Abstract
Background
We and others have shown that the gamma tocopherol (γT) isoform of vitamin E has multiple anti-inflammatory and antioxidant actions and that γT supplementation reduces eosinophilic and endotoxin (LPS)-induced neutrophilic airway inflammation in animal models and healthy human volunteers.
Objective
To determine if γT supplementation reduces eosinophilic airway inflammation and acute neutrophilic response to inhaled LPS challenge in volunteers with asthma.
Methods
Participants with mild asthma were enrolled in a double-blinded, placebo controlled crossover study to assess the effect of 1200 mg of γT daily for 14 days on sputum eosinophils, mucins and cytokines. We also assessed the effect on acute inflammatory response to inhaled LPS challenge following γT treatment, focusing on changes in sputum neutrophilia, mucins and cytokines. Mucociliary clearance was measured using gamma scintigraphy.
Results
Fifteen subjects with mild asthma completed both arms of the study. Compared to placebo, γT notably reduced pre-LPS challenge sputum eosinophils and mucins, including MUC5AC, and reduced LPS-induced airway neutrophil recruitment 6 and 24-hours after challenge. Mucociliary clearance was slowed 4-hours post-challenge in the placebo group but not in the γT treatment group. Total sputum mucins (but not MUC5AC) were reduced at 24-hours post-challenge during γT treatment compared to placebo.
Conclusion
γT supplementation for 14 days reduced inflammatory features of asthma, including sputum eosinophils and mucins, as well as acute airway response to inhaled LPS challenge when compared to placebo. Larger scale clinical trials are needed to assess the efficacy of γT supplements as a complementary or steroid-sparing treatment for asthma.
Keywords: asthma, vitamin E, tocopherol, endotoxin, lipopolysaccharide, neutrophil, eosinophil, mucin, mucociliary clearance
INTRODUCTION
Asthma is among the most prevalent chronic diseases in the U.S. (1) and represents a source of significant burden to patients and healthcare systems. Environmental pollutant exposure is a known trigger for asthma exacerbations, which are characterized by airway inflammation, bronchoconstriction, increased production of airway mucous, and decreased mucociliary clearance with formation of mucous plugs (2, 3). Endotoxin (the main component of which is lipopolysaccharide, or LPS) is commonly encountered in ambient air particulate matter as well as in domestic and occupational settings and has been linked to asthma severity (4–6). Endotoxin is a potent stimulator of the innate immune response (7), signaling through Toll-like receptor 4 on airway macrophages to stimulate production of inflammatory cytokines and eicosanoids, recruitment of granulocytes, and production of gel-forming airway mucins, including mucin 5AC (MUC5AC) (8).
Airway inflammation during acute exacerbations of asthma is often characterized by increase in both airway eosinophils and neutrophils (9). Neutrophilic airway inflammation is particularly evident in viral asthma exacerbations as well as in some chronic asthma phenotypes (10). Our group has shown that inhaled LPS challenge induces airway neutrophilia in human volunteers, and now employ this procedure as a model of acute exacerbation of airway disease against which potential therapies can be tested (11–13). We have previously demonstrated that inhaled fluticasone propionate administered for two weeks decreased sputum eosinophilia subsequent LPS-induced acute airway neutrophilia in asthmatics (14). In subsequent studies, we have shown that treatment with the IL-1 receptor antagonist, anakinra (15), and the vitamin E isoform, gamma tocopherol (γT) (16) also attenuated LPS-induced airway neutrophilia in healthy volunteers.
Our hypothesis that vitamin E supplementation decreases airway inflammation in asthma and allergy airway disease was inspired by epidemiologic evidence suggesting that increased dietary vitamin E intake is associated with reduced incidence of allergic disease (17–19) and asthma (20). Among the isoforms of vitamin E that have been suggested as asthma interventions are α-tocopherol (αT), which is commonly used as both a supplement and pharmaceutical product, and γT, the predominant isoform of vitamin E found in dietary sources. Intervention trials of αT in humans with asthma have been generally disappointing (21, 22).
γT has not been as vigorously studied for airway disease. γT and its primary metabolite 2,7,8-trimethyl-2-(B-carboxy-ethyl)-6-hydroxychroman (γ-CEHC) do have a have a number of unique anti-inflammatory actions (23, 24), including scavenging reactive nitrogen species to form 5-nitro-y-tocopherol (5-NO2-γT) (24) and inhibition of cyclooxygenase-2 (COX-2) and 5-lipooxygenase, reducing inflammatory eicosanoid production (25). We have pursued a program of preclinical and early phase clinical trials of γT as a novel therapeutic for treatment of airway inflammation (25–29). In a rodent model of evoked airway inflammation, γT reduced allergen-induced eosinophilia and mucin responses (27) as well as LPS-induced neutrophil, prostaglandin E2 (PGE2), and mucin responses (including MUC5AC) (29). We subsequently observed that one week of treatment with a γT-enriched mixed tocopherol preparation reduced the neutrophilic response to inhaled LPS challenge in a phase I randomized, double blinded, placebo-controlled crossover study of healthy adults (16). This report describes our next step in assessing γT as an intervention for asthma, in which we test the hypothesis that γT reduces eosinophilic airway inflammation and attenuate the neutrophilic airway response to inhaled LPS challenge in volunteers with mild asthma.
METHODS
Volunteer recruitment and inclusion criteria
We recruited subjects aged 18 to 50 years with a history of episodic wheezing or shortness of breath consistent with asthma or physician-diagnosed asthma classified as mild intermittent or mild persistent asthma as defined by the NHLBI guidelines for the Diagnosis and Management of Asthma (30). Exclusion criteria included any of the following: daily albuterol use, nighttime asthma symptoms more than once per week, or emergency treatment for asthma within the previous 12 months. As sputum inflammatory cell measures were a central endpoint in this study, all subjects were screened for their ability to provide an adequate induced sputum sample during their screening session, defined by >250,000 cells, >50% viability, and <40% squamous cells.
