Abstract
Background
Phospholipase Cɛ (PLCɛ), a member of the plc family, has been extensively studied to reveal its role in the regulation of different cell functions, but understanding of the underlying mechanisms remains limited. In the present study, we explored the effects of PLCɛ on PTEN (phosphatase and tensin homolog deleted on chromosome 10) in cell proliferation in prostate cancer cells.
Material/Methods
We assessed PLCɛ and PTEN expression in human benign prostate tissues compared to prostate cancer tissues by immunohistochemistry. Lentivirus-shPLCɛ (LV-shPLCɛ) was designed to silence PLCɛ expression in DU145 and PC3 cell lines, and the effectiveness was tested by qRT-PCR and Western blotting. MTT assay and colony formation assay were conducted to observe cell proliferation. Western blotting and immunofluorescence assays were used to detect changed PTEN expression in DU145.
Results
We observed that PLCɛ expression was reduced in human benign prostate tissues compared to prostate cancer tissues, while PTEN expression showed the opposite trend. Silencing of the PLCɛ gene significantly inhibited cell proliferation in DU145 and PC3 cell lines. DU145 is a PTEN-expressing cell, while PC3 is PTEN-deficient. After infection by LV-shPLCɛ, we noticed that PTEN expression was up-regulated in DU145 cells but not in PC3 cells. Furthermore, we found that PLCɛ gene knockdown decreased P-AKT protein levels, but AKT protein levels were not affected. Immunofluorescence assays showed that PTEN expression had an intracellular distribution change in the DU145 cell line, and Western blot analysis showed that PTEN was obviously up-regulated in cell nucleus and cytoplasm.
Conclusions
PLCɛ is an oncogene, and knockdown of expression of PLCɛ inhibits PCa cells proliferation via the PTEN/AKT signaling pathway.
MeSH Keywords: Cell Proliferation, Oncogene Protein v-akt, Phosphoinositide Phospholipase C, Prostatic Neoplasms, PTEN Phosphohydrolase
Background
Prostate cancer (PCa), with its high morbidity and mortality, has gradually become one of the most frequently diagnosed tumors in the urinary system among males in the malignant USA [1]. Statistical analysis shows that more than 250 000 people die of PCa worldwide, with at least 900 000 new cases each year [2]. Patients with PCa are mainly treated with surgery or drugs, but due to tumor recurrence or castration-resistance, it is necessary to develop a more effective therapeutic strategy or a prognostic marker. Hence, the present study explored the molecular mechanism leading to PCa development and progression.
Phospholipase Cɛ (PLCɛ), as a member of the human phospholipase C family, which was discovered by Song et al. in 2001. It is located on chromosome 10q23, and has been verified to play an important role in cell growth, differentiation, proliferation, and apoptosis [3,4]. As with other members of PLC families, PLCɛ can catalyze the hydrolysis of phosphatidylinositol 4,5-bisphosphate(PIP2) and generates 2 important downstream materials: diacylglycerol (DAG) and inositol1,4,5-trisphosphate (IP3). DAG acts through protein kinase C(PKC), influencing protein kinase D (PKD), and IP3 stimulates the Ca2+ signaling pathway, leading to various cellular events [5]. Furthermore, PLCɛ is involved in a great diversity of signaling pathways, such as the Ras signaling pathway and Rho signaling pathway, due to its Ras-associating domains at N-terminus and CDC25-like domain at the C-terminus [6]. Recently, many studies have shown that PLCɛ plays a pivotal role in development of various types of human cancers. Kataoka’s group explored the role of PLCɛ in Ras-triggered skin cancer using a transgenic mouse model, and found that PLCɛ acts as an oncogene [7]. Li et al. selected OE33 and CP-C ESCC cell lines, both of them highly expressed in PLCɛ, and found that knockdown of PLCɛ expression can modulate p53 expression via its promoter methylation [8]. In 2010, Ou et al. silenced PLCɛ expression in T24 bladder cancer cells and showed that the invasive cell potential was significantly decreased when MMP2, MMP9, and BCL2 gene expression was down-regulated [9]. Similarly, Ling et al. demonstrated that knockdown of PLCɛ expression inhibited cell proliferation in BIU-87 cells, and they noticed that BIU-87 cells accumulated in the G0/G1 phase of the cell cycle [10]. Taken together, these findings suggest that PLCɛ is involved in various signaling pathways and thus influences the tumor microenvironment and associated entities.
