Abstract
Objectives
While the majority of patients undergoing Total Knee Arthroplasty report substantial improvement in pain and function, a significant subset experience persistent post-surgical pain and dysfunction. Better understanding of the longitudinal postoperative course is needed, including the association between patient status following physical rehabilitation at 6 weeks post-TKA, to six month outcomes. This study aims to described the postoperative course of TKA and examine variables associated with change in pain and functioning between 6 weeks and 6 months post-TKA.
Methods
In this longitudinal study of 223 participants, assessments of analgesic intake, depression, anxiety, pain catastrophizing, dysfunction, resting and range of motion (ROM) pain, and pain sensitivity were completed at 6 weeks post-TKA. Analgesic intake, pain ratings and dysfunction data were also collected at 6 months post-TKA. Pain and dysfunction ratings were divided into none-mild and moderate–severe categories.
Results
Between 6 weeks and 6 months post-TKA, 75% of the sample stayed in the same pain category, 20% improved, and 5% worsened. In terms of functional changes between 6 weeks and 6 months, 65% of the sample stayed in the same functional category, while 31% improved, and 5% worsened.
Discussion
These findings demonstrate that the majority of patients’ pain and functioning remains stable between 6 weeks and 6 months post-TKA. However, a notable subset continues to improve or worsen in pain and functioning and the current study identifies variables associated with these changes.
Keywords: persistent postsurgical pain knee arthroplasty
Introduction
Total Knee Arthroplasty (TKA) is a prevalent procedure for patients with advanced osteoarthritis of the knee. As of 2010, approximately 2.5 million Americans underwent TKA [1]. As a result of increased prevalence of obesity and increased acceptance of surgical safety, these numbers are expected to increase substantially by 2020 [2]. Unfortunately, about 1 in 5 patients are dissatisfied following TKA [3] and approximately 20% of TKA patients report persistent pain following surgery [4]. Dissatisfaction following TKA is associated with both knee-related pain and dysfunction [3, 5–7]. Further, dissatisfaction at 3-months post-TKA predicts minimal improvement or worsening outcomes 12-months post-TKA [6].
Increased understanding of the longitudinal postoperative course following TKA could result in better understanding of how some patients develop poor long-term outcomes. Six weeks after surgery, when intensive physical therapy rehabilitation has been completed, improvements in pain can be detected [8]. Further, the first six weeks post-TKA is an important period of time during which, for most patients, pain decreases while functioning and quality of life increase. By 6 weeks post-TKA, quality of life is similar to that prior to surgery, suggesting that the acute impact of surgery has leveled out by this point in time for most patients [9]. Since initial improvement is expected by six weeks post-TKA, this may be an opportune time to assess for early indicators of longer-term outcomes.
We therefore examined the course of pain and functioning between 6 weeks and 6 months post-TKA and examined relationships between demographic and clinical variables (at 6 weeks post-TKA) and change in pain and function between 6 weeks and 6 months post-TKA. This study aimed to answer the following research questions: 1. How do levels of pain and functioning change between 6 weeks and 6 months post-TKA? and 2. What are the relationships between changes in pain and function between 6 weeks and 6 months and relevant demographic and clinical variables?
Methods
Design
This study employed a longitudinal design. Participants were initially recruited prior to TKA as part of a large randomized control trial (RCT) of transcutaneous electrical nerve stimulation (TENS) efficacy following TKA (TANK study: [10]). Sample size was calculated based on the parent study [10]; the current study provides a secondary analysis (which is appropriately powered for the statistics utilized). Data were collected preoperatively (demographics), at 6 weeks and 6 months post-TKA as part of the RCT. This study was approved by the local Institutional Review Board and participants completed informed consent procedures.
Participants
Participants were English speaking patients, aged 30 years or older, with osteoarthritis who received unilateral TKA and provided data preoperatively and at 6 weeks and 6 months post TKA. Exclusion criteria included: stroke/central nervous system disease or mental illness, unable to understand tests/measures, receiving treatment for chronic pain other than knee OA, sensory impairment, permanently or indefinitely wheelchair bound, TENS use in the last 5 years, a condition that precluded TENS use, and current incarceration (for further details see [10]).
