Abstract
A 59-year-old man with a history of peripheral vascular disease status post femoral popliteal bypass presented with critical limb ischaemia of the left leg. An arterial Doppler ultrasound showed an occluded graft requiring an above knee amputation. Five days after surgery, the patient developed fever, leucocytosis, significant stump swelling and pain, and serosanguinous discharge from his wound. Wound swab cultures from the stump grew Trichosporon asahii. A venous Doppler ultrasound revealed extensive thrombosis of the left lower extremity. Biopsy of the left thigh muscle showed necrotic thrombus with fungal hyphae in the clotted blood vessel. The left femoral vein was subsequently resected, and the excised venous tissue also grew T. asahii. The patient was successfully treated with voriconazole based on antifungal susceptibilities. This case describes an invasive fungal infection in the absence of typical immunosuppressive conditions commonly associated with Trichosporon spp. It also illustrates the role of a combination of antimicrobial and surgical management in achieving cure.
Keywords: infections, nosocomial infections, pathology, vascular surgery
Background
Trichosporon asahii is an emerging, life-threatening opportunistic pathogen. It is a yeast-like fungus, often found in soil, decomposing wood, air, rivers, lakes, seawater, cheese, scarab beetles, bird droppings, bats, pigeons and cattle.1 In humans, the fungus can occasionally colonise the skin, respiratory and gastrointestinal tracts including the oral cavity.2 The genus ‘Trichosporon’ currently consists of over 50 species and 16 of these can cause human disease. Trichosporon is distributed widely in nature and found predominantly in tropical and temperate areas of the world.
While several Trichosporon sp only cause skin and nail infections, invasive infections do rarely occur notably by T. asahii in patients who were immunocompetent, often with fatal outcomes. T. asahii septic thrombophlebitis is rare and only a limited number of reports describing infections of the peripheral vasculature are available.
Successful treatment of patients with invasive trichosporonosis remains a challenge because of limited data on the in vitro antimicrobial susceptibilities and in vivo clinical performance.
We present a case of T. asahii stump infection and septic thrombophlebitis in a patient who was immunocompetent with known peripheral vascular disease following above knee amputation who was successfully treated with voriconazole and surgical intervention. We also provide a review of relevant literature.
Case presentation
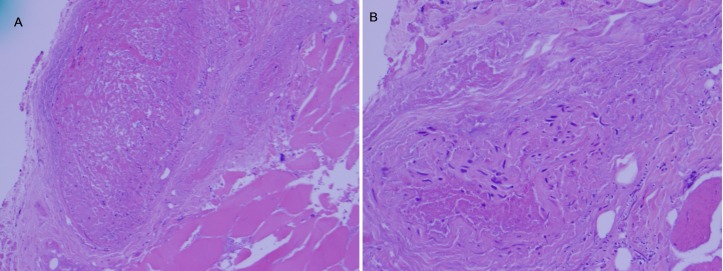
A 59-year-old man with a medical history of peripheral vascular disease status post femoral popliteal bypass a year prior, presented with critical limb ischaemia of the left leg. An arterial Doppler ultrasound suggested an occluded graft with no detected pulses. The patient underwent an above knee amputation, with wound vacuum-assisted closure (VAC) placement and the procedure was well tolerated. Five days after the surgery, the patient developed fever spikes, leucocytosis, significant stump swelling and pain, and serosanguinous discharge from his wound. He was started on clindamycin empirically and underwent repeated wound debridement with continuation of the wound VAC treatment. Cultures obtained from the wound initially grew coagulase-negative Staphylococcus, alpha-haemolytic Streptococcus and Corynebacterium spp. Clindamycin was subsequently discontinued, and ampicillin and gentamycin were started. T. asahii was identified from the tissue sample by a microscan rapid yeast identification panel and subsequent wound swab cultures from the stump later grew T. asahii 2 weeks after the amputation surgery. Infectious disease service was consulted for antimicrobial management of the stump infection. Evaluation showed swollen tender stump with copious brownish discharge. Empiric antibiotic coverage was broadened to include vancomycin, meropenem, voriconazole and amphotericin. Due to initial poor clinical response, a repeat venous Doppler ultrasound was done which revealed extensive thrombosis of the left lower extremity involving the external iliac, common femoral veins and extending into the superficial and deep femoral veins down to the level of the amputation. A CT scan of the lower extremity without contrast however showed no abscess collection or bone necrosis. A biopsy of the left thigh muscle in the proximity of the stump was done, which revealed necrotic thrombus (figure 1A) with fungal hyphae in the clotted blood vessel (figure 1B) and the surrounding muscles (figures 2 and 3).
