Abstract
Epithelial–mesenchymal transition (EMT), via activation of Wnt signaling, is prevailing in embryogenesis, but postnatally it only occurs in pathological processes, such as in tissue fibrosis and tumor metastasis. Our prior studies led us to speculate that EMT might be involved in the loss of limbal epithelial stem cells in explant cultures. To examine this hypothesis, we successfully grew murine corneal/limbal epithelial progenitors by prolonging the culture time and by seeding at a low density in a serum-free medium. Single cell-derived clonal growth was accompanied by a gradient of Wnt signaling activity, from the center to the periphery, marked by a centrifugal loss of E-cadherin and β-catenin from intercellular junctions, coupled with nuclear translocation of β-catenin and LEF-1. Large-colony-forming efficiency at central location of colony was higher than peripheral location. Importantly, there was also progressive centrifugal differentiation, with positive K14 keratin expression and the loss of p63 and PCNA nuclear staining, and irreversible EMT, evidenced by cytoplasmic expression of α-SMA and nuclear localization of S100A4; and by nuclear translocation of Smad4. Furthermore, cytoplasmic expression of α-SMA was promoted by high density cultures and their conditioned media, which contained cell density-dependent levels of TGF-β1, TGF-β2, GM-CSF, and IL-1α. Exogenous TGF-β1 induced α-SMA positive cells in a low density culture, while TGF-β1 neutralizing antibody partially inhibited α-SMA expression in a high density culture. Collectively, these results indicate that irreversible EMT emerges in the periphery of clonal expansion where differentiation and senescence of murine corneal/limbal epithelial progenitors occurs as a result of Smad-mediated TGF-β-signaling.
Keywords: cornea, epithelial, mesenchymal transition, limbus, senescence, stem cell, TGF-β, and Wnt/β-catenin pathway
Introduction
Epithelial–mesenchymal transition (EMT), a variant of transdifferentiation, ensues when expression of epithelial characteristics, such as intercellular junctions and polarity, mediated by cytokeratins and E-cadherin is gradually replaced by that of mesenchymal markers, such as S100A4 (Le Hir et al., 2005; Rossini et al., 2005) and α-SMA (Chagraoui et al., 2003; Masszi et al., 2003; Thiery and Sleeman, 2006; Willis et al., 2005). EMT is prevailing during embryonic morphogenesis (Kalluri and Neilson, 2003), but, postnatally, it is implicated in fibrosis of renal tissues(Iwano et al., 2002; Okada et al., 1997; Yang and Liu, 2001; Zeisberg et al., 2001), pulmonary tissues (Chilosi et al., 2003; Kim et al., 2006; Willis et al., 2005), and cancer metastasis (Kalluri and Neilson, 2003). Dissolution of E-cadherin-mediated adherens junctions results in liberation of β-catenin into the cytoplasm. Here it forms a complex with TCF/LEF-1, which enters the nucleus and transactivates genes via the canonical Wnt signaling pathway (Chilosi et al., 2003; Jamora et al., 2003; Kim et al., 2002). Therefore, that activation of Wnt/β-catenin signaling is an integral part of EMT (Nelson and Nusse, 2004; Savagner, 2001).
The enrichment of corneal epithelial stem cells (SCs) at the limbus, i.e., the anatomic junction between the cornea and the conjunctiva, renders it an ideal model to study regulation of adult somatic SCs (Lavker et al., 2004). Using rabbit limbal explants cultured at the air-medium interface, we have shown that EMT can be undertaken by limbal, but not corneal, basal epithelial progenitor cells, leading to their intrastromal invasion (Kawakita et al., 2005). As a result, the population of limbal epithelial SCs declines, which mimicked the pathologic state of limbal stem cell deficiency found in severe ocular surface diseases such as Stevens-Johnson syndrome, ocular cicatricial pemphigoid, and following chemical burns (Lavker et al., 2004). This EMT-mediated loss of limbal basal epithelial progenitor cells is recapitulated by human limbal tissues cultured on intact amniotic membrane (Li et al., 2007): a method for the ex vivo expansion of limbal epithelial SCs (Tseng et al., 2004). These results collectively prompted us to postulate that EMT may also be involved in epithelial SC senescence.
In cultures, EMT can be influenced by cell-cell contact and extracellular calcium concentrations ([Ca2+]). The expression of α-SMA and the nuclear accumulation of β-catenin are restricted to cells located at the edge of a wound created on a confluent culture of a pig proximal tubular epithelial cell line; or in cells treated by Ca2+-removal, where intercellular contacts are lost. In such models, EMT is facilitated by TGF-β1 (Masszi et al., 2004). E-cadherin is normally expressed in the intercellular junctions of epithelial cells under the correct [Ca2+] (Nagar et al., 1996): its expression can be downregulated by low [Ca2+], and by a low seeding density (Owens et al., 2000). Thus, one may expect the Wnt/β-catenin signaling pathway to be activated in culturing systems of epithelial progenitors isolated from the epidermis (Hager et al., 1999), cornea (Kruse and Tseng, 1992), and conjunctiva (Risse Marsh et al., 2002), where both maneuvers of a low seeding density and low [Ca2+] are used. Besides cell-cell contacts and [Ca2+], TGF-β has also been found to activate EMT in several cultured epithelial cells (Li et al., 2004; Saika et al., 2004; Yao et al., 2004). Because TGF-β is known to inhibit epithelial proliferation but promote epithelial differentiation (Barnard et al., 1988; McCartney-Francis and Wahl, 1994; Siegel and Massague, 2003), we postulate that additional activation of TGF-β signaling is necessary to render EMT irreversible so as to cause senescence during SC clonal expansion. In this study, we provide strong evidence supporting this hypothesis, and the significance of our findings is further discussed in the context of how to develop new strategies to achieve effective ex vivo expansion of epithelial progenitor cells.
Materials and Methods
Reagents
Amphotericin B, Defined Keratinocyte-SFM (KSFM), gentamicin, Hank's balanced salt solution (HBSS), HEPES-buffer, phosphate buffered saline (PBS), and 0.25% trypsin/1 mM EDTA were purchased from Gibco-BRL (Grand Island, NY). Dispase II powder was obtained from Roche (Indianapolis, IN). Tissue-Tek OCT compound and cryomolds were from Sakura Finetek (Torrance, CA). Anti-TGF-β neutralizing antibody was from R&D Systems (Minneapolis, MN). An ABC kit, Vectastain Elite, and anti-fading solution were from Vector Labs (Burlingame, CA). A DAB kit was from Dako (Carpinteria, CA). Other reagents and chemicals including transforming growth factor β1 (TGF-β1), cholera toxin, mouse-derived epidermal growth factor (EGF), sorbitol, and FITC-conjugated goat anti-mouse antibody, Dickkopf and BIO were purchased from Sigma (St. Louis, MO). All primary antibodies used in this study are summarized in the supplemental Table.
