Abstract
Purpose
Recent evidence has shown the involvement of inflammation in the development of diabetic peripheral neuropathy (DPN). MicroRNA-146a (miR-146a) is closely involved in the inflammatory response. However, the role of miR-146a in the inflammatory reaction in DPN has not been clarified. This study was designed to explore the role of miR-146a in the regulation of inflammatory responses in DPN.
Methods
Rats were randomly divided into three groups (n=6 per group): control group, type 2 diabetes mellitus (T2DM) group and DPN group. T2DM and DPN rats were intraperitoneally injected with streptozotocin. Sciatic nerve conduction velocity (NCV) was determined at the 6th week and the 12th week in each group. The expression of microRNAs was detected by quantitative real-time polymerase chain reaction in three sciatic nerves for each group of rats. Expression of inflammatory cytokines in nerve tissues and plasma was measured by Western blot and Bio-Plex Pro™ assays.
Results
The NCV and expression levels of miR-146a in the DPN group were significantly decreased (P<0.01) compared to the other two groups. Expression of tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in the DPN group was significantly increased compared with the control and T2DM groups (P<0.01). Pearson’s correlation analysis showed that the expression level of miR-146a was negatively correlated with the levels of IL-1β, TNF-α and NF-κB.
Conclusion
miR-146a is involved in the pathogenesis of DPN, and its expression level is closely related to the inflammatory responses that aggravate sciatic nerve injuries.
Keywords: type 2 diabetes mellitus, diabetic peripheral neuropathy, microRNA-146a, NF-κB, cytokines
Introduction
Diabetes mellitus (DM) is a group of metabolic disorders characterized by hyperglycemia and dysinsulinemia, which are responsible for the highest prevalence of chronic metabolic disease in the world. The main type of DM is T2DM, which commonly causes a diabetic peripheral neuropathy (DPN) complication. Approximately 30%–50% of T2DM patients are associated with peripheral neuropathy.1 The pathological mechanism of DPN is widely accepted as multifactorial and can include metabolic toxicity, vascular injury, neurotrophic factor deficiency, oxidative stress, autoimmune disorders and genetic susceptibility, along with other factors.2,3 Control of blood glucose and blood pressure, supplementation with neurotrophic factors, improvement of microcirculation and antioxidant therapies can all be applied in clinical practice; however, most patients still inevitably suffer from peripheral nerve damage. This suggests that DPN may have a different pathogenesis. Recent studies have illustrated that the inflammatory response plays a pivotal role in the development and progression of DPN.4–6 The pathological changes resulting from DPN are axonal degeneration in unmyelinated nerve fibers and segmental or diffuse crenation and demyelination in myelinated nerve fibers. Additionally, the inflammatory response caused by the exposure of the antigenic components of nerve tissues is the leading cause of demyelination. While in a state of long-term hyperglycemia, T cells produce various cytokines which can have toxic effects on neurons and glial cells, leading to demyelination changes. Molecular studies have revealed that abnormal expression of inflammatory cytokines in the microvasculature around peripheral nerves is found in DPN patients and relevant animal models.7,8 The precise process by which inflammatory damage to peripheral nerves occurs requires further clarification.
MicroRNAs (miRNAs) are endogenous noncoding, single-stranded RNAs ~22 nucleotides in length. A considerable number of miRNAs have been identified, most of which have the characteristics of regulating posttranscriptional gene expression.9 There has been speculation that miRNAs regulate one-third of human genes.10 miRNAs play a pivotal role in numerous biological processes such as cell proliferation, apoptosis, differentiation and metabolism, by mainly pairing with target-specific bases and causing target mRNA degradation or inhibiting its translation process. Participation of microRNA-146a (miR-146a) in the regulation of acute and chronic inflammatory responses has been extensively researched.11,12 Previous studies have elucidated that miR-146a plays an anti-inflammatory role in various immune cell types by repressing expression of the target gene nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).13,14 Intense induction of inflammatory biomarkers was observed in miR-146a-deficient mice. It was surmised that miR-146a significantly regulates the inflammatory response. However, the function of miR-146a in DPN requires further investigation. This study was designed to explore the role of miR-146a in regulating the inflammatory response in DPN rats.
