Abstract
Intestinal malformations are common defects of the newborn, treated in experienced centres. Reports on long-term follow-up and associated complications are scarce, possibly leading to misinterpretation of clinical signs and symptoms in adulthood. To prevent treatment errors, it is important that physicians are aware of long-term complications of intestinal malformations.
Keywords: paediatric surgery, gastrointestinal surgery
Background
Stenosis and atresia of the gastrointestinal tract are common defects of the newborn. They are defined by a narrowing of the lumen, a web in the lumen or a complete absence of sections of the intestine. Duodenal stenosis and atresia are the most frequent types of obstructing defects.1 Duodenal atresia appears in 1:7000 births and represents around 50% of small bowel atresias. Atresias or stenosis of other sections of the gut are found in 1:5000 of newborns.2 Within the jejunum and ileum, atresia is more frequent than stenosis and has been reported to occur at multiple sites in up to 6% of newborns with combined jejunal and ileal atresia.3 Typical clinical presentation of duodenal atresia consists of feeding intolerance, emesis, failure to thrive and upper abdominal distension.1
Treatment depends on the localisation of the malformation and generally requires early surgical intervention. The standard of care is resection of the atretic or stenosed bowel segment followed by an end-to-side or side-to-side anastomosis as extensively described in the literature.2 4 5
Different short-term and long-term complications following surgery for intestinal atresia have been reported depending on the localisation of the obstruction. In intestinal atresia, the most frequent early complications within the first month after the operation are anastomotic leakage and sepsis. The most frequent long-term complications, after the first month, are small bowel obstructions, stenoses and incisional hernias.6
In duodenal atresia, adhesive bowel obstruction, incisional hernias and gastro-oesophageal reflux disease are described.1 6 What stands out as a recurring complication in all studies following patients with duodenal atresia is an atonic megaduodenum due to duodenal dismotility.1 7 8 This complication was described in a case series of five patients in a period from 5 months up until 24 years after surgical intervention.9 10 In a 30-year review from Escobar et al,7 megaduodenum was present in 4.2% of 169 cases following surgery after duodenal obstruction. Patients with a megaduodenum have often been described to be asymptomatic. Therefore, only few reports show symptomatic cases in which feeding intolerance with frequent vomiting, poor weight gain, diarrhoea and abdominal pain were described.7 11 The megaduodenum causes a so-called functional stenosis characterised as an almost static and, in terms of resorption, non-functional part of the duodenum.11
However, most of these studies entail a short follow-up period after surgical intervention and documented long-term follow-up until adulthood is scarce. The clinical presentation and significance of findings in this patient group in adulthood remains unclear.
This is the case of a patient who suffered from duodenal atresia as a newborn and had a duodenal resection after birth. He presented to our clinic with complex symptoms at the age of 59 years. To our knowledge, this case represents the longest follow-up period after operation for symptomatic duodenal atresia in the literature.
Case presentation
A 59-year-old man admitted himself to our emergency department because of increasing weakness, dyspnoea and progressive weight loss. He reported short episodes of melena in the past, but never underwent gastroscopy or colonoscopy. His medical history was unremarkable except for an unknown abdominal surgery as a newborn and mild learning disabilities. Laboratory analysis showed a normocytic iron deficiency anaemia with a haemoglobin value of 5.5 g/dL (normal value (NV) 13.5–17.5 g/dL), a ferritin of 43 µg/L (NV 80–336 µg/L) and a transferrin saturation of 6% (NV 18%–45%). For further work-up of the suspected upper gastrointestinal bleeding, the patient was admitted to the medical ward and a total of five units of packed red blood cells were administered over 2 days. Further laboratory work-up indicated a coagulation disorder with spontaneous elevation of the international normalised ratio of prothrombin time up to 1.89 (NV 0.8–1.2) and an albumin of 36 g/L (NV 35–50 g/L), most likely caused by underlying malnutrition.
