Abstract
There is significant variability in the serum concentrations of tacrolimus attained early post-transplant due to drug interactions and genomic variation. We evaluated whether tacrolimus concentrations early post-transplant correlate with incidence of acute graft-versus-host disease in 120 consecutive patients allografted with a uniform reduced-intensity conditioning regimen. All patients received standard prophylaxis with oral tacrolimus and intravenous methotrexate. The primary variable of interest was mean weekly tacrolimus concentrations in the initial 4 weeks post-transplant. In multivariate analysis, week 1 tacrolimus concentration was an independent predictor of acute grade 2 – 4 graft-versus-host disease (HR, .90; 95% CI, .84 to .97; P < .01). This association was driven by a lower risk of acute grade 2 – 4 graft-versus-host disease in patients with week 1 tacrolimus concentrations >12 ng/mL (HR .47; 95% CI, .25 – .88; P = .02). Week 1 tacrolimus concentrations were not associated with chronic graft-versus-host disease, relapse, or overall survival. Lower tacrolimus concentrations at weeks 2, 3, and 4 were not associated with a higher incidence of graft-versus-host disease. In summary, we found that higher tacrolimus concentrations during the first week after allografting with a reduced-intensity conditioning regimen were associated with significantly reduced risk of acute grade 2 – 4 graft-versus-host disease without increasing risk of relapse.
Introduction
The development of reduced-intensity conditioning (RIC) regimens has led to the expanded use of allogeneic hematopoietic stem cell transplantation (HSCT), particularly in patients with advanced age or those with significant comorbidities. Although RIC regimens are characterized by reduced toxicity, acute graft-versus-host disease (GVHD) remains a leading cause of morbidity and mortality in this type of transplant.1 Despite standard prophylactic measures, the rates of acute GVHD are high, with incidence rates ranging from 25% to 68%.2–4 Therefore, preventing GVHD without impairing the graft-versus-tumor (GVT) effect remains a critical goal for successful HSCT.
Calcineurin inhibitors (CNI) are considered the backbone of GVHD prophylaxis in HSCT.5 Successful administration of CNI is complicated by their narrow therapeutic index and considerable intra- and inter-patient pharmacokinetic (PK) heterogeneity. The unpredictable PK profile associated with CNI is the result of drug interactions, genomic variation, hepatic and/or renal function, and binding capacity to blood and plasma proteins.6–9 The constellation of these factors lead to significant variability in CNI concentrations attained within the first week after HSCT, which may affect outcomes as preclinical models have demonstrated that the critical sequence of immunologic events that lead to acute GVHD occurs within the first few days after transplantation.10 Therefore, a delay in achieving therapeutic CNI concentrations within the first week post-transplant may result in a higher risk of acute GVHD.
Given these findings, we hypothesized that CNI concentrations attained early after transplant would affect clinical outcomes in RIC HSCT recipients. Previous studies have shown conflicting results regarding the associations between CNI concentrations and GVHD, possibly due to significant variability in the studied populations with respect to conditioning intensity, GVHD prophylaxis regimens, graft sources and CNI route of administration.11–14 To overcome some of these limitations and inform the management of immunosuppression early after RIC HSCT, we analyzed a uniform and large cohort of consecutive patients who received oral tacrolimus (TAC) after peripheral blood stem-cell transplantation with fludarabine+busulfan conditioning. The goal of this analysis was to evaluate whether early TAC concentrations correlated with incidence of GVHD, disease relapse, and survival.
