Abstract
Ferric carboxymaltose (FCM) is a novel iron formulation increasingly prescribed due to its effectiveness and fast infusion time. FCM administration can cause an asymptomatic hypophosphataemia secondary to fibroblast growth factor 23 (FGF23) dysregulation. In patients with chronic iron needs, however, a severe, long-lasting hypophosphataemia can lead to osteomalacia with associated bone pain. Lack of awareness of this complication results in delayed time to diagnosis and significant morbidity. We report a case of a patient with Crohn’s disease and chronic iron-deficiency anaemia receiving multiple doses of FCM who developed severe hypophosphataemic osteomalacia with urinary phosphate loss and increased FGF23. FGF23 excess and osteomalacia resolved only months after FCM discontinuation and aggressive phosphate repletion. Potential mechanisms of FGF23 dysregulation are discussed, with the aim of raising awareness of this significant side effect for prescribers of chronic intravenous iron supplementation, and to help guide future studies to determine the safety of FCM in all patient populations.
Keywords: contraindications and precautions, haematology (drugs and medicines), gastrointestinal system, calcium and bone
Background
The case demonstrates a critically emerging clinical bone problem accompanying the use of ferric carboxymaltose (FCM), a form of iron that promises to occupy an increasing share of iron dosing due to its efficacy, easy administration and tolerability. Here, our patient received multiple injections of FCM to treat long-standing iron deficiency anaemia due to Crohn’s disease. This led to a sustained increase in fibroblast growth factor 23 (FGF23) and debilitating osteomalacia. His diagnosis was delayed for almost a year before referral to one of us. The delay in diagnosis was accompanied by unnecessary treatment (ie, zolendronic acid).
While FCM is known to cause hypophosphataemia, published evidence has suggested that the hypophosphataemia is self-limited and associated with little morbidity. As demonstrated with our patient who required chronic iron supplementation, the hypophosphataemia can be sustained especially if the patient is given multiple doses and can lead to severe osteomalacia. Indeed, the patient’s bone pain was wrongly attributed to Crohn’s disease, and the coincident abnormal mineral metabolism due to the bowel disease further complicated diagnosis. His case is especially important for clinicians whose practice involves providing iron supplementation.
Case presentation
In October 2016, a 57-year-old man was referred for evaluation of a 1.5-year history of joint and bone pain. His medical history was characterised by a 40-year history of Crohn’s poorly responsive to multiple courses of steroids, biologic agents, and requiring two bowel resections in 1988 and 2001 for stricture. His course was further complicated by chronic iron deficiency anaemia requiring intravenous iron infusions since 2002, thought to be due to bleeding at the stricture site. In February 2014, he began treatment with injectable FCM. During this time, ferritin frequently fell to less than 20, at which point he was given intravenous iron. He received a total of 26 infusions (750 mg each) about monthly, before discontinuation in April 2016.
Before presentation to our clinic, the patient had undergone work-up and treatment for generalised bone pain that began in mid-2015, which was initially attributed to Crohn’s disease. Bone pain worsened during a course of prednisone such that he was unable to continue his exercise regimen. Consultant rheumatology ruled out Crohn’s-associated arthritis. Bone mineral density testing in late 2015 revealed osteoporosis (T score of −2.5 in the right femoral neck) leading to treatment with intravenous bisphosphonate. By early 2016, the patient was found to be both hypocalcaemic and hypophosphataemic, with low 25-dihydroxyvitamin D measured on multiple occasions. He was begun on calcitriol (Rocaltrol 0.25 μg twice daily) and oral calcium. FCM was discontinued in April 2016 and iron supported with monthly intravenous iron dextran. Severe bone pain in ribs, feet and shoulders persisted.
He was referred to our clinic for further evaluation in November 2016. The patient’s body mass index was normal with examination pertinent for waddling gait only.