An emergency gastroscopy was performed on the day of admission, which had to be interrupted due to a vasovagal-induced bradycardia. Postgastroscopy, the patient was transferred to the intensive care unit for further surveillance.
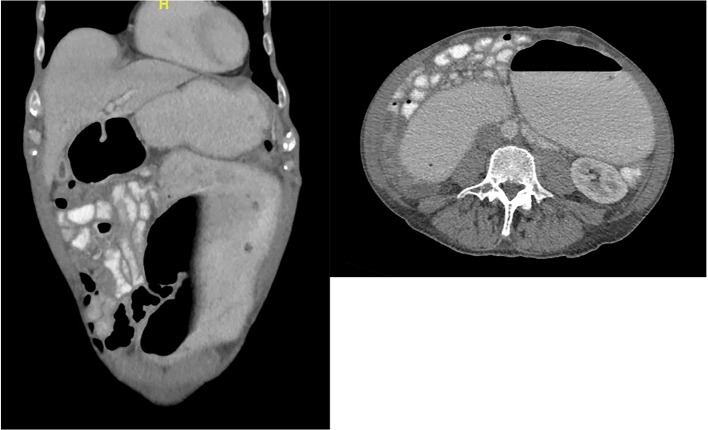
Two days later, the upper endoscopy was repeated and a colonoscopy was performed in order to find a possible source of bleeding. No signs of bleeding were detected. However, the endoscopy showed a distended duodenal lumen with normal mucosa. Subsequently, CT of the abdomen and pelvis with intravenous, rectal and oral contrast was performed revealing a massive dilatation of the stomach and duodenum, resembling the image of a mechanical ileus of unclear origin (figures 1 and 2). The patient was presented to the surgeon on call and the decision to perform an exploratory laparotomy was made.
Figure 1.
CT of the abdomen on the third day of hospitalisation showing a massively dilated duodenum. Images were interpreted as a mechanical obstruction causing these findings.
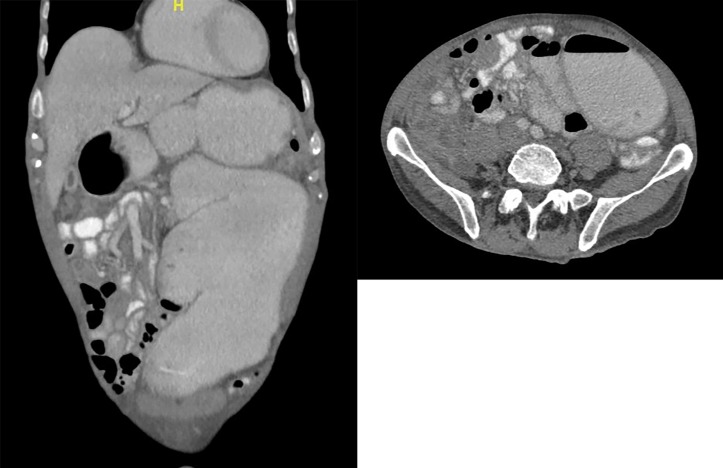
Figure 2.
CT of the abdomen on the third day of hospitalisation showing a massively dilated duodenum. Images were interpreted as a mechanical obstruction causing these findings.
The intraoperative findings revealed a massively dilated duodenum with a diameter of 20 cm (figures 3–5). A side-to-end duodenojejunostomy due to his operation as a newborn was identified, but after enterotomy, no mechanical obstruction or cause of bleeding were found. Furthermore, the remaining small intestine showed no anomaly. However, as constriction of the duodenojejunostomy seemed to be the most probable explanation for the present megaduodenum, the anastomosis was resected and a duodenojejunal side-to-end anastomosis was performed.