Materials and methods
Reagent
Streptozotocin (STZ) was purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Establishment of the DPN animal model and grouping
Eighteen male Sprague–Dawley rats (140–160 g) were purchased from the Department of Laboratory Animal Science of Fudan University. Animal use was in accordance with the Shanghai Animal Management Committee regulations. All animal experiments were approved by the Ethics Committee of Animal Care of Jinshan Hospital. Experimental methods were implemented according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were randomly divided into three groups with six rats in each group: control group, T2DM group and DPN group. The control group was given clean common feed. The T2DM group was fed common feed for 6 weeks, and then they were given a high-sugar and high-fat diet containing 10% lard, 20% sucrose and 70% regular feed for another 6 weeks. Then, the T2DM group was intraperitoneally injected with STZ at a dosage of 35 mg/kg.15 Fasting blood glucose levels (fasted for 12 hours, with water) were measured 72 hours after intraperitoneal injection. Rats with blood glucose levels >16.7 mmol/L were classified as T2DM rats. In the DPN group, the rats were administered STZ intraperitoneally after being fed the high-fat and high-sugar diet for 6 weeks, resulting in T2DM. The same rats were then fed the high-fat and high-sugar diet for an additional 6 weeks to establish a DPN model.16
Determination of nerve conduction velocity
The nerve conduction velocity (NCV) in all the rats was determined at the 6th week and the 12th week. Rats were anesthetized using 10% chloral hydrate (0.3–0.4 mL/100 g). The sensory nerve conduction velocity (SNCV) and motor nerve conduction velocity (MNCV) were measured and recorded as previously described in our publication.16
qRT-PCR analysis
TRIzol reagent (Clontech Laboratories Inc., Mountain View, CA, USA) was used to extract total RNA from sciatic nerve tissues according to the manufacturer’s protocol. cDNA of miR-146a was then synthesized using the Mir-X miRNA First-Strand Synthesis Kit (Clontech Laboratories Inc.). Quantitative real-time polymerase chain reaction (qRT-PCR) of miR-146a was conducted using the Mir-X miRNA qRT-PCR SYBR Kit (Clontech Laboratories Inc.) with the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The expression level of miRNA was detected using the 2−∆∆Ct system and normalized using U6 snRNA levels as an internal quantitative control.
Bio-Plex Pro™ assays
The magnetic bead-based multiplex Bio-Plex Pro™ assay kit (Bio-Rad Laboratories, Hercules, CA, USA) was used to measure interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) levels. Plasma samples were thawed and centrifuged twice at 4°C, after which IL-1β and TNF-α were detected with the Bio-Plex Pro™ assay kit according to the manufacturer’s instructions. Finally, the results were analyzed using the Bio-Plex 200 system and the Bio-Plex Manager software (Bio-Rad Laboratories).
Western blot assay
Total protein was isolated from sciatic nerve tissues with sodium dodecyl sulfate (SDS) lysis buffer (Beyotime, Shanghai, China) and mixed with 1% phenylmethylsulfonyl fluoride (Beyotime). Equal amounts of protein were analyzed with 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% nonfat milk at room temperature for 1 hour and then incubated with primary antibodies at 4°C overnight. The primary antibodies used were a monoclonal rabbit p65 antibody (1:1,000 dilution; Cell Signaling Technology, Danvers, MA, USA), p50 antibody (1:1,000 dilution; Cell Signaling Technology) and an anti-β-actin primary antibody (1:5,000 dilution; Proteintech Group Inc., Chicago, IL, USA). Suitable horseradish peroxidase-conjugated antibodies (1:5,000 dilution) were incubated with the membrane at room temperature for at least 2 hours. The membrane was covered with enhanced chemiluminescence solution (Merck Millipore, Billerica, MA, USA), and signals were detected using ECL-Plus (Merck Millipore) and quantified using ImageJ Software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation. Statistical analyses were performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA). One-way analysis of variance was performed for multiple-sample analyses. Pearson’s correlation analysis was carried out to clarify the relationship between miR-146a and inflammatory cytokines. All tests were considered significant with P<0.05.