Prior to study entry, subjects underwent a general health screen including a detailed medical history, physical exam, baseline laboratory evaluation, spirometry, and allergy skin testing to common aeroallergens including house dust mite, cockroach, tree mix, grass mix, weed mix, molds, cat, dog, guinea pig, rabbit, rat, and mouse allergens. A wheal size of 3 mm or greater than the negative control was considered positive. Subjects who were found to be pregnant, nursing an infant, regularly taking anti-inflammatory or immune-modulating medications, or with a history of abnormal blood coagulation parameters were excluded. This study was approved by the University of North Carolina Institutional Review Board and the U.S. Food and Drug Administration (IND 13004), and is listed on ClinicalTrials.gov, NCT02104505.
Study Design
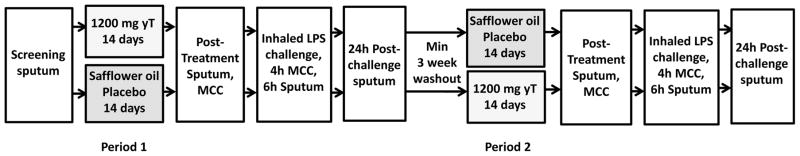
Subjects were randomized to 120 mg γT or placebo (safflower oil) treatment for 14 days (Figure 1), a study period similar to that we used to assess the effect of fluticasone propionate on airway response to LPS challenge in asthmatics. The γT supplement consisted of geltabs each containing 612 mg γ-tocopherol, 7 mg α-tocopherol, 28 mg β-tocopherol, and 8 mg δ-tocopherol (Callion Pharma, Inc), and subjects were instructed to take 2 geltabs once daily with a meal to maximize bile secretion and enhance absorption. Medication bottles were returned and any leftover pills were counted on the day of LPS challenge to ensure adherence. Twenty four to 48 hours prior to LPS challenge, subjects presented for sputum induction and gamma scintigraphy to measure mucociliary clearance (MCC). On day 14 of dosing, subjects underwent inhaled LPS challenge with 20,000 endotoxin units (EU) of Clinical Center Reference Endotoxin (CCRE), with MCC measurement performed 4-hours post-challenge and sputum induction at 6 and 24-hours post-challenge. Sputum was analyzed for granulocytes, inflammatory cytokines, and mucin content as previously described (31–34). After a minimum three-week washout to allow for clearance of inflammatory cells from the airways, subjects were crossed over to the alternate treatment group. Venipuncture was performed at regular intervals for assessment of PT, aPTT, and INR.
Figure 1. Phase IIa crossover study design in volunteers with mild asthma (n=15).
Randomized, placebo controlled crossover study of γT supplement or safflower oil placebo in 15 subjects with mild asthma. Subjects were challenged with inhaled LPS followed 4 hours later by gamma scintigraphy to measure mucociliary clearance (MCC) and 6 hours later by sputum induction.
Randomization and Masking
The randomization list was prepared by a biostatistician using SAS© and provided to the UNC Investigational Drug Service. Only the pharmacist and statistician had access to the randomization schedule. Subjects were randomized to treatment groups 1:1 using permuted blocks of four. γT and safflower oil (placebo) geltabs were identical in appearance and were dispensed as a 7-day supply from the Investigational Drug Service to the study staff. Subjects returned for a follow up visit to receive the additional 7-day supply of investigational drug for that period.
Endotoxin inhalation challenge
CCRE, referred to as LPS, was provided by the National Institutes of Health Clinical Center. All doses were prepared by the Investigational Drug Service and inhaled by subjects as a nebulized preparation using a DeVilbiss Ultraneb 99 ultrasonic nebulizer (12, 16).
Sputum induction, processing, and mediator measurement
Each subject provided seven induced sputum samples (Figure 1): screening (prior to placebo or active treatment), 24–48 hours prior to each LPS challenge session (post-treatment sputum), and 6 and 24-hours after each LPS challenge session (post-challenge sputum). Induced sputum samples were processed as previously described (31–34). Cell viability (trypan blue exclusion) and total cell counts were assessed in a Neubauer hemacytometer, and differential cell counts were performed on cytocentrifuged cells stained with a modified Wright stain (Hema-Stain-3; Fisher Scientific, Hampton, NH). Cytokines from sputum supernatants were measured using multiplex technology (Meso Scale Discovery/MSD, Gaithersburg, MD, USA). Each sample was analyzed with the V-PLEX Human Proinflammatory Panel II kit. Even though ability to provide adequate sputum for analysis was an entrance criterion, there were instances during the study in which a subject was not able to provide a sputum sample or provided a poor-quality sputum sample. In these instances, these volunteers were excluded from analysis of the effect of active treatment on airway inflammation. These volunteers were included in assessment of MCC and safety endpoints for the study.
Gamma scintigraphy for measurement of mucociliary clearance
The procedure used for measuring MCC in humans has been described in detail previously (35, 36). Briefly, volunteers inhaled an aerosol of 99mTc-sulfur colloid (99mTc -SC) using a slow inhalation (80 mL/sec), large particle (9.5 μm mass median aerodynamic diameter) method (37). Immediately following inhalation of the radioaerosol (duration of less than five minutes), an initial deposition scan was recorded (sum of two two-minute images) and then continuous two-minute images were recorded for a period of two hours to monitor clearance of particles from the lung as the subject remained seated in front of the gamma camera. Subjects returned the following day after the radiolabeled aerosol exposure to obtain a 30-minute scan of 24-hour lung activity/retention.