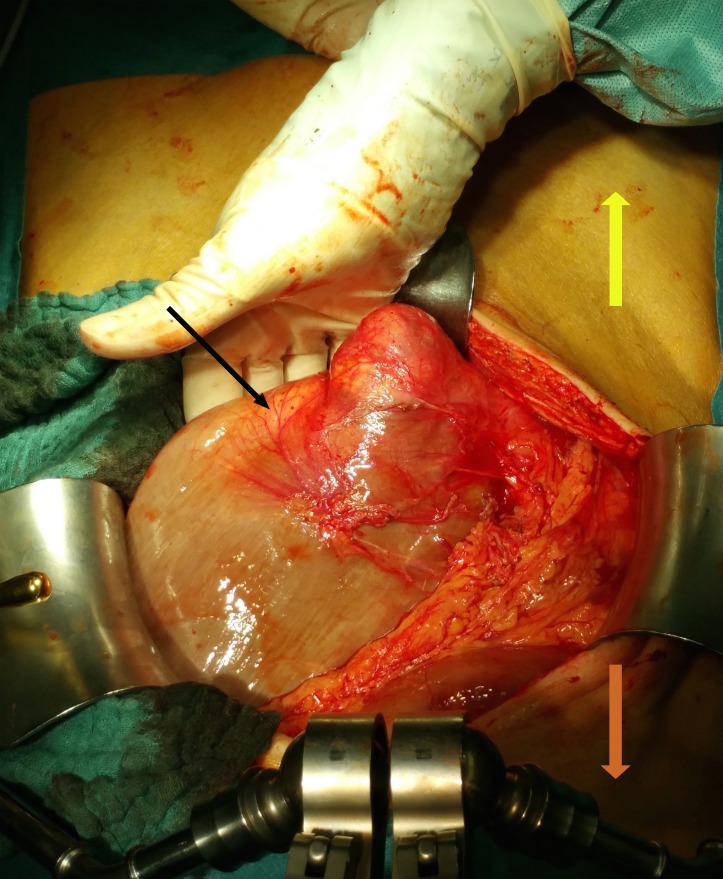
Figure 3.
First intraoperative finding after opening the abdomen. The black arrow is pointing at the megaduodenum. The orange arrow is pointing towards the head, the yellow one towards the feet of the patient.
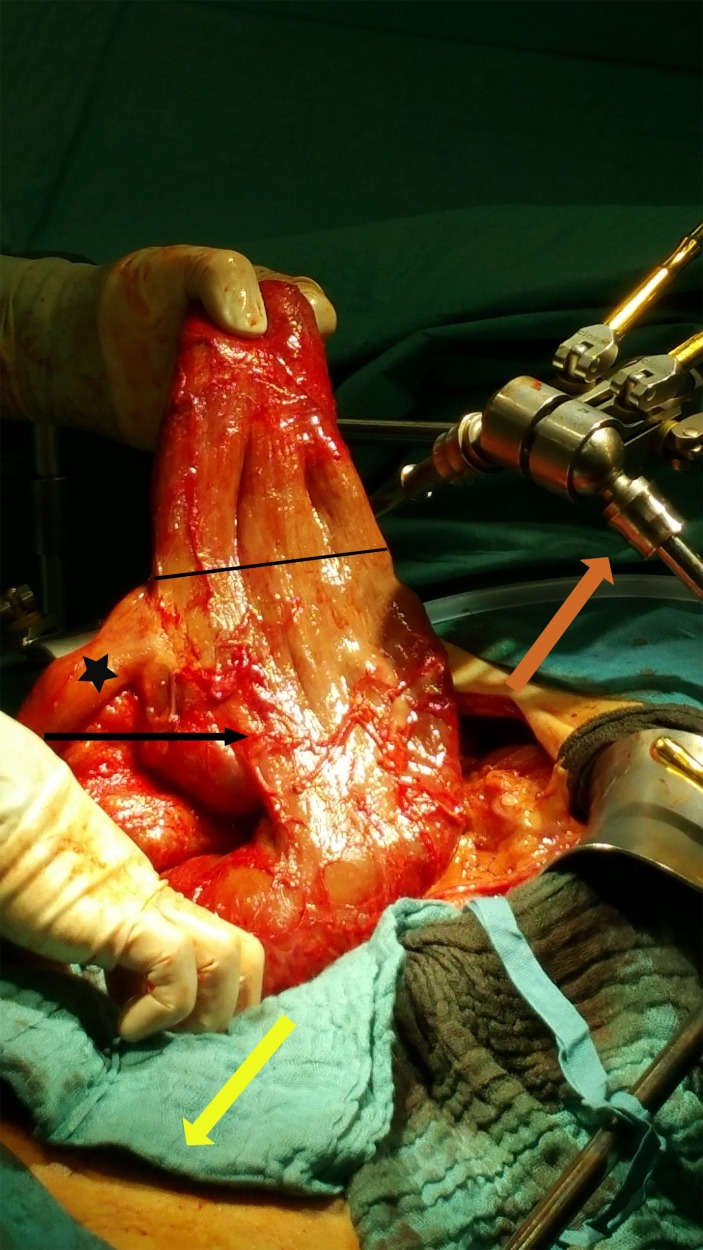
Figure 4.
