Abstract
Shewanella algae is a rare pathogen related to water exposure in temperate climates. It is commonly associated with skin and soft tissue infections, peritonitis and bacteraemia. We report the first-ever case of S. algae infective endocarditis in a patient with previous splenectomy and explore the difficulties in treatment as well as highlight the importance of this organism as an emerging pathogen.
Keywords: infectious diseases, travel medicine, valvar diseases
Background
Shewanella species are motile Gram-negative bacilli found mostly in marine environments. The first description of the species was in 1931 by Derby and Hammer who isolated the bacterium from water supplies of dairies and named it Achromobacter putrefaciens. In 1941, the species was transferred to the genus Pseudomonas, and in 1985, phylogenetic studies reclassified the species into the family Vibrionaceae and a new genus, Shewanella. In 1997, further DNA sequencing led to the proposal of a new family, Shewanellaceae with 30 subspecies.1
The most common clinical manifestations of Shewanella algae infection include skin and soft tissue infection associated with exposure to seawater, ear infections, hepatobiliary disease and bacteraemia. A case series in Martinique reviewed all cases of Shewanella over a 14-year period. Sixteen of the 21 isolates were considered pathogenic. More than half of the infections were polymicrobial and the most common infections were skin and soft tissue followed by arthritis, peritonitis and bacteraemia.2 Recently, there have been increasing reports of invasive disease caused by S. algae.3 This is likely representative of improved microbiology testing methods as well as the possible emergence of more virulent strains. To our knowledge, this is the first reported case of S. algae infective endocarditis (IE).
Case presentation
A 69-year-old man presented to the Sunshine Coast University Hospital, Queensland, Australia, with a 2-week history of fever, nausea and an episode of presyncope leading to a fall. One month prior, he was admitted to another hospital with lower limb cellulitis which he developed subsequent to fishing in salt water. He was treated with a course of intravenous and oral antibiotics (details unknown) but reported ongoing fever since discharge from hospital.
His medical history included coronary artery bypass grafting and bioprosthetic mitral and aortic valve replacements 2 years prior, a pacemaker for symptomatic heart block and prostate cancer with widespread bony metastases on hormonal therapy. Thirty years prior, he had also had a traumatic splenectomy but was not on any antibiotic prophylaxis.
On examination, his temperature was 39°C, respiratory rate 26 breaths per minute, BP 106/50 mm Hg, heart rate 96 beats per minute and oxygen saturation 96% on 2 L oxygen via nasal prongs. He had scarring and discolouration on his left lower limb lateral to the medial malleolus consistent with recent soft tissue infection without any open lesion or signs of septic arthritis. Heart sounds were dual with a pansystolic murmur graded 2/6, heard loudest in the mitral area. There were bibasal fine crackles on auscultation and mild peripheral oedema. Neurological examination revealed left-sided weakness and hyper-reflexia with preserved sensation as well as mild dysphasia.
Investigations
A CT scan of his brain showed a right frontal lobe subarachnoid haemorrhage (SAH) and multiple hypodense lesions in the cerebellum and caudate nucleus concerning for embolic phenomena. After 8.9 hours of incubation, four sets of blood cultures (aerobic and anaerobic) flagged positive as Gram-negative, motile bacilli (figure 1). Pure growth of non-lactose fermenting, oxidase positive colonies were observed on the MacConkey agar after overnight incubation at 35°C CO2 (figure 2). The mucoid, brown, pigmented colonies had a green discolouration on blood agar (figure 3). The isolate was identified as S. algae using matrix-assisted laser desorption ionisation-time of flight (VITEK MS, bioMérieux, Tokyo, Japan) spectrometry and confirmed with the VITEK 2 Gram-negative (GN) identification card (bioMérieux, Durham, North Carolina, USA). On further investigation, we became aware that he had isolated S.algae with the same antibiogram from his blood cultures during his previous admission when he had presented with lower limb cellulitis. A CT chest, abdomen and pelvis showed no other occult sites of infection. A transoesophageal echocardiogram showed a large mobile vegetation (1.3×0.7 cm) attached to the posterior leaflet of the prosthetic mitral valve and small vegetations attached to the prosthetic aortic valve (0.6×0.5 cm). There were no paravalvular abscesses. The left ventricular ejection fraction was normal.
Figure 1.