Measures
Demographic and Medical Variables
Participants provided demographic information preoperatively including: Sex, age, marital status, racial background, and education level. OA grade (Kellgren and Lawrence scale) was determined from the medical record.
Medication use
Participants recorded postoperative opioid and non-opioid analgesic medication use. At six weeks postoperatively, participants provided doses of opioid and non-opioid medications taken the hour prior to assessment and at 6 months post-TKA, participants provided opioid and non-opioid medications and dosages taken per day.
Pain Intensity (Resting and Range of Motion)
Pain Intensity (Resting and Range of Motion) was measured using a 21-point numeric rating scale (0–20 NRS) where 0 represents “no pain” and 20 represents “the most intense pain imaginable”. The 21-point NRS was used based on data that it is comparable to the 0–10 scale [11], provides a greater range of possible responses which increases sensitivity to change and reduces tool failures (e.g. circling 2 numbers or marking between numbers that is not interpretable accurately). The NRS was used to assess pain at rest and during active flexion and extension of the knee. Resting pain was assessed first, while participants were seated. Participants were given a laminated version of the pain scale to look at and asked to give a number that best described the intensity of pain they were experiencing. For pain during active extension of the knee, participants lay flat on an examination table with a rolled towel placed under the ankle of the surgical knee and straightened their leg as far as possible by pressing the knee down toward the exam table. Participants were asked to rate their pain during maximum extension while looking at the laminated pain scale. Pain during active flexion was assessed by participants bending their surgical knee as far as possible while keeping their foot flat on the exam table. Participants were asked to rate their pain during maximum flexion while looking at the laminated pain scale. Range of motion (ROM) pain was determined by averaging their flexion and extension pain ratings. Participants were defined as having moderate to severe ROM pain of their surgical knee if their average ROM pain was between 8–20 on the 21-point scale (equivalent to 4–10 on a 0–10 scale, based on standard cut offs in the literature) and none-mild pain if their average ROM pain was between 0–7 on the 21 point scale [12, 13].
Function (Dysfunction
Function (Dysfunction) was assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS), which is a self-report measure of knee-related problems [14]. The KOOS subscale: Function in Daily Living (KOOS ADL) was used to assess dysfunction. This KOOS ADL sub-scale has demonstrated good psychometric properties when used with patients with knee OA including internal consistency (αs = 0.78–0.97) and reliability (Inter-class correlations = 0.84–0.94) [15]. The KOOS has also demonstrated responsiveness in patients with knee OA following TKA [16]. Participants were defined as having moderate to severe dysfunction if their KOOS ADL Scale was < 75 [17].
Anxiety
Anxiety was assessed using the Trait scale of the State-Trait Anxiety Inventory (STAI). This scale has twenty statements, rated on a 4-point scale from “almost never” to almost always” [18]. The Trait Anxiety scale assesses how participants respond to stressful stimuli in their environment. This widely used measure has strong psychometric properties [19] and it has been used in other studies of post-TKA patients [20].
Depression
Depression was assessed using the Geriatric Depression Scale. This measure is a 5-item depression screening tool; endorsing 2 or more items screens positive. This measure has demonstrated good inter-rater reliability (kappa = 0.81 – 0.88), sensitivity (0.94 – 0.97), and specificity (0.81 – 0.85: [21, 22].
Pain Catastrophizing
Pain Catastrophizing was assessed with the Pain Catastrophizing Scale (PCS) [23]. The PCS is a 13-item self-report measure that produces a total score and three subscale scores: Rumination (tendency to repetitively think of negative emotional experience), Magnification (effects of one’s experiences are intensified), and Helplessness (passive submission to the painful stimuli). The PCS has good internal consistency (α = 0.87) and has been used to predict post-surgical pain [24].