Investigations
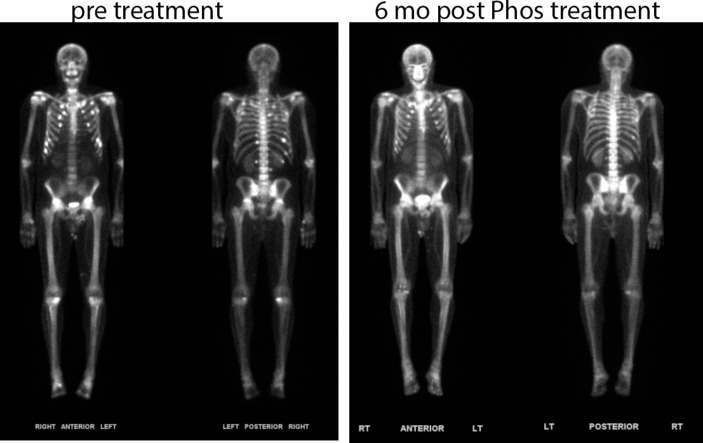
Laboratory values showed normocalcaemia with elevated alkaline phosphate and parathyroid hormone, and low-normal phosphate levels (table 1). Tubular maximum reabsorption of phosphate to glomerular filtration rate (TmP/GFR) was measured to evaluate renal phosphate transport: after an overnight fast, the second void was collected revealing a low reabsorption of phosphate of 1.0 (normal >3 mg/dL), suggesting renal phosphate wasting. Iron studies during this time reveal variable values, with total iron generally low (table 2). A bone scan revealed findings compatible with pseudofractures and confirmed the clinical diagnosis of osteomalacia (figure 1). We were unable to obtain an FGF23 at this visit. Re-evaluation 3 months later in February 2017 showed persistently low-normal phosphate levels despite significant oral supplementation of 25-dihydroxyvitamin D as well as calcitriol. Parathyroid hormone (PTH) had normalised. C-terminal FGF23 was elevated at 592 (normal <180 RU/mL).
Table 1.
Patient laboratories during course of treatment
| Visit 1 (11/16) |
+4 months (3/17) | +8 months (5/17) | Normal | |
| Calcium | 8.5 | 8.5 | 9.7 | 8.5–10.2 mg/dL |
| Phosphate | 2.7 | 2.4* | 3.9 | 2.4–4.5 mg/dL |
| Alkaline phosphatase | 159 | 178 | 98 | 38–126 U/L |
| PTH | 180 | 68 | 62 | 12.0–72.0 pg/mL |
| Vitamin D | 25 | 23 | 23 | |
| 1,25(OH)2D3† | 92 | 20–80 ng/mL | ||
| TmP/GFR, fasting | 1.0 | 2.7‡ | >3 | |
| cFGF23 | 592 | 69 | <180 |
*Oral phosphate (‘sod phos di, mono-K phos mono’ tab) had been instituted 2 months earlier at 250 mg three times a day.
†Patient was taking 0.25 μg calcitriol twice daily.
‡Measured when serum phosphate was normal >18 months after cessation of ferric carboxymaltose.
Table 2.
Patient iron studies during course of treatment
| 2/15 | 8/16 | 11/16 | 3/17 | Normal | |
| TIBC | 230 | 275 | 250–450 μg/dL | ||
| Total iron | 63 | 50 | 65–175 μg/dL | ||
| % Saturation | 27 | 18 | 20–40 | ||
| Ferritin | 202 | 77 | 43 | 54 | 20–400 ng/mL |
Figure 1.
Whole-body bone scan (Tc-99m MDP) prephosphate and 6 months postphosphate treatment. The pretreatment scan demonstrates multiple scattered foci along anterior and posterior aspects of the rib cage, with significant improvement shown postphosphate therapy.
With the prolonged elevation in FGF23 at 10 months after the last FCM dose, whole-body PET with 18F-FDG was performed and showed no evidence of FDG-avid tumour. Phosphate repletion was continued.
Differential diagnosis
The differential diagnosis included FCM-induced hypophosphataemia versus tumour-induced osteomalacia (TIO) and autosomal dominant hypophosphataemic rickets (ADHR) precipitated by iron deficiency. When the patient’s cFGF23 was found to be high 10 months after stopping FCM, we considered that he might have TIO, which is occult and usually difficult to diagnose. The negative FDG scan, together with the cFGF23 level falling into the normal range at subsequent visits, points to FCM as the causative agent of hypophosphataemia.
Treatment
Oral phosphate twice daily was initiated, with symptomatic relief of bone pain by 2 weeks, and thence increased to three times daily. The patient’s iron requirements were replenished with an alternate form of iron therapy.
Outcome and follow-up
Repeat cFGF23 in April 2017 fell within normal limits (90 RU/mL). Repeat cFGF23 in May 2017 remained normal, and a recent TmP/GFR was nearly normal at 2.71. The patient’s joint and bone pain had fully resolved at this time with normalisation of alkaline phosphate. Repeat bone scan was compatible with healing bone lesions (figure 1).