Materials and Methods
Study Population
We conducted a retrospective cohort study of 120 consecutive adult patients undergoing first allogeneic HSCT for a malignant hematologic disorder at the University of Pennsylvania between January 2009 and January 2014. All patients received a uniform RIC regimen of fludarabine (120 mg/m2) and busulfan (6.4 mg/kg), followed by infusion of T-cell replete, granulocyte colony-stimulating factor–mobilized peripheral-blood stem cells from either a related or unrelated donor. All patients received standard GVHD prophylaxis with oral TAC (0.06 mg/kg/day) in two divided doses starting 3 days before HSCT and intravenous methotrexate (MTX) at a dose of 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11. Antithymocyte globulin was not used in any of the patients. The dose of TAC was adjusted to a target trough level of 5 – 15 ng/mL and was continued through 100 days post-HSCT and then tapered. The standard at our institution is to obtain the first TAC trough concentrations 1 day prior to HSCT and then at least twice weekly and at least 72 hours after any dosage change to allow drug levels to achieve steady state. TAC whole blood concentrations were measured by liquid chromatography-mass spectrometry. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Clinical Outcomes
The primary outcome of interest was to assess the correlation between mean weekly TAC concentrations and acute grade 2 – 4 GVHD. We calculated the mean TAC concentration for each of the first four weeks post-HSCT by adding all of the available TAC concentrations from initiation of therapy until the last day of the given week and dividing the sum by the total number of available measurements. Secondary end-points included acute grade 3 – 4 GVHD, chronic GVHD, relapse, overall survival (OS), relapse-free survival (RFS) and acute kidney injury (AKI). Acute GVHD was graded according to the modified Glucksberg criteria and guidelines for data collection recently published by the MAGIC Consortium.15,16 Chronic GVHD was graded according to the National Institutes of Health Consensus Criteria.17 Disease relapse was defined as morphological, cytogenetic, or radiological evidence of disease demonstrating pre-transplant characteristics. We used the Disease Risk Index (DRI) stratification system to classify patients according to disease type and disease status.18 OS was defined as the time interval between date of HSCT and death from any cause or censored at last follow-up. RFS was defined as the time from date of HSCT to death or relapse/progression, whichever came first or censored at last follow-up. AKI was defined as at least a two-fold increase in serum creatinine or a reduction in glomerular filtration rate by greater than 50% from baseline, as per the Risk, Injury, Failure, Loss and End-stage (RIFLE) kidney disease criteria.19
Statistical Analysis
Baseline and treatment characteristics were analyzed with descriptive statistics. Associations between covariates and the cumulative incidence of acute grade 2 – 4 GVHD were determined using Fine and Gray proportional hazards regression. Death was considered a competing event. Associations of covariates with OS and RFS were analyzed using Cox regression models. Mean weekly TAC levels were included in the analyses as continuous variables and then divided into tertiles. The following variables were examined as potential covariates: patient and recipient age, patient and recipient sex, disease type, donor source, DRI, degree of human leukocyte antigen (HLA) match, and presence or absence of cytomegalovirus. Multivariable analysis was performed on variables with a univariate P value <.1. A two-sided P-value of ≤.05 was considered significant for all other analyses. Competing risks regression analyses were also conducted to identify predictors of relapse, allowing for death as a competing event. Patients were censored at the time of donor lymphocyte infusion for GVHD analyses. The associations between TAC concentrations and AKI were conducted using t-tests.
Results
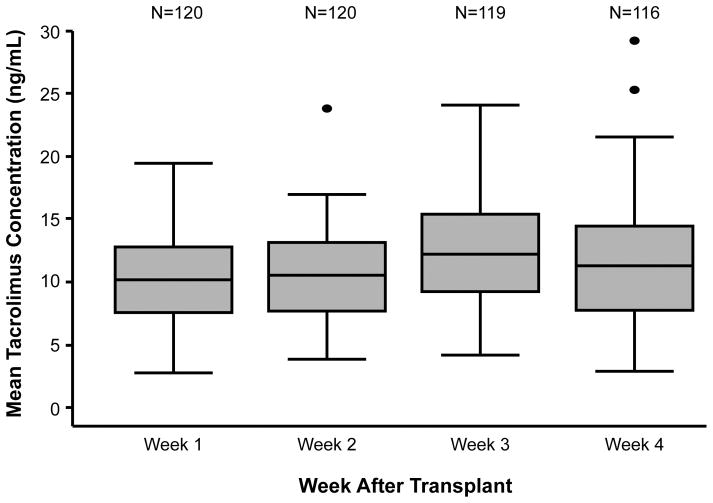
Patient and transplant characteristics of the 120 subjects are presented in Table 1. The median follow-up was 14.3 months (range, 0.7 to 66.3 months). The mean weekly TAC concentrations at weeks 1, 2, 3, and 4 were 10.2 (range, 2.8 to 19.4), 10.6 (range, 3.9 to 23.9), 12.7 (range, to 4.2 to 24.1), and 11.9 (range, to 2.9 to 29.2) ng/mL, respectively (Figure 1). Within this cohort, 115/120 (95.8%) patients had complete TAC concentration data available for all 4 weeks of the analysis. In the remaining 5 patients, the missing TAC concentration values were confined to weeks 3 (n=1) and 4 (n=4) of the study period. The majority of patients (111/120; 92.5%) received all four scheduled MTX doses.