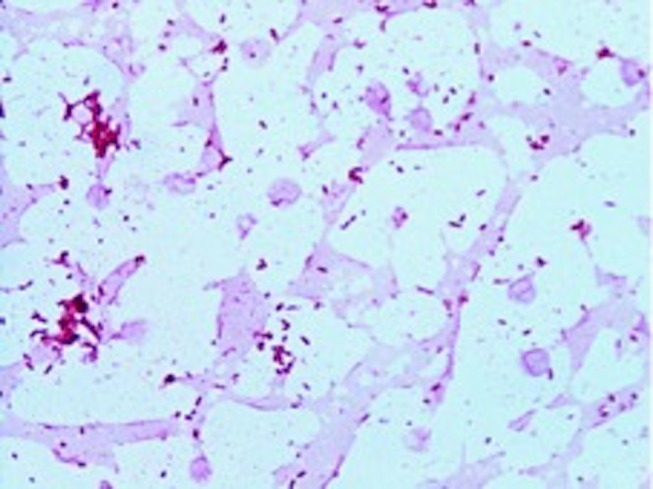
Shewanella algae on Gram stain.
Figure 2.

Shewanella algae on MacConkey agar.
Figure 3.
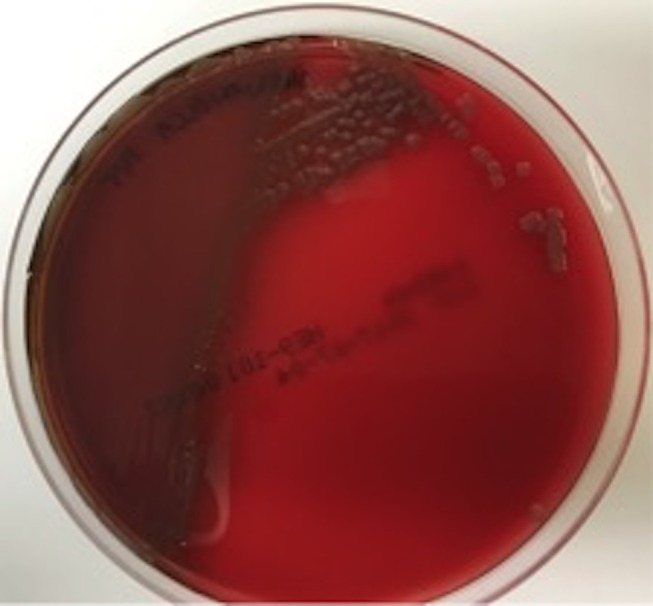
Shewanella algae on horse blood agar.
Treatment
The patient was treated with intravenous high-dose ceftriaxone (2 g two times daily) due to the additional concern for central nervous system infection and oral ciprofloxacin 500 mg twice a day (adjusted for renal function) for biofilm activity. The ceftriaxone minimum inhibitory concentration (MIC) by E-test was 0.25 µg/mL (S <8 µg/mL). The ceftriaxone serum trough level was considered adequate at 11.9 mg/L. The ciprofloxacin MIC by E-test was 0.125 µg/mL (S <1 µg/mL). Further susceptibilities were completed using the bioMérieux VITEK 2 GN card and the isolate was confirmed susceptible to aminoglycosides, fourth-generation cephalosporins and carbapenems. Due to the SAH and poor functional status, the patient was deemed not to be a surgical candidate and continued with medical management. He remained afebrile and continued to improve functionally and was admitted for inpatient rehabilitation on week 2 of his admission while continuing antibiotic treatment.
Outcome and follow-up
On week 5 of treatment, he developed new signs of heart failure. Repeat transthoracic echocardiography demonstrated new mobile echodensities on the prosthetic mitral and aortic valves with worsening regurgitation, as well as a new mobile element on the pacing wire in the right atrium. He also developed Coomb’s positive haemolytic anaemia presumed secondary to ceftriaxone and was switched to ciprofloxacin monotherapy. He was then discharged to a palliative care unit for supportive care and deceased within 2 weeks due to progression of his infection.
Discussion
Shewanella species are found around the world mainly in temperate marine environments. S. algae and Shewanella putrefaciens are the most commonly isolated species in clinical specimens. These organisms are often isolated with other bacteria so their pathogenicity has been controversial.4 In the past, it has been difficult to distinguish between the two species because automated identification systems did not include S. algae, and 16S rRNA gene sequencing and phenotypic tests were required. S. algae colonies are often mucoid, asaccharolytic, can cause beta-haemolysis on sheep blood agar and also grow at 42° on 6% NaCl in contrast to S. putrefaciens..4 The bioMérieux VITEK 2 automated system database now includes S. algae although the accuracy of identification is unknown.