Pain Sensitivity (Quantitative Sensory Testing)
Heat Pain Thresholds (HPT)
Heat Pain Thresholds (HPT) were assessed using a computer-controlled TSA-II Neurosensory Analyzer (Medoc, Israel) and a Peltier thermode, size 16 X 16 mm. The thermode was placed against the participant’s skin at 35°C, with the temperature rising 1°C/s to a maximum of 52°C. Participants pressed a button to indicate when the heat was first perceived as painful. HPTs were conducted on the surgical knee and contralateral tibia.
Pressure Pain Thresholds (PPT)
Pressure Pain Thresholds (PPT) were assessed using a hand-held pressure algometer (Somedic AB, Farsta, Sweden) with a 1cm2 probe applied at 40kPa/S perpendicularly to the participant’s skin. Participants pressed a button when the pressure was first perceived as painful (this was the PPT). PPTs were assessed on the surgical and contralateral tibia.
HPT and PPT measurements were performed on the surgical knee on three sites, using a standardized template for placement. The template was centered on the patellar midline incision. Three sites were identified 4 centimeters medial to the patellar midline incision, each site was 4 cm apart from one another, running in a line parallel to the patellar midline incision. The standardized template for the anterior tibialis muscle was placed on the tibial crest and three sites were marked 2 cm lateral to the tibial crest of the non-surgical leg. The three sites ran vertically next to the tibial crest and were each 4 cm apart from one another. The average of the 3 scores was used as a final value for pain sensitivity at the surgical knee and tibia (see Rakel et al, 2014 for further details on how outliers were identified and averages determined). The pain sensitivity variables were included for examination in relation to pain severity.
Data Collection Protocol
Participants provided demographic data preoperatively. At six-weeks and six-months post-TKA, participants completed survey measures including report of opioid and other analgesic medication use, and were asked to rate their pain on a 0 to 20 NRS at rest (seated) and during active flexion and extension of the surgical knee, then a research assistant conducted the QST measures.
Data Analyses
All continuous variables were checked for normality and subsequent analyses chosen accordingly. Descriptive statistics for demographic, six week, and six month post-TKA variables were presented as percentages for categorical variables, and mean ± SD or median (25th–75th percentile) for continuous variables.
Research question 1, (How do levels of pain and functioning change between 6 weeks and 6 months post-TKA?) was investigated by determining the number of participants with no pain (NRS = 0), mild pain (NRS > 0 – 7), moderate pain (NRS > 7 – 14), and severe pain (NRS > 14) at 6 weeks and 6 months post-TKA and by determining the percentage of participants with none-mild dysfunction (KOOS ADLs ≥ 75) and those with moderate–severe dysfunction (KOOS ADLs < 75) at 6 weeks and 6 months post-TKA. The following change categories were calculated: improved (from moderate-severe pain or dysfunction at 6 weeks to none-mild pain or dysfunction at 6 months), worsened (from none-mild pain or dysfunction at 6 weeks to moderate-severe pain or dysfunction at 6 months), remained stable/none-mild at both (none-mild pain or dysfunction at both time points), and remained stable/moderate-severe at both (moderate-severe pain or dysfunction at both time points). Percentages of participants who improved, remained stable, or worsened in pain and function between time points were then calculated.
For research question 2, (What are the relationships between changes in pain and function between 6 weeks and 6 months and relevant demographic and clinical variables?), continuous variables were calculated for change in pain and dysfunction. For pain, 6 month ROM pain was subtracted from 6 week ROM pain and for dysfunction, 6 week dysfunction was subtracted from 6 month dysfunction. As such, positive change scores for both pain and dysfunction indicted improvement. Univariate analyses were calculated between the demographic and 6 week clinical variables and the change variables (pain and dysfunction). All analyses assessing demographic and 6 week clinical variables associated with change in pain, adjusted for 6 week ROM pain and parallel analyses for change in dysfunction, adjusted for 6 week dysfunction. Correlation and ANCOVA analyses were used depending on the type of data. Variables that were significant in univariate analyses at p < .05, were entered into multivariate linear regression analyses. Separate models were run for change in pain and for change in functioning, controlling for 6 week pain and 6 week functioning (respectively).