Isolation and Culture of Murine Corneal/limbal Epithelial Cells
CD-1 albino mice of more than 3 weeks-old (Charles River., Boston, MA) were handled according to the ARVO guidelines for animal care. Murine corneal/limbal epithelial sheets were isolated in the same manner as previously reported (Kawakita et al., 2004). In brief, more than one hundred eyes were enucleated by forceps, washed profusely in PBS, stored in KSFM, and then transported at 4°C to reach the laboratory within 16 hours. These eyes were digested at 4°C for 18 h in KSFM containing 10 mg/ml dispase II. KSFM containing 0.07 mM [Ca2+] was supplemented with 10 ng/ml EGF and 10−10 mM cholera toxin as described previously (Caldelari et al., 2000; Kawakita et al., 2004). Subsequently, each murine eye was restrained, by suction applied to the posterior pole using a transfer pipette, and was gently shaken in KSFM to loosen the ocular surface epithelial sheet.
Single cells were obtained from the above corneal/limbal epithelial sheets by 0.25% trypsin/1 mM EDTA in HBSS for 10 min and vigorous pipetting, and were seeded at a density of 20,000 cells/cm2, on plastic containing KSFM. In one week, cells reached confluence and they were then subcultured by trypsin/EDTA at a 1:3 split to Passage 1 (P1) cultures. When cells reached up to 60-70 % confluence, they were subcultured at a 1:3 split in 4 weeks, i.e., 3 weeks beyond confluence, and subsequently allowed to pass beyond P100.
Culture Manipulations
In P12 cultures, cells seeded at 20,000 cells/cm2 were cultured for 1 week ± 5% FBS and 0.9 mM [Ca2+]. For both P10 and P15 cultures, 1 × 103 cells were seeded on each 60 mm plastic dish (n=3), and cultured in KSFM for 4 weeks. Epithelial clones were visualized by staining with crystal violet or subjected to Immunostaining. P21 cells were seeded at a density of 50, 500, 5,000, or 50,000 cells/cm2, and cultured in KSFM for 2 weeks (n=5). In parallel, 5 ng/ml of TGF-β1 was added to the low seeding density (50 cells/cm2) culture when some clones developed on day 7, while 10 μg/ml of anti-TGF-β neutralizing antibody was added to cultures immediately when seeded at 50,000 cells/cm2.
Immunostaining
Immunostaining was carried out using standard methods of immunohistochemistry or immunofluorescence staining using appropriate dilutions of primary antibodies as summarized in the supplementary table. Secondary antibodies were used in accordance with methods previously described (Kawakita et al., 2004). For anti-p63 antibody, we used clone 4A4, which recognizes all six p63 isotypes (Yang et al., 1998). Substitution of primary antibody with PBS served as a negative-staining control. For double-immunostaining, the cells were subsequently incubated with the second antibody for 1 h. Detection was performed using immunofluorescence. Images were photographed with a NikonTe-2000u Eclipse epi-fluorescent microscope (Nikon., Tokyo, Japan).
Colony-forming efficiency
Colony-forming efficiency was performed to confirm whether such EMT in the periphery of the clonal growth might have started with a loss of progenitor cell status or not. Therefore cells from central area, as well as those from peripheral area of colony were reseeded separately as a density of 1000 cells on each 6 well-culture dish. Large-colony- forming efficiency (the number of colonies more than 5mm diameter / number of seeded cells).
Cytokine Assays
Cell culture supernatant (50 μl/sample) was analyzed using the Bio-Plex™ analysis system and a Luminex 100TM analyzer (BioRad. Hercules, CA) according to manufacturer's instructions.
Inhibitor Assays
P100 cells were seeded at 5,000 cells/cm2 for 5 days and stimulated by 5 ng/ml TGF-β1 for 48 h, and they were then immunostained for the comparison of potential markers for EMT, which included: Snail, α-SMA, MMP7, and MMP9. To confirm the promotion of putative EMT markers (α-SMA, Snail, and Slug) by TGF-β stimulation, we performed RT-PCR as described below. To confirm the contribution of Wnt/β-catenin signaling, 200 ng/ml of Dickkopf (a Wnt/β-catenin inhibitor) or 1 ng/ml of BIO (a Wnt/β-catenin activator) were applied for 6 hours to examine the expression pattern of α-SMA, Snail, and MMP2, following modulation of Wnt/β-catenin signaling by these reagents.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from expanded cells, stratified epithelial sheets, mouse skin and mouse corneal epithelium using the SV total RNA isolation system, according to the manufacturer's recommendations. We generated cDNA using oligo (dT) priming and AVM reverse transcriptase XL by incubation of a 25 μl mixture at 41°C for 1 h. RT-PCR was performed using oligonucleotide primers specific to each gene (Table 2) in 1 μl cDNA (total reaction volume = 50 μl). The amplification cycle was 95°C for 30 sec, 53°C for 30 sec, and 72°C for 20 sec. A total of 20 cycles were done using the Takara EX Taq DNA polymerase (Takara). Using GAPDH as an internal control, PCR amplified products were separated by electrophoresis on a 1.5 % agarose gel. Sequences for primers were as follows: α-SMA, Fw:GAACCCTGAGACGCTGCTCCAGCTATGTG, and Rv:CAGTAGTCACGAAGGAATAGCCACGC, matrix metalloproteinase (MMP)-2, Fw:AACTACAACTTCTTCCCCCGCAAGC and Rv:ACCCATGGTAAACAAGGCTTCATGG; Snail, Fw:CGCTCTGAAGATGCACATCCGAAG and Rv:TGTGTCCAGAGGCTACACCTCATG; Slug, Fw:CGCCTTCCTCTGACACTTCATCCAA and RV:CCAGACTCCTCATGTTTATGCAGA.
Statistical Analyses
Data are presented as the mean ± SD for each experiment number (n). Statistical significance was determined by the Student's t-test or analysis of variance. A P-value of less than 0.05 is considered to be statistically significant.
Results
Progressive differentiation occurred in the periphery of single cell-derived clonal growth
When seeded at 500 cells/cm2 in KSFM, both P10 and P15 cells generated several clones with a smooth contour. Each clone consisted of uniformly small cells in the center (Fig. 1A and 1B), but had some enlarged vacuolated cells in the periphery (Fig. 1C). All small cells showed strong p63-positive staining in the nucleus (Fig. 1D), but the larger cells had weak or negative p63 nuclear staining (Fig. 1E, arrows). Cytoplasmic staining for K14 keratin (green) was negligible or scanty in most small cells and only slightly noticeable in some occasional cells in the center (Fig. 1F, arrows). In contrast, strong positive cytoplasmic staining was noted in all cells in the periphery (Fig. 1G). These results indicate that the cells gradually enlarged and differentiated, and this was associated with a gradual loss of proliferative activity in the periphery of the single cell-generated clonal growth. Large-colony-forming efficiency was significantly more in central location (5.3±0.6%) than peripheral location (3.1±0.5%) of the colony (Fig.1 H p<0.01), which also supported differentiation and loss of proliferative activity at peripheral location.