Results
Change of NCV in DPN rats
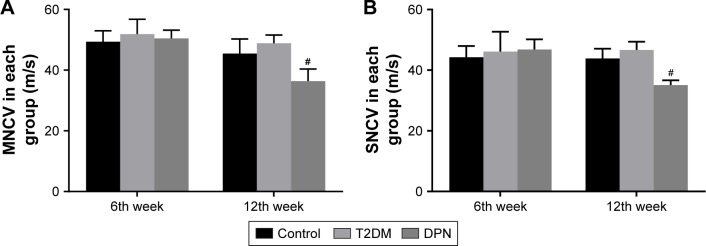
To evaluate differences in nerve electrophysiology among groups, the MNCV and SNCV were determined. As shown in Figure 1, no significant differences existed in either MNCV or SNCV among any groups at the sixth week (P>0.05). Over time, the MNCV and SNCV gradually decreased in the DPN group compared to the control and T2DM groups (P<0.01). However, the MNCV and SNCV of the control and T2DM groups remained statistically unchanged compared to the previous detection values (P>0.05). In addition, we found that there was no significant difference in either the MNCV or SNCV between control and T2DM groups at the 12th week (P>0.05). This indicates that MNCV and SNCV were not affected in the early stages of T2DM.
Figure 1.
NCV values for each group (m/s). (A) MNCV values among groups at different times. (B) SNCV values among groups at different times. #P<0.01 versus DPN group.
Abbreviations: NCV, nerve conduction velocity; MNCV, motor nerve conduction velocity; SNCV, sensory nerve conduction velocity; T2DM, type 2 diabetes mellitus; DPN, diabetic peripheral neuropathy.
Expression of miR-146a in each group
To identify the miRNA expression levels in the three groups of rats, we performed qRT-PCR. As shown in Table 1, qRT-PCR analysis verified that miR-146a expression was downregulated in the sciatic nerves of the rats in the DPN group compared to the control group (P<0.01), while it was upregulated in the T2DM group (P<0.05). Moreover, miR-146a expression in the T2DM group was higher than that in the DPN group (P<0.01). This showed that miR-146a expression was increased during early stages of T2DM but decreased when DPN developed.
Table 1.
Differential expression of miR-146a in sciatic nerves of each group, measured using qRT-PCR analysis
Notes:
P<0.05 versus control group.
P<0.01 versus DPN group. The data is shown as mean ± SD.
Abbreviations: miR-146a, microRNA-146a; qRT-PCR, quantitative real-time polymerase chain reaction; T2DM, type 2 diabetes mellitus; DPN, diabetic peripheral neuropathy.
Expression of cytokines in each group
The level of inflammatory cytokines in the plasma of each group was determined using the Bio-Plex Pro™ assay. No statistical significance existed in the expression levels of TNF-α and IL-1β between the control and T2DM groups. However, the expression of TNF-α and IL-1β in the DPN group was higher than that in the control and T2DM groups, suggesting that rats in the DPN group had an intense inflammatory reaction (P<0.01, Table 2).
Table 2.
Expression of cytokines in each group
| Cytokine | Control | T2DM | DPN |
|---|---|---|---|
| IL-1β | 116.056±1,119.052# | 173.253±13.719# | 13.502±15.308 |
| TNF-α | 921.868±129.594# | 933.066±67.38# | 1,749.034±289.706 |
Notes:
P<0.01 versus DPN group. The data is presented as mean ± SD in pg/mL.
Abbreviations: T2DM, type 2 diabetes mellitus; DPN, diabetic peripheral neuropathy; IL-1β, interleukin 1 beta; TNF-α, tumor necrosis factor alpha.
Expression of NF-κB p65 and p50 in each group
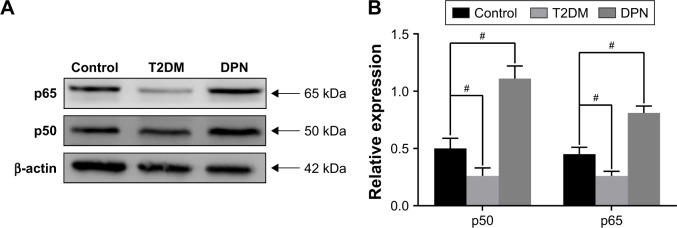
The total protein from sciatic nerve tissues of the control, T2DM and DPN groups was extracted and detected by Western blot. Dramatically decreased expression levels of NF-κB p65 and p50 were observed in the T2DM group compared to the control group (P<0.01). Expression of NF-κB p65 and p50 in the DPN group was significantly higher than that in the control and T2DM groups (P<0.01, Figure 2). This indicates that the component of NF-κB signaling that includes p65 and p50 in the sciatic nerve of the DPN group was increased but decreased in the T2DM group.