To our knowledge, this case is the first reported case of S. algae IE. There have been two previous case reports of Shewanella species causing IE; both these reports were of S. putrefaciens. The first report was in 1998 in India of a case of an immunocompetent woman with rheumatic heart disease who developed a polymicrobial bacteraemia with viridans group Streptococcus and S. putrefaciens associated with a vegetation on the mitral valve. This case was treated with intravenous gentamicin and penicillin for 6 weeks.5 The second report was in Brazil in 2014 of a 40-year-old immunocompetent man presenting with fever and upper limb thromboembolic disease 2 weeks after suffering a burn to his medial thigh. Subsequent investigation revealed mitral valve IE. He underwent a valvectomy and was treated with intravenous cefepime and gentamicin. Six sets of blood cultures were positive for S. putrefaciens but the valve cultures were negative.6 These cases as well as many others involving different organ systems highlight the lack of consistency and variability in treatment regimens owing to paucity of evidence available.
In the current case, the source of infection was likely salt water contamination of the original fishing wound 1 month prior. The patient was immunosuppressed due to the previous splenectomy and metastatic prostate cancer, although there was no recent chemotherapy. This report demonstrates that S. algae can be a virulent and pathogenic organism and is difficult to treat in the setting of IE. There currently are no microbiological guidelines to determine breakpoints for susceptibilities for this organism, and the Clinical and Laboratory Standards Institute breakpoints for non-Enterobacteriaceae were referred to for guidance in this case.7 In addition, there are no guidelines for the antimicrobial treatment of these conditions, and in this case, the isolate was susceptible to many antimicrobials. Despite this, the condition remained refractory to treatment. Interestingly, other reports have demonstrated the emergence of multiresistant strains of S. algae including carbapenem resistance.8 S. algae as an environmental reservoir for transmitted resistance has been suggested due to the discovery of the blaoxa55 and qnrA gene variants associated with mobile genetic elements in some isolates.9
This report of S. algae IE supports recent speculation of the emerging pathogenicity of this organism, and clinicians must be aware of its ability to cause invasive disease.
Learning points.
Shewanella algae is an emerging pathogen with the ability to cause invasive disease particularly in the immunocompromised host.
There currently are no standard treatment guidelines for the management of this organism due to the paucity of evidence available, but susceptibility to third-generation and fourth-generation cephalosporins as well as quinolones and aminoglycosides is common, though despite this can remain difficult to treat.
In a patient with Shewanella bacteraemia, endovascular infection should be considered in those with prosthetic material and disseminated disease.
Footnotes
Contributors: NLD was involved in design by writing the article and revising the drafts as well as care of the patient. KW and SS contributed to the design of the work and the revised draft. JM contributed to the design of the work as well as acquisition of data and draft review. All the authors were involved in the final approval of the version submitted and agreed to the accountability to all aspects of the work.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Holt HM, Gahrn-Hansen B, Bruun B. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect 2005;11:347–52. 10.1111/j.1469-0691.2005.01108.x [DOI] [PubMed] [Google Scholar]
- 2.Vignier N, Barreau M, Olive C, et al. Human infection with Shewanella putrefaciens and S. algae: report of 16 cases in Martinique and review of the literature. Am J Trop Med Hyg 2013;89:151–6. 10.4269/ajtmh.13-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma KK, Kalawat U. Emerging infections: shewanella - a series of five cases. J Lab Physicians 2010;2:61–5. 10.4103/0974-2727.72150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khashe S, Janda JM. Biochemical and pathogenic properties of Shewanella algae and Shewanella putrefaciens. J Clin Microbiol 1998;36:783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhawan B, Chaudhry R, Mishra BM, et al. Isolation of Shewanella putrefaciens from a rheumatic heart disease patient with infective endocarditis. J Clin Microbiol 1998;36:2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constant J, Chernev I, Gomez E. Shewanella putrefaciens infective endocarditis. Braz J Infect Dis 2014;18:686–8. 10.1016/j.bjid.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI. Performance standards for antimicrobial susceptibility testing: 20th informational supplement, CLSI document M100-S20. Wayne, PA: Clinical and Laboratory Standards Institute, 2010. [Google Scholar]
- 8.Srinivas J, Pillai M, Vinod V, et al. Skin and soft tissue infections due to Shewanella algae—an emerging pathogen. J Clin Diagn Res 2015;9:16–20. 10.7860/JCDR/2015/12152.5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousfi K, Bekal S, Usongo V, et al. Current trends of human infections and antibiotic resistance of the genus Shewanella. Eur J Clin Microbiol Infect Dis 2017;36:1353–62. 10.1007/s10096-017-2962-3 [DOI] [PubMed] [Google Scholar]