A whole lung region of interest bordering the right lung (created from a Co57 transmission lung scan (37)) was used to determine, by computer analysis, the whole lung retention (Rt) (decay and background corrected) as a fraction of the initial counts in the right lung, over the two-hour clearance period at 10-minute intervals. MCC was calculated and expressed as average clearance in percent over the two-hour period (35). Because measures of MCC can be influenced by the initial, regional lung deposition of the inhaled radioaerosol, we also calculated 1) the central to peripheral (C/P) ratio of activity and 2) the skew of the counts/pixel vs. number of pixels histogram for the initial two-minute image from each study visit (35, 38).
Analysis of serum tocopherols and γ-CEHC
αT, γT, δT, and 5-NO2-γT were measured by a HPLC assay with electrochemical detection (39), and γ-CEHC was analyzed using liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described (40).
Analysis of sputum mucins
To measure total mucins, a 100 μL aliquot of induced sputum was solubilized in 6MGuHCl and subjected to differential refractometry (tREX, Wyatt Technology, Goleta, CA) coupled with size exclusion chromatography as described previously (41). Individual concentration of MUC5AC was measured by labeled mass spectrometry method using deuterium labeled MUC5AC peptide standards.
Statistical analysis
We employed methods similar to those used in our initial study of the effect of γT in healthy volunteers (16). The primary endpoints of the study were airway eosinophilia (defined as the difference in sputum eosinophils present in post-treatment samples) and LPS-induced airway neutrophilia (defined as the change in induced sputum neutrophils (PMNs) from post-treatment to 6-hours post-challenge), comparing γT treatment to placebo.
In planning this study, we were guided by the results of our previous study of γT-enriched supplementation on airway PMN response to LPS challenge in 13 healthy volunteers (16). Based on these data, we estimated that a sample size of 30 volunteers would be adequate for this study, with an a priori plan to undertake an interim analysis after 15 volunteers completed this study. As planned and approved by IRB and FDA review, the interim analysis would lead us to stop the study due to demonstration of futility or statistically significant support of the hypotheses that γT inhibits airway eosinophilia and LPS-induced neutrophilia, or continuation of the study to n=30 due to inconclusive interim results.
For initial post-treatment vs. post-challenge comparisons of sputum endpoints and MCC within each treatment group, paired t-tests or Wilcoxon signed rank tests (depending on whether the normality assumption was met) were employed. Data that were not normally distributed were transformed using Box-Cox transformation. Given the crossover design of our study, we next determined the gT treatment effect (compared to placebo) on post-treatment sputum endpoints and on LPS-induced changes (Δ post-challenge – post-treatment) in sputum endpoints using a linear mixed model approach described by Jones and Kenward (42) that considers the above individual tests in a global, unified way where all data are used at the same time. SAS PROC MIXED was used to fit the linear mixed model. Criterion for significance was taken to be p ≤ 0.05.
RESULTS
Subject Demographics
Twenty-three subjects with mild asthma were enrolled and underwent randomization. Based on frequency of daytime and nighttime symptoms and use of rescue albuterol, 22 subjects were classified as having mild intermittent asthma. One subject was classified as mild persistent asthma and was using montelukast daily at the time of enrollment. However, this subject withdrew from the study prior to inhaled LPS exposure. No subjects were using inhaled corticosteroids at the time of enrollment or at any point during the study. The majority of participants were atopic (74%) based on the results of skin prick testing. Demographic characteristics of the study population are shown in Table 1. Fifteen subjects completed both arms of the crossover study (Figure E1 in the article’s Online Repository at www.jacionline.org), with 13 providing adequate sputum for assessment of the primary sputum inflammatory endpoints for both treatment periods.
Table 1.
Demographic characteristics of enrolled study volunteers (N=23).
| Age (years), median (range) | 26 (20–47) |
| Sex (Female/Male) | 19/4 |
| Race | |
| Caucasian | 15 |
| African American | 4 |
| Asian | 2 |
| Native American | 2 |
| Ethnicity | |
| Hispanic | 1 |
| Non-Hispanic | 22 |
| Atopic, N (%) | 17 (74%) |
| BMI (kg/m2), median (range) | 26 (20–42) |
| FEV1 (L), median (range) | 3.3 (2.4–4.4) |
| FEV1 % predicted, median (range) | 97 (83–109) |
γT supplementation increased serum y-tocopherol and γ-CEHC concentrations
Serum γT and γ-CEHC concentrations rose significantly from baseline values in the active treatment group only (p<0.0001 for both) (Table 2). Conversely, αT concentrations decreased from baseline in the active treatment group (p=0.003).
Table 2.
Serum concentrations of tocopherols and γ-CEHC from 18 volunteers with mild asthma.
| Baseline | Placebo Treatment Period | Active Treatment Period | |
|---|---|---|---|
| γT (μM) | 2.6 (1.43–6.22) | 3.69 (1.65–8.82) | 19.8* (2.49 –50.15) |
| αT (μM) | 25.41 (14.72–37.81) | 25.22 (18.48–52.65) | 19.04* (11.07–36.52) |
| δT(μM) | 0.09 (0.01–0.68) | 0.12 (0.04–0.4) | 0.26 (0.06–0.6) |
| γ-CEHC (μM) | 0.14 (0.05–0.45) | 0.17 (0.07–1.4) | 3.11* (0.08–7.82) |
| 5-NO2-γT (μM) | 0.01 (0–0.07) | 0.02 (0–0.07) | 0.01 (0–0.05) |
Data represented as median (range).
p<0.05 comparing baseline concentrations to those obtained after 14 days of γT supplementation. Analyses were performed using paired t tests for γT and αT data and by Wilcoxon Matched Pairs Signed Rank Test for δT and γ-CEHC data as they were not normally distributed.
