Abstract
Background
Although transfusion is a life-saving intervention, it may be associated with significant morbidity in injured patients. We hypothesize that stored red blood cells induce pro-inflammatory activation of human pulmonary microvascular endothelium (HMVECs) resulting in neutrophil (PMN) adhesion and predisposition to acute lung injury (ALI).
Methods
Ten units of red blood cells (RBCs) were collected and 50% (by weight) were leukoreduced (LR-RBCs) and the remainder was unmodified and stored in additive solution-5 (AS-5). Another 10 units of RBCs were collected, leukoreduced, and stored in AS-3. HMVECs were incubated with [10–40%]FINAL of the supernatants day (D)1-D42 of storage, lipid extracts and purified lipids. Endothelial surface expression of ICAM-1, IL-8 release, and PMN adhesion to HMVECs were measured. HMVEC signaling via the BLT2 receptor was evaluated. Supernatants and lipids were also employed as the first event in a two-event model of ALI.
Results
The supernatants [10–40%]FINAL from D21 LR-RBCs and D42 RBCs and LR-RBCs and the lipids from D42 stored in AS-5 induced increased ICAM-1 surface expression on endothelium, IL-8 release, and PMN adhesion. In addition, the supernatants [20–40%]FINAL from D21 and D42 RBCs in AS-5 also increased endothelial surface expression of ICAM-1. D42 supernatants and lipids also caused co-precipitation of β-arrestin-1 with BLT2, PKCβI, and PKCδand served as the first event in a two-event rodent model of ALI. In conclusion, lipids that accumulate during RBC storage activate endothelium and predispose to ALI, which may explain some of the adverse events associated with the transfusion of critically injured patients.
Keywords: Massive transfusion, red blood cells, traumatic injury, pulmonary endothelium, liver sinusoidal endothelium, acute lung injury
Introduction
Pro-inflammatory activation of vascular endothelium results in the release of chemokines and the increased surface expression of adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1), in the lung microvasculature.1–3 Chemokine release induces phenotypic alterations of neutrophils (PMNs) in the capillary beds of the lung such that they become captured, via selectins, and then firmly adherent through binding of the PMN β2-integrin CD11b/CD18 and endothelial ICAM-1.1–3 These sequestered, primed PMNs are hyper-reactive such that mediators that normally do not induce activation of the microbicidal arsenal, cause the assembly of the NADPH oxidase, which elicits the production and release of reactive oxygen species and the release of proteolytic enzymes from granules resulting in PMN-mediated endothelial damage, capillary leak, and organ injury.4,5
Although it is a life-saving intervention, the transfusion of stored red blood cells (RBCs) has been linked to increased morbidity and mortality in the critically ill.6–13 Injured patients, irrespective of the mechanism: blunt, penetrating, or a combination of the two, are prone to acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS).10–12 Post-injury ALI, and ARDS are postulated to be the result of at least two clinical insults, as demonstrated in animal models.10–12 In a prospective study, controlled for the amount of blood transfused, transfusion of stored RBCs, versus fresh, was an independent predictor for the development of ALI and multiple organ failure (MOF) in injured patients.11 Similar data were found using pre-storage leukoreduced RBCs (LR-RBCs) such that the transfusion of older, stored LR-RBCs was associated with increased mortality.10,14 Despite the incidence of post-injury MOF declining, ALI/ARDS remain significant causes of morbidity and mortality with 64% of severely injured patients developing ALI with 46% progressing to ARDS.15 Thus, the injury itself plus the transfusion of stored RBCs appear to comprise the first event associated with the development of post-injury ALI/ARDS. During routine storage of LR-RBCs, pro-inflammatory lipids accumulate, which prime PMNs and can serve as the second event in two-event PMN-mediated animal models of ALI.4,16,17 Therefore, we hypothesize that the supernatant from stored RBCs causes pro-inflammatory activation of human pulmonary microvascular endothelial cells (HMVECs) and predisposes patients to ALI.
Materials and Methods
Materials
Unless otherwise indicated all chemicals were purchased from Sigma Chemical Corporation (St. Louis, MO). Buffers were made from sterile water or 0.9% saline for human injection (Baxter Healthcare Corp., Deerfield, NY) and sterilely filtered.17 Arachidonic acid (AA), 5-hydroxyeicosotetranoic acid (5-HETE), 12-HETE, and 15-HETE, and antibodies to the leukotriene B4 receptor-1 (BLT1) and BLT2 were purchased from Cayman Chemical (Ann Arbor, MI), siRNA oligo duplexes and Accell© siRNA agents were obtained from Dharmacon Inc. (Lafayette, CO). Antibodies for immunoblots and immunoprecipitations were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enzyme-linked immunosorbent assays (ELISA) for interleukin-8 (IL-8) were obtained from R&D Systems (Minneapolis, MN)
Isolation of Blood Components
After obtaining informed consent through a protocol approved by the Colorado Multi-Institutional Review Board (COMIRB), one unit of whole blood was collected from 20 healthy donors, per industry standards (AABB/FDA). Plasma was separated from RBCs by centrifugation followed by expression, employing an automated closed system, Compomat G4 (Fresenius-Kabi, Schweinfurt, Germany) and additive solution-5 (AS-5, Optisol) or AS-3 (Haemonetics, Braintree, MA) were added to a final hematocrit of 60–65%. The estimated amount of residual plasma was 5–10 ml/unit based upon values previously published.18 For the RBC units stored in AS-5, 50% of each unit was pre-storage leukoreduced via filtration (LR-RBCs) using a Haemonetics BPF4 leukoreduction filter (Braintree, MA) by weight with the other 50% left unmodified (RBCs). The 10 AS-3 stored RBC units were separated from whole blood and prestorage leukoreduced. The hematocrits ranged from 58–65%. The RBCs and LR-RBCs were stored at 1–6°C, and sterile couplers were used to obtain samples on day (D) 1, D14, D21, and D42 (the last day a unit can be transfused) from the RBC and LR-RBC units. The supernatant was isolated via centrifugation (5000×g for 7 min) followed by a second spin at 12,500×g for 5 min to remove platelets and cellular debris.17
Apheresis platelet concentrates (PCs) were collected from healthy donors using a Terumo Trima apheresis device (Lakewood, CO) per industry standards, samples were obtained via sterile couplers, and the supernatant isolated as described above. Prior to use in tissue culture or in vivo the plasma controls or the supernatants from all cellular components were heated to 56°C for 30 min to obviate human complement and fibrinogen and in the tissue culture experiments, heparin (10 units/ml) was added in selected experiments, to prevent activation of the clotting cascade.4
Lipid extractions
Lipids were extracted from the supernatants of the PRBCs and resuspended in the identical starting volume of either 1.25% fatty acid-free human albumin (albumin) for HMVEC experiments or hexane/isopropanol/water for HPLC separation.17,19,20 Lipids were separated by reverse phase high performance liquid chromatography (HPLC) developed for phospholipid class separation, and the individual fractions were dried and resuspended in 1.25% albumin and assayed for their ability to activate HMVECs.4,16,17,19
Pro-inflammatory HMVEC Activation
HMVECs (Lonza, Allendale, NJ) were stimulated (2–24 hours) with media or heat-treated supernatants [1–40%]FINAL from D1, D21, D28, & D42 RBCs/LR-RBCs, the lipid extractions from D1 & D42 RBCs/LR-RBCs, or the individual purified non-polar lipids (NLs) at identical concentrations in D1 or D42 RBCs/LR-RBCs.5,17 The tissue culture supernatant was aspirated and stored at −80°C, and HMVECs were trypsinized and the surface expression of ICAM-1 (CD54) was measured using a PE-conjugated antibody to CD54 (BD BioSciences, San Diego, CA) via flow cytometry. IL-8 was measured in HMVEC supernatants by commercial ELISAs. Isolated PMNs were added to HMVECs and allowed to adhere for 60 min, and adherence was measured as described.5
PMN isolation
Blood was collected from healthy volunteers using informed consent under a protocol approved by the COMIRB. The PMNs were isolated as previously described.17
siRNA knock down of BLT2
Knockdown of BLT2 in HMVECs was done as previously reported.21 Transfections were completed 72 hours before functional assays were performed.