Figure 2.
Expression of NF-κB in the sciatic nerve of each group. (A) Western blot analysis showed the expression of NF-κB p65 and p50 in the sciatic nerve of each group. (B) The relative expression of NF-κB p65 and p50 normalized to β-actin (#P<0.01).
Abbreviations: NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; T2DM, type 2 diabetes mellitus; DPN, diabetic peripheral neuropathy.
Analysis of the association between miR-146a, cytokines and NF-κB
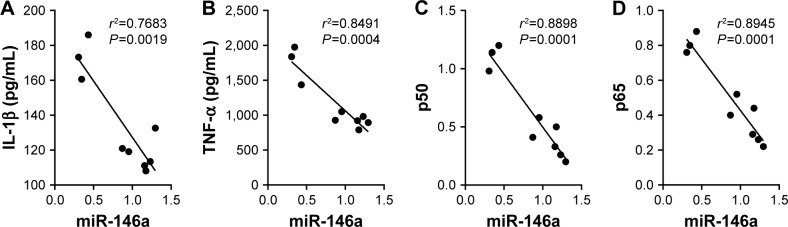
The correlation between miR-146a and cytokines was examined by Pearson’s correlation analysis. As Figure 3 shows, there was a negative correlation between the expression of miR-146a and NF-κB p65 and p50, consistent with the correlation between the expression of miR-146a and TNF-α and IL-1β. This demonstrated that the expression of TNF-α and IL-1β increased gradually with decrease in the expression of miR-146a.
Figure 3.
The association between miR-146a, cytokines and NF-κB. Correlation between (A) miR-146a and IL-1β (r2=0.7683, P<0.01), (B) TNF-α (r2=0.8491, P<0.01), (C) NF-κB p50 (r2=0.8898, P<0.01) and (D) NF-κB p65 (r2=0.8945, P<0.01).
Abbreviations: miR-146a, microRNA-146a; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL-1β, interleukin 1 beta; TNF-α, tumor necrosis factor alpha.
Discussion
The inflammatory response is likely critical for the development and progression of DPN, despite the existence of various other acceptable pathogenetic factors, which may explain the nerve damage observed in DPN rats.17 In recent years, studies have shown that TNF-α is closely related to DPN and is upregulated in the sciatic nerve of DPN rats.18 The upregulation of TNF-α promotes a series of downstream inflammatory responses, which leads to damage and demyelination of the nerve cells, resulting in the final peripheral nerve dysfunction.19,20 The inhibitor of TNF-α, rhTNFR:FC, alleviates nerve damage in DPN rats.16 There are many inflammatory factors in addition to TNF-α including interleukin 6, interferon-γ, C-reactive protein, adhesion factor, interleukin 1, interleukin 10, interleukin 12 and monocyte chemoattractant protein 1 which are involved in the progression of DPN. It reveals that inflammatory response plays an important role in the development of DPN.
According to previous research, alloxan and STZ could be used to induce T2DM rats. Alloxan leads to high mortality of rats, which limits its use in inducing T2DM rats.21 However, STZ has no effect on the conduction velocity of the sciatic nerve in mice and is of no neurotoxicity, which is suitable for the study of the nervous system complications involved in DM.22 T2DM can be induced by two different doses of STZ:23 rats fed with common diet could be made into T2DM models at a dose of 50 mg/kg, while rats fed with high-fat and high-glucose diet and treated with a dose of 35 mg/kg could also serve as T2DM models.15 The latter was chosen to produce T2DM model in the present study. DPN rats are characterized by an obvious decline in MNCV and SNCV, which is similar to clinical conditions. We have observed that 6 weeks of uncontrolled diabetes reduced the miR-146a expression level in the sciatic nerves of DPN group rats. However, the expression level of NF-κB was increased in the DPN group. This indicates that miR-146a and NF-κB may be involved in the pathophysiological mechanism of DPN.