γT treatment reduced post-treatment sputum eosinophils and mucins
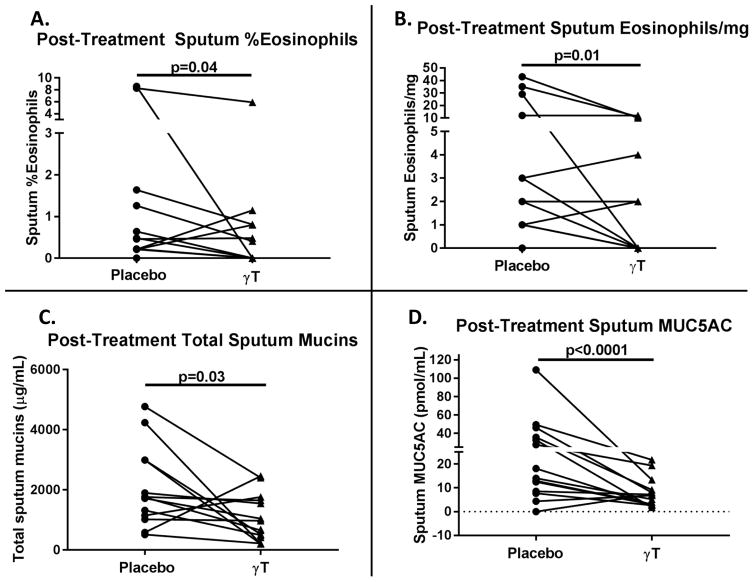
Using the linear mixed model approach, we found that γT treatment significantly reduced post-treatment sputum %eosinophils (p=0.04) and eosinophils/mg of sputum (p=0.01) compared to placebo (Figure 2a–b). Likewise, γT treatment significantly reduced post-treatment total mucins (p=0.03) and MUC5AC content (p<0.0001) compared to placebo (Figure 2c–d).
Figure 2. γT reduced post-treatment sputum eosinophils and mucins (n=13).
A) Sputum %eosinophils, B) sputum eosinophils/mg, C) total sputum mucins were reduced in post-treatment sputum samples during active treatment compared to placebo treatment.
γT treatment attenuated LPS-induced sputum neutrophilia
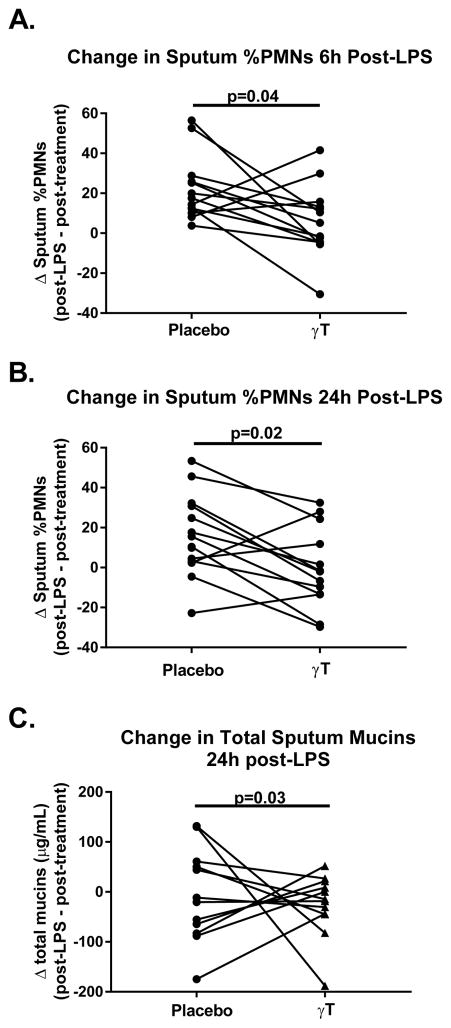
Inhaled LPS challenge significantly increased sputum percent neutrophils (%PMNs) (p=0.003) and neutrophils per mg (PMNs/mg) of sputum (p=0.01) at 6 hours compared to post-treatment sputum during the placebo period. The increase during the placebo period (%PMNs: Δ 20.1% ± 16.5 SD, p<0.01; PMNs/mg Δ 384.1 ± 531.2 SD, p<0.01) was greater than that seen during the active period (% PMNS Δ 11.7% ± 20.7 SD, p=0.04; PMNs/mg Δ 236.2 ± 692.7 SD, p=0.2). Linear mixed modeling demonstrated that γT treatment (compared to placebo) significantly attenuated sputum %PMNs at both 6 (p=0.04) and 24 hours (p=0.02) after LPS challenge (Figure 3a–b). There was no effect of inhaled LPS challenge or γT treatment on any measure of sputum eosinophilia following LPS challenge.
Figure 3. γT attenuated LPS-induced sputum neutrophilia and mucin production (n=13).
A) Sputum %PMNs at 6-hours and B) 24-hours post-challenge were significantly reduced during active treatment compared to placebo. C) Total sputum mucins at 24-hours post-challenge were significantly reduced during active treatment compared to placebo.
γT effects on airway mucin production and mucociliary clearance
MUC5AC content was significantly increased from post-treatment levels in both treatment groups 6 hours after inhaled LPS challenge [p=0.001 (placebo), p=0.0004 (active)]. By 24 hours post-LPS challenge, total sputum mucins decreased in both treatment groups compared to prior to LPS challenge, though not significantly. Using linear mixed modeling to assess for a treatment affect, we detected significantly less total sputum mucins during the active treatment period compared to placebo (Figure 3c, p=0.03). We found no significant difference in MUC5AC concentrations between the treatment groups at the same time point (data not shown).