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) was initially identified as a tumor suppressor candidate in 1997 [11], and many papers about PTEN have been published, gradually confirming its tumor-suppressor function. PTEN exhibits phosphatase activity, which hydrolyzes phosphatidylinositol 3,4,5-trisphosphate (PIP3) to PIP2 [12], thus antagonizing the PI3K/AKT signaling pathway. Phosphatidylinositol 3-kinase (PI3K) catalyzes the conversion of PIP2 to PIP3, which mediates downstream signaling molecule activation such as serine/threonine kinases AKT. Accumulation of P-AKT promotes cell survival, proliferation, growth, angiogenesis, and cellular metabolism [13]. Many publications have reviewed PTEN gene mutation in various types of tumors, including prostate cancer. It has been confirmed that the contribution of PTEN loss is associated with activation of the PI3K/AKT signaling pathway [13]. Genetic mutation or loss of PTEN is a frequent event that promotes tumorigenesis, but upstream regulatory mechanisms include transcriptional modulation, post-translational modulation, decreased protein levels, and function destruction [14–16]. Considerable progress has been made during the last 2 decades in defining the role of PTEN in tumor suppression, but its specific mechanism is still unclear.
In the present study, we investigated the expression of PLCɛ and PTEN in 40 prostate cancer tissues and 15 benign prostatic hyperplasia (BPH) samples by immunohistochemistry (IHC). We found that PLCɛ expression was up-regulated in prostate cancer tissues and PTEN expression levels were down-regulated, but for benign prostate tissues, it showed the opposite result. By using lentivirus-mediated shPLCɛ knockdown of PLCɛ expression in DU145 and PC3 prostate cancer cells, our research showed that PLCɛ knockdown up-regulates PTEN expression and influences cells proliferation via the PTEN/AKT signaling pathway in the DU145 cell line. Importantly, the immunofluorescence assay revealed that PTEN expression is obviously up-regulated in cell nucleus and cytoplasm after knockdown of PLCɛ expression. Taken together, our results suggest that PLCɛ acts as an oncogene and promotes cells proliferation, with potential to become a new therapy target for clinical use.
Material and Methods
Tissue specimens
A total of 15 benign prostatic hyperplasia (BPH) tissue samples and 40 prostate cancer (PCa) tissue samples were obtained from patients who underwent biopsy or radical prostatectomy at the Department of Urology in the First Affiliated Hospital of Chongqing Medical University between July 2015 and June 2017. There was little difference between groups in socioeconomic status. We obtained patient informed consent. None of them had received androgen deprivation therapy or neoadjuvant chemotherapy, thus avoiding non-correlation interference. All carcinoma samples and BPH samples were confirmed to be carcinoma or BPH before the experiment, then they were stored at −80°C until required. This study was approved by the Ethics and Research Committees of our institution.
Immunohistochemistry
All the formalin-fixed and paraffin-embedded tissue samples were cut into 5-um–thick sections. A standard immunoperoxidase staining procedure was used to perform immunohistochemical staining (PLCɛ,1: 50; PTEN,1: 100, Santa Cruz, USA). The expression status of immunostaining was reviewed and scored by 2 pathologists based on the proportion of positive cells and staining intensity. Scoring for percentage was as follows: 0, 0%; 1, <5%; 2, 5–50%; and 3, >50%. Staining intensity was scored as 0 (negative), 1 (weak), 2 (intermediate), or 3 (strong). The product of percentage score and intensity score comprising negative (0), weak (1–2), moderate (3), and strong (4–6) was the final criterion for the evaluation. For statistical purposes, samples were categorized as either positive (SI≥3) or negative (SI<3).
Cell culture and transfection
The human prostate cancer cell line PC3 was obtained from the American Type Culture Collection (ATCC), and the DU145 cell line was purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells was cultured in DMEM/F-12 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 100 units/mL penicillin, and 100 ug/mL streptomycin (Gibco). Lentivirus-shRNA targeting human PLCɛ (5′-GGTTCTCTCCTAGAAGCAACC-3′) and the negative control (5′-TTCTCCGAACGTGTCACGT-3′) were purchased from Shanghai GenePharma Corporation (Shanghai, China). For transfection, 3×105 cells were cultured into each well of a 6-well plate overnight before transfection. When cells were at 60~70% confluence, we replaced the medium and infected it with 15 uL LV-shPLCɛ or LV-HK stock solution with 2 ul Polybrene. Application of the antibiotic puromycin (1 ug/mL) selected stable cells.
Reverse transcription and quantitative RT-PCR (qRT-PCR)
Total RNA was extracted from DU145 and PC3 cell lines by TRIzol (Takara, Japan), the Prime Script RT reagent kit (Takara, Tokyo, Japan) was used to generate cDNA, and all the experiments were performed according to the manufacturer’s Instructions. The primer sequences of PLCɛ, PTEN, and β-actin were: PLCɛ forward primer, 5′-GCAACTACAACGCTGTCATGGAG-3′ and reverse primer, 5′-GCAACTACAACGCTGTCATGGAG-3′; PTEN forward primer, 5′-TTTGAAGACCATAACCCACCA-C-3′ and reverse primer, 5′-ATTACACCAGTTCGTCCCTTTC-3′; β-actin forward primer, 5′-GGGACCTGACTGACTACCTC-3′ and reverse primer, 5′-ACGAGACCACCTTCAACTCCAC-3′. qRT-PCR was performed on an CFX Connect q-PCR system (BIO-RAD, USA) with the SYBR Premix Ex Taq II kit (Takara, Japan). Expression was calculated using the 2−ΔΔCT method and calibrated by β-actin expression.