Figure 1. Progressive differentiation in the periphery of clonal growth.
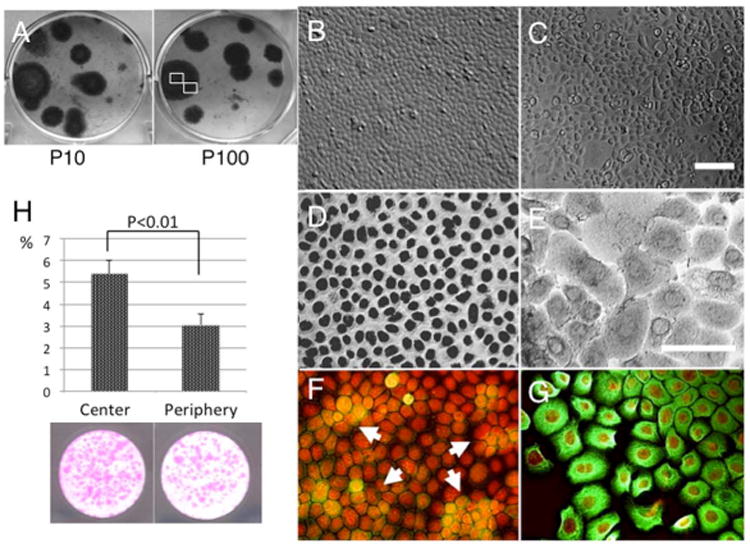
Murine corneal/limbal epithelial progenitor cells exhibited clonal growth when seeded at 500 cells/cm2 in KSFM. Clones were round for both Passage 10 and 100 cultures (A = P10 and P100). In each P15 clone, cells in the center were uniformly small (A and B), but those in the periphery were enlarged and showed vacuolation (C). p63 nuclear staining was positive in all small cells in the center (D), but became weaker and also negative in larger cells in the periphery (E, marked by arrows). Expression of K14 keratin (green) was scanty in most small cells, but there was some sporadic staining of cells, particularly in the center (F, arrows). This was more uniformly positive in the cytoplasm of cells in the periphery (G). Bar represents 50 μm. Large-colony-forming efficiency (more than 0.5 mm diameter) was significantly higher in central location of the colony (5.38±0.6%) to comared with peripheral location (3.05±0.5%). (H, p<0.01)
Progressive differentiation in the periphery was accompanied by activation of the Wnt/β-catenin pathway
Previously, it has been shown that activation of the canonical Wnt-mediated signaling pathway is involved in EMT in a number of epithelial cells (Savagner, 2001). In the rabbit limbal explant model, we also noted that the Wnt signaling was activated during EMT of limbal basal epithelial progenitor cells (Kawakita et al., 2005). To determine whether the Wnt signaling pathway was similarly activated or not, P15 clones were subjected to immunostaining. The results showed that E-cadherin was predominantly expressed in intercellular junctions by cells in the center of clones (Fig. 2A), but the pattern of staining became more cytoplasmic for the cells in the mid-periphery (Fig. 2B); and for cells in the periphery of the clone, the staining was exclusively located in the perinuclear cytoplasm (Fig. 2C). β-catenin was also located intercellularly in cells in the center (Fig. 2D), but in the mid-periphery it became located in the perinuclear cytoplasm of cells (Fig. 2E), and in the periphery, it was exclusively present in the nucleus (Fig. 2F). LEF-1 was in the cytoplasm of cells in the center (Fig. 2G), but the pattern of staining became nuclear for some cells in the mid-periphery, and all cells in the periphery (Figures 2H and 2I). Collectively, these results indicated that the canonical Wnt pathway, mediated by nuclear translocation of β-catenin and LEF-1, was also activated in the periphery of clonal expansion.
Figure 2. Activation of Wnt signaling during clonal expansion.
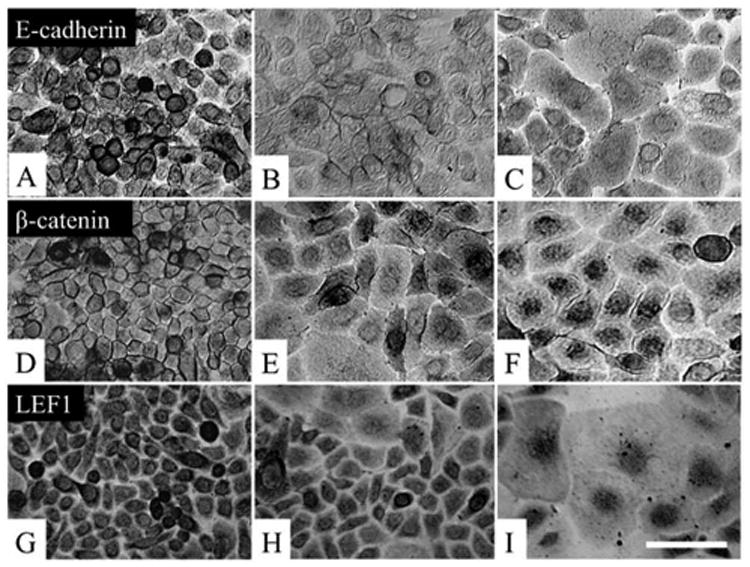
P15 clones were examined for activation of the Wnt signaling, Immunostaining to E-cadherin showed strong positive staining at the intercellular junctions by cells in the center (A). E-cadherin expression became more in the cytoplasm for some cells in the mid-periphery (B), and it was located exclusively in the perinuclear cytoplasm in the periphery of the clone (C). β-catenin was also located intercellularly in the center (D), but it became located in the perinuclear cytoplasm in the mid-periphery (E), and it was exclusively in the nucleus in the periphery (F). LEF-1 was in the cytoplasm in cells in the center (G), but staining became nuclear for some cells in the mid-periphery (H), and for all cells in the periphery (I). Bar represents 50 μm.