Figure 1.
(A) The section shows a large vessel on the left and muscle on the right and necrotic thrombus is noted in the vessel (×10). (B) H&E stain showing fungal elements in the vessel and muscle (×20).
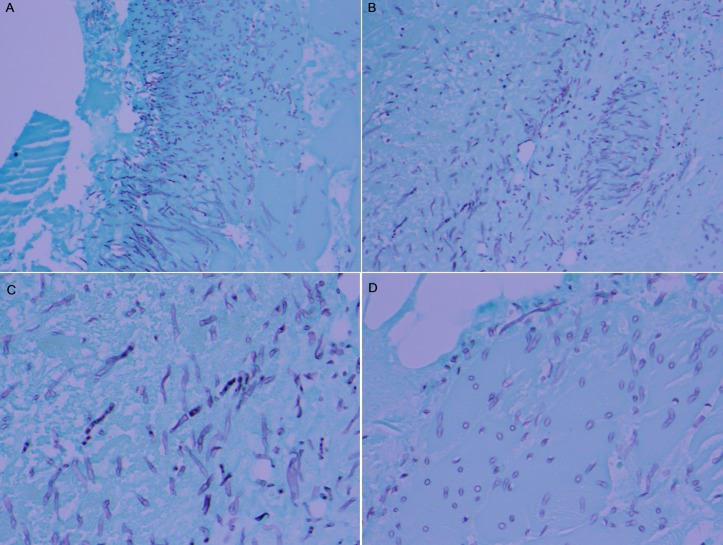
Figure 2.
The Grocott’s methenamine silver (GMS) stained section revealing fungal elements: (A) in the vessel (×20), (B) in the vessel (×20), (C) in the muscle (×40), (D) in the vessel (×40). GMS
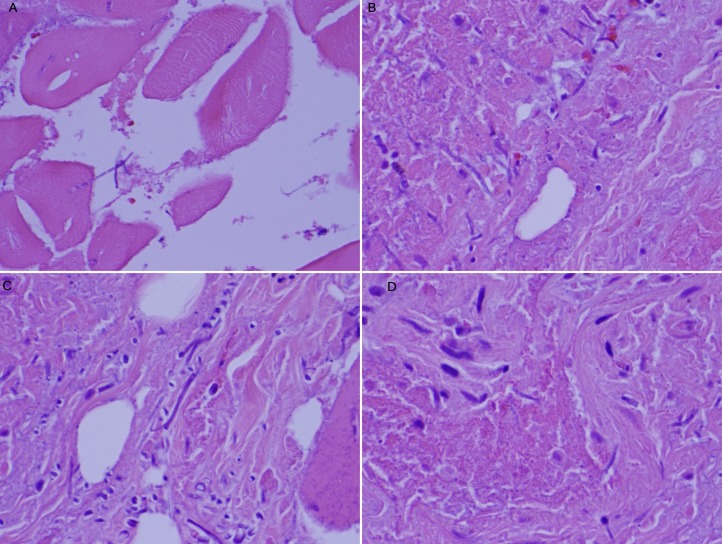
Figure 3.
H&E stain showing fungal elements: (A) in the muscle (×40), (B) fungal elements (×60), (C) in the connective tissue (×60), (D) in the vessel (×60).
The patient was taken back to surgery and the left femoral vein was resected 6 cm in length from the infected margins. The excised venous tissue also grew T. asahii and fungal susceptibilities with minimal inhibitory concentration (MIC) are shown in table 1. Antifungal susceptibilities was done by a colorimetric microdilution susceptibility test. Each plate was dosed with appropriate dilutions of antifungal agents and a colorimetric indicator. After inoculation with a standardised suspension of organisms in an inoculum medium and incubation at 35°C for 24–48 hours, the MIC for the test organism were determined by observing the lowest antifungal concentration showing inhibition of growth as evidenced by no colour change. Amphotericin B was discontinued and intravenous voriconazole was continued based on susceptibility results. The patient eventually required multiple debridement of the wound for three additional weeks. After 1 month of debridement and antimicrobial therapy, subsequent bone and tissue culture from the stump did not reveal any new fungal organisms.