Protein extraction and Western blotting assay
The total protein was extracted from cells by using RIPA reagent containing protease inhibitor PMSF and phosphatase inhibitors NaF and Na3VO4. The cytoplasm and nuclear protein were separated by use of Beyotime cytoplasmic nuclear extraction reagents (Jiangsu, China). The CBA protein assay kit (Beyotime Institute of Biotechnology) was used to detect the intensity levels. The proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes was immersed in a blocking solution TBS-T (Tris-buffered saline with Tween-20) containing 5% non-fat milk for 2 h and then incubated with the primary antibodies at 4°C overnight. The antibodies used were: PTEN and PLCɛ (1: 300 Santa Cruz, CA), AKT (1: 500 WanleiBio, China), P-AKT (S473) (1: 500 Abcam, MA, USA), GAPDH (1: 2000 ImmunoWay, USA), Histone H3 (1: 500 WanleiBio, China). After incubation with horseradish peroxidase-conjugated secondary antibody for 1 h at 37°C, the bands were exposed and developed using an enhanced chemiluminescence kit (Beyotime). All the experiments were performed 3 times.
MTT assay
Initially, 2×103 cells were seeded into 96-well plates with 100 ul growth medium at each well. After cell attachment, MTT (5 mg/mL, Sigma) was added and incubated for 4 at 37°C, followed by removal of the culture medium and addition of 150 ml dimethyl sulphoxide (DMSO) (Sigma). Absorbance was measured on a microplate reader at a 490-nm wavelength every day for 4 days. Triplicates independent experiments were performed.
Colony formation assay
DU145 and PC3 (Blank group, LV-shHK group, and LV-shPLCɛ group) were plated in each 6-well plate (400 cells/well), and 14 days later the cells were washed twice with 1 mL of PBS, fixed with 4% paraformaldehyde for 15 min, and stained with 0.1% crystal violet for 20 min. The number of colonies was independently counted. The experiment was performed 3 times for each cell line.
Immunofluorescence
DU145 cell lines were independently seed on sterile glass coverslips for 48 h, washed with PBS 3 times and fixed with 4% paraformaldehyde for 20 min, then cells were blocked in 5% normal goat serum for 1 h at 37°C. The primary antibody anti-PTEN (1: 100, Santa Cruz, CA) was used to incubate cells overnight at 4°C. After incubating with secondary antibody (goat anti-rabbit) (Zhongshan Goldenbridge Biotechnology, China), nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired by photomicrography (Nikon, Tokyo, Japan) at 400× magnification. All experiments were performed 3 times.
Statistical analysis
GraphPad Prism version 7.0 and SPSS 20.0 software were used to process data. Significant associations among categorical variables were analyzed by one-way ANOVA, chi-square test, Cohen’s kappa, and Student’s t test. Measurement data are expressed as mean ± standard deviation (SD). Statistical significance was set at a value of p<0.05, and extreme statistical significance was set at a value of p<0.01.
Results
Increased PLCɛ expression is associated with decreased PTEN expression in prostate cancer tissues
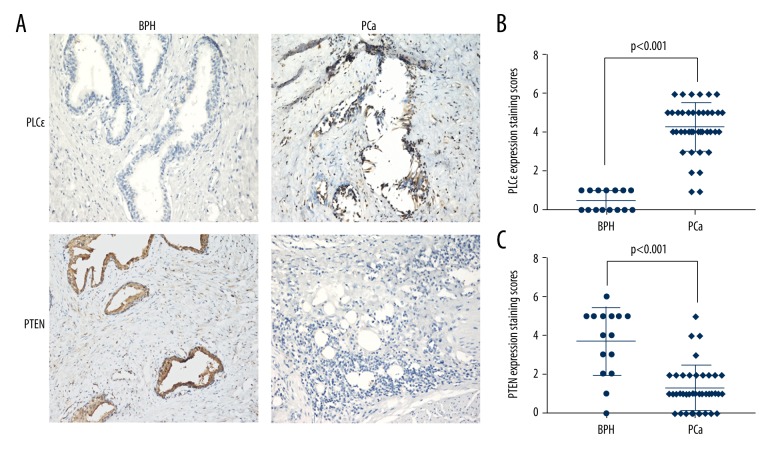
Many studies have demonstrated that PLCɛ plays an important role in tumor growth, differentiation, proliferation, and apoptosis. We collected 40 samples of human prostate cancer tissues and 15 cases of BPH tissues and analyzed them using IHC. The results showed a higher expression of PLCɛ in approximately 90% of the PCa tissue samples compared to BPH tissues. PTEN was identified as a tumor suppressor in prostate cancer and we also observed that the expression of PTEN was strongly up-regulated in approximately 73.3% of BPH tissues, but PTEN showed a low or undetectable level in PCa tissue samples (Figure 1A–1C, P<0.05). Furthermore, we respectively analyzed the relationship between the various clinical parameters and the expression of PLCɛ or PTEN in the PCa tissues. As shown in Table 1, we noticed that high PLCɛ expression was associated with histological stage (P=0.027), but for age or Gleason grade, there was no difference (P>0.05). We found that the expression level of PTEN was not associated with histological stage, age, or Gleason grade (P>0.05) (Table 2). In addition, the correlation between increased PLCɛ and decreased PTEN in PCa tissue was analyzed using Cohen’s kappa, and the results indicated a strong level of agreement between these 2 alterations (Table 3, k=0.444, p=0.0049).