Progressive differentiation in the periphery led to EMT and was accompanied by activation of Smad-mediated TGF-β signaling
Previously, several studies have demonstrated that EMT of cultured epithelial cells (from murine kidney (Forino et al., 2006), and porcine lens (Saika et al., 2004)), is mediated, in part, by activation of Smad-mediated TGF-β signaling (Yook et al., 2005). Because cells in the periphery of clonal growth showed more differentiation (Fig. 1) and activation of Wnt/β-catenin signaling (Fig. 2), we wondered whether this process was associated with EMT. To do so, we immunostained P15 clones with antibodies against α-SMA and S100A4, which have been shown to be markers of EMT (Kalluri and Neilson, 2003). The results showed that cytoplasmic expression of α-SMA was absent in all cells in the center and the mid-periphery of the clone (Figures 3A and 3B), but was vividly observed in some cells in the periphery of the clone (Fig. 3C). S100A4 was weakly expressed in the cytoplasm by cells in the center (Fig. 3D) and by most cells in the mid-periphery (Fig. 3E). S100A4 expression became strongly positive in the nucleus by some cells in the mid-periphery (Fig. 3E), but by nearly all cells in the periphery of the clone (Fig. 3F). Previously, it has been reported that nuclear translocation of S100A4 is indicative of myofibroblast differentiation by corneal fibroblasts (Ryan et al., 2003), and S100A4 has also been used as marker for myofibroblast differentiation, but nuclear translocation has not been reported. (Le Hir et al., 2005; Rossini et al., 2005) Double immunostaining confirmed that some cells in the periphery indeed co-expressed α-SMA in the cytoplasm and S100A4 in the nucleus (Fig. 3F, inset). These results collectively showed that cells in the periphery of the clonal growth exhibited characteristics indicative of EMT. Importantly, immunostaining to Smad4 revealed weak cytoplasmic expression by cells in the center (Fig. 3G) but positive nuclear expression in some cells of the mid-periphery (Fig. 3H); and in nearly all cells in the periphery of the clone (Fig. 3I). Because nuclear translocation of Smad4 is indicative of activation of TGF-β signaling (Reguly and Wrana, 2003; ten Dijke and Hill, 2004), we speculated that EMT might occur in the periphery of clonal expansion as a result of activation of Smad-mediated TGF-β signaling.
Figure 3. Correlation of positive α-SMA/S100A4 expression and activation of Smad-mediated signaling.
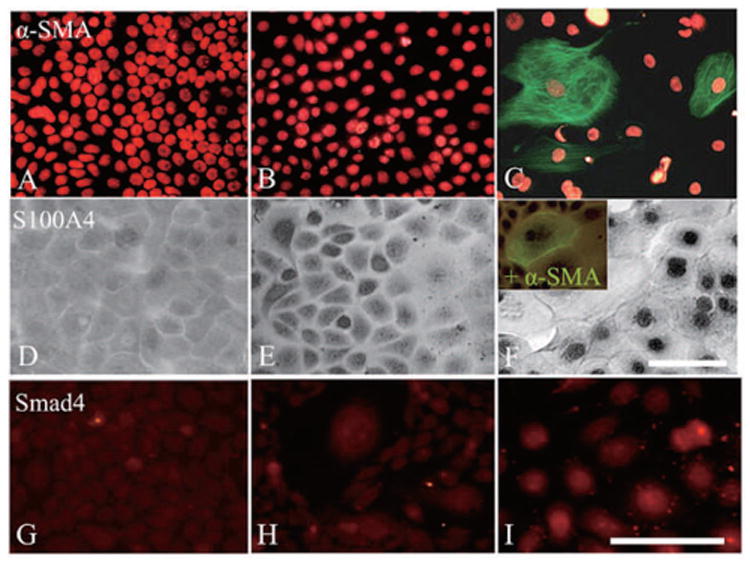
P15 clones were immunostained with α-SMA and S100A4 for evidence of EMT, and this correlated with Smad4 as a marker for activation of TGF-β signaling activation. The results showed that α-SMA-positive cells were not found in the center (A) or the mid-periphery (B), but only observed in the periphery of the clone (C). S100A4 was weakly expressed in the cytoplasm by cells in the center (D), but it gradually moved into the nucleus in cells in the mid-periphery (E), and then became universally nuclear in the periphery of the clone (F). Double labeling showed some cells co-expressing both α-SMA and S100A4 (F, inset). Smad4 expression was weak in the cytoplasm in the center (G) and became nuclear in some cells of the mid-periphery (H), but all cells in the periphery of the clone expressed Smad4 (I). Bar represents 50 μm.
EMT was associated with a loss of proliferative activity
Consistent with Figure 3, α-SMA was indeed expressed by large squamous cells in the cytoplasm as prominent stress fibers (Fig. 4A, marked by *), but it was also sporadically expressed in the cytoplasm without stress fibers by some small to intermediate cells in the region (Fig. 4A, marked by arrows). We therefore wondered whether such EMT in the periphery of the clonal growth might have started with the loss of progenitor cell status. To examine this possibility, we performed double immunostaining to α-SMA (green) and p63 (brown) in P15 clones and noted that part of α-SMA-expressing small/intermediate cells still expressed nuclear p63 (Fig. 4B). To further demonstrate whether such a process was accompanied by the loss of cellular proliferation, we performed double immunostaining to α-SMA (green) and PCNA (brown). These α-SMA-expressing small/intermediate cells in the mid-periphery still showed positive nuclear staining for PCNA (Fig. 4C), but as shown in Figure 1, large squamous cells that expressed α-SMA had lost proliferative activity as evidenced by negative nuclear staining of PCNA (Fig. 4D). These results indicated that small p63-expressing epithelial progenitors began to express α-SMA earlier, but eventually they lost the proliferative activity in the direction of EMT.
Figure 4. Loss of proliferative activity during EMT.
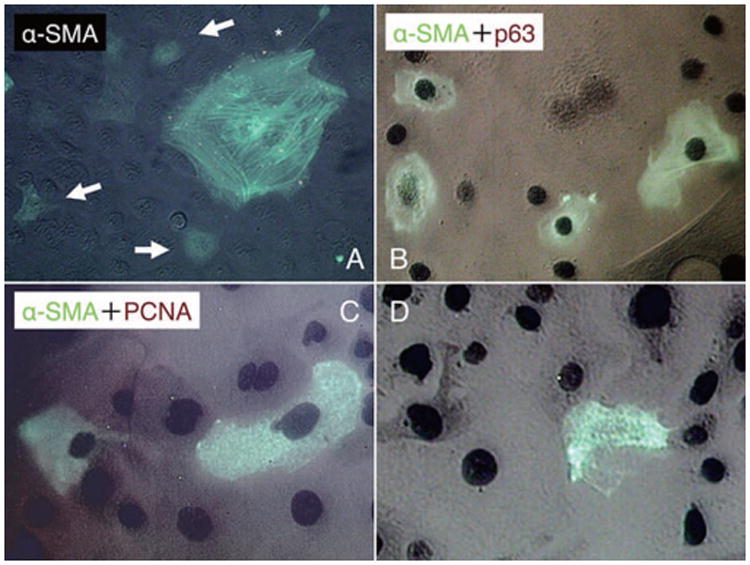
In P15 clones, α-SMA expression was observed in association with cytoplastic stress fibers in large squamous cells (A, marked by *), and also, it was found in the cytoplasm in some small to intermediate cells without stress fibers (A, marked by arrows). Double immunostaining to α-SMA (green) and p63 (brown) showed that some of the α-SMA-expressing small/intermediate cells still expressed nuclear p63 (B). Double immunostaining to α-SMA (green) and PCNA (brown) also showed that α-SMA-positive cells in the mid-periphery also had a positive nuclear staining for PCNA (C). Cells in periphery lost their nuclear staining for PCNA (D). Bar represents 50 μm.