Results
A total of 223 participants completed measures preoperatively, 6 weeks and 6 months post-TKA. At both post-operative time points, some participants had missing data on specific measures; these participants were maintained in the study and simply excluded from the analyses for which that variable was missing (see Table 1 for the n of each measure). The mean age of the sample was 62 years (SD = 9.2), the sample was 56% female, 69% were married or cohabiting, and the mean BMI was 34.7 (SD = 7.1). At 6 weeks post-TKA, mean ROM pain was 5.42 (SD = 4.20), and mean KOOS ADLs was 75 (SD = 15). The median anxiety score at 6 weeks post TKA was 28 (IQR: 23 – 35), 15% of the sample screened positive for depression, and the median pain catastrophizing score = 3 (IQR: 1–9). Median PPTs were 261.17 (IQR: 196.83 – 350.17) for the surgical knee and 316.33 (IQR: 247.33 – 450.58) for the contralateral tibia. Mean HPTs were 43.27 (SD = 3.08) for the surgical knee and 43.95 (SD = 2.78) for the contralateral tibia. At six-months post-TKA, 16% of the sample had moderate to severe ROM pain (as reported previously [13]), 23% of the sample had moderate to severe dysfunction (< 75 on KOOS ADLs), and 10% of the sample had both moderate to severe ROM pain and dysfunction.
Table 1.
Sample Description (N = 223).
| Variable | Mean (SD) or Median [IQR] or count (%) |
|---|---|
| Sex (Male) | 98 (44%) |
| Preoperative age | 61.62 (9.18) |
| Preoperative BMI | 34.65 (7.14) |
| Married or Co-habiting | 144 (69%) (n = 208) |
| Racial background (Caucasian) | 207 (93%) |
|
| |
| Education | (n = 207) |
| Some high school/high school graduate | 65 (31%) |
| Some college | 61 (30%) |
| College graduate/Post graduate education | 81 (39%) |
|
| |
| OA grade | |
| 2 | 4 (2%) |
| 3 | 60 (27%) |
| 4 | 159 (71%) |
|
| |
| 6 weeks post-TKA | |
| Opioid dose: hour prior to testing | 0.16 [0.00 – 1.44] |
| Non-opioid analgesic: hour prior to testing | 77.67 [3.33 – 176.67] |
| Resting pain | 1.00 [0.00 – 3.00] (n = 216) |
| Range of Motion pain (moderate – severe) | 66 (31%) (n = 216) |
| Dysfunction (moderate – severe) | 106 (49%) (n = 217) |
| Depression (screened positive) | 33 (15%) (n = 222) |
| Anxiety | 28.00 [23.00 – 35.00] (n = 221) |
| Pain Catastrophizing | 3.00 [1.00 – 9.00] (n = 220) |
| Pressure Pain Threshold (PPT) surgical knee | 261.17 [196.83 – 350.17] (n = 220) |
| PPT contralateral tibia | 316.33 [247.33 – 450.58] (n = 218) |
| Heat Pain Threshold (HPT) surgical knee | 43.27 (3.08) (n = 180) |
| HPT contralateral tibia | 43.95 (2.78) (n = 179) |
|
| |
| 6 months post-TKA | |
| Opioid mg/day | 0.00 [0.00 – 0.00] (n = 222) |
| Non-opioid mg/day | 0.00 [0.00 – 975.00] (n = 222) |
| Range of Motion pain (moderate – severe) | 31 (16%) (n = 191) |
| Dysfunction (moderate – severe) | 49 (23%) (n = 212) |
Where no n is indicated it is equal to 223.
SD = Standard deviation; IQR = Interquartile Range
Research Question 1: Pain change across time points
Between 6 weeks and 6 months post-TKA, 75% of the sample stayed in the same pain category (64% reported none-mild pain at both time points and 11% reported moderate to severe pain at both time points) 20% improved, and 5% worsened. See table 2 for variability within each change category and figure 1 for changes across time between no pain, mild, moderate, and severe pain categories.