Figure 1.
Up-regulated PLCɛ expression was associated with down-regulated of PTEN expression in human PCa tissues. (A) immunohistochemical stainings in 40 human prostate cancer tissue samples and 15 BPH tissue samples. Magnification 200×. (B) PLCɛ expression staining scores in BPH and PCa tissues. (C) PTEN expression staining scores in BPH and PCa tissues.
Table 1.
Relationship between PLCɛ expression and the clinicopathological parameters in prostate cancer patients.
| Numbers | Number of patients (%) | P-value | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Total number | 40 | 36 (90.00) | 4 (10.00) | |
| Age (year) | ||||
| <60 | 13 (32.50) | 10 (27.77) | 3 (75.00) | 0.056 |
| ≥60 | 27 (67.50) | 26 (72.23) | 1 (25.00) | |
| Histological stage | ||||
| Ta–T1 | 19 (47.50) | 15 (41.66) | 4 (100) | 0.027* |
| T2–T4 | 21 (52.50) | 21 (58.33) | 0 (0) | |
| Gleason grade | ||||
| <7 | 11 (27.50) | 10 (41.66) | 1 (25.00) | 0.906 |
| ≥7 | 29 (72.50) | 26 (58.33) | 3 (75.00) | |
Statistically significant.
Table 2.
Relationship between PTEN expression and the clinicopathological parameters in prostate cancer patients.
| Numbers | Number of patients (%) | P-value | ||
|---|---|---|---|---|
| Positive | Negative | |||
| Total number | 40 | 4 (10.00) | 36 (90.00) | |
| Age (year) | ||||
| <60 | 13 (32.50) | 2 (50.00) | 11 (30.55) | 0.431 |
| ≥60 | 27 (67.50) | 2 (50.00) | 25 (69.44) | |
| Histological stage | ||||
| Ta–T1 | 19 (47.50) | 3 (75.00) | 16 (44.44) | 0.246 |
| T2–T4 | 21 (52.50) | 1 (25.00) | 20 (55.56) | |
| Gleason grade | ||||
| <7 | 11 (27.50) | 3 (75.00) | 10 (16.67) | 0.056 |
| ≥7 | 29 (72.50) | 1 (25.00) | 26 (72.22) | |
Table 3.
Correlation between PLCɛ, and PTEN in prostate cancer patients.
| No. specimens (%) | PTEN | Kappa | P | |
|---|---|---|---|---|
| PLCɛ | Negative | Positive | ||
| Positive | 34 | 2 | 0.444 | 0.0049* |
| Negative | 2 | 2 | ||
Statistically significant.
Lentivirus-shPLCɛ inhibited expression of PLCɛ in DU145 and PC3 PCa cells
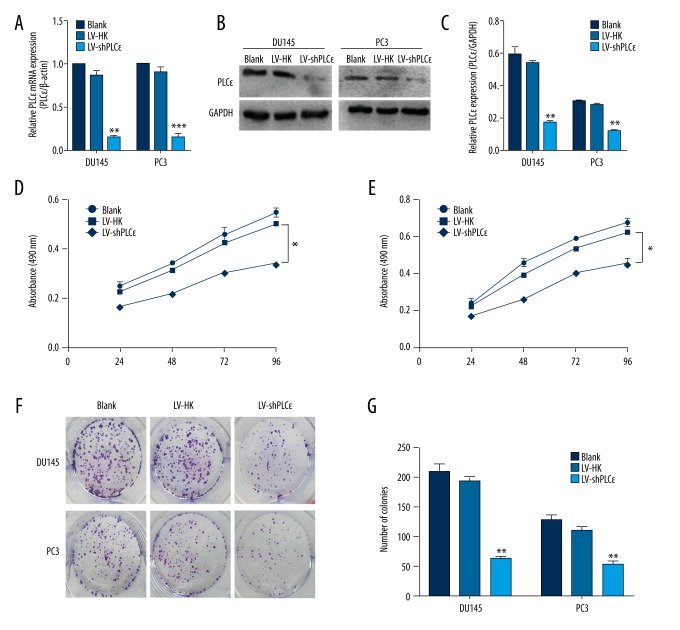
To investigate whether PLCɛ is involved in regulation of PCa cell lines (DU145 and PC3), we examined PLCɛ expression by qRT-PCR and Western blot analysis and observed a high level in both DU145 and PC3 cells. Then, we lentivirus-shPLCɛ to interfere with PLCɛ expression and developed stable interfering cell lines. We also established a blank and negative control of both cell lines. Similarly, we used Western blot and qRT-PCR to examine the expression of PLCɛ. Our data show that LV-shPLCɛ significantly down-regulated PLCɛ expression (Figure 2A–2C).