Discussion
FCM is a novel iron complex that has been increasingly prescribed due to its ease of use and effectiveness in treating iron deficiency anaemia, with correction of iron deficiency in less than 15 min1 and a profile of patient satisfaction including less gastrointestinal side effects than with oral supplementation.2 While hypophosphataemia is a documented side effect of iron infusion, increasing reports in the last 5 years have demonstrated a strong relationship between FCM and phosphate metabolism.3 4 The mechanism, though poorly understood, is thought to be driven by complex interactions between iron formulations and FGF23.5
Despite reports in the literature, FCM-induced hypophosphataemia remains widely underappreciated. When symptoms go unrecognised, and there is a chronic need for iron infusion, the severity of hypophosphataemia associated with FCM administration can result in significant pathology, poor outcomes and decreased patient quality of life. Here, we discuss a case of FGF23-induced hypophosphataemia resulting in severe osteomalacia following administration of FCM.
This case represents a cautionary tale for doctors using repeated iron infusions to treat refractory anaemia. Despite presenting with classic hypophosphataemic osteomalacia, our patient’s symptomatic hypocalcaemia, likely due to insufficient vitamin D associated with Crohn’s malabsorption, and the secondary rise in parathyroid hormone, led to a delay in diagnosis. During this time, the patient continued to receive FCM, compromising his skeleton. His bone scan, showing multiple fractures and pseudofractures, and decreased urine phosphate resorption were consistent with osteomalacia due to renal phosphate wasting. FGF23 was threefold above the upper limit of normal at the time of measurement. As FCM infusion had been discontinued 10 months before, it is likely that FGF23 levels were even higher prior during the 2 years when FCM was dosed monthly. While iron deficiency alone can cause increased FGF23, the level of C-terminal FGF23 measured in our patient at his initial visit was almost twice as high in the most iron-deficient patient described in our prior report.6 As such, iron deficiency alone is unlikely to have caused such extreme elevation in FGF23 in our patient. Further, in our patient, ferritin frequently fell to less than 20, at which point he was given intravenous iron; it is therefore difficult to equate oscillating iron levels with chronically elevated FGF23. It would have been most appropriate to measure intact FGF23 to understand the contribution of iron deficiency to the increased FGF23, but this was not possible for our patient.
Our patient’s case is similar to others appearing in recent literature,7–9 but his phosphate wasting appeared to have persisted longer than other reported cases. With our case, we found that, despite normal creatinine, elevated FGF23 persisted long after cessation of FCM. FGF23 did fall into the normal range in the next year despite the necessary continuance of alternate forms of iron supplementation that have not been reported to cause increases in FGF23. With his extended course of FCM treatment and hypophosphataemia, our patient developed a substantial burden of osteomalacic pseudofractures. Importantly, the patient’s bone pain responded within several weeks to phosphate therapy, and his bone scan showed progress towards resolution by 12 months after cessation of FCM. Our patient’s clinical course provides strong support for a significant relationship between iron formulations, FGF23 and the risk to skeletal health.
The discovery of FGF23 in the 2000s revolutionised the understanding of phosphate metabolism. Originally discovered for its role in ADHR,10 it has since been associated with a number of diseases, including various forms congenital hypophosphataemic rickets and osteomalacia.11 FGF23 has also been implicated in TIO, a paraneoplastic disease, where tumour production of FGF23 results in phosphate wasting and defects in bone mineralisation.12 13
Regulation and action of FGF23, a 32-kDa protein secreted by bone osteocytes, is complex.14–16 It acts primarily on the kidney to downregulate the expression of sodium-phosphate cotransporters to stimulate phosphaturia,15 but may have direct effects on the skeleton. FGF23 function underwrites the physiology of hypophosphataemic osteomalacia, including ADHR’s gain of function FGF23 mutation and the excess FGF23 production of TIO.12 16 Although these rare diseases have been well studied, much of FGF23 physiology remains to be elucidated, in particular the cues for transcription and secretion from osteocytes.
Interestingly, clinical observations and mouse models demonstrate that iron deficiency increases osteocyte FGF23 mRNA and protein expression; the increased FGF23 protein is, however, cleaved to generate inactive C-terminal and N-terminal FGF23 fragments.6 17 18 As such, increases in FGF23 transcription due to iron deficiency should be negated by its increased degradation. Indeed, studies show that women with iron deficiency respond to infusion of iron with decreased FGF23 expression, but also with reduced FGF23 cleavage.4 5 As such, the net total of FGF23 activity should be unchanged and phosphate homeostasis preserved.