Immunoblotting and immunoprecipitation
HMVECs were stimulated with the D1 or D42 LR-RBC plasma and the individual NLs at D1 and D42 concentrations for 1–15 min. The media was removed, HMVECs lysed with 1% Triton, and the lysates saved for immunoprecipitations. A BCA protein assay was completed to ensure that equivalent amounts of protein were loaded, followed by immunoblotting and immunoprecipitations as described.22
A two-event in vivo model of ALI
Male Sprague Dawley rats (Harlan, Indianapolis, IN) were treated via a protocol approved by the Institutional Animal Care and Use Committee, UCD.4 Following anesthesia and cannulation of the femoral vessels, 10% of the blood volume was removed and the animals were infused with an identical volume of saline, heat-treated plasma from D1 or D42 LR-RBCs, or the NLs in HSA. After 6 hours, rats were injected intravenously (IV) with LPS (100 µg/kg), and returned to their cages for 2 hours. Blood was drawn, the rats euthanized, and a bronchoalveolar lavage (BAL) was performed to measure the Evans Blue dye (EBD) leak.4
Statistics
The data is presented as the mean ± the standard error of the mean (SEM) and were analyzed with independent or repeated measures ANOVA with a post-hoc Bonferroni or Newman-Keuls test for multiple comparisons based upon the equality of variance.
Results
Supernatants from stored RBCs/LR-RBCs but not PCs caused pro-inflammatory activation of HMVECs
Plasma from units for transfusion [10–20%]FINAL or supernatants from PCs [10–20%]FINAL D1 or D5, the last day PCs may be transfused, did not cause HMVEC activation, as quantified by ICAM-1 surface expression (Table 1). The lipid extracts [10–20%]FINAL from the PC supernatants or plasma also did not cause HMVEC activation at 6 hours. In contrast, the supernatants from D42 RBC and D21 and D42 LR-RBC units stored in AS-5 [10%]FINAL caused increased surface expression of ICAM-1 on HMVECs vs. D1 supernatants from the identical units and the media-treated controls (Table 2). Final concentrations of 20% and 40% also induced significantly increased surface expression of ICAM-1 vs D1 and media controls (Table 2). The HMVEC ICAM-1 surface expression was durable (24 hours) and was not significantly increased compared to the 6 hour incubations (D42 PRBC supernatant at 24 hours: 10%: 20.4±3.8, 20%: 27.0±4.6 vs. 6 hours: 10%: 21.3±3.4, 20%: 34.6±1.3, p>0.05, n=8). Lesser concentrations (1–5%) of D42 PRBCs/LR-RBCs or incubation times shorter than 6 hours did not affect ICAM-1 surface expression, and heparinized RBC supernatants elicited similar results (data not shown). In addition, the supernatants from D42 RBCs and D21 and D42 LR-RBCs, but not D1, caused significant release of IL-8 and increased PMN adhesion at final concentrations of 10–40% versus D1 and media controls (Table 2).
Table 1.
Plasma and platelet concentrates do not induce HMVEC pro-inflammatory activation
| ICAM-1 (MFI) | ||||
|---|---|---|---|---|
| 10% | 20% | |||
| FFP and Plt Supernatants | Controls | MC (20%) | 9.6±1.1 | |
| Plasma | 7.5±1.6 | 9.8±2.7 | ||
| Platelets | D1 | 9.6±1.2 | 9.4±1.3 | |
| D5 | 8.6±2.2 | 9.6±2.8 | ||
| Lipid Extracts | Controls | AC (20%) | 5.6± 1.1 | |
| Lyso-PCs | 4.5 µM | 9.4±4.0 | 9.2±2.8 | |
| Platelets | D1 | 5.6±0.9 | 5.8±0.9 | |
| D5 | 5.4±0.8 | 7.9±0.8 | ||
Data are expressed as the mean ± SEM. 10% and 20% denote the final supernatant concentration.
MC= media control, AC=albumin control, and*=p<.05 vs. MC, AC, and D1.
Table 2.
The supernatants and lipids from stored RBC and LR-RBC units (AS-5) cause pro-inflammatory HMVEC activation.
| Treatment Groups | ICAM-1 (MFI) | IL-8 (pg/ml) | MPO (% Adherent PMNs) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 20% | 40% | 10% | 20% | 10% | 20% | 40% | |||
| Supernatants | C | MC | 6.1±1.1 (40%) | 485±25 (20%) | 0.6±0.3 (40%) | |||||
| LPS | 30.6±2.6† | 1452±303† | 18.8±2.1† | |||||||
| RBC | D1 | 5.3±1.4 | 4.7±1.9 | 6.0±1.0 | 539±42 (20%) | 1.4±0.4 | 1.2±0.5 | 1.0±0.6 | ||
| D14 | 5.9±1.0 | |||||||||
| D21 | 7.6±0.7 | |||||||||
| D42 | 15.1±2.1* | 20.3±3.4* | 24.6±6.2* | 2082±1166* | 2182±76* | 8.9±1.0* | 11.7±1.1* | 15.3±0.8* | ||
| LR-RBC | D1 | 6.4±0.6 | 6.9±1.8 | 6.1±0.4 | 412±45 (20%) | 1.6±0.2 | 1.5±0.3 | 1.3±0.2 | ||
| D14 | 8.8±1.0 | |||||||||
| D21 | 10.2±1.1* | 18.7±2.3* | ||||||||
| D42 | 13.0±2.0* | 24.3±4.6* | 22.8±1.3* | 786±68* | 810±93* | 9.1±1.3* | 14.0±1.2* | 17.8±1.0* | ||
| Lipids | C | AC | 5.6±1.8 (40%) | 454±147 (20%) | ||||||
| RBC | D1 | 7.6±0.6 | 7.7±0.9 | 8.0±0.9 | 497±63 (20%) | |||||
| D42 | 14.9±3.2* | 18.6±1.0* | 20.6±3.4* | 880±62* | 816±35* | |||||
| LR-RBC | D1 | 5.1±0.6 | 5.8±0.7 | 5.2±0.8 | 564±62 (20%) | |||||
| D42 | 12.8±1.7* | 19.2±1.1* | 21.6±1.3* | 860±57* | 812±15* | |||||
=p<.05 vs. MC or AC and D1.
= p<.05 vs. vehicle controls.
MC = media control, AC = albumin control, D = Day. MFI = mean fluorescence intensity.
Lipids from D42 RBCs and LR-RBC activated HMVECs
The lipid extracts from the D42 RBC supernatants at [10, 20, and 40%]FINAL significantly increased ICAM-1 surface expression on HMVECs as compared to the media, albumin, and the lipids from both plasma and D1 LR-RBCs (p<0.05) (Table 2). These lipid extracts also caused significant release of IL-8 and PMN adhesion versus D1 extracts and albumin-treated controls (Table 2).