Figure 2.
PLCɛ down-regulation suppresses PCa cells proliferation in DU145 and PC3 PCa cell lines. (A). Relative mRNA expression level of PLCɛ was examined by qRT-PCR, and β-actin served as loading control. The results are represented as the mean ±SD. * P<0.05 vs. LV-HK; ** P<0.01 vs. LV-HK; *** P<0.001 vs. LV-HK. (B, C) Relative PLCɛ protein expression was determined by Western blot analysis, and GAPDH served as loading control. The results are represented as the mean ±SD.** P<0.01 vs. LV-HK. (D, E) MTT assays revealed that down-regulation of PLCɛ reduced cell growth of DU145 and PC3 cell lines. (F, G) Colony forming assay was used to determine the colony forming efficiency of DU145 and PC3. The results are represented as the mean ±SD.* P<0.05 vs. LV-HK; ** P<0.01 s.LV-HK.
PLCɛ down-regulation suppresses PCa cells proliferation
Uncontrolled proliferation is a characteristic of tumor cells. To investigate the biological function of PLCɛ in the DU145 and PC3 PCa cell lines, we conducted MTT and colony formation analysis to reveal the growth rate and proliferation rate. MTT showed that LV-shPLCɛ markedly reduced the proliferation ability of transfected cells. However, for the blank group and LV-HK group, there was no obvious difference. The process was time-dependent manner and we observed a significant difference at 4 days after plating (Figure 2D, 2E, P<0.01). Colony formation assay demonstrated that the proliferative capacities of DU145 and PC3 cells were significantly decreased by LV-shPLCɛ (Figure 2F, 2G, P<0.01). Taken together, our data confirm the regulatory role of PLCɛ on cell proliferation and suggest that knockdown of PLCɛ expression can inhibit tumor growth and proliferation.
PLCɛ knockdown up-regulates PTEN expression in PCa cell lines
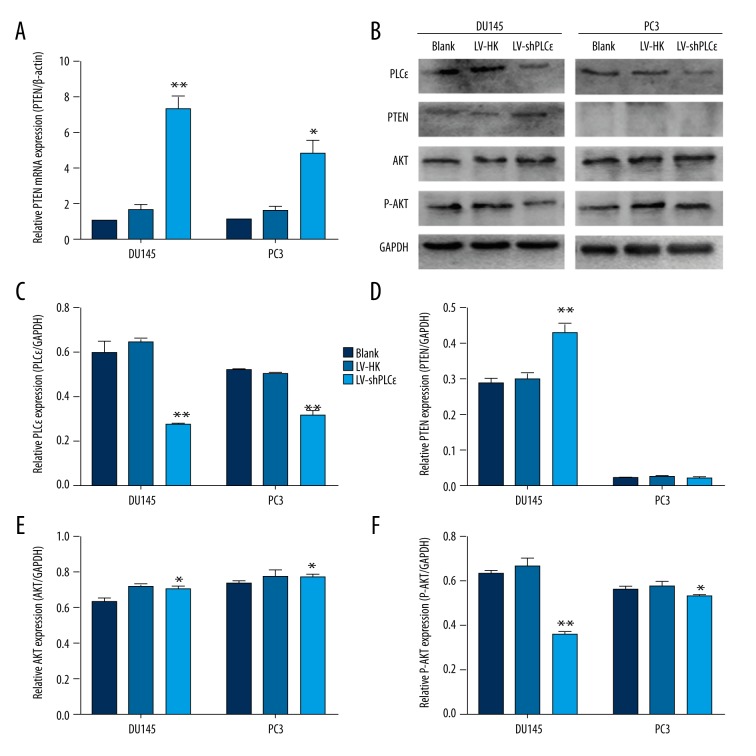
PTEN has been identified to be involved in cell growth and proliferation. Since found the regulatory role of PLCɛ in promoting cell growth and proliferation, we surmised that PLCɛ may influence PTEN expression in PCa. Thus, we used qRT-PCR and Western blot analysis to determine whether PTEN is modulated by PLCɛ. The experimental results showed that PTEN expression is up-regulated in the LV-shPLCɛ DU145 cell line after being simultaneously tested by qRT-PCR and Western blot analysis (Figure 3A, 3B, 3D, P<0.01). The DU145 cell line has been reported to express PTEN, but in PTEN-deficient PC3 cells, we observed that PTEN expression was up-regulated only in the qRT-PCR results, and there was no obvious difference at protein levels (Figure 3A, 3B, 3D, P<0.01). These findings suggest that PLCɛ plays a role in controlling PTEN transcription and translation in prostate cancer cell lines.
Figure 3.