Table 1.
Patient and Transplant Characteristics (N = 120)
| Characteristic | Value |
|---|---|
|
| |
| Recipient age, median in years (range) | 62 (28 – 72) |
|
| |
| Recipient sex, male/female (n) | 70/50 |
|
| |
| Donor age, median in years (range) | 44 (18 – 72) |
|
| |
| Donor sex, male/female (n) | 64/56 |
|
| |
| Sex-mismatch, n (%) | 55 (46) |
|
| |
| Female donor to male recipient transplant, n (%) | 28 (24) |
|
| |
| Diagnosis, n (%) | |
| Acute myeloid leukemia | 49 (41) |
| Myelodysplastic syndromes | 33 (27) |
| Non-Hodgkin lymphoma | 22 (18) |
| Acute lymphoblastic leukemia | 8 (7) |
| Othera | 8 (7) |
|
| |
| Donor source, n (%) | |
| Matched sibling | 53 (44) |
| Matched unrelated | 50 (42) |
| Single-allele mismatched unrelated | 17 (14) |
|
| |
| Disease Risk Index, n (%) | |
| Low | 10 (8) |
| Intermediate | 80 (67) |
| High/very high | 30 (25) |
Other includes: chronic myeloid leukemia (n=2), Hodgkin lymphoma (n=2), multiple myeloma (n=1), myelodysplatic/myeloproliferative neoplasm (n=1), myelofibrosis (n=2)
Figure 1.
Significant variability in TAC concentrations attained early after RIC HSCT. Box-and-whisker plot showing the distribution of mean weekly TAC concentrations during the first 4 weeks after transplantation.
GVHD
The primary outcome of interest was acute grade 2 – 4 GVHD. The cumulative incidence of acute grade 2 – 4 GVHD was 21.3% (95% confidence interval [CI], 14.9% to 29.9%) at day 100 and 42.8% (95% CI, 34.3% to 52.4%) at day 180 post-HSCT. To assess whether early TAC concentrations were predictive of acute grade 2 – 4 GVHD, we analyzed mean weekly TAC concentrations as continuous variables up to four weeks post-HSCT. We first examined the effect of TAC concentrations at each week independently, and then constructed a multivariable model for predicting risk of acute grade 2 – 4 GVHD. The hazard ratios (HR) reflect the increased or decreased risk of acute grade 2 – 4 GVHD for each 1 ng/mL of difference in mean TAC concentration.
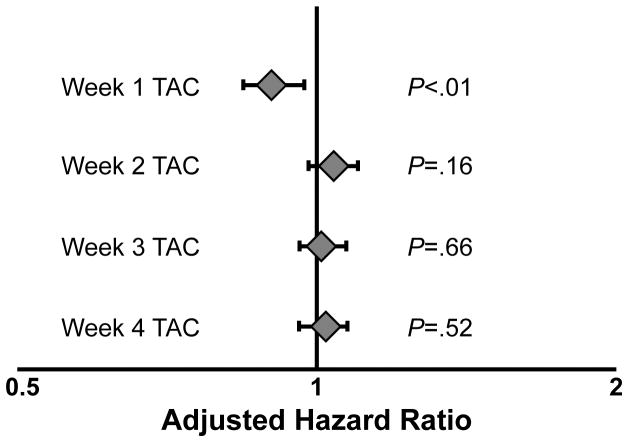
In univariable analysis, week 1 TAC concentrations were inversely associated with acute grade 2 – 4 GVHD (HR, .92; 95% CI, .85 to .99; P = .03). Other variables associated with a higher risk of acute grade 2 – 4 GVHD that met our threshold for modeling were the presence of a single-allele HLA mismatch (HR, 2.14; 95% CI, 1.20 – 3.84; P < .01) and lymphoid malignancies as opposed to myeloid malignancies (HR, .56; 95% CI, .30 – 1.03; P = .06). When incorporating these covariates into a multivariable model, higher week 1 TAC concentrations remained independently associated with a lower risk of acute grade 2 – 4 GVHD (HR, .90; 95% CI, .84 to .97; P < .01), as shown in Figure 2. We found no correlations between lower TAC concentrations at weeks 2, 3, or 4 and increased incidence of acute grade 2 – 4 GVHD. In addition, TAC concentrations on the day prior to HSCT were not associated with acute grade 2 – 4 GVHD.