Density-dependent increase of TGF-β, GM-CSF, and IL-1α levels in conditioned media, which promoted α-SMA expression
The above findings prompted us to speculate that EMT-mediated senescence, noted in the periphery of expanded clones, might result from paracrines secreted by an increasing cell density, which then promoted TGF-β signaling. We collected conditioned media from cultures seeded at 500, 5,000, and 50,000 cells/cm2 in KSFM for 72 h, and subjected them to Bioplex™ assays. The results showed that there was a density-dependent increase of GM-CSF and IL-1α levels in these conditioned media (Figures 5A and 5B, respectively). Similarly, levels of TGF-β1 and TGF-β2, but not TGF-β3, were detected only in conditioned media collected from cells seeded at 50,000 cells/cm2 (Fig. 5C). When the latter conditioned media was added at 40% (V/V) to this highest density culture for 7 days, the percentage of α-SMA-expressing cells was significantly promoted similar to that by 5 ng/ml TGF-β1 as compared with the control (Fig. 5D, p <0.05).
Figure 5. Density-dependent increase of GM-CSF, IL-1α, TGF-β1, and TGF-β2 levels in conditioned media, which promoted α-SMA expression.
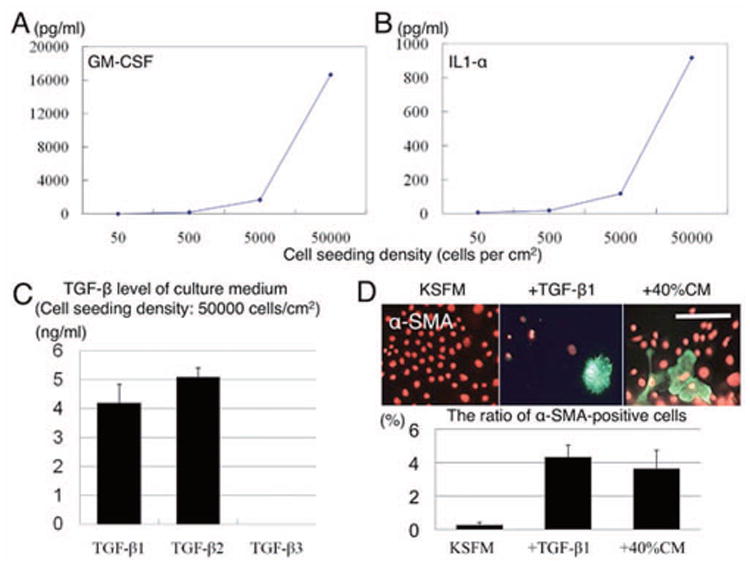
Bioplex™ assays showed that there was a density-dependent increase of GM-CSF and IL-1α in the conditioned media harvested from cells seeded at 500, 5,000 and 50,000 cells/cm2 for 72 h (A and B). Similarly, levels of TGF-β1 and TGF-β2, but not TGF-β3, were detected only in conditioned media collected from cells seeded at 50,000 cells/cm2 (C). When such conditioned media was added at 40% to this highest density culture, the percentage of α-SMA-expressing cells was similarly promoted by 5 ng/ml TGF-β1 (D). Bar represents 100 μm.
Density-dependent promotion of α-SMA expression could be blocked by TGF-β neutralizing antibody
As stated above, TGF-β plays an important role in upregulating α-SMA expression in the rat kidney (Li et al., 2004), and lung epithelial cells (Willis et al., 2005; Yao et al., 2004). Because levels of TGF-β1 and TGF-β2 were measurable in the conditioned medium of high seeding density cultures (Fig. 5), we speculated that expression of α-SMA, an indicator for EMT, was promoted in both density-dependent and culture time-dependent manners due to a progressive autocrine and/or paracrine TGF-β influence. To test this hypothesis, P15 cells were seeded at 500, 5,000, and 50,000 cells/cm2 for 1 week, and immunostained for α-SMA on days 1 and 7 after culturing (Fig. 6). On day 1, less than 0.33% of cells were α-SMA-positive in cultures seeded at 500 cells/cm2, but this increased to 1.26% in cultures seeded at 5,000 cells/ cm2, and 1.70% in cultures seeded at 50,000 cells/cm2. By day 7, such α-SMA expression was found to have increased to 0.4, 1.77, and 4.7 %, respectively (p <0.01* = only for 50,000 cells/cm2). In cultures seeded at 500 cells/cm2, addition of 5 ng/ml TGF-β for 48 h significantly promoted the expression of α-SMA with prominent stress fibers (p <0.01*) In contrast, addition of 10 μg/ml of anti-TGF-β neutralizing antibody for 48 h significantly inhibited α-SMA expression in cultures seeded at 50,000 cells/cm2 (p <0.01*). Collectively, these results supported the notion that TGF-β signaling was promoted in both density-dependent and culture time-dependent manners and was necessary to promote α-SMA-expressing EMT.
Figure 6. Inhibition of density-dependent promotion of α-SMA expression by TGF-β neutralizing antibody.
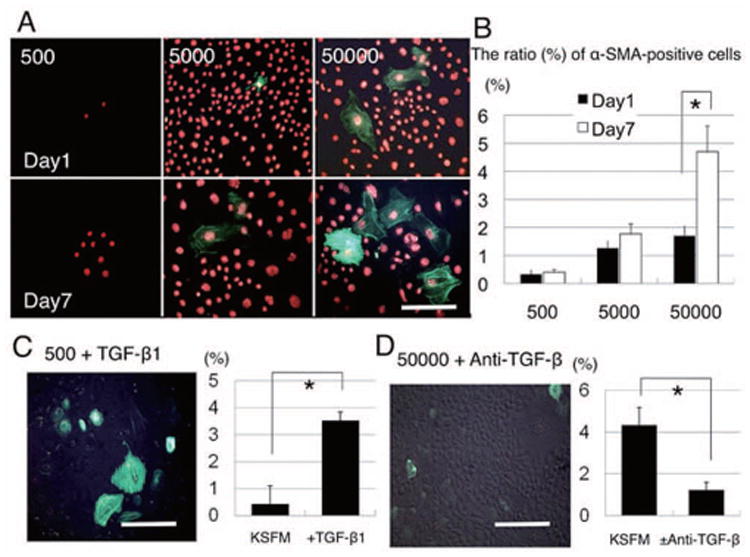
P15 cells were seeded at 500, 5,000 and 50,000 cells/cm2 for 1 week, and immunostained for α-SMA on day 1 (upper panel) and day 7 (lower panel). On day 1, fewer than 0.33% of cells were α-SMA-positive at 500 cells/cm2, but increased to 1.26% at 5000 cells/cm2, and 1.70% at 50,000 cells/cm2. By day 7, such α-SMA expression was increased to 0.4, 1.77, and 4.7 %, respectively (p <0.01* only for 50,000 cells/cm2). At 500 cells/cm2, addition of 5 ng/ml TGF-β for 48 h significantly promoted α-SMA expression, associated with prominent stress fibers (p <0.01*) Addition of 10 μg/ml of anti-TGF-β neutralizing antibody for 48 h significantly inhibited α-SMA expression at 50,000 cells/cm2 (p <0.01*). Bar represents 100 μm.