Down-regulation of PLCɛ increased PTEN expression at mRNA and protein levels, and the PTEN/AKT signaling pathway is involved in the oncogenic effect of PLCɛ. (A) qRT-PCR was performed to show that down-regulation of PLCɛ increased PTEN expression at the mRNA level. (B–F) Western blot analysis showed that down-regulation of PLCɛ increased PTEN expression at protein levels only in DU145 cells and had no obvious effect on PC3 cells. P-AKT expression was significantly reduced in DU145 cells and slightly reduced in PC3 cells, showing the oncogenic effect of PLCɛ via the PTEN/AKT signaling pathway. The results are represented as the mean ±SD. * P<0.05 vs. LV-HK; ** P<0.01 vs. LV-HK.
The effect of PLCɛ in tumor proliferation via PTEN/AKT signaling pathway
Published studies suggest that the PTEN/AKT signaling pathway is involved in many types of biological functions, including growth and proliferation. Taken together, our results show a correlation between PLCɛ and PTEN, and suggest that PLCɛ promotes tumor growth and proliferation via activating the AKT pathway. Our Western blot results showed no clear difference in total AKT expression between the LV-shPLCɛ group and the LV-HK and blank groups of both cell lines. Surprisingly, after we silenced PLCɛ expression by LV-shPLCɛ, the phosphorylation level of AKT was significantly reduced at protein levels (Figure 3B–3F, P<0.01) in DU145 cells but was slightly reduced in PC3 cells. Results in the literature confirm that transactivity of p-AKT is often abrogated due to up-regulated PTEN expression in prostate cancer. p-AKT down-regulation indicates that PLCɛ function activates the AKT pathway via inhibiting PTEN expression in DU145 cells, but for PTEN-deficient PC3 cells, the slight change suggests that PLCɛ regulates the AKT signaling pathway, mainly though inhibiting PTEN and partly though other ways.
PLCɛ silencing changed PTEN intracellular distribution
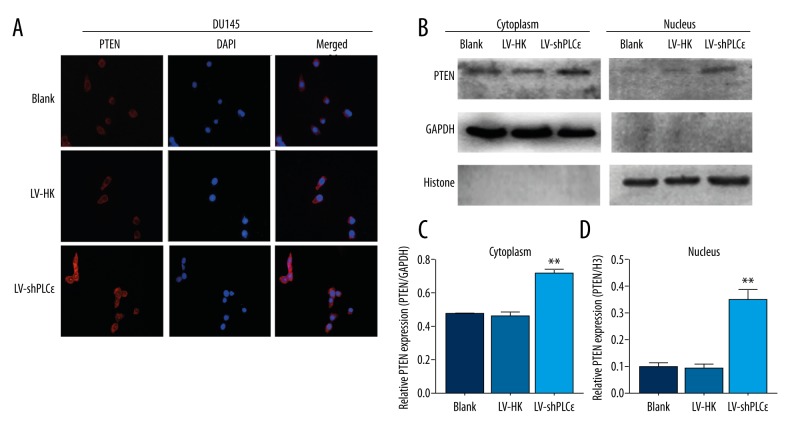
PLCɛ-mediated regulation of the PTEN/AKT signaling pathway needs to be elucidated. We conducted an immunofluorescence assay to determine the role of PLCɛ and PTEN. We selected the PTEN-expression PCa cell line DU145. After infected interference and setting-up negative controls (blank group and LV-HK group), we detected that PTEN expression was up-regulated in cytoplasm of the LV-shPLCɛ group, but in control groups there was no significant difference (Figure 4A). More interesting, we found that PTEN expression showed a higher level in cell nuclei in the LV-shPLCɛ group. Specifically, PTEN was predominantly localized to the cytoplasm, but in our LV-shPLCɛDU145 cells, PTEN appeared to be a nuclear import, and this the phenomenon was also confirmed by Western blot analysis (Figure 4B–4D, P<0.01). The absence of nuclear PTEN is associated with many types of cancer. PTEN acts as a tumor suppressor, at least in part due to its nuclear function [17]. Thus, PLCɛ may play a critical role in prostate cancer through affecting PTEN intracellular distribution.
Figure 4.
Down-regulation of PLCɛ induced PTEN intracellular distribution changes in DU145 cells. (A) Immunofluorescence staining demonstrated PTEN intracellular distribution changes, and PTEN expression showed a nuclear import. Magnification: 400×. (B–D) Western blot analysis showed that PTEN expression was significantly up-regulated in the nucleus and cytoplasm. The results are represented as the mean ±SD. * P<0.05 vs. LV-HK; ** P<0.01 vs. LV-HK.