Table 1.
Antifungal susceptibilities by colorimetric microdilution susceptibility test for T. asahii including MIC
| Drug | MIC (µg/mL) |
| Itraconazole | 0.25 |
| Posaconazole | 0.25 |
| Voriconazole | 0.12 |
| Amphotericin B | 0.5 |
| Micafungin | >8 |
| Caspofungin | >8 |
| Fluconazole | 8 |
There are no CLSI or published breakpoints for this genus of the yeast and the only MIC in µg/mL is reported.
CLIS, Clinical & Laboratory Standards Institute; MIC, minimal inhibitory concentration; T. asahii, Trichosporon asahii.
Investigations
CT scan of the left lower extremity.
CT scan of the abdomen and pelvis.
Blood bacterial and fungal cultures.
Wound tissue bacterial and fungal cultures.
Doppler ultrasound the left lower extremity.
Significant laboratory values include white blood cell count: 16.59 (reference range: 3.6–11.2 K/μL), neutrophils absolute: 14.71 (reference range: 1.8–7.8 K/μL), serum creatinine: 0.95 (reference range: 0.70–1.30 mg/dL), procalcitonin: 0.12 ng/mL (>1.9 represents a high risk of severe sepsis or septic shock), hemoglobinA1c: 5.8 (reference range: 3.9%–6.1%) and fourth generation HIV 1 testing: negative.
Differential diagnosis
Gram positive aerobes such as Staphylococcal infections.
Gram-negative rod infection.
Mycobacterial infections.
Other fungal infections such as aspergillosis and mucor.
Treatment
Voriconazole 200 mg orally two times per day for 6 weeks.
Outcome and follow-up
At the time of discharge the infection had subsided and the wound was showing good evidence of healing. The patient was discharged on voriconazole 200 mg orally two times per day for additional 4 weeks. At his 6-month follow-up, the left stump skin graft was completely healed and the patient was considered ready for limb prosthesis.
Discussion
T. asahii is an emerging fungal pathogen generally associated with superficial infections in immunocompetent patients and invasive infections in immunocompromised hosts.
T. asahii is the most common species causing invasive trichosporonosis, and consists of nine different genotypes, based on the intergenic spacer 1 (IGS1) sequence.3 It is relatively easy to grow on most standard fungal media even though species identification and taxonomy have been rather challenging.
Opportunistic fungal pathogens retain several factors that allow their growth and permit the establishment of disease and their dissemination within the host.4 Risk factors related to impaired host immunity are often encountered in the background of invasive fungal infections including those caused by Trichosporon. A recent systematic review of invasive Trichosporon infections over the last two decades revealed most infections occurred in patients suffering from haematological malignancies, followed by solid organ transplantation, solid tumours, autoimmune disorders and postoperative complications. Trichosporon has the ability to adhere to and form biofilms on implanted devices, and this unique characteristic can account in part for its virulence and resistance to antifungal drugs.5 Another important virulence factor possessed by Trichosporon is the ability to produce and secrete enzymes which helps in scavenging nutrients from the environment. Proteases and phospholipases produced by Trichosporon also contribute in cleaving host proteins and promote disruption of host cell membranes.