The lipids from D42 LR-RBC units were separated by normal phase HPLC (NP-HPLC). The individual fractions, solubilized in 1.25% albumin, were incubated with HMVECs at a final concentration of 10%. Only fraction 2 demonstrated increased surface expression of ICAM-1 on HMVECs by 2.3-fold vs. media controls and fractions 1 & 3–14 (data not shown). Fraction 2, the void volume from NP-HPLC contains the non-polar lipids, which are not retained on the column, were then run on reverse phase HPLC and found to elute at the retention time of the controls, namely: arachidonic acid and 5-, 12-, and 15-HETE (results not shown).17 These lipids were further identified by gas chromatography/quadropole tandem mass spectroscopy and the purified lipids were combined at the concentrations present in the average of 10 units of D42 LR-RBCs and added to HMVECs at a concentration identical to that [10–20%]FINAL of the supernatant.17,23 These purified lipids caused significant increases in ICAM-1 surface expression on HMVECs vs. albumin-treated controls (Table 2). In contrast, lysophosphatidylcholines, which prime PMNs and also accumulate during routine storage of PCs and unmodified RBCs, did not increase ICAM-1 surface expression (Table 1).19,24
D42 LR-RBC supernatant activated HMVECs through the BLT2 receptor
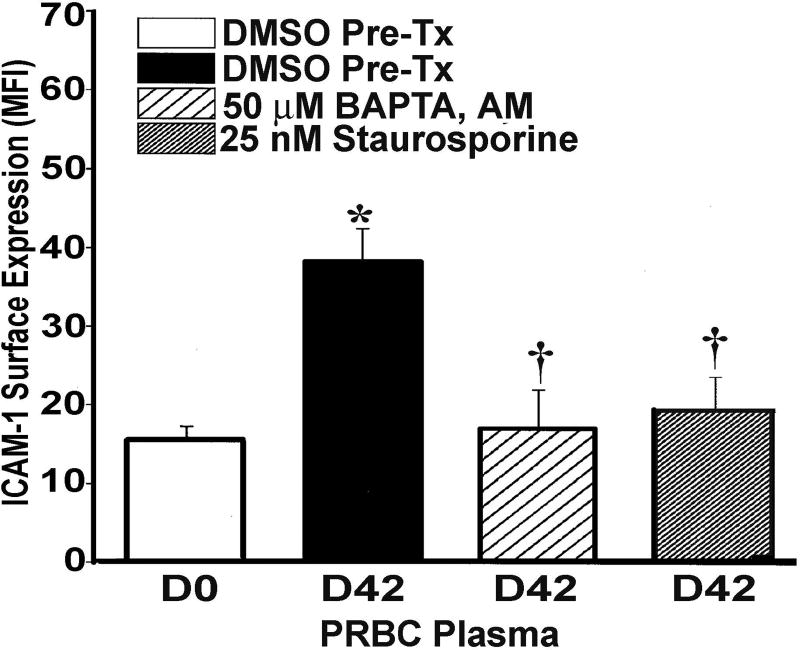
Both the D42 LR-RBC supernatant and the lipid mixture induced co-precipitation of BLT2 with β-arrestin-1 at 1 min which was still present at 5 min (Fig.1 Panel IA). The individual lipids which comprise this mixture, specifically arachidonic acid (AA) and 5-, 12-, and 15-HETEs elicited similar co-precipitation of BLT2 with β-arrestin-1 beginning at 1 min with some showing maximal co-precipitation at 5–15 min (Fig.1, panel I, B-E). In addition, AA, the most abundant lipid, induced co-precipitation of both the classical protein kinase C (cPKC) PKCβ-I at 1 min (Fig.1, panel IIA) and the non-classical PKC (nPKC) PKCδ at 1 min (Fig.1, panel IIB) with β-arrestin-1.17 To confirm that PKC activity was required, HMVECs were pre-treated with 25 nM staurosporine (5 min), a pan-PKC antagonist, which significantly inhibited the increase in ICAM-1 surface expression by D42 PRBC supernatant by 94±7% and the amount of IL-8 released by 95±12% in response to the non-polar lipid mixture as compared to albumin-treated controls (n =5 for both ICAM-1 and IL-8, p<0.05) (Fig.2 and data not shown).25 BAPTA,AM a rapid intracellular Ca2+ chelator also inhibited the D42-mediated increase in ICAM-1 surface expression by 98±4% through eliminating the increases in cytosolic Ca2+ (Fig.2).25
Figure 1. Non-polar lipids induce co-precipitation of the BLT-2 receptor with β-arrestin-1 and AA causes co-precipitation of β-arrestin-1 with PKC isoforms.
Panel I shows representative immunoblots of HMVECs stimulated with the NLs (the mixture and the individual lipids: arachidonic acid (AA) and 5-, 12-, and 15-HETE). The mixture (A) and the individual compounds (B–E) caused co-precipitation of the BLT2 receptor with β-arrestin-1, (n=3). The co-precipitation of β-arrestin-1 was initially visualized at 1 min and became maximal at 5–10 min. Panel II is a representative immunoblot demonstrating that AA, the most abundant NL to accumulate during LR-RBC storage, caused co-precipitation of β-arrestin-1 with PKCβI and PKCδ (n=3).
Figure 2. PKC inhibition and cytosolic Ca2+ chelation inhibit D42 PRBC supernatant-mediated increases in ICAM-1 surface expression.
ICAM-1 surface expression as quantified by flow cytometry, mean fluorescence intensity (MFI) is shown as a function of treatment group. As compared to the supernatant [10%]FINAL from D0 RBC units the supernatant [10%]FINAL from D42 RBC units + DMSO vehicle significantly increased ICAM-1 surface expression (*=p<.05, n=5). Pretreatment with BAPTA,AM [50 nM] an intracellular Ca2+ chelator or staurosporine [25 nM], a pan-PKC antagonist, significantly inhibited the increased ICAM-1 surface expression induced by the D42 RBC supernatant [10%]FINAL (†=p<.05, n=5).
Silencing of BLT2 decreased ICAM-1 surface expression
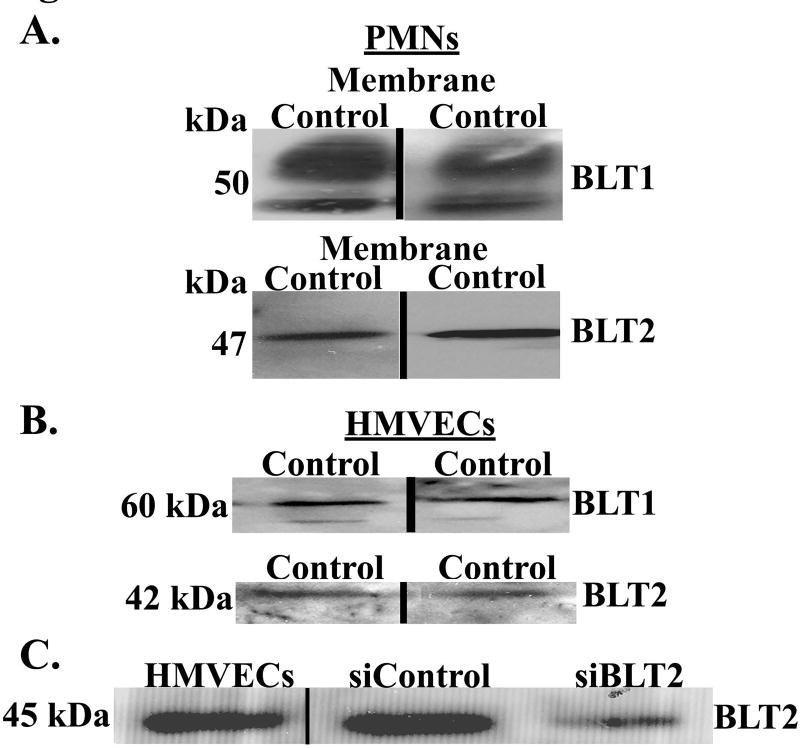
Extracts of PMNs (positive controls) and HMVECs demonstrated immunoreactivity for both BLT1 and BLT2 (Panels A&B, Fig.3). The siRNA oligo sequences to BLT2 (siBLT2) selectively reduced BLT2 immunoreactivity in HMVECs (Panel C, Fig.3). In addition, silencing of BLT2 inhibited the D42 LR-RBC supernatant [20%]FINAL mediated increase in ICAM-1 surface expression by 52±8% vs. HMVECs treated with non-targeting siRNA (n=5 for both ICAM-1 and adherence, p<0.05).