Table 2.
Pain and dysfunction change between 6 weeks and 6 months post-TKA (positive scores indicate improvement).
| None-mild pain (≤ 7) at both time points | Worsened (none-mild pain to mod-severe pain) | Mod-severe pain (> 7) at both time points | Improved (mod – severe pain to none-mild pain) | |
|---|---|---|---|---|
| n (%) | 122 (64%) | 10 (5%) | 21 (11%) | 38 (20%) |
| Mean change score (SD) | 1.12 (2.23) | −5.65 (2.94) | 1.19 (4.30) | 7.80 (2.62) |
| Minimum | −5.50 | −11.50 | −11.00 | 3.50 |
| Maximum | 6.50 | −2.00 | 7.50 | 17.00 |
| None-mild dysfunction (≥75) at both time points | Worsened (none-mild dysfunction to mod-severe dysfunction) | Mod-severe dysfunction (<75) at both time points | Improved (mod – severe dysfunction to none-mild dysfunction) | |
|---|---|---|---|---|
| n (%) | 99 (47%) | 10 (5%) | 39 (18%) | 64 (30%) |
| Mean change score(SD) | 6.14 (7.53) | −28.08 (22.45) | 2.21 (12.20) | 20.87 (9.20) |
| Minimum | −16.10 | −83.80 | −38.20 | 3.00 |
| Maximum | 23.30 | −4.40 | 22.10 | 45.50 |
Figure 1.
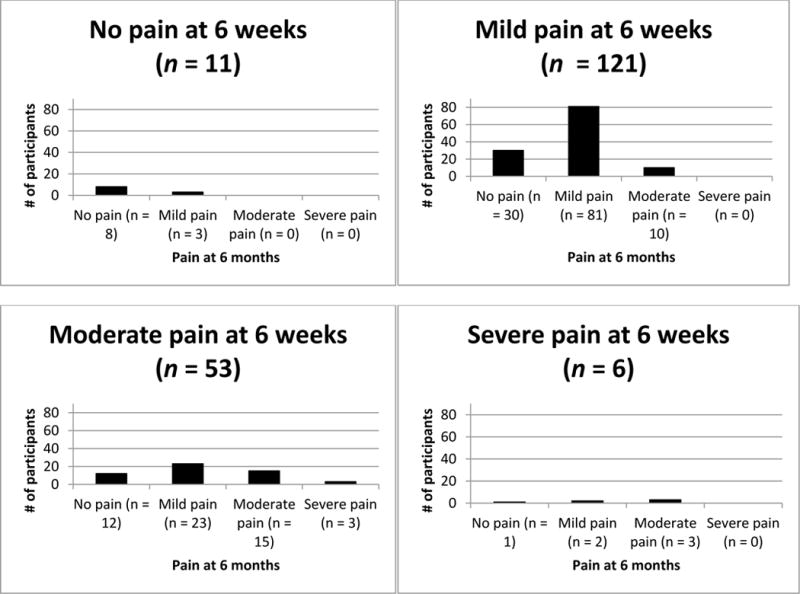
Range of motion pain severity between 6 weeks and 6 months post-TKA.
Function change across time points
Between 6 weeks and 6 months, 65% of the sample stayed in the same category: 47% reported none-mild dysfunction at both time points (≥ 75 on KOOS ADLs) and 18% reported moderate-severe dysfunction at both time points (< 75 on KOOS ADLs). A further 30% improved (moving from moderate-severe dysfunction, to none-mild dysfunction) and 5% worsened (moving from none-mild dysfunction to moderate-severe dysfunction categories) between 6 weeks and 6 months post-TKA (see table 2 and figure 2).
Figure 2.