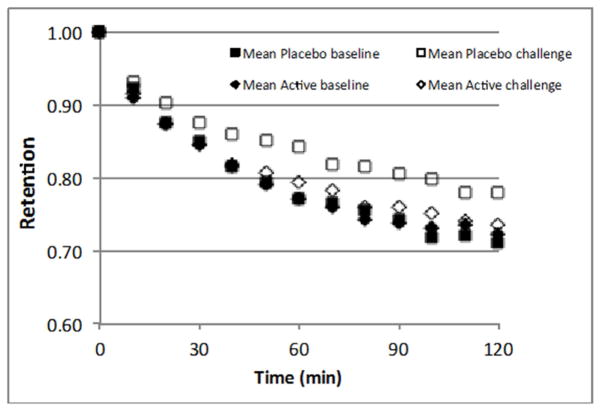
As an exploratory measure, we assessed how γT intervention may impact MCC. MCC was measured prior to and 4 hours after LPS challenge. There were no differences in regional deposition indices (C/P or skew) nor in 24-hour retention between post-treatment and post-challenge measurements for either treatment period. MCC was significantly slowed following LPS challenge compared to post-treatment measurements for the placebo treatment period (MCC = 16.3 ± 9.3% post-challenge vs. 21.4 ± 6.9% post-treatment, p < 0.01) (Figure 4). In contrast, there was no such slowing of MCC by LPS challenge during active treatment (MCC = 20.2 ± 8.0% post-challenge vs. 21.4 ± 9.7% post-treatment, p=0.6). However, for this new endpoint, the sample size was not adequate to definitively ascribe a treatment effect for γT on LPS-induced slowing of MCC when accounting for period and carryover effects.
Figure 4. γT was associated with attenuation of LPS-induced changes in MCC (n=15).
MCC is represented as mean retention versus time at post-treatment and 4-hours post-challenge for each treatment group. Inhaled LPS challenge resulted in significant slowing of MCC after placebo treatment, but no significant effect on MCC was seen after γT treatment.
γT treatment did not impact LPS-induced changes in sputum TH1 cytokines
During the placebo period, sputum IL-1β and IL-8 concentrations were significantly increased 6 hours post-LPS challenge compared to post-treatment values (p=0.002 and p=0.01, respectively), while no significant LPS-induced increase was observed during the active treatment period (p=0.07 and p=0.40, respectively). There was no significant LPS-induced change in sputum IL-6 concentration during either treatment period. Compared to placebo treatment, we did not detect a significant γT treatment effect on LPS-induced inflammatory cytokine concentrations in sputum following LPS challenge.
Adverse events
No serious adverse events occurred during the study period. The most commonly reported symptoms were gastrointestinal in nature. During the active treatment period, 21.7% of subjects reported nausea and 26% reported diarrhea or loose stools, compared to 8.7% and 4.3% during the placebo treatment period, respectively. These symptoms were typically transient and tended to occur during the first or second day of treatment then self-resolved. One subject chose to discontinue study participation due to intolerable diarrhea during the active treatment period. No significant change in PT, aPTT, or INR was observed, and there were no reported bleeding events during the study. After completion of 14 days of active treatment, no clinically or statistically significant changes were seen in FEV1 or FEV1/FVC from measurements taken during the initial baseline visit.
DISCUSSION
The primary goal of this proof-of-concept study was to determine if γT supplementation in adults with asthma decreases airway eosinophilia as well as the inflammatory response to inhaled LPS, a model of neutrophil-predominant asthma exacerbation. Our results demonstrate that asthmatics treated with γT supplementation for 14 days had significantly reduced eosinophils in sputum when compared to placebo treatment. These findings suggest that γT may reduce baseline TH2-mediated airway inflammation, which could be beneficial for eosinophilic asthma phenotypes. We also found that γT treatment was associated with lower post-treatment sputum mucins compared to placebo, including the inducible mucin glycoprotein, MUC5AC, which has been found in high concentrations in mucous plugs from fatal asthma cases (43). Ex vivo γT treatment was previously found to inhibit IL-13-induced secretion of eotaxin from airway epithelial cells, a potent chemotactic factor for eosinophils (44). Given that mucin production is enhanced by IL-13, similar mechanisms may account for the impact of γT on mucin production as well.
We also found that γT treatment attenuated neutrophilic airway response to inhaled LPS challenge. Neutrophilic airway inflammation is often less responsive to corticosteroid treatment (45), and there is a great unmet need for non-steroidal therapies that target this specific type of inflammation. While we found no significant difference in sputum mucins between the treatment periods at 6 hours post-LPS challenge, total mucins were significantly lower at 24 hours with γT treatment, which could suggest faster recovery from mucin hypersecretion following an acute inflammatory challenge.
We observed a significant impairment of MCC following inhaled LPS challenge during the placebo treatment period but not during the γT treatment period. We have previously found a slowing of MCC by LPS challenge in healthy non-smokers (35) and a trend towards slowing in mild asthmatics that was confounded by regional deposition differences between baseline and post-LPS challenge MCC (unreported) (38). While this study was not powered to detect a significant treatment effect of γT on MCC, our results do suggest that γT reduces LPS-induced slowing of MCC, and warrants further study. The mechanism by which inhaled LPS slows MCC is not understood, but may be related to quantity or quality of sputum mucins, epithelial tethering of mucins, direct effects on ciliary function, or a combination of these factors (43).
The γT supplement used in our study given daily over a 2-week period resulted in significantly increased serum concentrations of γT and its primary active metabolite γ-CEHC but with reduced serum αT concentrations by an unknown mechanism. This finding is consistent with previous studies of γT supplementation effects on reducing αT plasma concentrations (46), including one conducted by our group that demonstrated reduced αT concentrations in serum following a 3-dose regimen of an identical γT supplement administered over a 24-hour period (47). It is unknown whether continued γT supplementation would result in further decline in αT concentrations, nor is it known what the long-term physiologic consequences of this decline would be.