Figure 3. HMVECs contain BLT1 and BLT2 immunoreactivity and BLT2 silencing decreases its immunoreactivity.
Panel A: PMNs are used as a positive control and display immunoreactivity for both BLT1 and BLT2. Panel B: HMVECs also contain immunoreactivity for both BLT1 and BLT2. The presented data are representative of three separate experiments. Panel C: siRNA oligo sequence treatment of BLT2 in HMVECs selectively reduced BLT2 immunoreactivity as compared to siRNA-treated HMVECs that received a non-targeting sequence (NTS) (n=4).
D42 LR-RBC supernatant and the non-polar lipid mixture may serve as the first event in a two-event, in vivo model of ALI
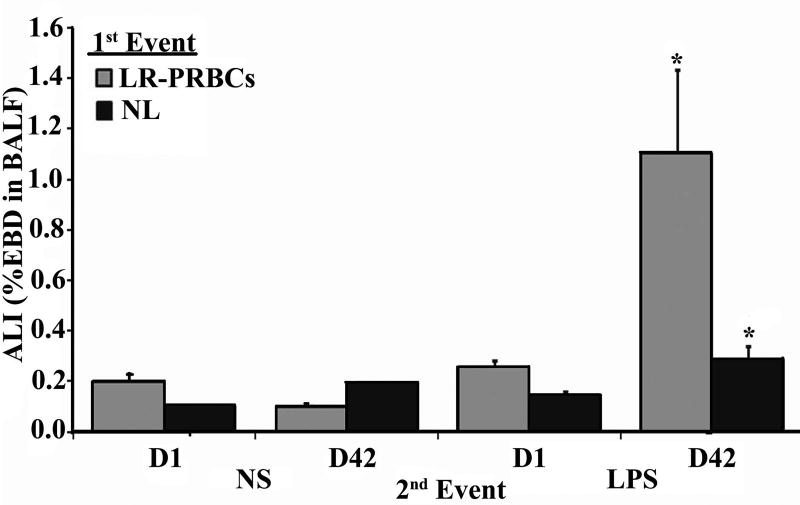
Rats infused with 10% supernatant or purified lipids from D1 LR-RBCs or saline, incubated for 6 hours, and then given IV saline or 100 µg/kg of LPS IV did not develop ALI (Fig.4). In addition, rats infused with 10% supernatant or purified lipids from D42 LR-RBCs, incubated for 6 hours, and given intravenous saline did not evidence ALI. However, rats infused with 10% supernatant or lipids from D42 LR-RBCs, incubated for 6 hours, and infused with 100 µg/kg LPS demonstrated significant EBD leak consistent with ALI (Fig.4).
Figure 4. The supernatant and NLs from D42 LR-RBCs serve as the first event in a two-event model of ALI.
Evans Blue dye leak (EBD) is shown as a function of treatment group. Rats infused with saline, D1 or D42 plasma from LR-RBCs, or the D1 or D42 NLs from LR-RBCs (first event), infused with saline (second event) did not evidence EBD leak/ALI. Furthermore, animals who received saline or the D1 plasma or lipids who then received IV LPS as the second event also did not experience significant EBD/ALI. However, rats infused with the plasma or lipids from D42 LR-RBCs (first event) that then received IV LPS (second event) evidenced significant EBD/ALI (*=p<0.05 vs. D1 LR-RBC plasma/LPS or D1 NL/LPS, n≥4).
Discussion
The supernatant [10–40%]FINAL from D42 RBCs and D21 and D42 LR-RBCs stored in AS-5, but not D1, induced pro-inflammatory activation of pulmonary HMVECs as evidenced by increased ICAM-1 surface expression. The supernatant [10–40%]FINAL from AS-3 stored D21 and D42 LR-RBCs also caused similar increased ICAM-1 HMVEC surface expression vs. D1 and media controls. Such pro-inflammatory activation also induced the release of IL-8, and together with the increased ICAM-1 surface expression resulted in significant PMN adherence. In contrast, neither the plasma nor the supernatant from PCs elicited HMVEC pro-inflammatory activation. NLs released during RBC and LR-RBC storage are one of the responsible mediators as both lipid extracts and purified NLs caused similar pro-inflammatory activation.17 Incubation of HMVECs with these NLs caused ligation of the BLT2 receptor with recruitment of β-arrestin-1, and AA induced co-precipitation of two isoforms of PKC, PKCβI and PKCδ, with β-arrestin-1. Both silencing of the BLT2 receptor and inhibition of PKC activity significantly inhibited D42 supernatant-driven, pro-inflammatory HMVEC activation. The supernatant and lipids from D42 LR-RBCs, but not D1, also served as the first event in an in vivo two-event rodent model of ALI demonstrating possible clinical ramifications for the transfusion of stored LR-RBCs.