The black arrow is pointing at the megaduodenum. The hand is holding the blind loop section of the megaduodenum with the line marking the transition to the functional lumen. The star illustrates the normal jejunum postduodenojejunostomy. The orange arrow is pointing towards the head, the yellow one towards the feet of the patient.
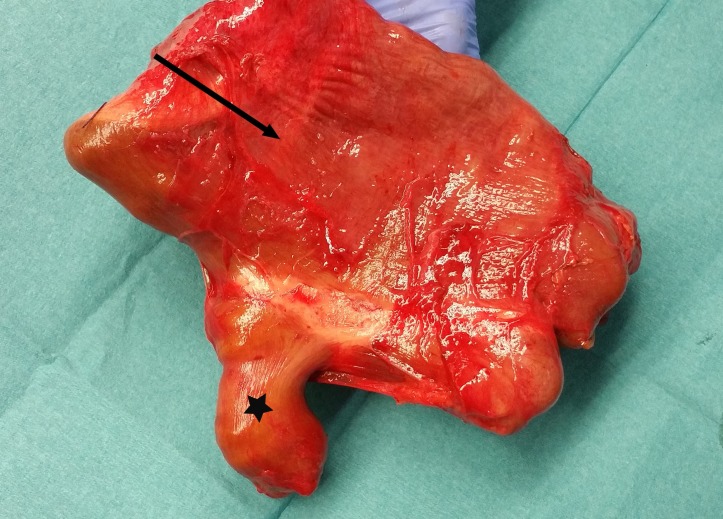
Figure 5.
This is the resected segment containing the initial anastomosis. The arrow is pointing at the megaduodenum containing the blind loop, the star at the normal jejunum.
Postoperative recovery was uneventful. Iron and nutritional deficiency such as vitamins were substituted and general state of health improved rapidly. The haemoglobin value remained stable at 9 g/dL (NV 13.5–17.5 g/dL), and after having organised a medical rehabilitation, the patient was discharged on the 14th postoperative day. During follow-up examination 7 weeks postoperatively, the patient had no further symptoms and was in a good general state of health. We, therefore, ceased follow-up examinations in our outpatient clinic and referred the patient back to his general practitioner (GP). The haemoglobin value was checked again 10 weeks postoperatively and remained stable at 13.3 g/dL (NV 13.5–17.5 g/dL) and the patient continued to be asymptomatic.
Regarding the further work-up, histological analysis of the resected segment showed no evidence of malignancy or other pathology. Thorough survey of the personal medical history was challenging due to a mild learning disabilities present since birth and unfortunately the paediatric medical records were no longer available. In this regard, it was of particular interest that the patient was still in close relationship with the daughter of the surgeon who had operated on him 59 years ago. After obtaining informed consent from the patient, the daughter provided us with the helpful information that our patient had multiple operations for duodenal atresias as a newborn.