Figure 2.
Significant association between week 1 TAC concentrations and acute grade 2 – 4 GVHD. Multivariable analysis showing adjusted hazard ratios (aHRs) for acute grade 2 – 4 GVHD based on mean TAC concentrations at weeks 1, 2, 3 and 4 after RIC HSCT. The aHRs reflect the increased or decreased risk of acute grade 2 – 4 GVHD for each 1 ng/mL of difference in mean TAC concentration.
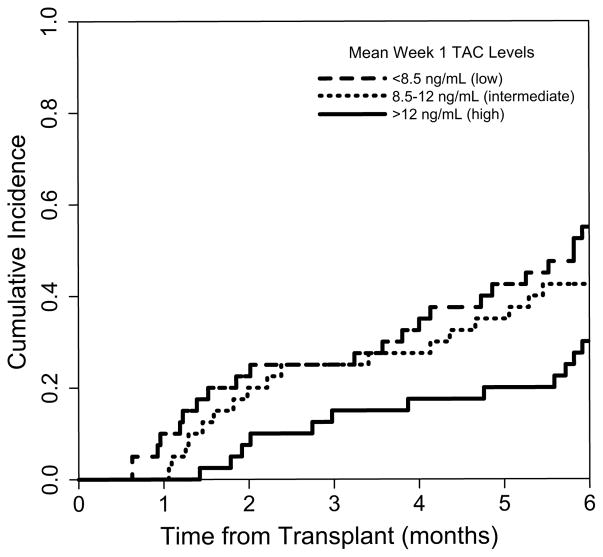
To further characterize the relationship between week 1 TAC concentrations and acute grade 2 – 4 GVHD, we examined the effect of week 1 TAC concentrations categorized in tertiles (< 8.5, 8.5 – 12, and > 12 ng/mL). Interestingly, the inverse association between week 1 TAC concentrations and acute grade 2 – 4 GVHD was driven by a lower risk in the upper tertile (> 12 ng/mL), as shown in Figure 3. Patients in the upper tertile had a lower risk of acute grade 2 – 4 GVHD compared to those in the lower tertile (HR .47; 95% CI, .25 – .88; P = .02). There was no difference in risk of acute grade 2 – 4 GVHD when comparing the intermediate and lower tertiles (HR 1.1; 95% CI, .60 – 1.93; P = .80).
Figure 3.
Lower risk of acute grade 2 – 4 GVHD in patients with mean week 1 TAC > 12 ng/mL. Cumulative incidence plots showing acute grade 2 – 4 GVHD according to mean week 1 TAC concentrations. Patients in the lower tertile (< 8.5 ng/mL), middle tertile (8.5 – 12 ng/mL), and upper tertile (> 12 ng/mL) are represented by the blue, red and green solid lines, respectively.
We then analyzed the effect of week 1 TAC concentrations on acute grade 2 – 4 GVHD in recipients of grafts from related and unrelated donors separately. The cumulative incidence of acute grade 2 – 4 in related donor HSCT at day 100 and day 180 was 11.3% (95% CI, 2.5% to 20.1%) and 32.1% (19.1% to 45.1%), respectively. Week 1 TAC concentrations were not predictive of acute grade 2 – 4 GVHD following related donor HSCT (adjusted HR 0.96; 95% CI, 0.85 – 1.07; P = .46). In recipients of grafts from unrelated donors, the cumulative incidence of acute grade 2 – 4 GVHD at day 100 was 31.3% (95% CI, 19.9% to 42.7%) and at day 180 was 50.7% (38.3% to 63.1%). In unrelated donor HSCT, higher week 1 TAC concentrations were associated with a lower risk of acute grade 2 – 4 GVHD (adjusted HR 0.88; 95% CI, 0.81 – 0.96; P = .003).
Furthermore, we evaluated whether TAC concentrations within the first month post-HSCT impacted the risk of acute grade 3 – 4 GVHD. The cumulative incidence of acute grade 3 – 4 GVHD was 6.8% (95% CI, 3.2% to 10.4%) at day 100 and 16.6% (95% CI, 10.2% to 23.0%) at day 180 post-HSCT. We found no association between TAC concentrations and this outcome (data not shown). We then examined the associations between TAC concentrations and acute grade 3 – 4 GVHD when recipients of related and unrelated grafts were analyzed separately. In related donor HSCT, the cumulative incidence of acute grade 3 – 4 at day 100 was 5.8% (95% CI, 0.6% to 11.0%) and 15.2% (5.8% to 24.6%) day 180. The cumulative incidence of acute grade 3 – 4 GVHD in recipients of grafts from unrelated donors was 7.9% (2.9% to 12.9%) at day 100 and 18.9% (10.1% to 27.7%) at day 180. Week 1 TAC concentrations were not predictive of acute grade 3 – 4 GVHD when patients were analyzed separately according to donor source. Similar analyses were conducted for chronic GVHD and no associations were found (data not shown).