Discussion
PLCɛ, which differs from other members of the PLC family, has been reported to be involved in various carcinomas since it was found in 2001. Our group has demonstrated that PLCɛ is involved in different types of biological functions and acts as a modulator. In 2015, DU et al. showed that PLCɛ is involved in PKCα/β/TBX3/E-cadherin signaling and regulates bladder cancer cell migration and invasion [18]. Yang et al. found that PLCɛ expression was linked to STAT3 signaling in transitional cell carcinoma of the bladder (TCCB) [19]. In 2014, Wang et al. reported that PLCɛ knockdown suppresses the Notch signaling pathway and inhibits AR nuclear translocation in prostate cancer cells [20]. In summary, PLCɛ has previously been proven to regulate cell growth, proliferation, invasion, and migration in urinary tract cancers, but the specific mechanisms underlying the PCa cell growth and proliferation have not been clearly elucidated.
PTEN, a tumor repressor, has been demonstrated to undergo expression deletion in various types of tumors, including prostate cancer. It has been shown that the classical tumor suppressor is subject to several regulatory mechanisms, including phosphorylation, acetylation, oxidation, and ubiquitination. These modifications influence the activity of PTEN, thus leading to tumorigenesis. PTEN modulates the PI3K/AKT signaling pathway, thus inhibiting tumor survival, growth, and proliferation [13]. Loss-of-function of PTEN, the key protein in the PI3K/AKT pathway, contributes to tumor development. The phosphorylation level of AKT is a prognosis indicator. Campbell et al. and Redfern et al. demonstrated that PTEN dephosphorylates PIP3 to PIP2, and a certain concentration of PIP2 helps maintain the function of PTEN [21,22]. PLCɛ can catalyze the hydrolysis of PIP2 and generates 2 downstream materials (DAG and IP3), thus reducing the concentration of PIP2. PLCɛ can cause PTEN expression decrease or function loss. We hypothesize that PLCɛ-mediated tumorigenesis is associated with the PTEN/AKT signaling pathway.
In the present study, we utilized immunohistochemistry assay and observed that PLCɛ expression is significantly elevated in most human prostate cancer tissue specimens, while PTEN expression was low or was lost. However, PTEN was expressed at a higher level compared to PLCɛ in benign prostatic hyperplasia tissues. These results demonstrate that PLCɛ is involved in tumorigenesis of PCa and there is a potential connection between PLCɛ and PTEN. PLCɛ, as an oncogene, has been confirmed to have a proliferation-promoting role in renal carcinoma and bladder carcinoma [9,10]. PTEN loses its tumor suppressive function and leads to uncontrolled proliferation in many types of cancer [23]. We found that PLCɛ silencing inhibited the growth and proliferation in DU145 and PC3 cell lines. We therefore hypothesize that PLCɛ may be involved in PTEN-mediated tumor proliferation.
PLCɛ, because of its particular Ras-interactive domains (CDC25 and Ras-associating domains) differ from other members of the PLC family [24]; it does not just act like a phospholipase, and the function is amplified in tumorigenesis. In our previous study, we observed that PLCɛ mediates types of downstream effectors such as E-cadherin, NF-κB, and Notch1, and we confirmed it is an oncogene involved in urinary tract cancers. Here, we observed PTEN expression is up-regulated in the DU145 PCa cell line at protein and mRNA levels after knockdown of PLCɛ expression. Furthermore, the phosphorylation level of AKT is significantly reduced, while the total protein level of AKT appears to be similar to that found in the DU145 PCa cell line. The results show that PLCɛ may be a regulator through inhibiting PTEN expression to activate the AKT signaling pathway. Western blot analysis demonstrated that PLCɛ silencing has no effect on PTEN PC3 cells, and P-AKT was only slightly reduced. Because PC3 is a PTEN-deficient prostate cancer cell line, we hypothesize that PLCɛ activates the AKT signaling pathway by inhibiting PTEN.
Similarly, immunofluorescence results show down-regulated PLCɛ promotes PTEN translocation from cytoplasm to nucleus, and plasma and nuclear protein was extracted and confirmed by Western blot analysis. Specifically, PTEN protein is predominantly localized in the cytoplasm and the absence of nuclear PTEN is associated with some aggressive diseases, including various types of cancers [25,26]. The literature shows that PTEN tumor suppressive activity is not restricted to its cytoplasmic function, and nuclear import is suggested to be another approach to suppressing tumors. In 2006 Chung et al. reported that PTEN regulates the cell cycle by limiting cyclin-D1 nuclear accumulation [27], and Gil et al. observed it may promote tumor cell apoptosis by its nuclear accumulation [28]. Unfortunately, the mechanisms for nuclear import of PTEN are unclear, and there may be some upstream regulatory factors. In the present study, we showed that PLCɛ silencing leads to PTEN protein accumulation in nuclei and inhibits PCa cell proliferation. We assume that PLCɛ is not the only factor activating the AKT signaling pathway in the cytoplasm; it also inhibits PTEN nuclear import, but the mechanisms should be further explored.