Discussion
The presented case reports the longest follow-up period of a patient with a very late complication after surgery for duodenal atresia as a newborn in the literature.
No report describing complications after this kind of surgery with a follow-up period greater than 23 years has been found1 7 12 and we want to highlight the uniqueness of this case on various levels.
The patient was born in a time period when survival after duodenal atresia and its repair was not common. In their publication on a 25-year review, Wesley et al13 analysed 72 cases of newborns with congenital duodenal obstruction from 1951 to 1975 at the Children’s Hospital of Los Angeles. Mean overall survival following operation was 67%. From 1951 to 1966, overall survival was merely 55%. In the cases from 1966 to 1975, overall survival increased to 88%. Furthermore, results from two recently published studies demonstrated that survival increased from 60% in the 1960s to almost 100% nowadays.1 14 The improved survival is due to multiple factors such as advances in prenatal diagnostics, better surgical outcomes due to improved surgical techniques and surveillance in the neonatal intensive care unit, as well as advances in paediatric anaesthetic techniques.
Assessing this case retrospectively, it is debatable if the patient needed exploratory surgery in adulthood.
The main symptoms at presentation were weakness, loss of weight and dyspnoea. Only on precise questioning in the emergency department—which could have induced a suggested answer in the patient— did he describe having had episodes of melena. During hospitalisation, melena was never observed. In addition, gastroscopy, colonoscopy and intraoperative exploration did not reveal any cause of bleeding. It is, therefore, likely that the patient never had any active bleeding. The laboratory findings (ie, anaemia, iron deficiency, etc) can clearly be interpreted in the context of his malnutrition. This is supported by various studies in which blind loop syndrome and malnutrition were put in a causal relation to a megaduodenum.7 8 15 Our patient never expressed abdominal discomfort. In cases of symptomatic megaduodenum, standard of care procedure is duodenal tapering or plication, in which the exceeding part of the duodenal circumference is removed.9 10 16 The exact technical procedure for the tapering can be found in the paper by Adzick et al.16
Unlike the described cases of symptomatic megaduodenum, it can be questioned whether a surgical approach is necessary in asymptomatic patients. In this specific case, surgery had a curative effect, the patients’ nutritional deficiency was reversed and he no longer complained of any discomfort, fatigue or dyspnoea during the follow-up consultation.
This case is the longest follow-up in the literature after surgery for duodenal atresia, illustrating the relevance of complications years after intervention. It shows how a complication of a paediatric disease can be misinterpreted as a mechanical obstruction leading to surgical intervention in adulthood, when presented to physicians who are not familiar with the course of this pathology. Therefore, we strongly believe that it is imperative for the GP, internist, general surgeon, radiologist and other specialists working in related fields to be familiar with symptoms, the general picture and the course of patients who underwent surgery for intestinal atresia.
Long-term follow-up protocols have already been recommended in various studies. Unfortunately, no such protocol has yet been established for these patients.
The here reported case with the longest follow-up in the medical literature highlights the need for such a protocol and in addition illustrates the relevance of complications even years after surgery. A long-term protocol would facilitate the correct interpretation of clinical and radiological findings, avoiding unnecessary procedures in future patients.
Learning points.
Patients who underwent surgery for intestinal malformation in early childhood can present with a variety of symptoms and complications even many years later.
It is imperative for doctors of all specialties to be familiar with these complications in order to interpret them accurately and avoid unnecessary treatment.
Patient who underwent surgery in early childhood for intestinal malformation need long-term follow-up and a specialist to go to in case of complications even in adulthood.