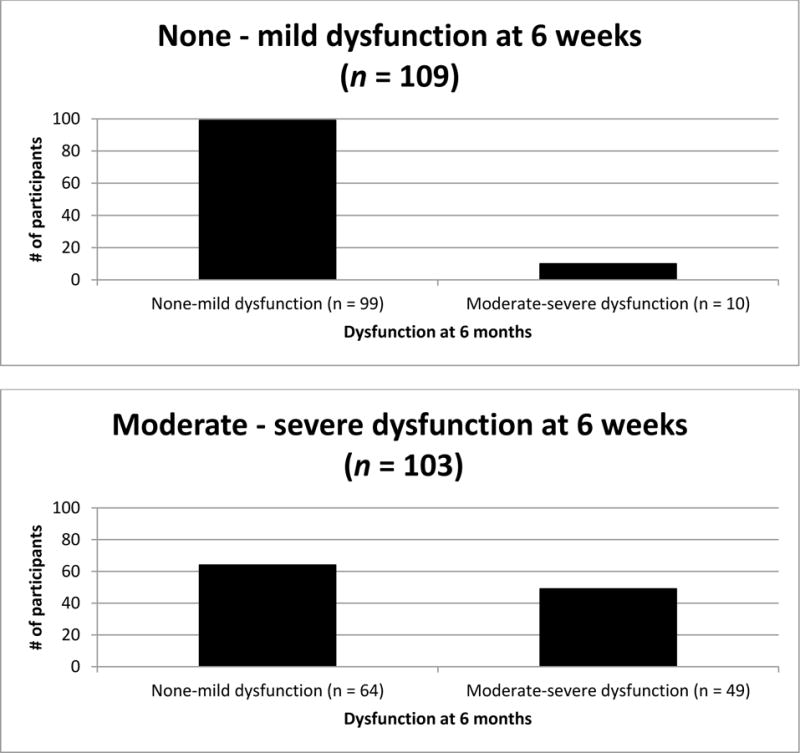
Dysfunction between 6 weeks and 6 months post-TKA.
Research Question 2: Variables associated with change in pain between 6 weeks and 6 months post-TKA
Univariate analyses identified that 6 week anxiety (r = −.20, p < .05) and 6 week pain catastrophizing (r = −.24, p < .05) were significantly associated with change in pain when adjusting for 6 week ROM pain. See table 3 for further details on all non-significant findings.
Table 3.
Association of demographic and clinical variables to change in movement pain (adjusted for 6 week movement pain) and to change in dysfunction (adjusted for 6 week dysfunction).
| Pain | Change | Dysfunction | Change | |
|---|---|---|---|---|
|
| ||||
| Variable | Correlation (r) or ANCOVA (F) | p value | Correlation (r) or ANCOVA (F) | p value |
| Sex | F = 1.97 | 0.16 | F = 32.64* | 0.01 |
| Age | r = 0.06 | 0.46 | r = 0.07 | 0.34 |
| 6 weeks post-TKA | ||||
| Opioid dose hour prior to testing | r = 0.08 | 0.34 | r = 0.14 | 0.07 |
| Non-Opioid dose hour prior to testing | r = −0.10 | 0.21 | r = 0.07 | 0.36 |
| Range of motion pain | — | — | r = −0.12 | 0.09 |
| Depression – positive screen | F = 0.28 | 0.60 | F = 19.48* | 0.00 |
| Anxiety | r = −0.20* | 0.02 | r = −0.22* | 0.00 |
| Pain Catastrophizing | r = −0.24* | 0.01 | r = −0.31* | 0.00 |
| Dysfunction (KOOS ADLs) | r = −0.16 | 0.06 | — | — |
| PPT surgical knee | r = 0.02 | 0.78 | — | — |
| PPT contralateral tibia | r = −0.09 | 0.30 | — | — |
| HPT surgical knee | r = −0.04 | 0.65 | — | — |
| HPT contralateral tibia | r = 0.01 | 0.86 | — | — |
| 6 months post-TKA | ||||
| Opioid mgs per day | r = −0.06 | 0.45 | r = −0.10 | 0.22 |
| Non-opioid mgs per day | r = −0.04 | 0.64 | r = −0.06 | 0.44 |
p < .05
A multiple linear regression model controlling for 6 week ROM pain, found that both 6 week anxiety and pain catastrophizing each accounted for a significant amount of unique variance in change in pain (between 6 weeks and 6 months). The full model was significant: F (3, 183) = 38.81, p < .05, adjusted R2 = 0.38 (See table 4 for individual beta weights). Of note however, the first step (including only 6 week ROM pain) produced an adjusted R2 = 0.32, indicating that the addition of both anxiety and pain catastrophizing to the model only accounted for an additional 6% of variance. Overall, findings indicate that higher levels of anxiety and pain catastrophizing were associated with less improvement and that higher levels of 6 week ROM pain severity were associated with greater improvement (this was likely an artifact of a greater possible range of change scores available to those with higher pain at 6 weeks post-TKA and the previously discussed findings that fewer participants worsened during this time period then improved).