The presented data differ from other studies of endothelial activation in that primary HMVECs were employed. HMVECs express both the BLT1 and BLT2 receptors on their surface and increase ICAM-1 and release chemokines in response to pro-inflammatory stimuli resulting in PMN adhesion.21,26–30 Previous data demonstrated that leukotriene B4 (LTB4)-mediated HMVEC activation occurred via the BLT2 receptor in congruence with the presented data, and the BLT2 receptor has been implicated in other pulmonary diseases and angiogenesis.21,31–33 Furthermore, the concentration of LPS employed as a positive control may also seem excessive; however, it is based upon the human response to injected LPS in which concentrations of 10 ng/ml injected induced hypotensive shock and a concentration of 500 ng/ml induced both shock and signs of ALI, and not just fevers and changes in cytokine profiles.34–36 The percentage of PMNs that adhered to the activated HMVECs may appear low, but these assays inverted the tissue culture plates (with the adherent cells) and centrifuged them at 200×g for 10 min; thus, firm PMN adherence is required to remain adherent after exposure to centripetal acceleration of 200×g. The animal model of ALI employed has been published multiple times and requires a first event which elicits pro-inflammatory activation of the pulmonary endothelium, resulting in PMN adherence followed by a second event, which activates these primed, adherent PMNs.4,37,38 In the presented data the infusion of RBC supernatant or the NLs was substituted for LPS as the first event, and LPS was employed as the second event.4,37,38 This animal model has demonstrated ALI via 5 different types of measurements, which correlate with lung leak as quantified by Evans blue dye.4
The D42 RBC supernatants, the lipid mixture, and the purified lipids caused co-precipitation of the BLT2 receptor with β-arrestin-1. Ligand occupancy of G-protein-linked cellular receptors (GPCR) initiated cellular signaling through recruitment of β-arrestins and other mediators with β-arrestins responsible for both transduction and termination of signaling events from the activated receptor.22,39–41 The β-arrestin-1 scaffold also allows for the interaction of the cPKC isozyme PKCβI and the nPKC isoform PKCδ to become activated and propagate the signal to increase ICAM-1 surface expression possibly through NFκB, although activation of this transcription factor has not been reported downstream of PKC in HMVECs. There is little data to assume that the NL-mediated increases in ICAM-1 surface expression are downstream of the NADPH oxidase proteins (Nox). The BLT2 receptor also has not been linked to Nox proteins in endothelium, although endothelial Nox proteins are linked to organ invasion by Pseudomonas aeruginosa or cancer cells, and HNA-3a antibody-induced TRALI; however, RBC supernatant alone did not induce ALI.33,42–46
Clinical studies of injured patients have determined that blood transfusion is an independent predictor of the development of post-injury MOF and that the infusion of older, stored RBC units correlated with the development of ALI/MOF, which was unaffected by leukoreduction.10–13 A secondary analysis of the “Age of Blood Evaluation” (ABLE) trial suggested a “volume-dependent, threshold effect of prolonged red cell storage” such that “further studies on the effect of RBC unit storage age should be focused on patients anticipated to require more than 5 RBC transfusions”.47 In addition, numerous investigators have concluded that the resuscitation of injured patients with older, stored RBC units may result in increased mortality, infections, ALI/ARDS and post-injury MOF, and such clinical complications may be due to the numbers of units transfused and the “dose” of pro-inflammatory agents in the stored RBCs.6,10,11,14,48–59 The presented in vitro and in vivo studies of pro-inflammatory activation of HMVECs by stored RBCs/LR-RBCs support these observations, and it is important to note that such activation only occurred with the transfusion of “>4”, “>8”, and “>16” units of RBCs, depending upon the weight of the patient and their inherent plasma volume, analogous to the final concentration of 10–40% of D42 LR-RBC supernatant. These lipids increase during routine storage and large to massive transfusions may allow for exposure to large concentrations of LR-RBC supernatants and the NLs which they contain. Numerous studies of injured patients, with competent immunity, who required massive transfusions (>6 units of RBCs in the first 12 hours), demonstrated independent associations between RBC transfusions and the development of MOF, ALI/ARDS, post-injury infections, and mortality.7–9,49,60–62 Furthermore, the age of transfused RBCs further correlates to ALI/MOF development, infections, and mortality.10,11,14,51–59,63 Therefore, the concentration of circulating pro-inflammatory agents is important and to reach such concentrations a significant number of RBC units must be administered. One would not expect such pro-inflammatory changes in patients receiving simple, chronic, or small volume transfusions. Lastly, the animal model may not perfectly mimic an injured patient who received a massive transfusion; however a number of points must be taken into account. The pathophysiology of both MOF, which invariably begins with ALI, and ALI/ARDS are at least two events.64,65 Both ALI and MOF start 48–72 hours post-injury and have been linked to the transfusion of older units of blood in patients requiring >6 units of RBCs in the first 12 hours.8,9,62 Thus, the transfusion of older, stored RBCs can be seen as an inciting (first) event inducing endothelial pro-inflammatory activation rather than the precipitating (second) event, such as in TRALI, in which these lipids activate adherent PMNs inducing endothelial cell damage. Massive transfusion has been linked to ALI/ARDS and the presented in vivo data demonstrates the infusion of older, stored RBC supernatant may induce ALI as a first, durable event.62,66–68 Liver transplantation may also result in massive transfusion support; however, these patients are usually on immunosuppressive medications and are immunosuppressed which affects ALI.69–71 Thus, these in vivo studies demonstrate that the supernatant from older, stored RBC units may serve as one of the inciting events for ALI.
NLs accumulate in the supernatant during the routine storage of both RBCs and LR-RBCs and have inherent pro-inflammatory activity which changes the cellular physiology of both the vascular endothelium and circulating granulocytes.17 Conversely, only lysophosphatidylcholines accumulate in PCs and unmodified RBCs and not in LR-RBCs, indicating that their source is likely the platelet because pre-storage leukoreduction also removes platelets.17,38,72 The NLs are generated through the action of an atypical phospholipase, peroxiredoxin-6, which is released and increased during storage of RBCs/LR-RBCs and is active via T-phosphorylated (T177). This releases AA, and 5-lipoxygenase which converts 5-hydroperoxyeicosatetraenoic acid (5-HpETE) to 5-HETE.72–74 and, although leukotrienes are downstream of 5-HpETE, the leukotriene synthase to produce LTA4 and the LTA4 hydrolase do not accumulate during RBC storage, like both peroxoredoxin-6 and 5-LO..73 Importantly, there were no leukotrienes, save the HETEs, found by mass spectroscopy, and leukoreduction of RBC units removes the major leukocyte sources of LTB4 and the cysteinyl leukotrienes, with the few short-lived granulocytes rapidly (days) becoming apoptotic. Experimental filtration with a leukoreduction filter that also removed 2 logs of IgG also decreased the production of 5-HETE, the relative amount of lipid priming activity and the ability of stored RBC supernatant to induce ALI as the second event.38 This decrease in 5-HETE was due to virtual elimination as demonstrated by western blotting of two of the enzymes responsible for %-HETE synthesis and accumulation from AA.38,72 These enzymes, 5-lipoxygenase activating protein and 5-lipoxygenase, share 10% and 17% sequence homology with IgG allowing their clearance from the blood by the experimental filter.38,72 There may be other pro-inflammatory mediators (proteins), which elicit pro-inflammatory activation of the vascular endothelium predisposing injured patients to PMN-mediated organ injury as has been demonstrated in vitro for α-enolase.75
Pro-inflammatory activation of HMVECs occurred through activation of the BLT2 receptor. There is little data with regard to the physiologic function of BLT2 on endothelium except for vascular endothelial growth factor-induced angiogenesis.33 Moreover, both the lipid mixture and each individual lipid moiety caused recruitment of β-arrestin-1 to BLT2.33 Silencing of BLT2 confirmed the importance of the BLT2 receptor in lipid activation of HMVECs.21,33 Parallel silencing approaches were employed with HMVECs because they are sensitive to both transfection and viral infections and to ensure that the inhibition was not due to siRNA delivery alone. These data support a physiologic role for the BLT2 receptor in NL-induced pro-inflammatory activation of HMVECs.