miRNAs bind to the 3′-untranslated regions of target mRNA and cause degradation or repression of the translational process by pairing with target-specific bases. miR-146a has been identified as being expressed in 20 normal human tissues and organs, particularly the thymus and spleen, which are rich in lymphocytes.24 Multiple studies have confirmed that miR-146a is involved in the inflammatory response.25 Upregulated miR-146a inhibits the production of proinflammatory cytokines in degenerative arthritic cartilage.26 In macrophages, upregulation of miR-146a decreases inflammatory cytokine secretion induced by oxidized low-density lipoprotein.27 The expression of interleukin 1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) is inhibited, and the expression level of inflammatory cytokines is low in mice transfected with miR-146a lentivirus, revealing that miR-146a alleviates the inflammatory response.28
With the tremendous development of molecular research, it has been revealed that the pathogenetic pathway of the inflammatory response is mainly orchestrated through a transcription factor called NF-κB.29,30 Recent studies demonstrated that the expression of miR-146a is regulated by several transcription factors such as transcription factor PU.1, protein C-ets, NF-κB and activator protein 1.31 Moreover, a variety of microbial components and proinflammatory cytokines, including lipopolysaccharides (LPSs), TNF-α and interferon alpha, can induce miR-146a expression. IRAK1 and TRAF6 are two vital molecules in the downstream pathway of toll-like receptors (TLRs). miR-146a mainly inhibits target genes IRAK1 and TRAF6 at posttranscriptional levels.32 Low expression levels of miR-146a cause less inhibition of IRAK1 and TRAF6, resulting in the excessive expression of cytokines. Another study found that miR-146a promotes the binding of a transcription inhibitor RelB to the TNF-α promoter, leading to the selective decreased expression of acute inflammatory cytokines.33 Similar to previous studies, we observed a high expression of IL-1β and TNF-α, with a decrease in the expression of miR-146a in DPN group rats. miR-146a expression levels were high in T2DM group rats, while the expression levels of IL-1β and TNF-α were relatively low compared with DPN group rats. Pearson’s correlation revealed that miR-146a is negatively correlated with IL-1β and TNF-α. We believe that miR-146a may have indirectly inhibited the expression of the inflammatory cytokines IL-1β and TNF-α in the present study.
Previous research has confirmed that NF-κB plays a key role in the transcription of miR-146a induced by LPS, TNF-α and IL-1β. The NF-κB-binding site in the miR-146a promoter also confirms that miR-146a is NF-κB dependent.34 NF-κB, a well-known proinflammatory transcription factor, is found in all cell types and is believed to be comprising five protein subunits: RelA (p65), RelB, C-Rel, NF-κB1 (p50 and its precursor molecule p105) and NF-κB2 (p52 and its precursor molecule p100). These protein subunits have an amino terminus of ~300 amino acids with a high degree of homology. This region is called the Rel homologous region, and it interacts with DNA to form a dimer. In the nonactivated state, NF-κB is located in the cytosol in a complex with the inhibitory protein I-κBα. When the cells are stimulated by stressors, I-κBα is rapidly cleaved and released. The free NF-κB in the cytoplasm is then transported into the nucleus and becomes involved in gene transcriptional regulation activities, such as cell proliferation, apoptosis and the cellular inflammatory response. The combination of TNF-α and interleukins binding to the TLRs has been proposed to activate TRAF6 and IRAK1, which in turn promotes the release of NF-κB and the subsequent stimulation of inflammatory gene transcription in the nucleus.35 In the present study, it was observed that NF-κB p65 and p50 expression levels were increased in the sciatic nerves of DPN group rats and decreased in those of T2DM group rats. Expression of NF-κB p65 and p50 in the sciatic nerves of rats in each group was consistent with IL-1β and TNF-α expression. NF-κB p65 and p50 expression increased when miR-146a expression was inhibited, contrary to what was observed in the T2DM group. According to the previous research, we speculate that hyperglycemia may activate NF-κB and in turn quickly activate the expression of miR-146a. miR-146a then inhibits NF-κB through the downregulation of IRAK1 and TRAF6, resulting in decreased expression levels of IL-1β and TNF-α. However, the expression of miR-146a decreased under conditions of long-term hyperglycemia, causing a weakened inhibition of NF-κB p65 and p50 and resulting in increased levels of IL-1β and TNF-α expression (Figure 4).
Figure 4.