In contrast, iron-induced hypophosphataemic osteomalacia has been reported with several formulations of parenteral iron since the 1980s, including iron saccharide,19 iron polymaltose8 and most recently as in our patient, FCM.7 A recent randomised controlled trial comparing iron dextran to FCM in women with iron deficiency anaemia secondary to heavy uterine bleeding demonstrated that although FCM causes similar decreases in inactive cFGF23, 58% of patients receiving FCM experienced a transient increase in iFGF23 and subsequent hypophosphataemia.4 The authors propose that while iron reduces FGF23 transcription, the carbohydrate moieties in certain iron preparations, like FCM, may disproportionately inhibit FGF23 degradation to ultimately result in increased FGF23 activity and phosphate wasting.4 17
As FCM and novel iron formulations gain traction in patients requiring chronic supplementation due to their ease of use,20 we may see more cases of FGF23-induced hypophosphataemia and associated osteomalacia. Wolf et al4 demonstrate that the majority of women who receive FCM have transient hypophosphataemia. Recent prospective studies suggest that FCM is associated with a substantially higher risk of asymptomatic hypophosphataemia than other formulations.5 21 Based on previous reports in the literature, which demonstrate transient hypophosphataemia and resolution by 12 weeks,22 it appears that a single dose may be well tolerated. However, as with our patient, significant and symptomatic hypophosphataemia results in a subpopulation of patients requiring chronic iron infusions.5 8 9
What determines the susceptibility of patients to iron-induced hypophosphataemia remains speculative. It is possible that it is the accumulated dose of the FCM in patients continuing to require iron supplementation. Additionally, patients with a combination of inflammation, malnutrition, renal insufficiency or genetic predisposition may develop more severe and long-lasting hypophosphataemia.8 23 Alternatively, genetic variants in genes other than FGF23 may affect predisposition towards hypophosphataemia. Certainly, these circumstances may have contributed to the severity of skeletal manifestations observed in our patient. Our patient’s inflammatory bowel disease contributed both a degree of both malnutrition, including vitamin D deficiency, and baseline inflammation. His low bone density, which led to treatment with bisphosphonate, and his altered mineral homeostasis delayed his diagnosis and appropriate therapy. However, many patients receiving chronic FCM infusions have a similar inflammatory milieu, and it is therefore critical to recognise FCM as an iatrogenic cause of hypophosphataemic osteomalacia. Therefore, we suggest that in patients who will be receiving more than a single FCM dose, plasma phosphate, TmP/GFR and 1,25-dihydroxyvitamin D should be measured before continued dosing to diagnose FCM-induced hypophosphataemia. Measurement of FGF23, particularly iFGF23 if available, will be interesting to further correlate the disease with this important phosphate regulator.
Patient’s perspective.
I suffered from debilitating bone pain and broken bones for almost 2 years. My initial care team could not solve for the underlying cause of my pain and fractures, other than to say Crohn’s and prednisone had destroyed my bones and that taking more and more medicine was my solution. Thankfully I was referred to the authors of this paper as my care team. They took the time and showed the intellectual curiosity to figure out what was really happening to me. The timeline for my pain correlated strongly with my being switched to the fast-acting iron infusions, which I was getting every 2 months.
When I started seeing my new endocrinologist, I was using a cane, could not climb steps without severe bone pain and I had multiple fractured ribs. I could not sleep in a prone position at night.
Once we were able to determine that fast-acting iron infusions were harming me and I switched back to the old slow but safe iron infusion treatment, took k-phos and rocalcitrol, I was able to rapidly recover my quality of life. In less than 12 months, I was working out again, playing full contact basketball with my sons and stopped taking pain medications. I was also walking pain free. I was shocked that my original haematologist had not figured the iron infusion connection out and that I had been suffering needlessly. I was even more dismayed to learn that none of my regular physicians had heard of any issues with that type of iron infusion and bone pain. Clearly, I’m not the only one who has suffered and information which can be disseminated to other health providers to be on the lookout for this situation is crucial.
Learning points.
Ferric carboxymaltose (FCM)-induced hypophosphataemia remains widely underappreciated in the communities most commonly prescribing iron replenishment. Hypophosphataemia, thought to be driven by complex interactions between iron formulations and fibroblast growth factor 23, can lead to osteomalacia presenting as occult bone pain. We urge physicians to be vigilant in addressing bone pain in patients receiving iron infusions to reduce time to diagnosis and consequent morbidity.
In patients who will be receiving more than a single FCM dose, phosphate and TmP/GFR and 1,25-dihydroxyvitamin D should be measured before dosing. Low TmP/GFR and low 1,25-dihydroxyvitamin D in the setting of hypophosphataemia can confirm ongoing renal phosphate wasting induced by iFGF23.
If phosphate reabsorption remains persistently low, and FGF23 is abnormal, FCM should be exchanged for a non-maltose iron formulation.