Inhibition of the Wnt/β-catenin pathway partially inhibits EMT
In comparison to the control, 5ng/ml TGF-β1 promoted the cytoplasmic expression of Snail, α-SMA, MMP7, and MMP9 (Fig. 7A). Such promotion of α-SMA, Snail, and Slug by 1, and 5 ng/ml TGF-β was also confirmed by RT-PCR (Fig. 7B). The Wnt/catenin inhibitor, Dickkopf, did not inhibit Snail expression, but it inhibited both MMP2 and α-SMA (Fig. 7C). Furthermore, the Wnt/β-catenin activator, BIO, also did not promote Snail, but it promoted MMP2 and maintained the expression of α-SMA.
Figure 7. Inhibition of Wnt/β-catenin pathway partially inhibits EMT.
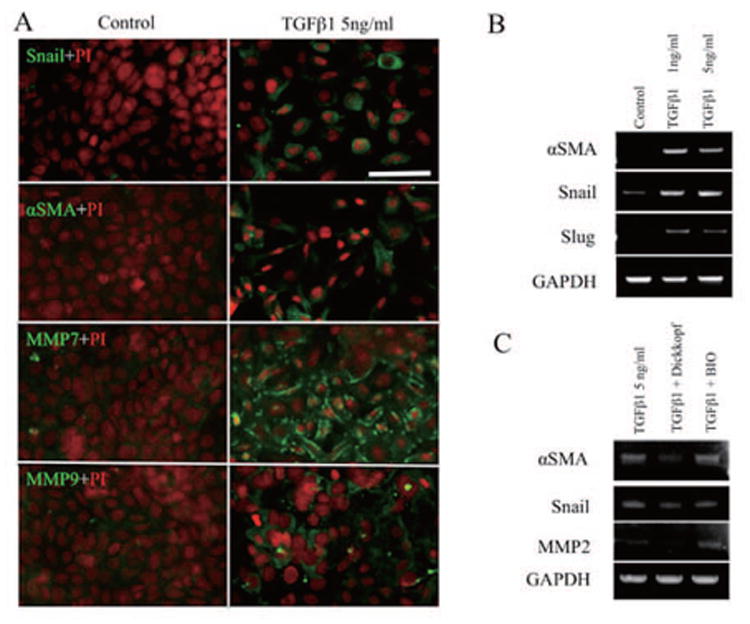
, P100 cells were seeded at 5,000 cells/cm2 and stimulated by 5 ng/ml TGF-β1 for 48 h, and then immunostained for Snail, α-SMA, MMP7 and MMP9. (A) To compare vs. control, TGF-β1 was used to promote cytoplasmic expression of Snail, α-SMA, MMP7 and MMP9, which was also confirmed by RT-PCR. (B) The Wnt/catenin inhibitor, Dickkopf, did not inhibit Snail, but it blocked the expression of MMP2 and α-SMA. BIO (a Wnt/catenin activator) also did not promote Snail, but promoted MMP2 and maintained α-SMA expression. Bar represents 50 μm.
Discussion
Many studies have shown that ex vivo expansion of epithelial progenitor cells requires the use of a serum-free, low [Ca2+] medium (Yuspa et al., 1988) supplemented with growth factors if fibroblast feeder layers are not used (Heng et al., 2005). Using such a culturing medium, we have already succeeded in expanding murine limbal/corneal epithelial progenitor cells by two methods: a low seeding density, and a prolonged culturing time beyond the life span of transit amplifying cells (manuscript submitted). These expanded progenitor cells could undergo clonal growth and generate a progeny consisting of mostly uniformly small cells that expressed nuclear staining to PCNA and p63 (Fig. 4), which is indicative of preservation of proliferating epithelial progenitor cells for stratified epithelia. These small epithelial progenitor cells could still undergo normal terminal differentiation into large squamous cells expressing K12 keratin upon either an increase of [Ca2+], addition of FBS, or a combination of both (supplemental data). In such single cell-derived clonal growth, there was a gradual activation of the Wnt/β-catenin signaling pathway as evidenced by dissolution of E-cadherin and β-catenin from intercellular junctions, followed by the nuclear translocation of β-catenin and LEF-1 in the periphery of the clone (Fig. 2). Several studies have suggested that activation of Wnt/β-catenin signaling pathway is involved in the turnover of epithelial stem cells in the intestines, [44, (He et al., 2004) and mammary glands (Liu et al., 2005), as well as hematopoietic stem cells (Reya et al., 2003). In our culturing system, we found that a low [Ca2+] (i.e., 0.07 mM), low seeding density, and supplementation of growth factors in KSFM medium can all help prevent the formation of intercellular junctions and facilitate activation of Wnt/β-catenin signaling.
Continuous clonal growth did require activation of Wnt/β-catenin signaling as shown in the mid-periphery of the clone (Fig. 2), and this was eventually accompanied by progressive differentiation in the periphery: as illustrated by a gradual increase of cell sizes and positive cytoplasmic staining for K14 keratin (Fig. 1), a marker for the basal epithelial phenotype of stratified epithelia. Such progressive cellular differentiation also correlated with the loss of p63 nuclear staining (Fig. 1), and loss of large-colony-forming efficiency, suggesting the loss of progenitor status. This process was accompanied by the emergence of α-SMA-containing stress fibers in the cytoplasm (Figures 3 and 4) and positive nuclear staining for S100A4 (Fig. 3), which is indicative of EMT. Previously, cytoplasmic expression of α-SMA (Chagraoui et al., 2003; Masszi et al., 2003; Thiery and Sleeman, 2006; Willis et al., 2005) or S100A4 (Le Hir et al., 2005; Rossini et al., 2005) was found to marks EMT in several different types of cultured epithelial cells. However, nuclear translocation of S100A4, which correlates with α-SMA expression in rabbit corneal fibroblasts during their myofibroblast differentiation (Ryan et al., 2003), has not previously been reported in EMT. Nuclear translocation of S100A4 is thought to be a pre-apoptotic event, because of its potential binding with p53 (Grigorian et al., 2001). The notion that EMT could lead to cellular senescence was supported by our finding that the emergence of α-SMA expression in the cytoplasm preceded the loss of nuclear expression of p63 and PCNA (Fig. 4). Given that activation of Wnt/β-catenin signaling plays an important role in stem cell self-renewal (Kuhnert et al., 2004)46] and is an integral part of EMT (Thiery and Sleeman, 2006), we, therefore, speculated that there must be an important trigger during EMT for stem cells to move from turnover to differentiation/senescence.
