Viñas M, Nikaido H. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J Bacteriol. 1997;179:6855–6857. doi: 10.1128/jb.179.21.6855-6857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean CR, Narayan S, Daigle DM, Dzink-Fox JL, Puyang X, Bracken KR, et al. Role of the acrAB-tolC efflux pump in determining susceptibility of Haemophilus influenzae to the novel peptide deformylase inhibitor LBM415. Antimicrob Agents Chemother. 2015;49:3129–3135. doi: 10.1128/AAC.49.8.3129-3135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trepod CM, Mott JE. Identification of the Haemophilus influenzae tolC gene by susceptibility profiles of insertionally inactivated efflux pump mutants. Antimicrob Agents Chemother. 2004;48:1416–1418. doi: 10.1128/AAC.48.4.1416-1418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaczmarek FS, Gootz TD, Dib-Haji F, Shang W, Hallowel S, Cronan M. Genetic and molecular characterization of β-lactamase negative ampicillin resistant Haemophilus influenzae with unusually high resistance to ampicillin. Antimicrob Agents Chemother. 2004;48:1630–1639. doi: 10.1128/AAC.48.5.1630-1639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seyama S, Wajima T, Nakaminami H, Noguchi N. Molecular mechanism of epidemic clarithromycin-resistant β-lactamase-non-producing ampicillin-resistant Haemophilus influenzae in Japan. Antimicrob Agents Chemother. 2016;60:3207–3210. doi: 10.1128/AAC.00163-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witherden EA, Bajance-Lavado MP, Tristram SG, Nunes A. Role of inter-species recombination of the ftsI gene in the dissemination of altered penicillin-binding-protein-3-mediated resistance in Haemophilus influenzae and Haemophilus haemolyticus. J Antimicrob Chemother. 2014;69:1501–1509. doi: 10.1093/jac/dku022. [DOI] [PubMed] [Google Scholar]
- 32.Doren GV, Brueggemann AB, Pierce G, Jolley HP, Jr, Rauch A. Antibiotic resistance among clinical isolates of Haemophilus influenzae in the United States in 1994 and 1995 and detection of β-lactamase-positive strains resistant to amoxicillin-clavulanate: results of a national multicenter surveillance study. Antimicrob Agents Chemother. 1997;41:292–297. doi: 10.1128/aac.41.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Reference sequences used in this study.
Additional file 2. Antibiotic minimum inhibitory concentrations (MICs) for β-lactamase non-producing H. influenzae examined in this study.
Additional file 3. AcrB mutations and their relationship with ABPC MICs for each genotype.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.