Relapse
Because the pathogenesis of GVHD is closely intertwined with the GVT effect, we examined whether early TAC concentrations after RIC HSCT influenced risk of disease relapse. The cumulative incidence of disease relapse was 29.2% (95% CI, 21.8% to 37.9%) at day 180 and 38.3% (95% CI, 30.1% to 47.3%) at 1 year. In univariable analyses, disease relapse was not associated with mean TAC concentrations at week 1 (HR, .98; 95% CI, .89 – 1.06; P = .58), week 2 (HR, .98; 95% CI, .91 – 1.05; P = .52), week 3 (HR, .97; 95% CI, .91 – 1.03; P = .29), or week 4 (HR, 1.04; 95% CI, .98 – 1.11; P = .15). We also analyzed the relationship between TAC concentrations and risk of relapse with adjustment for the DRI and found no significant associations (data not shown). In addition, mean TAC concentrations did not correlate with donor-recipient whole blood or T-cell chimerism levels at days 30, 60, 100, and 1 year after RIC HSCT.
RFS and OS
The 2-year estimated rates of RFS and OS were 25.8% (95% CI, 18.8% to 34.4%) and 34.2% (95% CI, 26.3% to 43.0%), respectively. In univariable analysis, higher week 3 TAC concentrations were associated with improved RFS (HR, .95; 95% CI, .90 – 1.00; P = .06) but did not meet our threshold for statistical significance. With adjustment for the DRI, this correlation remained non-significant (HR, .95; 95% CI, .90 – 1.01; P = .08). TAC concentrations at weeks 1, 2, and 4 were not predictive of RFS.
We conducted a similar analysis to identify associations between early TAC concentrations and OS. In univariable analysis, higher week 3 TAC concentrations were associated with improved OS (HR, .95; 95% CI, .89 – 1.00; P = .07) but did not reach statistical significance. With adjustment for disease type, recipient age, and DRI, the relationship between higher week 3 TAC concentrations and improved OS remained non-significant (HR, .94; 95% CI .89 – 1.00; P = .06). TAC concentrations at weeks 1, 2, and 4 were not associated with OS.
Kidney Injury
As nephrotoxicity is a commonly reported complication of TAC, we examined whether TAC concentrations early post-transplant were predictive of AKI in this cohort. Renal impairment, defined by the RIFLE kidney disease criteria, which is a standard criterion in pharmacological studies, occurred in 20 (16.7%) patients during the first 4 weeks after RIC HSCT. Week 1 TAC concentrations were higher in patients who developed AKI by week 2 post-transplant compared to those who did not (12.7 vs. 10.0 ng/mL; P = .01). We did not observe an association between TAC concentrations at any other time points and AKI within the first month post-transplant. No patients required hemodialysis within the first 4 weeks of HSCT. We also examined whether TAC concentrations early after RIC HSCT were associated with chronic renal impairment. Of the 71 patients alive at 1 year after HSCT, no one had a serum creatinine > 2 mg/dL.
Discussion
In this study, we demonstrated that there is significant variability in the serum concentrations of TAC attained early post-RIC HSCT. In addition, we found that higher TAC concentrations during the first week after RIC HSCT were associated with significantly reduced risk of acute grade 2 – 4 GVHD without increasing risk of relapse. For each 1 ng/mL increase in TAC concentration, there was a 10% decrease in risk of acute grade 2 – 4 GVHD. Importantly, this association was driven by a lower risk of acute grade 2 – 4 GVHD in patients with mean week 1 TAC concentrations greater than 12 ng/mL. To the best of our knowledge, this study is the first to characterize the importance of achieving higher therapeutic TAC concentrations within the first week after RIC HSCT.
As expected, we observed a direct association between week 1 TAC concentrations and incidence of AKI occurring by day 14 post-transplant. The incidence of AKI has been previously reported to be proportionally related to TAC concentrations in HSCT recipients.20 AKI was reversible and long-term renal complications were not observed.