Our study identified one key trigger for the activation of Smad-mediated TGF-β signaling. We demonstrated that the nuclear translocation of Smad4 is indicative of activation of TGF-β signaling (Massague and Wotton, 2000), which appeared as a gradient from the mid-periphery to the periphery (Fig. 3). Because α-SMA expression increased proportionally according to the cell seeding density, and the culture time from Day 1 to Day 7 (Fig. 6), one likely mechanism for turning on Smad4-mediated TGF-β signaling was thought to be via a density-dependent autocrine and paracrine influence. This interpretation was supported by detecting 4-6 ng/ml of TGF-β1 and TGF-β2, but not TGF-β3, in conditioned media of high, but not low, density cultures (Fig. 5), and also, by the finding that addition of such condition media or exogenous TGF-β1 at a comparable level to both high (Fig. 5) and low density cultures (Fig. 6), upregulated α-SMA expression. Finally, such an upregulation was partly abolished by addition of a TGF-β neutralizing antibody in the aforementioned conditioned medium (Fig. 6).
Activation of Smad-mediated TGF-β signaling has been shown to facilitate irreversible EMT by working in concert with Wnt/β-catenin signaling (Thiery and Sleeman, 2006). TGF-β1 further downregulates E-cadherin (Fan et al., 1999; Tian et al., 2003) and ZO-1 (Miettinen et al., 1994), rendering rat tubular and mouse mammary epithelial cells susceptible to EMT. Although the complex of β-catenin/LEF-1 directly regulates the SMA promoter (Masszi et al., 2004), an additional complex with Smad4 augments the transactivation (Nawshad and Hay, 2003). When human keratinocytes (HaCaT) are seeded at a high density, β-catenin translocates from the cytoplasm to the plasma membrane (Dietrich et al., 2002). Furthermore, TGF-β1 promotes the association of SMAD with β-catenin to recruit LEF-1 (Tian et al., 2003), and stimulates the association of LEF-1 with SMAD in MDCKII cells, augmenting transcription of snail via SMAD-binding elements (Medici et al., 2006).
Epithelial–mesenchymal transition by TGF-β mediated Wnt/β-catenin signaling activation was partially inhibited by Dickkopf, and partially promoted by BIO, which was confirmed by the MMP and α-SMA expression patterns. However, such Wnt/β-catenin signaling modulation did not regulate snail expression, which needs further study to clarify the molecular mechanisms responsible.
Consequently, continuous clonal growth inevitably gave rise to progressive differentiation and EMT-mediated senescence as a result of activation of TGF-β signaling. Schematic illustration was shown (Fig. 8) Besides TGF-β1 and TGF-β2, the conditioned media also contained GM-CSF and IL-1α, which are also reported to serve as autocrine and/or paracrine mediators of inflammation and apoptosis (Liu et al., 1996). Because secretion of TGF-β1 and TGF-β2 (Lucia et al., 1998), GM-CSF (Migliaccio et al., 1988) and IL-1α (Larsen et al., 1989) is promoted during epithelial differentiation, we also suspected that transit amplifying cells and terminally differentiated cells might exert a negative influence via these paracrines on stem cell expansion. This hypothesis helps to explain why we found that a prolonged cultivation time and a low seeding density were two useful techniques to successfully expand murine limbal/corneal epithelial progenitor cells. Further investigation into the pathogenic role of TGF-β signaling in triggering EMT in this clonal culture might unravel not only new strategies to further improve ex vivo expansion of adult epithelial stem cells, but also shed some light into why limbal stem cell deficiency develops in severe ocular surface diseases associated with tissue fibrosis.
Figure 8.
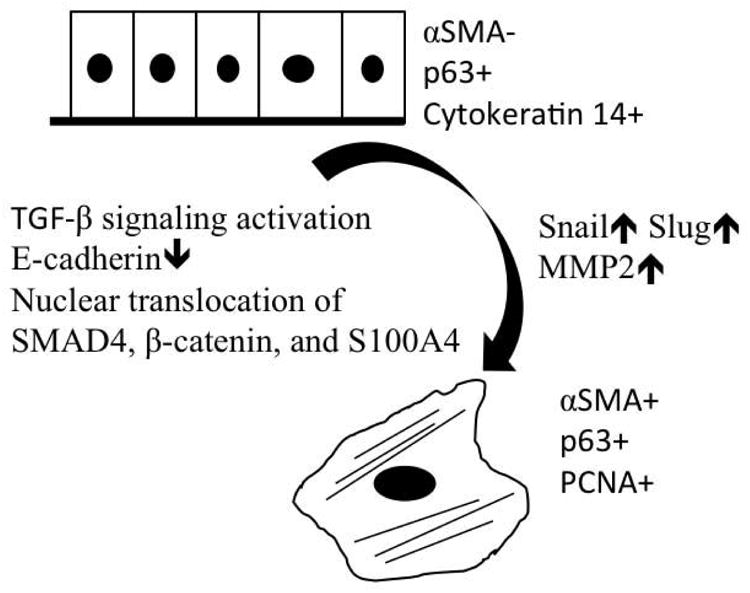
Schematic illustration of possible mechanism for epithelial-mesenchymal transition in peripheral location of epithelial colonies.
Supplementary Material
Acknowledgments
This work was supported by EY06819 and EY015735 grants from the National Eye Institute, National Institutes of Health, Bethesda, MD, a research grant from TissueTech, Inc., an unrestricted grant from the Ocular Surface Research and Education Foundation, Miami, FL; a Grant-in-Aid for Young Scientists (B) (18790131) from the Ministry of Education, Culture, Sports, Science and Technology, and a Grant-in-Aid for Scientific Research (H18-tissue engineering-young -002) from the Ministry of Health, Labour and Welfare.
Supported by RO1 EY06819 and RO1EY15735 grants (SCGT) from National Institutes of Health, National Eye Institute, Bethesda, MD, a research grant from TissueTech, Inc., Culture, Sports, Science and Technology, and Grant-in-Aid for Scientific Research (H18-regeneration-young-002) from the Ministry of Health and Welfare. There was no conflict of interest among researchers about this manuscript.
Footnotes
Presented in part at the annual meeting of Association of Research in Vision and Ophthalmology at Ft. Lauderdale, FL, in May 2007.