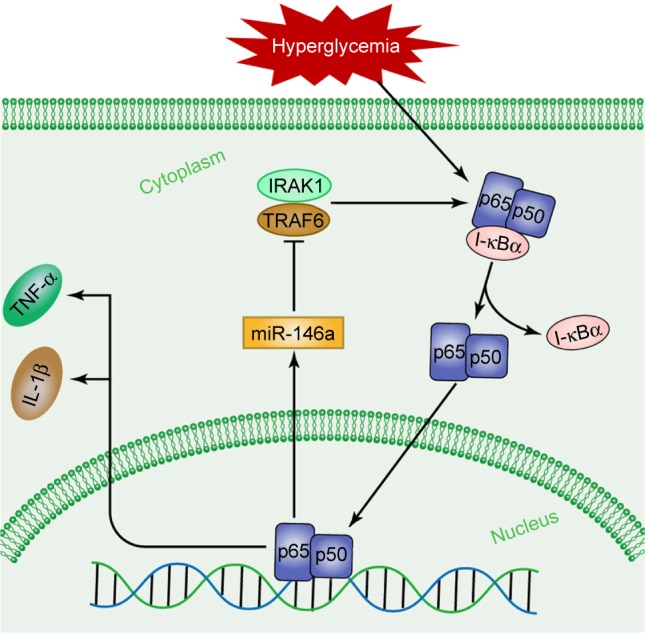
The potential pathway of miR-146a in the sciatic nerves of DPN group rats.
Abbreviations: miR-146a, microRNA-146a; DPN, diabetic peripheral neuropathy; IRAK1, interleukin 1 receptor-associated kinase 1; TRAF6, TNF receptor-associated factor 6; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin 1 beta.
Conclusion
We demonstrated that miR-146a is involved in DPN through the downregulation of the inflammatory reaction. This provides new insight into the molecular mechanisms of DPN and a potential basis for diagnosing DPN through the use of miR-146a. However, exogenous miR-146a intervention may still be implemented, and the mechanism by which miR-146a expression decreases in long-term hyperglycemia remains to be elucidated.
Acknowledgments
This work was supported by a grant from the Shanghai Municipal Commission of Health and Family Planning (201540075).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017;8(1):23–33. doi: 10.1007/s13167-017-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013;36(8):439–449. doi: 10.1016/j.tins.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Li B, Chen B, et al. Thymoquinone alleviates the experimental diabetic peripheral neuropathy by modulation of inflammation. Sci Rep. 2016;6:31656. doi: 10.1038/srep31656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL. Inflammation as a therapeutic target for diabetic neuropathies. Curr Diab Rep. 2016;16(3):29. doi: 10.1007/s11892-016-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Schmeichel AM, Iida H, Schmelzer JD, Low PA. Enhanced inflammatory response via activation of NF-kappaB in acute experimental diabetic neuropathy subjected to ischemia-reperfusion injury. J Neurol Sci. 2006;247(1):47–52. doi: 10.1016/j.jns.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Mu ZP, Wang YG, Li CQ, et al. Association between tumor necrosis factor-α and diabetic peripheral neuropathy in patients with type 2 diabetes: a meta-analysis. Mol Neurobiol. 2017;54(2):983–996. doi: 10.1007/s12035-016-9702-z. [DOI] [PubMed] [Google Scholar]
- 8.Chandramoorthy HC, Bin-Jaliah I, Karari H, et al. MSCs ameliorates DPN induced cellular pathology via [Ca2+ ]i homeostasis and scavenging the pro-inflammatory cytokines. J Cell Physiol. 2018;233(2):1330–1341. doi: 10.1002/jcp.26009. [DOI] [PubMed] [Google Scholar]
- 9.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 10.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann M, Mehta A, Zhao JL, et al. An NF-kappaB-microRNA regulatory network tunes macrophage inflammatory responses. Nat Commun. 2017;8(1):851. doi: 10.1038/s41467-017-00972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava A, Nikamo P, Lohcharoenkal W, et al. MicroRNA-146a suppresses IL-17-mediated skin inflammation and is genetically associated with psoriasis. J Allergy Clin Immunol. 2017;139(2):550–561. doi: 10.1016/j.jaci.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Jahromi SSM, Jamshidi MM, Farazmand A, Aghazadeh Z, Yousefi M, Mirshafiey A. Pharmacological effects of β-d-mannuronic acid (M2000) on miR-146a, IRAK1, TRAF6 and NF-κB gene expression, as target molecules in inflammatory reactions. Pharmacol Rep. 2017;69(3):479–484. doi: 10.1016/j.pharep.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 14.West C, McDermott MF. Effects of microRNA-146a on the proliferation and apoptosis of human osteochondrocytes by targeting TRAF6 through the NF-κB signalling pathway. Biosci Rep. 2017;37(4):BSR20170180. doi: 10.1042/BSR20170180. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Shi X, Chen Y, Nadeem L, Xu G. Beneficial effect of TNF-alpha inhibition on diabetic peripheral neuropathy. J Neuroinflammation. 