Footnotes
Contributors: All authors contributed to the case report equally. JR and BW conducted the research and wrote the case report. MZ critically analysed and suggested improvements. OS helped us to analyse and revise the images.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dalla Vecchia LK, Grosfeld JL, West KW, et al. Intestinal atresia and stenosis: a 25-year experience with 277 cases. Arch Surg 1998;133:490–6. [DOI] [PubMed] [Google Scholar]
- 2.Christoper P, Coppola A, Scorpio RJ, et al. Pediatric surgery diagnosis and treatment. Switzerland: Springer International Publishing, 2014. [Google Scholar]
- 3.Baglaj M, Carachi R, Lawther S. Multiple atresia of the small intestine: a 20-year review. Eur J Pediatr Surg 2008;18:13–18. 10.1055/s-2007-965771 [DOI] [PubMed] [Google Scholar]
- 4.Kimura K, Tsugawa C, Ogawa K, et al. Diamond-shaped anastomosis for congenital duodenal obstruction. Arch Surg 1977;112:1262–3. 10.1001/archsurg.1977.01370100116026 [DOI] [PubMed] [Google Scholar]
- 5.Weber TR, Lewis JE, Mooney D, et al. Duodenal atresia: a comparison of techniques of repair. J Pediatr Surg 1986;21:1133–6. 10.1016/0022-3468(86)90025-4 [DOI] [PubMed] [Google Scholar]
- 6.Stollman TH, de Blaauw I, Wijnen MH, et al. Decreased mortality but increased morbidity in neonates with jejunoileal atresia; a study of 114 cases over a 34-year period. J Pediatr Surg 2009;44:217–21. 10.1016/j.jpedsurg.2008.10.043 [DOI] [PubMed] [Google Scholar]
- 7.Escobar MA, Ladd AP, Grosfeld JL, et al. Duodenal atresia and stenosis: long-term follow-up over 30 years. J Pediatr Surg 2004;39:867–71. 10.1016/j.jpedsurg.2004.02.025 [DOI] [PubMed] [Google Scholar]
- 8.Kokkonen ML, Kalima T, Jääskeläinen J, et al. Duodenal atresia: late follow-up. J Pediatr Surg 1988;23:216–20. 10.1016/S0022-3468(88)80725-5 [DOI] [PubMed] [Google Scholar]
- 9.Ein SH, Shandling B. The late nonfunctioning duodenal atresia repair. J Pediatr Surg 1986;21:798–801. 10.1016/S0022-3468(86)80371-2 [DOI] [PubMed] [Google Scholar]
- 10.Ein SH, Kim PC, Miller HA. The late nonfunctioning duodenal atresia repair-a second look. J Pediatr Surg 2000;35:690–1. 10.1053/jpsu.2000.6007 [DOI] [PubMed] [Google Scholar]
- 11.Spigland N, Yazbeck S. Complications associated with surgical treatment of congenital intrinsic duodenal obstruction. J Pediatr Surg 1990;25:1127–30. 10.1016/0022-3468(90)90746-V [DOI] [PubMed] [Google Scholar]
- 12.Stauffer UG, Irving I. Duodenal atresia and stenosis--long-term results. Prog Pediatr Surg 1977;10:49–60. [PubMed] [Google Scholar]
- 13.Wesley JR, Mahour GH. Congenital intrinsic duodenal obstruction: a twenty-five year review. Surgery 1977;82:716–20. [PubMed] [Google Scholar]
- 14.Rescorla FJ, Grosfeld JL. Intestinal atresia and stenosis: analysis of survival in 120 cases. Surgery 1985;98:668–76. [PubMed] [Google Scholar]
- 15.Grosfeld JL, Rescorla FJ. Duodenal atresia and stenosis: reassessment of treatment and outcome based on antenatal diagnosis, pathologic variance, and long-term follow-up. World J Surg 1993;17:301–9. 10.1007/BF01658696 [DOI] [PubMed] [Google Scholar]
- 16.Adzick NS, Harrison MR, deLorimier AA. Tapering duodenoplasty for megaduodenum associated with duodenal atresia. J Pediatr Surg 1986;21:311–2. 10.1016/S0022-3468(86)80191-9 [DOI] [PubMed] [Google Scholar]