Conclusions
Our study has shown that PLCɛ expression knockdown can up-regulate PTEN expression in PCa cell lines and suppresses tumor cell proliferation via the PTEN/AKT signaling pathway. These findings indicate that PLCɛ acts as an oncogene in prostate cancer and provides a diagnostic marker for PCa therapy.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the National Natural Science Foundation of China (Grant No. 81072086), and the Scientific and Technological Research Program of Chongqing Municipal Education Committee (Grant No. KJ110305)
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. In J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Dusaban SS, Purcell NH, Rockenstein E, et al. Phospholipase C epsilon links G protein-coupled receptor activation to inflammatory astrocytic responses. Proc Natl Acad Sci USA. 2013;110:3609–14. doi: 10.1073/pnas.1217355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song C, Hu CD, Masago M, et al. Regulation of a novel human phospholipase C, PLCɛ, through membrane targeting by Ras. J Biol Chem. 2001;276:2752–57. doi: 10.1074/jbc.M008324200. [DOI] [PubMed] [Google Scholar]
- 5.Yang YR, Follo MY, Cocco L, Suh PG, et al. The physiological roles of primary phospholipase C. Adv Biol Regul. 2013;53(3):232–41. doi: 10.1016/j.jbior.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Lopez I, Mak EC, et al. A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J Biol Chem. 2001;276(4):2758–65. doi: 10.1074/jbc.M008119200. [DOI] [PubMed] [Google Scholar]
- 7.Bai Y, Edamatsu H, Maeda S, et al. Crucial role of phospholipase C epsilon in chemical carcinogen-induced skin tumor development. Cancer Res. 2004;64(24):8808–10. doi: 10.1158/0008-5472.CAN-04-3143. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, An J, Huang S, et al. PLCE1 suppresses p53 expression in esophageal cancer cells. Cancer Invest. 2014;32(6):236–40. doi: 10.3109/07357907.2014.905588. [DOI] [PubMed] [Google Scholar]
- 9.Ou L, Guo Y, Luo C, et al. RNA interference suppressing PLCE1 gene expression decreases invasive power of human bladder cancer T24 cell line. Cancer Genet Cytogenet. 2010;200(2):110–19. doi: 10.1016/j.cancergencyto.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Ou L, Tang M, et al. Knockdown of PLC epsilon inhibits inflammatory cytokine release via STAT3 phosphorylation in human bladder cancer cells. Tumour Biol. 2015;36(12):9723–32. doi: 10.1007/s13277-015-3712-8. [DOI] [PubMed] [Google Scholar]
- 11.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 12.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Ann Rev Biochem. 2001;70(1):247–79. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 13.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–14. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Patel L, Pass I, Coxon P, et al. Tumor suppressor and anti-inflammatory actions of PPAR gamma agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11:764–68. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 15.Meng F, Henson R, Lang M, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–29. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 16.Kwon J, Lee SR, Yang KS, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101:16419–24. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133(3):403–14. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Du HF, Ou LP, Yang X, et al. A new PKCα/β/TBX3/E-cadherin pathway is involved in PLCɛ-regulated invasion and migration in human bladder cancer cells. Cell Signal. 2014;26(3):580–93. doi: 10.1016/j.cellsig.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Ou L, Tang M, et al. Knockdown of PLCɛ inhibits inflammatory cytokine release via STAT3 phosphorylation in human bladder cancer cells. Tumor Biol. 2015;36:9723–32. doi: 10.1007/s13277-015-3712-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Wu X, Ou L, et al. PLCɛ knockdown inhibits prostate cancer cell proliferation via suppression of Notch signalling and nuclear translocation of the androgen receptor. Cancer Lett. 2015;362(1):61–69. doi: 10.1016/j.canlet.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Campbell RB, Liu FH, Ross AH. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278:33617–20. doi: 10.1074/jbc.C300296200. [DOI] [PubMed] [Google Scholar]
- 22.Redfern RE, Redfern D, Furgason ML, et al. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry. 2008;47:2162–71. doi: 10.1021/bi702114w. [DOI] [PubMed] [Google Scholar]
- 23.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 24.Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C(epsilon): A novel Ras effector. EMBO J. 2001;20:743–54. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tachibana M, Shibakita M, Ohno S, et al. Expression and prognostic significance of PTEN product protein in patients with esophageal squamous cell carcinoma. Cancer. 2002;94:1955–60. doi: 10.1002/cncr.0678. [DOI] [PubMed] [Google Scholar]
- 26.Perren A, Weng L-P, Boag AH, et al. Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol. 1999;155:1253–60. doi: 10.1016/S0002-9440(10)65227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung JH, Ostrowski MC, Romigh T, et al. The ERK1/2 pathway modulates nuclear PTEN-mediated cell cycle arrest by cyclin D1 transcriptional regulation. Hum Mol Genet. 2006;15:2553–59. doi: 10.1093/hmg/ddl177. [DOI] [PubMed] [Google Scholar]
- 28.Gil A, Andrés-Pons A, Fernández E, et al. Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: Involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol Biol Cell. 67(9):4002–13. doi: 10.1091/mbc.E06-05-0380. 200. [DOI] [PMC free article] [PubMed] [Google Scholar]