2013;10(1):69. doi: 10.1186/1742-2094-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox AA, Sagot Y, Hedou G, et al. Low-Dose Pulsatile Interleukin-6 As a Treatment Option for Diabetic Peripheral Neuropathy. Front Endocrinol (Lausanne) 2017;8:89. doi: 10.3389/fendo.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Zang Y, Zhou L, Gui W, Liu X, Zhong Y. The role of TNF-alpha/NF-kappa B pathway on the up-regulation of voltage-gated sodium channel Nav1.7 in DRG neurons of rats with diabetic neuropathy. Neurochem Int. 2014;75:112–119. doi: 10.1016/j.neuint.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Zhu T, Meng Q, Ji J, Lou X, Zhang L. Toll-like receptor 4 and tumor necrosis factor-alpha as diagnostic biomarkers for diabetic peripheral neuropathy. Neurosci Lett. 2015;585:28–32. doi: 10.1016/j.neulet.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Hussain G, Rizvi SA, Singhal S, Zubair M, Ahmad J. Serum levels of TNF-alpha in peripheral neuropathy patients and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes Metab Syndr. 2013;7(4):238–242. doi: 10.1016/j.dsx.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Khajuria DK, Joseph S, Razdan R, Mahapatra DR, Kachroo M, Pai R. Novel eudragit coated alloxan nanoparticle based formulation for inducing type 2 diabetes mellitus in rats. J Nanopharm Drug Deliv. 2013;1(4):394–403. [Google Scholar]
- 22.Davidson E, Coppey L, Lu B, et al. The roles of streptozotocin neurotoxicity and neutral endopeptidase in murine experimental diabetic neuropathy. Exp Diabetes Res. 2009;2009:431980. doi: 10.1155/2009/431980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao GS, Karimian H, Razavi M, et al. Antinociceptive effect of terminalia bellirica in diabetic peripheral neuropathy: a comparison with fluoxetin, imipramine and quercetin. Lat Am J Pharm. 2012;31(4):520–525. [Google Scholar]
- 24.Sonkoly E, Wei T, Janson PC, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007;2(7):e610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saba R, Sorensen DL, Booth SA. MicroRNA-146a: a dominant, negative regulator of the innate immune response. Front Immunol. 2014;5:578. doi: 10.3389/fimmu.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu SX, Li X, Hamilton JL, et al. MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc. Gene. 2015;555(2):80–87. doi: 10.1016/j.gene.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang K, He YS, Wang XQ, et al. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011;585(6):854–860. doi: 10.1016/j.febslet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Gao M, Wang X, Zhang X, et al. Attenuation of cardiac dysfunction in polymicrobial sepsis by microRNA-146a is mediated via targeting of IRAK1 and TRAF6 expression. J Immunol. 2015;195(2):672–682. doi: 10.4049/jimmunol.1403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron JE, Yin Q, Fewell C, et al. Epstein-Barr virus latent membrane protein 1 induces cellular microRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol. 2008;82(4):1946–1958. doi: 10.1128/JVI.02136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou J, Wang P, Lin L, et al. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183(3):2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 33.El Gazzar M, Church A, Liu T, McCall CE. MicroRNA-146a regulates both transcription silencing and translation disruption of TNF-α during TLR4-induced gene reprogramming. J Leukoc Biol. 2011;90(3):509–519. doi: 10.1189/jlb.0211074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. Signaling to NF-kappaB by toll-like receptors. Trends Mol Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]