There are a number of limitations in the presented data. Not each and every storage time point was tested for their ability to induce pro-inflammatory activation of HMVECs; rather, selected storage times were employed which were found to induce PMN priming because we hypothesized that these lipids would have duality in function: the ability to both prime PMNs and activate endothelium, similar to LPS.16 In addition, AS-1 stored RBCs and LR-RBCs were not tested. AS-1(Adsol) and AS-5 (Optisol) are very similar and differ only by minor changes in NaCl, adenine, and mannitol, although AS-1 has >2-fold more dextrose.76 AS-5 stored RBCs were compared to AS-3 (Nutricell) because AS-3 contains NaH2PO4, citric acid, sodium citrate, and the CP2D anticoagulant with <50% of the NaCl of either AS-1 or AS-5. “Omics” analyses of RBCs stored in AS-3 have demonstrated that AS-3 preserves RBC function and physiology better than AS-1, AS-5 or even saline-adenine-glucose-mannitol (SAGM).77–83 Longer storage was required for lipid accumulation in AS-3 stored LR-RBCs. Lastly, the two-event model of ALI employed has been questioned because humans who received endotoxin did not manifest injury when transfused with older, stored blood RBCs.84,85 However, the dose of endotoxin (2 ng/kg; 40–50 pg/ml) used in these experiments was much less than the intravenous doses (≥1 mg) for the treatment of neuro-syphilis, which was completed in most cases without significant mortality.35,72,84,85 Aside from becoming febrile with a mild increase in the respiratory rate the subjects were not as ill as the LPS-treated rats.35,72,84,85 Moreover, LPS does not induce pro-inflammatory changes in human PMNs and HMVECs at concentrations <20 ng/ml in vitro, which is orders of magnitude higher than the in vivo dosing.16,35,75,86
These data support prior clinical reports that identified stored RBC transfusion as an independent risk factor for post-injury ALI/ARDS, MOF, infections, and mortality.10,11,14,51,52,55–57,59 Changes in RBC storage to decrease the accumulation of pro-inflammatory compounds in the supernatant are needed. The presented data has illustrated possible adverse events associated with transfusion; however, these data should not detract from the overall positive impact RBC transfusion has made, allowing for surgical intervention, organ transplantation, and increased survival from cancer.
Acknowledgments
Funding
This work was supported by Bonfils Blood Center and grants P50-GM049222 from NIGMS, NIH and HL59355 from NHLBI, NIH.
Footnotes
Disclosure: The authors have no financial or other conflicts of interest.
Authorship
CCS designed the studies, analyzed the data, and wrote the manuscript. MRK performed experiments, constructed the figures, and aided in manuscript preparation. SYK, NJDM, and DJE performed experiments. AB provided critical insight and data analysis. KJE and JB performed experiments. FBW oversaw all blood component accrual, helped to write the manuscript, and provided critical insight into the experimental design. SK helped with data analysis and to write the manuscript
References
- 1.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994 May;8(8):504–12. [PubMed] [Google Scholar]
- 2.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J.Leukoc.Biol. 1994 May;55(5):662–75. [PubMed] [Google Scholar]
- 3.Smith CW. 3. Adhesion molecules and receptors. J.Allergy Clin.Immunol. 2008 Feb;121(2 Suppl):S375–S379. doi: 10.1016/j.jaci.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Kelher MR, Masuno T, Moore EE, Damle S, Meng X, Song Y, Liang X, Niedzinski J, Geier SS, Khan SY, et al. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009 Feb 26;113(9):2079–87. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyman TH, Bjornsen AJ, Elzi DJ, Smith CW, England KM, Kelher M, Silliman CC. A two-insult in vitro model of PMN-mediated pulmonary endothelial damage: requirements for adherence and chemokine release. Am.J.Physiol Cell Physiol. 2002 Dec;283(6):C1592–C1603. doi: 10.1152/ajpcell.00540.2001. [DOI] [PubMed] [Google Scholar]
- 6.Holena DN, Netzer G, Localio R, Gallop RJ, Bellamy SL, Meyer NJ, Shashaty MG, Lanken PN, Kaplan S, Reilly PM, et al. The association of early transfusion with acute lung injury in patients with severe injury. J.Trauma Acute.Care Surg. 2012 Oct;73(4):825–31. doi: 10.1097/TA.0b013e318256de38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SV, Kidane B, Klingel M, Parry N. Risks associated with red blood cell transfusion in the trauma population, a meta-analysis. Injury. 2014 Oct;45(10):1522–33. doi: 10.1016/j.injury.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch.Surg. 1994 Jan;129(1):39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 9.Sauaia A, Moore FA, Moore EE, Norris JM, Lezotte DC, Hamman RF. Multiple organ failure can be predicted as early as 12 hours after injury. J.Trauma. 1998 Aug;45(2):291–301. doi: 10.1097/00005373-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg JA, McGwin G, Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW., III Duration of red cell storage influences mortality after trauma. J.Trauma. 2010 Dec;69(6):1427–31. doi: 10.1097/TA.0b013e3181fa0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am.J.Surg. 1999 Dec;178(6):570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 12.Watkins TR, Rubenfeld GD, Martin TR, Nester TA, Caldwell E, Billgren J, Ruzinski J, Nathens AB. Effects of leukoreduced blood on acute lung injury after trauma: a randomized controlled trial. Crit Care Med. 2008 May;36(5):1493–9. doi: 10.1097/CCM.0b013e318170a9ce. [DOI] [PubMed] [Google Scholar]
- 13.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N.Engl.J.Med. 2008 Mar 20;358(12):1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg JA, McGwin G, Jr, Griffin RL, Huynh VQ, Cherry SA, III, Marques MB, Reiff DA, Kerby JD, Rue LW., III Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J.Trauma. 2008 Aug;65(2):279–82. doi: 10.1097/TA.0b013e31817c9687. [DOI] [PubMed] [Google Scholar]
- 15.Howard BM, Kornblith LZ, Hendrickson CM, Redick BJ, Conroy AS, Nelson MF, Callcut RA, Calfee CS, Cohen MJ. Differences in degree, differences in kind: characterizing lung injury in trauma. J.Trauma Acute.Care Surg. 2015 Apr;78(4):735–41. doi: 10.1097/TA.0000000000000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyman TH, Bjornsen AJ, Elzi DJ, Smith CW, England KM, Kelher M, Silliman CC. A two-insult in vitro model of PMN-mediated pulmonary endothelial damage: requirements for adherence and chemokine release. Am.J.Physiol Cell Physiol. 2002 Dec;283(6):C1592–C1603. doi: 10.1152/ajpcell.00540.2001. [DOI] [PubMed] [Google Scholar]
- 17.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011 Dec;51(12):2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silliman CC, Kelher M, Ambruso DR. Bioactive lipids from stored cellular blood components: in vitro method is crucial for proper interpretation. Transfusion. 2012 May;52(5):1155–7. doi: 10.1111/j.1537-2995.2012.03564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silliman CC, Dickey WO, Paterson AJ, Thurman GW, Clay KL, Johnson CA, Ambruso DR. Analysis of the priming activity of lipids generated during routine storage of platelet concentrates. Transfusion. 1996 Feb;36(2):133–9. doi: 10.1046/j.1537-2995.1996.36296181925.x. [DOI] [PubMed] [Google Scholar]