Table 4.
Multivariate linear regression model: Change in pain between 6 weeks and 6 months.
| Variable and Step | β | p - value | |
|---|---|---|---|
| Step 1 | |||
| Range of motion (ROM) pain | 0.57* | 0.00 | |
|
| |||
| F (1, 185) = | 86.61, p =.00, | Adjusted R2 = 0.32 | |
|
| |||
| Step 2 | |||
| ROM pain | 0.64* | 0.00 | |
| Anxiety | −0.15* | 0.02 | |
| Pain Catastrophizing | −0.18* | 0.01 | |
|
| |||
| F (3, 183) = | 38.81, p =.00, | Adjusted R2 = 0.38 | |
p < .05
Variables associated with change in dysfunction between 6 weeks and 6 months post-TKA
When adjusting for 6 week dysfunction, univariate analyses identified that the following variables were significantly associated with change in dysfunction between 6 weeks and 6 months: sex (F = 32.64, p < .05), depression screen (F = 19.48, p < .05), anxiety (r = −.22, p < .05), and pain catastrophizing (r = −.31, p < .05). See table 3 for further details on all non-significant findings.
A multiple linear regression model controlling for 6 week dysfunction, found that sex, depression, and pain catastrophizing, but not anxiety, each accounted for a significant amount of unique variance in change in dysfunction (between 6 weeks and 6 months). The full model was significant: F (5, 204) = 16.28, p < .05, adjusted R2 = 0.27 (See table 5 for individual beta weights). The first step (including only 6 week dysfunction) produced an adjusted R2 = 0.13, so the additional variables in the second step accounted for an additional 14% of the variance in change in dysfunction. Overall, findings indicate that male sex (mean change for males = 5.13, SD = 17.00; mean change for females = 10.69, SD = 12.40), higher levels of pain catastrophizing, and positive depression screen (mean change for positive depression screen = 2.70, SD = 24.85 and for negative screen = 9.23, SD = 12.06) were associated with less functional improvement. Further, higher 6 week dysfunction was associated with greater improvement, which was likely an artifact of a greater possible range of change scores available to those with higher dysfunction at 6 weeks post-TKA.
Table 5.
Multivariate linear regression model: Change in dysfunction between 6 weeks and 6 months.
| Variable and Step | β | p - value | |
|---|---|---|---|
| Step 1 | |||
| Dysfunction | −0.37* | 0.00 | |
|
| |||
| F (1, 208) = | 32.38, p =.00, | Adjusted R2 = 0.13 | |
|
| |||
| Step 2 | |||
| Dysfunction at 6 weeks | −0.50* | 0.00 | |
| Sex | 0.19* | 0.00 | |
| Depression | −0.20* | 0.01 | |
| Anxiety | −0.11 | 0.13 | |
| Pain Catastrophizing | −0.16* | 0.02 | |
|
| |||
| F (1, 208) = | 16.28, p =.00, | Adjusted R2 = 0.27 | |
p < .05
Discussion
This study examined the longitudinal postoperative course following TKA. Findings suggest that the majority of patients’ pain remained relatively stable from 6 weeks post-TKA to 6 months post-TKA, suggesting that 6 weeks post-TKA is an opportune time point to assess for early indicators of chronic pain. There is still, however, a significant subset (20%) of patients whose pain continued to improve after 6 weeks post-TKA. The current study helps identify these patients, suggesting that higher anxiety and pain catastrophizing 6-weeks post-TKA is associated with less improvement in pain by 6 months post-TKA. This is consistent with previous findings that pre-operative anxiety and pain catastrophizing predict postoperative pain severity [4, 13, 25, 26]. Function appeared to be somewhat more variable then pain from 6 weeks post-TKA, with nearly one-third (31%) of the sample continuing to improve by 6 months post-TKA. This fits with previous findings which suggest that functioning can continue to improve for at least 2 years post-TKA [27].