Footnotes
Contributors: KK made substantial contributions to the conception, analysis and interpretation of data; drafted the work and gave final approval of submission; and will be accountable for all aspects of work including accuracy and integrity. SA made substantial contributions to the conception, analysis and interpretation of data; reviewed draft and gave final approval of submission; and will be accountable for all aspects of work including accuracy and integrity. ME made substantial contributions to the analysis and interpretation of data, reviewed draft and gave final approval of submission, and will be accountable for aspects of work including accuracy and integrity. JER made substantial contributions to the conception, analysis and interpretation of data; drafted the work and gave final approval of submission; and will be accountable for all aspects of work including accuracy and integrity.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lyseng-Williamson KA, Keating GM. Ferric carboxymaltose: a review of its use in iron-deficiency anaemia. Drugs 2009;69:739–56. 10.2165/00003495-200969060-00007 [DOI] [PubMed] [Google Scholar]
- 2.Hardy S, Vandemergel X. Intravenous iron administration and hypophosphatemia in clinical practice. Int J Rheumatol 2015;2015:1–6. 10.1155/2015/468675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer B, Würtinger P, Finkenstedt A, et al. . Choice of high-dose intravenous iron preparation determines hypophosphatemia risk. PLoS One 2016;11:e0167146 10.1371/journal.pone.0167146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 2013;28:1793–803. 10.1002/jbmr.1923 [DOI] [PubMed] [Google Scholar]
- 5.Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens 2017;26:266–75. 10.1097/MNH.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 6.Imel EA, Liu Z, McQueen AK, et al. . Serum fibroblast growth factor 23, serum iron and bone mineral density in premenopausal women. Bone 2016;86:98–105. 10.1016/j.bone.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand G, Schmid C. Severe hypophosphataemia after intravenous iron administration. BMJ Case Rep 2017;2017:bcr2016219160 10.1136/bcr-2016-219160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schouten BJ, Doogue MP, Soule SG, et al. . Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem 2009;46(Pt 2):167–9. 10.1258/acb.2008.008151 [DOI] [PubMed] [Google Scholar]
- 9.Schouten BJ, Hunt PJ, Livesey JH, et al. . FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab 2009;94:2332–7. 10.1210/jc.2008-2396 [DOI] [PubMed] [Google Scholar]
- 10.ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 2000;26:345–8. 10.1038/81664 [DOI] [PubMed] [Google Scholar]
- 11.Econs MJ. Genetic diseases resulting from disordered FGF23/klotho biology. Bone 2017;100:56–61. 10.1016/j.bone.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 12.White KE, Jonsson KB, Carn G, et al. . The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab 2001;86:497–500. 10.1210/jcem.86.2.7408 [DOI] [PubMed] [Google Scholar]
- 13.Shimada T, Mizutani S, Muto T, et al. . Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 2001;98:6500–5. 10.1073/pnas.101545198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe PS. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr 2012;22:61–86. 10.1615/CritRevEukarGeneExpr.v22.i1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo YC, Yuan Q. Fibroblast growth factor 23 and bone mineralisation. Int J Oral Sci 2015;7:8–13. 10.1038/ijos.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razzaque MS. The FGF23–Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol 2009;5:611–9. 10.1038/nrendo.2009.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf M, White KE. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens 2014;23:411–9. 10.1097/01.mnh.0000447020.74593.6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imel EA, Peacock M, Gray AK, et al. . Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab 2011;96:3541–9. 10.1210/jc.2011-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada M, Imamura K, Fuchigami T, et al. . [2 cases of nonspecific multiple ulcers of the small intestine associated with osteomalacia caused by long-term intravenous administration of saccharated ferric oxide]. Nihon Naika Gakkai Zasshi 1982;71:1566–72. 10.2169/naika.71.1566 [DOI] [PubMed] [Google Scholar]
- 20.Aksan A, Işık H, Radeke HH, et al. . Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017;45:1303–18. 10.1111/apt.14043 [DOI] [PubMed] [Google Scholar]
- 21.Bager P, Hvas CL, Dahlerup JF. Drug-specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br J Clin Pharmacol 2017;83:1118–25. 10.1111/bcp.13189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evstatiev R, Marteau P, Iqbal T, et al. . FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011;141:846–53. 10.1053/j.gastro.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 23.Fierz YC, Kenmeni R, Gonthier A, et al. . Severe and prolonged hypophosphatemia after intravenous iron administration in a malnourished patient. Eur J Clin Nutr 2014;68:531–3. 10.1038/ejcn.2014.20 [DOI] [PubMed] [Google Scholar]