- 20.Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J.Clin.Invest. 1998 Apr 1;101(7):1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eun JC, Moore EE, Banerjee A, Kelher MR, Khan SY, Elzi DJ, McLaughlin NJ, Silliman CC. Leukotriene b4 and its metabolites prime the neutrophil oxidase and induce proinflammatory activation of human pulmonary microvascular endothelial cells. Shock. 2011 Mar;35(3):240–4. doi: 10.1097/SHK.0b013e3181faceb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J.Immunol. 2006 Jun 1;176(11):7039–50. doi: 10.4049/jimmunol.176.11.7039. [DOI] [PubMed] [Google Scholar]
- 23.Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J.Lab Clin.Med. 1994 Nov;124(5):684–94. [PMC free article] [PubMed] [Google Scholar]
- 24.Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J.Lab Clin.Med. 1994 Nov;124(5):684–94. [PMC free article] [PubMed] [Google Scholar]
- 25.Kelher MR, McLaughlin NJ, Banerjee A, Elzi DJ, Gamboni F, Khan SY, Meng X, Mitra S, Silliman CC. LysoPCs induce Hck- and PKCdelta-mediated activation of PKCgamma causing p47phox phosphorylation and membrane translocation in neutrophils. J.Leukoc.Biol. 2017 Jan;101(1):261–73. doi: 10.1189/jlb.3A0813-420RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinthamani S, Odusanwo O, Mondal N, Nelson J, Neelamegham S, Baker OJ. Lipoxin A4 inhibits immune cell binding to salivary epithelium and vascular endothelium. Am.J.Physiol Cell Physiol. 2012 Apr 1;302(7):C968–C978. doi: 10.1152/ajpcell.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia-Aguilar J, Hibbs ML, Springer TA. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) J.Cell Biol. 1990 Dec;111(6 Pt 2):3129–39. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El azreq MA, Bourgoin SG. Cytohesin-1 regulates human blood neutrophil adhesion to endothelial cells through beta2 integrin activation. Mol.Immunol. 2011 Jul;48(12–13):1408–16. doi: 10.1016/j.molimm.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Johansson AS, Haeggstrom JZ, Hultenby K, Palmblad J. Subcellular localization of leukotriene receptors in human endothelial cells. Exp.Cell.Res. 2010 Oct 15;316(17):2790–6. doi: 10.1016/j.yexcr.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005 Jul 15;106(2):584–92. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho KJ, Seo JM, Shin Y, Yoo MH, Park CS, Lee SH, Chang YS, Cho SH, Kim JH. Blockade of airway inflammation and hyperresponsiveness by inhibition of BLT2, a low-affinity leukotriene B4 receptor. Am.J.Respir.Cell Mol.Biol. 2010 Mar;42(3):294–303. doi: 10.1165/rcmb.2008-0445OC. [DOI] [PubMed] [Google Scholar]
- 32.Hicks A, Monkarsh SP, Hoffman AF, Goodnow R., Jr Leukotriene B4 receptor antagonists as therapeutics for inflammatory disease: preclinical and clinical developments. Expert.Opin.Investig.Drugs. 2007 Dec;16(12):1909–20. doi: 10.1517/13543784.16.12.1909. [DOI] [PubMed] [Google Scholar]
- 33.Kim GY, Lee JW, Cho SH, Seo JM, Kim JH. Role of the low-affinity leukotriene B4 receptor BLT2 in VEGF-induced angiogenesis. Arterioscler.Thromb.Vasc.Biol. 2009 Jun;29(6):915–20. doi: 10.1161/ATVBAHA.109.185793. [DOI] [PubMed] [Google Scholar]
- 34.Sauter C, Wolfensberger C. Interferon in human serum after injection of endotoxin. Lancet. 1980 Oct 18;2(8199):852–3. doi: 10.1016/s0140-6736(80)90189-0. [DOI] [PubMed] [Google Scholar]
- 35.Taveira da Silva AM, Kaulbach HC, Chuidian FS, Lambert DR, Suffredini AF, Danner RL. Brief report: shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. N.Engl.J.Med. 1993 May 20;328(20):1457–60. doi: 10.1056/NEJM199305203282005. [DOI] [PubMed] [Google Scholar]
- 36.Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J.Infect.Dis. 2010 Jan 15;201(2):223–32. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silliman CC, Khan SY, Ball JB, Kelher MR, Marschner S. Mirasol Pathogen Reduction Technology treatment does not affect acute lung injury in a two-event in vivo model caused by stored blood components. Vox Sang. 2010 May;98(4):525–30. doi: 10.1111/j.1423-0410.2009.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silliman CC, Kelher MR, Khan SY, LaSarre M, West FB, Land KJ, Mish B, Ceriano L, Sowemimo-Coker S. Experimental prestorage filtration removes antibodies and decreases lipids in RBC supernatants mitigating TRALI in vivo. Blood. 2014 May 29;123(22):3488–95. doi: 10.1182/blood-2013-10-532424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003 Mar 6;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 40.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005 Apr 22;308(5721):512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin NJ, Banerjee A, Khan SY, Lieber JL, Kelher MR, Gamboni-Robertson F, Sheppard FR, Moore EE, Mierau GW, Elzi DJ, et al. Platelet-activating factor-mediated endosome formation causes membrane translocation of p67phox and p40phox that requires recruitment and activation of p38 MAPK, Rab5a, and phosphatidylinositol 3-kinase in human neutrophils. J.Immunol. 2008 Jun 15;180(12):8192–203. doi: 10.4049/jimmunol.180.12.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim EY, Seo JM, Kim C, Lee JE, Lee KM, Kim JH. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic.Biol.Med. 2010 Sep 15;49(6):1072–81. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Kim GY, Lee JW, Ryu HC, Wei JD, Seong CM, Kim JH. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J.Immunol. 2010 Apr 1;184(7):3946–54. doi: 10.4049/jimmunol.0901735. [DOI] [PubMed] [Google Scholar]
- 44.Lee JW, Kim JH. Activation of the leukotriene B4 receptor 2-reactive oxygen species (BLT2-ROS) cascade following detachment confers anoikis resistance in prostate cancer cells. J.Biol.Chem. 2013 Oct 18;288(42):30054–63. doi: 10.1074/jbc.M113.481283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ.Res. 2012 Apr 27;110(9):1217–25. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 46.Bayat B, Tjahjono Y, Sydykov A, Werth S, Hippenstiel S, Weissmann N, Sachs UJ, Santoso S. Anti-human neutrophil antigen-3a induced transfusion-related acute lung injury in mice by direct disturbance of lung endothelial cells. Arterioscler.Thromb.Vasc.Biol. 2013 Nov;33(11):2538–48. doi: 10.1161/ATVBAHA.113.301206. [DOI] [PubMed] [Google Scholar]
- 47.Mack JP, Kahn SR, Tinmouth A, Fergusson D, Hebert PC, Lacroix J. Abstract 96 Dose-Dependent Effect of Stored Red Blood: Results of a Sub-Group Analysis of the Age of Blood Evaluation (ABLE) Trial. Blood Meeting Abstracts 128[22] 2016 [Google Scholar]
- 48.Balvers K, Wirtz MR, van DS, Goslings JC, Juffermans NP. Risk factors for trauma-induced coagulopathy- and transfusion-associated multiple organ failure in severely injured trauma patients. Front Med (Lausanne) 2015;2:24. doi: 10.3389/fmed.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, Rivara FP. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology. 2009 Feb;110(2):351–60. doi: 10.1097/ALN.0b013e3181948a97. [DOI] [PubMed] [Google Scholar]
- 50.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch.Surg. 2005 May;140(5):432–8. doi: 10.1001/archsurg.140.5.432. [DOI] [PubMed] [Google Scholar]
- 51.Hassan M, Pham TN, Cuschieri J, Warner KJ, Nester T, Maier RV, Shalhub S, O'Keefe GE. The association between the transfusion of older blood and outcomes after trauma. Shock. 2011 Jan;35(1):3–8. doi: 10.1097/SHK.0b013e3181e76274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juffermans NP, Vlaar AP, Prins DJ, Goslings JC, Binnekade JM. The age of red blood cells is associated with bacterial infections in critically ill trauma patients. Blood Transfus. 2012 Jul;10(3):290–5. doi: 10.2450/2012.0068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009 Jul;49(7):1384–94. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 54.Mostafa G, Gunter OL, Norton HJ, McElhiney BM, Bailey DF, Jacobs DG. Age, blood transfusion, and survival after trauma. Am.Surg. 2004 Apr;70(4):357–63. [PubMed] [Google Scholar]