While our work has consistently demonstrated a beneficial effect of γT on airway inflammation, others have proposed a pro-inflammatory role for γT based primarily on human observational or animal model studies. In a cross-sectional study of young adults enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) cohort, higher serum αT levels were associated with higher lung function values (FEV1 and FVC), while higher serum γT levels were associated with lower FEV1 and FVC values (48). While the results of this epidemiological study are intriguing, the correlation between serum γT levels and lung function may reflect γT as a risk factor or biological marker for lung function. Furthermore, others have shown that dietary vitamin E, at least 70% of which is comprised of γT, was associated with increased FEV1 in older adults (49) and may be protective against adult-onset asthma (20). It is important to emphasize that these studies were not intervention trials, and several potential confounding factors could have influenced their results, including differences in intake of dietary fats. For example, γT-rich oils tend to have higher levels of polyunsaturated fatty acids, which may contribute to certain disease states, whereas αT-rich oils contain predominantly monounsaturated fatty acids, which have more health benefits (50). Although further studies are needed to address the long-term impact of γT supplementation on airway inflammation, our 2-week dosing regimen with γT had no impact on spirometry measurements.
There are very few published human trials of γT supplementation in the context of airway inflammation prevention and/or treatment. Vitamin E has been studied for prevention and treatment of many chronic health conditions (51–54), yet human trials in asthma have yielded conflicting results and have focused on treatment with αT, the most abundant tocopherol isoform in widely available supplements. In contrast to the results presented here, studies utilizing murine models found that γT supplementation exacerbated eosinophilic inflammation, while αT supplementation conferred protection (55, 56). It is possible that these conflicting reports reflect species-dependent differences in the anti-inflammatory effects of γT. Previous work from our group demonstrates that γT supplementation reduces airway eosinophilia in humans (16) and rodents (27, 28). This is in agreement with our current study, in which short term dosing with γT exhibits acute anti-eosinophilic and anti-inflammatory properties in human subjects. These results, coupled with evidence that γT has unique anti-inflammatory properties compared to aT (including the ability to scavenge reactive nitrogen species (24) and inhibit cyclooxygenase-2 (COX-2) and 5-lipooxygenase (25)) supports the use of γT-enriched Vitamin E preparations as a potential intervention for acute exacerbation, pollution induced disease, and possibly even chronic allergic diseases. These findings support conducting larger trials with γT supplementation in volunteers with asthma to further evaluate its role in modulating features of asthma.
This early phase clinical trial has several limitations. Our participants were predominantly female which could reduce the generalizability of our results. The study population was somewhat heterogeneous with both atopic (74%) and non-atopic (26%) participants, and based on our safety criteria to undergo inhaled LPS challenges, had mild asthma. Given that the supplementation period only lasted two weeks, the longer-term effect of reduced serum αT levels noted with γT supplementation will have to be further studied for safety and efficacy in treating chronic airway inflammation. Our dosing regimen was generally well-tolerated, though early, transient gastrointestinal symptoms occurred in about one-fourth of participants studied. Additionally, we saw no prolongation of blood coagulation measurements, and no significant bleeding events were reported. The occurrence of early GI side effects and potential need for both long term and short term treatment regimens suggests that dose ranging studies be done. Finally, the impact of BMI on driving treatment responses to LPS will have to be further evaluated, given that we have previously shown that increased BMI is associated with sputum neutrophil recruitment to inhaled LPS among atopic subjects with asthma (57).
In conclusion, we have shown that a 14-day course of γT supplementation resulted in reduced eosinophilic inflammation of the airways and reduction in sputum mucins including MUC5AC. Additionally, γT supplementation reduced LPS-induced neutrophilic airway inflammation and mucinous content of sputum following inhalation challenge and was associated with reduced impact of LPS on MCC. Overall, our results with two weeks of γT supplementation were similar to the effects of two weeks of treatment with inhaled fluticasone propionate on both post-treatment sputum eosinophilia and acute neutrophilic response to inhaled LPS challenge (14). Taken together, these observations indicate that γT has potential to treat multiple features of asthma, including airway inflammation, mucous production, and clearance of mucous from the airways, and should be studied further in larger-scale clinical trials to investigate the efficacy of γT for improving asthma outcomes.
Supplementary Material
KEY MESSAGES.
γT supplementation in mild asthmatics reduced sputum eosinophils and mucins compared to placebo in a fashion similar to that of inhaled fluticasone propionate and may have a role in reducing TH2-mediated inflammation.
γT reduced the neutrophilic inflammatory response to inhaled LPS challenge compared to placebo and may represent a useful therapy for neutrophil-predominant asthma exacerbation.
Acknowledgments
The authors are grateful to Jihong Wu and Dr. John Lay, PhD, for their assistance in acquisition and analysis of MCC data. We are also grateful to Martha Almond, RRT, for her assistance with study visits and regulatory documentation.
Sources of support: This work was supported by NIEHS grants R01ES023349, K23-ES021745, and P30ES010126. AJB is supported by T32GM086330. CGD is supported by T32ES007126-33. HZ and YP are supported by R01ES021900. MLH was supported by the AAAAI Foundation.
Abbreviations
- αT
α-Tocopherol
- γT
γ-Tocopherol
- γ-CEHC
2,7,8-trimethyl-2-(β-carboxyethyl)-6- hydroxychroman
- PMN
neutrophil
- COX
cyclooxygenase
- LPS
lipopolysaccharide
- IL
interleukin
- LC-MS
liquid chromatography tandem mass spectrometry
- INR
international normalized ratio
- PT
prothrombin time
- aPTT
activated partial thromboplastin time
- MCC
mucociliary clearance
- PGE2
prostaglandin E2
- 5-NO2-γT
5-nitro-gamma- tocopherol
- MUC5AC
mucin 5AC
Footnotes
Conflicts of Interest: None.