References
- Barnard JA, Bascom CC, Lyon RM, Sipes NJ, Moses HL. Transforming growth factor-キ in the control of epidermal proliferation. 1988;31:159–163. doi: 10.1097/00000441-198809000-00003. [DOI] [PubMed] [Google Scholar]
- Caldelari R, Suter MM, Baumann D, De Bruin A, Muller E. Long-term culture of murine epidermal keratinocytes. 2000;114(5):1064–1065. doi: 10.1046/j.1523-1747.2000.00960-4.x. [DOI] [PubMed] [Google Scholar]
- Chagraoui J, Lepage-Noll A, Anjo A, Uzan G, Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003;101(8):2973–2982. doi: 10.1182/blood-2002-05-1341. [DOI] [PubMed] [Google Scholar]
- Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162(5):1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C, Scherwat J, Faust D, Oesch F. Subcellular localization of beta-catenin is regulated by cell density. Biochem Biophys Res Commun. 2002;292(1):195–199. doi: 10.1006/bbrc.2002.6625. [DOI] [PubMed] [Google Scholar]
- Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56(4):1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Forino M, Torregrossa R, Ceol M, Murer L, Vella MD, Prete DD, D'Angelo A, Anglani F. TGFbeta1 induces epithelial-mesenchymal transition, but not myofibroblast transdifferentiation of human kidney tubular epithelial cells in primary culture. Int J Exp Pathol. 2006;87(3):197–208. doi: 10.1111/j.1365-2613.2006.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian M, Andresen S, Tulchinsky E, Kriajevska M, Carlberg C, Kruse C, Cohn M, Ambartsumian N, Christensen A, Selivanova G, Lukanidin E. Tumor suppressor p53 protein is a new target for the metastasis-associated Mts1/S100A4 protein: functional consequences of their interaction. J Biol Chem. 2001;276(25):22699–22708. doi: 10.1074/jbc.M010231200. [DOI] [PubMed] [Google Scholar]
- Hager B, Bickenbach JR, Fleckman P. Long-term culture of murine epidermal keratinocytes. 1999;112(6):971–976. doi: 10.1046/j.1523-1747.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Heng BC, Cao T, Liu H, Phan TT. Directing stem cells into the keratinocyte lineage in vitro. Exp Dermatol. 2005;14(1):1–16. doi: 10.1111/j.0906-6705.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. 2002;110(3):341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422(6929):317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol. 2005;167(2):381–393. doi: 10.1016/S0002-9440(10)62983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45(10):3507–3512. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. 2002;26(5):463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse FE, Tseng SCG. Proliferative and differentiative response of corneal and limbal epithelium to extracellular calcium in serum-free cultures. 1992;151:347–360. doi: 10.1002/jcp.1041510216. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101(1):266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. 2004;78(3):433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol. 2005;123(4-5):335–346. doi: 10.1007/s00418-005-0788-z. [DOI] [PubMed] [Google Scholar]
- Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164(4):1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17(1):26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7(3):86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Striker GE, Stetler-Stevenson M, Fukushima P, Patel A, Striker LJ. TNF-alpha and IL-1 alpha induce mannose receptors and apoptosis in glomerular mesangial but not endothelial cells. Am J Physiol. 1996;270(6 Pt 1):C1595–1601. doi: 10.1152/ajpcell.1996.270.6.C1595. [DOI] [PubMed] [Google Scholar]
- Lucia MS, Sporn MB, Roberts AB, Stewart LV, Danielpour D. The role of transforming growth factor-beta1, -beta2, and -beta3 in androgen-responsive growth of NRP-152 rat prostatic epithelial cells. J Cell Physiol. 1998;175(2):184–192. doi: 10.1002/(SICI)1097-4652(199805)175:2<184::AID-JCP8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19(8):1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2003;284(5):F911–924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol. 2004;165(6):1955–1967. doi: 10.1016/s0002-9440(10)63247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney-Francis NL, Wahl SM. Transforming growth factor キ: a matter of life and death. 1994;55:401–409. doi: 10.1002/jlb.55.3.401. [DOI] [PubMed] [Google Scholar]
- Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17(4):1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127(6 Pt 2):2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G, Migliaccio AR, Adamson JW. In vitro differentiation of human granulocyte/macrophage and erythroid progenitors: comparative analysis of the influence of recombinant human erythropoietin, G-CSF, GM-CSF, and IL-3 in serum-supplemented and serum-deprived cultures. Blood. 1988;72(1):248–256. [PubMed] [Google Scholar]
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380(6572):360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Nawshad A, Hay ED. TGFbeta3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J Cell Biol. 2003;163(6):1291–1301. doi: 10.1083/jcb.200306024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. 1997;273(4 Pt 2):F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- Owens DW, McLean GW, Wyke AW, Paraskeva C, Parkinson EK, Frame MC, Brunton VG. The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell-cell contacts. Mol Biol Cell. 2000;11(1):51–64. doi: 10.1091/mbc.11.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguly T, Wrana JL. In or out? The dynamics of Smad nucleocytoplasmic shuttling. Trends Cell Biol. 2003;13(5):216–220. doi: 10.1016/s0962-8924(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Risse Marsh BC, Massaro-Giordano M, Marshall CM, Lavker RM, Jensen PJ. Initiation and characterization of keratinocyte cultures from biopsies of normal human conjunctiva. 2002;74(1):61–69. doi: 10.1006/exer.2001.1099. [DOI] [PubMed] [Google Scholar]
- Rossini M, Cheunsuchon B, Donnert E, Ma LJ, Thomas JW, Neilson EG, Fogo AB. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int. 2005;68(6):2621–2628. doi: 10.1111/j.1523-1755.2005.00734.x. [DOI] [PubMed] [Google Scholar]
- Ryan DG, Taliana L, Sun L, Wei ZG, Masur SK, Lavker RM. Involvement of S100A4 in stromal fibroblasts of the regenerating cornea. 2003;44(10):4255–4262. doi: 10.1167/iovs.03-0578. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WW, Roberts AB. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164(2):651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. BioEssays. 2001;23(10):912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. 2003;3(11):807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29(5):265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Tian YC, Fraser D, Attisano L, Phillips AO. TGF-beta1-mediated alterations of renal proximal tubular epithelial cell phenotype. 2003;285(1):F130–F142. doi: 10.1152/ajprenal.00408.2002. [DOI] [PubMed] [Google Scholar]
- Tseng SCG, Espana EM, Kawakita T, Di Pascuale MA, Wei ZG, He H, Liu TS, Cho TH, Gao YY, Yeh LK, Liu CY. How does amniotic membrane work? The Ocular Surface. 2004;2(3):177–187. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166(5):1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. 1998;2(3):305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159(4):1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HW, Xie QM, Chen JQ, Deng YM, Tang HF. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004;76(1):29–37. doi: 10.1016/j.lfs.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280(12):11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Hennings H, Tucker RW, Jaken S, Kilkenny AE, Roop DR. Signal transduction for proliferation and differentiation in keratinocytes. Ann N Y Acad Sci. 1988;548:191–196. doi: 10.1111/j.1749-6632.1988.tb18806.x. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. 2001;159(4):1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