T. faecale and T. mycotoxinovorans are potential human pathogens of invasive infections in immunocompromised patients. T. asteroides, and T. mucoides also are known to cause invasive infections. T. ovoides, T. inkin and T. cutaneum which can cause white piedra while the latter can also cause allergic pneumonia.6 More recently, Trichosporon cutaneum has been transferred into a new genus named Cutaneotrichosporon. T. asahii has been implicated in the causation of invasive infections in immunocompromised patients7 such as catheter-associated fungaemia and disseminated infections in patients with neutropaenia.8 9
Rastogi and Nirwan reported an invasive infection in a healthy young adult male diagnosed with T. asahii meningoencephalitis and pneumonia.10 Zhang et al studied the skin of 380 healthy Japanese using a nested PCR assay, detecting T. asahii in 128 of 200 men, and in 42 of 180 women.11
Infections caused by invasive Trichosporon spp are frequently associated with central venous catheters, vesical catheters and peritoneal catheter-related devices. As alluded to earlier, biofilm coating of these devices is paramount to the fungus ability to evade host defences and promote virulence and relative resistance to antifungal drugs. Colombo et al reported that T. asahii biofilms were resistant to all antifungals tested (MIC 1024 g/mL) and the isolates were up to 16 000 times more resistant to voriconazole than planktonic cells (MIC 0.06 g/mL).12
The number of reported cases of invasive Trichosporon infection has gradually increased over time, especially over the past decade. This increase is likely due to improved cancer diagnosis, invasive procedures and increased use of chemotherapy. Compromised immunity especially related to haematological malignancies have been implicated as a major risk factor for trichosporonosis, occurring in about 30%–40% of patients notably during the neutropaenic phase.13 Patients in intensive care unit (ICU) with invasive procedures are at a high risk for invasive trichosporonosis. Unfortunately, majority of the invasive Trichosporon infections reported often resulted in a negative outcome with mortality close to 80% especially in the immunocompromised hosts.14
Even though compromised immunity in trichosporonosis is very common, our literature review revealed a few cases of invasive infections attributable to T. asahii in healthy individuals with no risk factors for immune suppression. Chan et al described a case of endometritis in a healthy woman with no risk factors.15 Wolf et al also reported six ICU cases related to Trichosporon including four skin infections and two invasive infections which were successfully treated. The first invasive infection was a case of purulent peritonitis associated with peritoneal dialysis catheter, successfully cured by removal of the peritoneal dialysis catheter and amphotericin B therapy (duration was not mentioned). The second case was a woman with penetrating trauma, multiple rib fractures and pulmonary contusion on ventilator support who developed numerous necrotic skin nodules. Skin biopsy and urine cultures grew T. asahii. She was also successfully treated with amphotericin B for 2 weeks, with resolution of fever and cutaneous lesions. In a similar case, T. asahii was isolated from the urine of an apparently immunocompetent elderly woman with a history of atrial fibrillation and peripheral vascular disease who was admitted to the ICU for head trauma.16
Despite these favourable outcomes, fatal outcomes are common with invasive Trichosporon infection. Baraboutis et al described a case of a severely ill patient with no cancer and no neutropaenia but who developed an ultimately lethal T. asahii mediastinitis and osteomyelitis after sternotomy. The patient had been previously colonised with the fungus. Therapy with liposomal amphotericin failed to sterilise the bloodstream despite favourable in vitro susceptibility results. Even though the addition of voriconazole cleared the fungaemia, the patient still had a fatal outcome.17
Kim et al described the first case of T. asahii spondylodiscitis that developed in a healthy woman at the site of an open lumbar discectomy. The drained abscess grew a yeast, identified as T. asahii that was successfully treated with 28-day fluconazole based on susceptibility results.18
Our case report illustrates the fact that T. asahii can both complicate stump infection and cause septic thrombophlebitis. As source control is highly important in any invasive fungal surgical site infection, we believe aggressive source control by repeated debridement played a crucial role in the recovery of this patient. The same principle should be adopted for invasive T. asahii infection under similar scenarios.
Amphotericin B is also often unsuccessful, and this observation is further corroborated by the high MICs in our case.19 20 Using the European Committee on Antimicrobial susceptibility testing (EUCAST) methodology, lowest MICs were observed for voriconazole. The MIC results obtained for the azoles, either using the Clinical and Laboratory Standards Institute (CLSI) or the EUCAST method, are quite similar with the possible exception of voriconazole for which higher MICs were observed using the EUCAST method in one of two studies (0.21–0.29 mg/L for T. inkin and 0.61–0.69 mg/L for T. mucoides/dermatis). Conversely, a wide range of MIC geometric means (GMs) of fluconazole against T. mucoides/dermatis has been reported, ranging from 0.25 to 7 mg/L. Two of three studies described higher fluconazole MICs of T. mucoides/dermatis isolates in comparison with the MICs of T. asahii isolates.20
Voriconazole, posaconazole and isavuconazole are extended spectrum triazoles that have demonstrated good efficacy for Trichosporon in in vitro studies.21 Among the azole compounds, fluconazole is the most extensively analysed. MIC distribution of fluconazole is heterogeneous, with MIC50s, MIC90s and MIC GMs ranging between 0.5 and 16, 1 and 64 and 0.8 and 17.1 mg/L, respectively. Echinocandins have no role in the treatment of invasive Trichosporon infection as almost all T. asahii isolates are intrinsically resistant.22
Due to the limited, small case series often involving single institutions, no comprehensive analysis is available to guide our understanding of the epidemiology, optimal therapeutic approaches and prognostic factors associated with trichosporonosis.23 Only molecular methods, such as IGS region sequencing, allow the complete identification of Trichosporon isolates at the species level. Methods for the diagnosis of invasive trichosporonosis include PCR-based methods, Luminex xMAP technology and, more recently, proteomics. Matrix-assisted laser desorption ionisation time of flight mass spectrometry (MALDI-TOF MS), VITEK 2 enable rapid and reliable identification of clinically important yeasts.24
Duration of therapy for invasive Trichosporon infection is often unclear since most published studies are from case reports/series. Several of these hosts are often neutropaenic and careful monitoring preferably in the ICU, until neutrophilic recovery is often necessary. Serum galactomannan was not done as fungal infections were not on our top differentials in the immediate postoperative period infection in this patient who was immunocompetent. However, it would have been helpful for prognostic purpose and to monitor treatment response.