- 55.Murrell Z, Haukoos JS, Putnam B, Klein SR. The effect of older blood on mortality, need for ICU care, and the length of ICU stay after major trauma. Am.Surg. 2005 Sep;71(9):781–5. doi: 10.1177/000313480507100918. [DOI] [PubMed] [Google Scholar]
- 56.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch.Surg. 2002 Jun;137(6):711–6. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 57.Spinella PC, Carroll CL, Staff I, Gross R, Mc QJ, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012 Jun;52(6):1184–95. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinberg JA, Barnum SR, Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion. 2011 Apr;51(4):867–73. doi: 10.1111/j.1537-2995.2011.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long K, Heaney JB, Simms ER, McSwain NE, Duchesne JC. When enough is enough: impact of packed red blood cells in massive transfusion outcomes. Am.Surg. 2013 Aug;79(8):810–4. [PubMed] [Google Scholar]
- 61.Sadjadi J, Cureton EL, Twomey P, Victorino GP. Transfusion, not just injury severity, leads to posttrauma infection: a matched cohort study. Am.Surg. 2009 Apr;75(4):307–12. [PubMed] [Google Scholar]
- 62.Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, Maier RV, Burlew CC. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J.Trauma Acute.Care Surg. 2014 Mar;76(3):582–92. doi: 10.1097/TA.0000000000000147. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Offner PJ. Age of blood: does it make a difference? Crit Care. 2004;8(Suppl 2):S24–S26. doi: 10.1186/cc2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Partrick DA, Moore FA, Moore EE, Barnett CC, Jr, Silliman CC. Neutrophil priming and activation in the pathogenesis of postinjury multiple organ failure. New Horiz. 1996 May;4(2):194–210. [PubMed] [Google Scholar]
- 65.Salzer WL, McCall CE. Primed stimulation of isolated perfused rabbit lung by endotoxin and platelet activating factor induces enhanced production of thromboxane and lung injury. J.Clin.Invest. 1990 Apr;85(4):1135–43. doi: 10.1172/JCI114545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brattstrom O, Granath F, Rossi P, Oldner A. Early predictors of morbidity and mortality in trauma patients treated in the intensive care unit. Acta Anaesthesiol.Scand. 2010 Sep;54(8):1007–17. doi: 10.1111/j.1399-6576.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- 67.Brown LM, Kallet RH, Matthay MA, Dicker RA. The influence of race on the development of acute lung injury in trauma patients. Am.J.Surg. 2011 Apr;201(4):486–91. doi: 10.1016/j.amjsurg.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharpe JP, Weinberg JA, Magnotti LJ, Fabian TC, Croce MA. Does plasma transfusion portend pulmonary dysfunction? A tale of two ratios. J.Trauma Acute.Care Surg. 2013 Jul;75(1):32–6. doi: 10.1097/TA.0b013e318294672d. [DOI] [PubMed] [Google Scholar]
- 69.Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW, Jr, Hakala TR, Rosenthal JT, et al. Evolution of liver transplantation. Hepatology. 1982 Sep;2(5):614–36. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD, Esquivel CO. Immunosuppression and other nonsurgical factors in the improved results of liver transplantation. Semin.Liver Dis. 1985 Nov;5(4):334–43. doi: 10.1055/s-2008-1040630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wall WJ. Liver transplantation: current concepts. CMAJ. 1988 Jul 1;139(1):21–8. [PMC free article] [PubMed] [Google Scholar]
- 72.Silliman CC, Burke T, Kelher MR. The accumulation of lipids and proteins during red blood cell storage: the roles of leucoreduction and experimental filtration. Blood Transfus. 2017 Mar;15(2):131–6. doi: 10.2450/2017.0314-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dzieciatkowska M, Silliman CC, Moore EE, Kelher MR, Banerjee A, Land KJ, Ellison M, West FB, Ambruso DR, Hansen KC. Proteomic analysis of the supernatant of red blood cell units: the effects of storage and leucoreduction. Vox Sang. 2013 Oct;105(3):210–8. doi: 10.1111/vox.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A(2) activities. Antioxid.Redox.Signal. 2011 Aug 1;15(3):831–44. doi: 10.1089/ars.2010.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bock A, Tucker N, Kelher MR, Khan SY, Gonzalez E, Wohlauer M, Hansen K, Dzieciatkowska M, Sauaia A, Banerjee A, et al. alpha-enolase Causes Pro-Inflammatory Activation of Pulmonary Microvascular Endothelial Cells and Primes Neutrophils Through Plasmin Activation of Protease-Activated Receptor-2. Shock. 2015 May 4; doi: 10.1097/SHK.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for "omics" analyses. Blood Transfus. 2012 May;10(Suppl 2):s7–11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.D'Alessandro A, Hansen KC, Silliman CC, Moore EE, Kelher M, Banerjee A. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2015 Feb;108(2):131–40. doi: 10.1111/vox.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D'Alessandro A, Nemkov T, Kelher M, West FB, Schwindt RK, Banerjee A, Moore EE, Silliman CC, Hansen KC. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015 Jun;55(6):1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D'Amici GM, Mirasole C, D'Alessandro A, Yoshida T, Dumont LJ, Zolla L. Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus. 2012 May;10(Suppl 2):s46–s54. doi: 10.2450/2012.008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dzieciatkowska M, Silliman CC, Moore EE, Kelher MR, Banerjee A, Land KJ, Ellison M, West FB, Ambruso DR, Hansen KC. Proteomic analysis of the supernatant of red blood cell units: the effects of storage and leucoreduction. Vox Sang. 2013 Oct;105(3):210–8. doi: 10.1111/vox.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gevi F, D'Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J.Proteomics. 2012 Dec 5;76(Spec No.:168-80) doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 82.Roback JD, Josephson CD, Waller EK, Newman JL, Karatela S, Uppal K, Jones DP, Zimring JC, Dumont LJ. Metabolomics of ADSOL (AS-1) red blood cell storage. Transfus.Med Rev. 2014 Apr;28(2):41–55. doi: 10.1016/j.tmrv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D'Alessandro A, D'Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012 Jan;97(1):107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters AL, van Hezel ME, Cortjens B, Tuip-de Boer AM, van BR, de KD, Jonkers RE, Bonta PI, Zeerleder SS, Lutter R, et al. Transfusion of 35-Day Stored RBCs in the Presence of Endotoxemia Does Not Result in Lung Injury in Humans. Crit Care Med. 2016 Jun;44(6):e412–e419. doi: 10.1097/CCM.0000000000001614. [DOI] [PubMed] [Google Scholar]
- 85.Peters AL, Vervaart MA, van Bruggen R, de Korte D, Nieuwland R, Kulik W, Vlaar AP. Non-polar lipids accumulate during storage of transfusion products and do not contribute to the onset of transfusion-realted acute lung injury. Vox Sang. 2016 doi: 10.111/vox12453:1-8. [DOI] [PubMed] [Google Scholar]
- 86.Dudek SM, Munoz NM, Desai A, Osan CM, Meliton AY, Leff AR. Group V phospholipase A2 mediates barrier disruption of human pulmonary endothelial cells caused by LPS in vitro. Am.J.Respir.Cell Mol.Biol. 2011 Mar;44(3):361–8. doi: 10.1165/rcmb.2009-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]