ClinicalTrials.gov: NCT02104505
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention; Centers for Disease Control and Prevention. [Available from: https://www.cdc.gov/asthma/asthmadata.htm.
- 2.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, et al. Endotoxin Exposure: Predictors and Prevalence of Associated Asthma Outcomes in the United States. Am J Respir Crit Care Med. 2015;192(11):1287–97. doi: 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RL, Peden DB. Environmental effects on immune responses in patients with atopy and asthma. J Allergy Clin Immunol. 2014;134(5):1001–8. doi: 10.1016/j.jaci.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1641–6. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 5.Lai PS, Sheehan WJ, Gaffin JM, Petty CR, Coull BA, Gold DR, et al. School Endotoxin Exposure and Asthma Morbidity in Inner-city Children. Chest. 2015;148(5):1251–8. doi: 10.1378/chest.15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172(11):1371–7. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peden DB. The role of oxidative stress and innate immunity in O(3) and endotoxin-induced human allergic airway disease. Immunol Rev. 2011;242(1):91–105. doi: 10.1111/j.1600-065X.2011.01035.x. [DOI] [PubMed] [Google Scholar]
- 8.Song KS, Kim HJ, Kim K, Lee JG, Yoon JH. Regulator of G-protein signaling 4 suppresses LPS-induced MUC5AC overproduction in the airway. Am J Respir Cell Mol Biol. 2009;41(1):40–9. doi: 10.1165/rcmb.2008-0280OC. [DOI] [PubMed] [Google Scholar]
- 9.Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000;161(3 Pt 1):769–74. doi: 10.1164/ajrccm.161.3.9809071. [DOI] [PubMed] [Google Scholar]
- 10.Hekking PP, Bel EH. Developing and emerging clinical asthma phenotypes. J Allergy Clin Immunol Pract. 2014;2(6):671–80. doi: 10.1016/j.jaip.2014.09.007. quiz 81. [DOI] [PubMed] [Google Scholar]
- 11.Alexis NE, Brickey WJ, Lay JC, Wang Y, Roubey RA, Ting JP, et al. Development of an inhaled endotoxin challenge protocol for characterizing evoked cell surface phenotype and genomic responses of airway cells in allergic individuals. Ann Allergy Asthma Immunol. 2008;100(3):206–15. doi: 10.1016/S1081-1206(10)60444-9. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez ML, Harris B, Lay JC, Bromberg PA, Diaz-Sanchez D, Devlin RB, et al. Comparative airway inflammatory response of normal volunteers to ozone and lipopolysaccharide challenge. Inhal Toxicol. 2010;22(8):648–56. doi: 10.3109/08958371003610966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez M, Brickey WJ, Alexis NE, Fry RC, Rager JE, Zhou B, et al. Airway cells from atopic asthmatic patients exposed to ozone display an enhanced innate immune gene profile. J Allergy Clin Immunol. 2012;129(1):259–61. e1–2. doi: 10.1016/j.jaci.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexis NE, Peden DB. Blunting airway eosinophilic inflammation results in a decreased airway neutrophil response to inhaled LPS in patients with atopic asthma: a role for CD14. J Allergy Clin Immunol. 2001;108(4):577–80. doi: 10.1067/mai.2001.118511. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez ML, Mills K, Almond M, Todoric K, Aleman MM, Zhang H, et al. IL-1 receptor antagonist reduces endotoxin-induced airway inflammation in healthy volunteers. J Allergy Clin Immunol. 2015;135(2):379–85. doi: 10.1016/j.jaci.2014.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez ML, Wagner JG, Kala A, Mills K, Wells HB, Alexis NE, et al. Vitamin E, gamma-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic Biol Med. 2013;60:56–62. doi: 10.1016/j.freeradbiomed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA, Jr, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84(4):903–11. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devereux G, Turner SW, Craig LC, McNeill G, Martindale S, Harbour PJ, et al. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174(5):499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 19.Fogarty A, Lewis S, Weiss S, Britton J. Dietary vitamin E, IgE concentrations, and atopy. Lancet. 2000;356(9241):1573–4. doi: 10.1016/S0140-6736(00)03132-9. [DOI] [PubMed] [Google Scholar]
- 20.Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. Am J Respir Crit Care Med. 1995;151(5):1401–8. doi: 10.1164/ajrccm.151.5.7735592. [DOI] [PubMed] [Google Scholar]
- 21.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59(8):652–6. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoskins A, Roberts JL, 2nd, Milne G, Choi L, Dworski R. Natural-source d-alpha-tocopheryl acetate inhibits oxidant stress and modulates atopic asthma in humans in vivo. Allergy. 2012;67(5):676–82. doi: 10.1111/j.1398-9995.2012.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97(21):11494–9. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74(6):714–22. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 25.Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, et al. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free Radic Biol Med. 2007;43(8):1176–88. doi: 10.1016/j.freeradbiomed.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, et al. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic Biol Med. 2008;45(1):40–9. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, et al. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 2008;38(3):501–11. doi: 10.1111/j.1365-2222.2007.02855.x. [DOI] [PubMed] [Google Scholar]
- 28.Wagner JG, Harkema JR, Jiang Q, Illek B, Ames BN, Peden DB. Gamma-tocopherol attenuates ozone-induced exacerbation of allergic rhinosinusitis in rats. Toxicol Pathol. 2009;37(4):481–91. doi: 10.1177/0192623309335630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner JG, Birmingham NP, Jackson-Humbles D, Jiang Q, Harkema JR, Peden DB. Supplementation with gamma-tocopherol attenuates endotoxin-induced airway neutrophil and mucous cell responses in rats. Free Radic Biol Med. 2014;68:101–9. doi: 10.1016/j.freeradbiomed.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.3 EPR. Guidelines for the Diagnosis and Management of Asthma. 2007 [Available from: http://www.nhlbi.nih.gov/guidelines/asthma/