This case report is unique in the sense that it illustrates a rare invasive fungal infection likely contributing to thrombosis and septic thrombophlebitis in a patient who was immunocompetent with peripheral vascular disease. A thorough literature search did not show T. asahii as an aetiological agent responsible for a similar presentation in an immunocompetent host. Whether patients with underlying peripheral vascular disease are at an increased risk of vascular infection with this fungus is not clear currently. Future research to study such possible association is reasonable and will provide further insight into clinicians. We believe that this case report will contribute to current knowledge by increasing awareness, emphasise the importance of prompt diagnosis and assist in the optimal management of invasive Trichosporon infections.
Learning points.
Trichosporon asahii is an emerging, life-threatening opportunistic pathogen which is commonly associated with superficial infections of hair shaft (white piedra), allergic pneumonitis but can also cause invasive infections in immunocompromised patients.
Risk factors for Trichosporon infection are haematological malignancies, solid organ transplantation, solid tumours, auto immune disorders and postoperative complications.
Echinocandins have no role in the treatment of T. asahii infections. Triazoles have demonstrated good efficacy in in vitro studies and voriconazole is probably the drug of choice.
Footnotes
Contributors: PKM: design of the case report. FA: data acquisition. AL: analysis and interpretation of the data/images. JT: revised the manuscript for intellectual content and finalised it.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Walsh TJ, Melcher GP, Lee JW, et al. Infections due to Trichosporon species: new concepts in mycology, pathogenesis, diagnosis and treatment. Curr Top Med Mycol 1993;5:79–113. [PubMed] [Google Scholar]
- 2.Colombo AL, Padovan AC, Chaves GM. Current knowledge of Trichosporon spp. and trichosporonosis. Clin Microbiol Rev 2011;24:682–700. 10.1128/CMR.00003-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guého E, de Hoog GS, Smith MT. Neotypification of the genus Trichosporon. Antonie Van Leeuwenhoek 1992;61:285–8. 10.1007/BF00713937 [DOI] [PubMed] [Google Scholar]
- 4.Hogan LH, Klein BS, Levitz SM. Virulence factors of medically important fungi. Clin Microbiol Rev 1996;9:469–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Bonaventura G, Pompilio A, Picciani C, et al. Biofilm formation by the emerging fungal pathogen trichosporon asahii: development, architecture, and antifungal resistance. Antimicrob Agents Chemother 2006;50:3269–76. 10.1128/AAC.00556-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke M, Matthews JJ, Ambrosetti DR, et al. Trichosporon funguemia in a pediatric patient with acute lymphoblastic leukemia. Elsevier 2015;2:106–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jong IC, Hsu GJ, Hung PH, et al. Invasive trichosporonmucoides infection in a uremic patient with type 2 diabetes mellitus on maintenance hemodialysis. ActaNephrologica 2011;25:30–2. [Google Scholar]
- 8.Takamura S, Oono T, Kanzaki H, et al. Disseminated trichosporonosis with trichosporon asahii. Eur J Dermatol 1999;9:577–9. [PubMed] [Google Scholar]
- 9.Ruan SY, Chien JY, Hsueh PR. Invasive trichosporonosis caused by Trichosporon asahii and other unusual Trichosporon species at a medical center in Taiwan. Clin Infect Dis 2009;49:e11–e17. 10.1086/599614 [DOI] [PubMed] [Google Scholar]