The results of our study show the critical importance of achieving therapeutic TAC concentrations within the first week after RIC HSCT in order to optimally attenuate donor alloreactivity. This association is consistent with preclinical studies that demonstrated that the initiating events of acute GVHD occur very early after transplant.10,21 It was observed that alloreactive T-cell activation, proliferation, and migration to GVHD target organs occur within several days after stem cell infusion. In addition, ex vivo analyses of gastrointestinal tract tissue have shown alloreactive CD4+ donor lymphocyte infiltration of Peyer’s patches and mesenteric lymph nodes as early as 12 hours after HSCT.10 The importance of inhibiting alloreactivity within the first week after RIC HSCT has been further characterized with the emergence of novel approaches to GVHD prophylaxis, including proteasome inhibition with bortezomib and post-transplant cyclophosphamide. The efficacy of both of these novel strategies is critically dependent on the early timing of drug administration after HSCT.22,23 Taken together, our findings imply that achieving a mean TAC concentration greater than 12 ng/mL during the first week after RIC HSCT potentially mitigates the intense alloreactivity that occurs immediately after stem cell infusion and thereby reduces the risk of developing acute GVHD. In a subset analysis, this association appeared to be driven by the group of recipients of unrelated donor grafts, although the analysis in recipients of related grafts may have been inadequately powered due to a smaller sample size.
The optimal target concentration of CNI early after HSCT has been previously examined with conflicting results.11–14 The varying results are likely due to heterogeneity in studied populations, inclusion of multiple GVHD prophylaxis regimens, different routes of TAC administration, and heterogeneity in conditioning regimens and graft sources. In order to overcome these limitations, our analysis focused on a homogeneous patient population consisting of patients undergoing first allogeneic HSCT that were allografted with fludarabine/busulfan, the most commonly used RIC regimen according to the Center for International Blood and Marrow Transplant Research.1 In addition, all patients received a uniform GVHD prophylaxis regimen and received peripheral-blood stem cells. Another retrospective study that reported on a uniform patient population that underwent RIC HSCT found no associations between TAC concentrations and acute grade 2 – 4 GVHD, although week 2 TAC concentrations less than 10.5 ng/mL were associated with a higher risk of acute grade 3 – 4 GVHD. In contrast to our findings, there was no correlation between week 1 TAC concentrations and acute GVHD.11 Several differences between this report and our study, including the use of non-myeloablative conditioning (low dose total body irradiation +/− fludarabine) and the use of mycophenolate mofetil and not methotrexate, could potentially explain the different results of these studies.
Our data highlight the importance of developing novel methods to optimize the initial dosing of TAC in RIC HSCT recipients. One approach that may aid in achieving higher therapeutic concentrations rapidly is to incorporate a patient’s genotype into the formula for determining the initial starting dose rather than using the standard weight-based fixed-dose strategy. TAC is primarily metabolized by cytochrome P450 3A4 and 3A5, both highly polymorphic isoenzymes. In addition, a number of other enzymes responsible for TAC metabolism possess gene variants that have the propensity to influence TAC PK.24 Identifying patient-specific genotypes prior to initiating TAC may reduce the time it takes to achieve therapeutic concentrations of TAC and thereby reduce the incidence of acute GVHD.
In summary, we conclude that achieving higher TAC concentrations, and in particular levels higher than 12 ng/mL within the first week of RIC HSCT, may significantly reduce the risk of acute grade 2 – 4 GVHD without impairing the GVT effect. Although post-transplant AKI is more commonly seen in patients with high TAC concentrations, long-term renal complications are rare. These data highlight the importance of optimizing the initial dosing of TAC in RIC HSCT recipients. Prospective confirmation of our findings is warranted.
Acknowledgments
Financial disclosure: Supported by a Career Development Award from the Conquer Cancer Foundation (R.R.); Amy Strelzer Manasevit Award from the National Marrow Donor Program (R.R.); National Institutes of Health grants K23-CA178202 (R.R.) & U01-HL069286 (D.L.P.), and the Margie and Andy Rooke Fund for Leukemia Research (R.R. and D.L.P.). We thank Oren Litvin for help with preparation of the figures.
Footnotes
Conflict of Interest: None to report.