- 31.Alexis NE, Zhou H, Lay JC, Harris B, Hernandez ML, Lu TS, et al. The glutathione-S-transferase Mu 1 null genotype modulates ozone-induced airway inflammation in human subjects. J Allergy Clin Immunol. 2009;124(6):1222–8. e5. doi: 10.1016/j.jaci.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lay JC, Alexis NE, Kleeberger SR, Roubey RA, Harris BD, Bromberg PA, et al. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. J Allergy Clin Immunol. 2007;120(3):719–22. doi: 10.1016/j.jaci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Alexis NE, Eldridge MW, Peden DB. Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol. 2003;112(2):353–61. doi: 10.1067/mai.2003.1651. [DOI] [PubMed] [Google Scholar]
- 34.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, et al. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol. 2006;117(6):1396–403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Bennett WD, Alexis NE, Almond M, Herbst M, Zeman KL, Peden DB. Effect of inhaled endotoxin on mucociliary clearance and airway inflammation in mild smokers and nonsmokers. J Aerosol Med Pulm Drug Deliv. 2014;27(6):459–65. doi: 10.1089/jamp.2013.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett WD, Herbst M, Alexis NE, Zeman KL, Wu J, Hernandez ML, et al. Effect of inhaled dust mite allergen on regional particle deposition and mucociliary clearance in allergic asthmatics. Clin Exp Allergy. 2011;41(12):1719–28. doi: 10.1111/j.1365-2222.2011.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett WD, Wu J, Fuller F, Balcazar JR, Zeman KL, Duckworth H, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol (1985) 2015;118(12):1483–90. doi: 10.1152/japplphysiol.00404.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett WD, Herbst M, Zeman KL, Wu J, Hernandez ML, Peden DB. Effect of inhaled endotoxin on regional particle deposition in patients with mild asthma. J Allergy Clin Immunol. 2013;131(3):912–3. doi: 10.1016/j.jaci.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christen S, Jiang Q, Shigenaga MK, Ames BN. Analysis of plasma tocopherols alpha, gamma, and 5-nitro-gamma in rats with inflammation by HPLC coulometric detection. J Lipid Res. 2002;43(11):1978–85. doi: 10.1194/jlr.d200023-jlr200. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Q, Xu T, Huang J, Jannasch AS, Cooper B, Yang C. Analysis of vitamin E metabolites including carboxychromanols and sulfated derivatives using LC/MS/MS. J Lipid Res. 2015;56(11):2217–25. doi: 10.1194/jlr.D061663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124(7):3047–60. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones B, Kenward MG. Design and analysis of cross-over trials. 3. CRC Press; 2015. [Google Scholar]
- 43.Bonser LR, Zlock L, Finkbeiner W, Erle DJ. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest. 2016;126(6):2367–71. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Moreland M, Wagner JG, Ames BN, Illek B, Peden DB, et al. Vitamin E forms inhibit IL-13/STAT6-induced eotaxin-3 secretion by up-regulation of PAR4, an endogenous inhibitor of atypical PKC in human lung epithelial cells. J Nutr Biochem. 2012;23(6):602–8. doi: 10.1016/j.jnutbio.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–9. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshikawa S, Morinobu T, Hamamura K, Hirahara F, Iwamoto T, Tamai H. The effect of gamma-tocopherol administration on alpha-tocopherol levels and metabolism in humans. Eur J Clin Nutr. 2005;59(8):900–5. doi: 10.1038/sj.ejcn.1602154. [DOI] [PubMed] [Google Scholar]
- 47.Burbank AJ, Duran CG, Almond M, Wells H, Jenkins S, Jiang Q, et al. A short course of gamma-tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchese ME, Kumar R, Colangelo LA, Avila PC, Jacobs DR, Jr, Gross M, et al. The vitamin E isoforms alpha-tocopherol and gamma-tocopherol have opposite associations with spirometric parameters: the CARDIA study. Respir Res. 2014;15:31. doi: 10.1186/1465-9921-15-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dow L, Tracey M, Villar A, Coggon D, Margetts BM, Campbell MJ, et al. Does dietary intake of vitamins C and E influence lung function in older people? Am J Respir Crit Care Med. 1996;154(5):1401–4. doi: 10.1164/ajrccm.154.5.8912755. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287(24):3223–9. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 52.Johnson KC, Pan S, Mao Y Canadian Cancer Registries Epidemiology Research G. Risk factors for male breast cancer in Canada, 1994–1998. Eur J Cancer Prev. 2002;11(3):253–63. doi: 10.1097/00008469-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 53.de Gaetano G Collaborative Group of the Primary Prevention P. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357(9250):89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 54.Naziroglu M, Simsek M, Simsek H, Aydilek N, Ozcan Z, Atilgan R. The effects of hormone replacement therapy combined with vitamins C and E on antioxidants levels and lipid profiles in postmenopausal women with Type 2 diabetes. Clin Chim Acta. 2004;344(1–2):63–71. doi: 10.1016/j.cccn.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 55.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182(7):4395–405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook-Mills J, Gebretsadik T, Abdala-Valencia H, Green J, Larkin EK, Dupont WD, et al. Interaction of vitamin E isoforms on asthma and allergic airway disease. Thorax. 2016;71(10):954–6. doi: 10.1136/thoraxjnl-2016-208494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexis NE, Peden DB. Inflammatory response of the airway to inhaled endotoxin correlates with body mass index in atopic patients with asthma but not in normal volunteers. J Allergy Clin Immunol. 2006;117(5):1185–6. doi: 10.1016/j.jaci.2005.12.1305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.