- 10.Rastogi VL, Nirwan PS. Invasive trichosporonosis due to Trichosporon asahii in a non-immunocompromised host: a rare case report. Indian J Med Microbiol 2007;25:59–61. 10.4103/0255-0857.31065 [DOI] [PubMed] [Google Scholar]
- 11.Zhang E, Sugita T, Tsuboi R, et al. The opportunistic yeast pathogen trichosporon asahii colonizes the skin of healthy individuals: Analysis of 380 healthy individuals by age and gender using a nested polymerase chain reaction assay. Milton, Australia: The Societies and Blackwell Publishing Asia Ptu Ltd, 2010:483–8. [DOI] [PubMed] [Google Scholar]
- 12.Cox GM, Perfect JR. Cryptococcus neoformans var : Ajello L, Hay RJ, Neoformans and gattii and trichosporon species. In Topley and Wilson’s microbiology and microbial infections-medical mycology. 9th ed New York, N.Y: Oxford University Press, 1998:pp 461–84. [Google Scholar]
- 13.Tashiro T, Nagai H, Kamberi P, et al. Disseminated Trichosporon beigelii infection in patients with malignant diseases: immunohistochemical study and review. Eur J Clin Microbiol Infect Dis 1994;13:218–24. 10.1007/BF01974540 [DOI] [PubMed] [Google Scholar]
- 14.Ruan SY, Chien JY, Hsueh PR. Invasive trichosporonosis caused by Trichosporon asahii and other unusual Trichosporon species at a medical center in Taiwan. Clin Infect Dis 2009;49:e11-7 10.1086/599614 [DOI] [PubMed] [Google Scholar]
- 15.Chan RM, Lee P, Wroblewski J. Deep-seated trichosporonosis in an immunocompetent patient: a case report of uterine trichosporonosis. Clin Infect Dis 2000;31:621 10.1086/313968 [DOI] [PubMed] [Google Scholar]
- 16.Wolf DG, Falk R, Hacham M, et al. Multidrug-resistant Trichosporon asahii infection of nongranulocytopenic patients in three intensive care units. J Clin Microbiol 2001;39:4420–5. 10.1128/JCM.39.12.4420-4425.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baraboutis I, Belesiotou E, Platsouka E, et al. Poststernotomy sternal osteomyelitis and mediastinitis by Trichosporon asahii: a rare occurrence with a grave prognosis. Mycoses 2010;53:272–4. 10.1111/j.1439-0507.2009.01709.x [DOI] [PubMed] [Google Scholar]
- 18.Kim KW, Ha KY, Kim MS, et al. Postoperative trichosporon asahii spondylodiscitis after open lumbar discectomy: a case report. Spine 2008;33:E116–E120. 10.1097/BRS.0b013e3181642a7c [DOI] [PubMed] [Google Scholar]
- 19.Antachopoulos C, Papakonstantinou E, Dotis J, et al. Fungemia due to Trichosporon asahii in a neutropenic child refractory to amphotericin B: clearance with voriconazole. J Pediatr Hematol Oncol 2005;27:283–5. 10.1097/01.mph.0000164865.70522.d7 [DOI] [PubMed] [Google Scholar]
- 20.de Almeida Júnior JN, Hennequin C. Invasive trichosporon infection: a systematic review on a re-emerging fungal pathogen. Front Microbiol 2016;7:1629 10.3389/fmicb.2016.01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Gorman C, McMullan R, Webb CH, et al. Trichosporon asahii. Blood-stream infection in a non-cancer patient receiving combination antifungal therapy. Ulster Med J 2006;75:226–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh TJ, Melcher GP, Rinaldi MG, et al. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J Clin Microbiol 1990;28:1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao Y, Lu X, Yang S, et al. EpideMiology and outcome of trichosporon fungemia: a review of 185 reported cases from 1975 to 2014. Open Forum Infect Dis 2015;2:ofv141 10.1093/ofid/ofv141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto A, Halliday C, Zahra M, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry identification of yeasts is contingent on robust reference spectra. PLoS One 2011;6:e25712 10.1371/journal.pone.0025712 [DOI] [PMC free article] [PubMed] [Google Scholar]