References
- 1.Reshef R, Porter DL. Reduced-intensity conditioned allogeneic SCT in adults with AML. Bone Marrow Transplant. 2015;50:759–69. doi: 10.1038/bmt.2015.7. [DOI] [PubMed] [Google Scholar]
- 2.Jagasia M, Arora M, Flowers EM, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eapen M, Logan BR, Horowitz MM, Zhong X, Perales MA, Lee SJ, et al. Bone marrow or peripheral blood for reduced-intensity conditioning unrelated donor transplantation. J Clin Oncol. 2015;33:364–69. doi: 10.1200/JCO.2014.57.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston L. Acute graft-versus-host disease: differing risk with differing graft sources and conditioning intensity. Best Pract Res Clin Haematol. 2008;21:177–92. doi: 10.1016/j.beha.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus host disease. Nat Rev Clin Oncol. 2014;11:536–47. doi: 10.1038/nrclinonc.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson P, Ng J, Ratanatharathorn V, Uberti J, Brundage RC. Factors affecting the pharmacokinetics of tacrolimus (FK506) in hematopoietic cell transplant (HCT) patients. Bone Marrow Transplant. 2001;28:753–58. doi: 10.1038/sj.bmt.1703224. [DOI] [PubMed] [Google Scholar]
- 7.Onizuka M, Kunii N, Toyosaki M, Machida S, Ohgiya D, Ogawa Y, et al. Cytochrome P450 genetic polymorphisms influence the serum concentration of calcineurin inhibitors in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2011;46:1113–7. doi: 10.1038/bmt.2010.273. [DOI] [PubMed] [Google Scholar]
- 8.Brown NW, Gonde CE, Adams JE, Tredger JM. Low hematocrit and serum albumin concentrations underlie the overestimation of tacrolimus concentrations by microparticle enzyme immunoassay versus liquid chromatography-tandem mass spectrometry. Clin Chem. 2005;51:586–92. doi: 10.1373/clinchem.2004.043950. [DOI] [PubMed] [Google Scholar]
- 9.Miano TA, Ganetsky A, Porter DL, Reshef R. Serum hemoglobin is a predictor of tacrolimus whole blood concentration in hematopoietic stem cell transplant patients. 2015 American Society for Clinical Pharmacology and Therapeutics Annual Meeting; Abstract 620. [Google Scholar]
- 10.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–22. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ram R, Storer B, Mielcarek M, Sandmaier BM, Maloney DG, Martin PJ, et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:414–22. doi: 10.1016/j.bbmt.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Offer K, Kolb M, Jin Z, Bhatia M, Kung AL, George D, et al. Efficacy of tacrolimus/mycophenolate mofetil as acute graft-versus-host disease prophylaxis and the impact of subtherapeutic tacrolimus levels in children after matched sibling donor allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:496–502. doi: 10.1016/j.bbmt.2014.11.679. [DOI] [PubMed] [Google Scholar]
- 13.Mori T, Kato J, Shimizu T, Aisa Y, Nakazato T, Yamane A, et al. Effect of early posttransplantation tacrolimus concentration on the development of acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation from unrelated donors. Biol Blood Marrow Transplant. 2012;18:229–234. doi: 10.1016/j.bbmt.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Rogosheske JR, Fargen AD, DeFor TE, Warlick E, Arora M, Blazar BR, et al. Higher therapeutic CsA levels early post-transplantation reduces risks of acute graft-versus-host disease and improves survival. Bone Marrow Transplant. 2014;49:122–125. doi: 10.1038/bmt.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995:825–828. [PubMed] [Google Scholar]
- 16.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multi-center standardization of acute graft-versus-host disease clinical data collection: a report from the MAGIC consortium. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2015.09.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Przepiorka D, Nash RA, Wingard JR, Zhu J, Maher RM, Fitzsimmons WE, et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol Blood Marrow Transplant. 1999;5:94–7. doi: 10.1053/bbmt.1999.v5.pm10371361. [DOI] [PubMed] [Google Scholar]
- 21.Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4:154–60. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 22.Sun K, Wilkins DEC, Anver MR, Sayers TJ, Panoskaltsis-Mortari A, Blazar BR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–99. doi: 10.1182/blood-2004-11-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical BMT. Semin Oncol. 2012;39:1–16. doi: 10.1053/j.seminoncol.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53:123–39. doi: 10.1007/s40262-013-0120-3. [DOI] [PubMed] [Google Scholar]