Multiple variables were identified that impact the trajectory of change in functioning between 6 weeks and 6 months post-TKA. Male sex, pain catastrophizing, and positive depression screen were significantly related to less improvement. These findings fit with previous findings that pre-operative depression is associated with less functional improvement 2 years post-TKA [28] and lower function scores 5 years post-TKA [29]. This is also consistent with findings that greater preoperative pain catastrophizing is associated with worse functional outcomes 6 weeks post-TKA [30]. Male sex as a risk factor for dysfunction fits with Kauppila et al.’s (2011) finding that males had smaller improvements in function compared to females following TKA [31]. However, previous research has also identified female sex as a risk factor for persistent post-TKA pain [25, 32]. This discrepancy emphasizes the importance of investigating how variables relate differently to pain and functional outcomes following TKA.
The current study did not find pain sensitivity related to pain change trajectories between 6 weeks and 6 months post-TKA. While recent pain sensitivity literature has identified that measures of central sensitization (widespread hyperalgesia and temporal summation) impact postoperative outcomes [33, 34], the current study suggests that pain sensitivity (local and widespread hyperalgesia) 6 weeks post-TKA does not relate to changes in pain between 6 weeks and 6 months post-TKA.
This study was not without limitations. The sample was primarily Caucasian, which may limit the generalizability of findings [35]. In addition, the measure of depression utilized was a screen rather than a full diagnostic assessment. Finally, larger samples are needed to disentangle whether the same variables impact improvement compared to worsening during this postoperative time period.
In conclusion, the current findings suggest that the majority of patients stay in the same pain and functioning categories between six weeks and six months post-TKA; however, a notable subset continued to improve while only a few participants worsened in this timeframe. Further, with the exception of pain catastrophizing, different variables were associated with change in pain then were associated with change in functioning.
Acknowledgments
We would like to thank Katharine Geasland, BS, RN and Jennie Embree, MS for their contributions to this project.
This work was funded by The National Institutes of Nursing Research (R01 NR009844), the National Institute of Neurological Disorders and Stroke (T32 NS045549), the University of Iowa College of Nursing, and DJO, Inc. Dr. Sluka serves as a consultant for DJO, Inc.
Footnotes
There are no other conflicts of interest to report.
References
- 1.Maradit-Kremers H, et al. Prevalence of Total Hip (THA) and Total Knee (TKA) Arthroplasty in the United States, in. American Academy of Orthopaedic Surgeons. 2010 [Google Scholar]
- 2.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis & Rheumatism-Arthritis Care & Research. 2008;59(4):481–488. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- 3.Bourne RB, et al. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis GN, et al. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114(4):551–61. doi: 10.1093/bja/aeu441. [DOI] [PubMed] [Google Scholar]
- 5.Becker R, et al. Expectation, satisfaction and clinical outcome of patients after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1433–41. doi: 10.1007/s00167-011-1621-y. [DOI] [PubMed] [Google Scholar]
- 6.Williams DP, et al. Early postoperative predictors of satisfaction following total knee arthroplasty. Knee. 2013;20(6):442–6. doi: 10.1016/j.knee.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Scott CE, et al. Patient expectations of arthroplasty of the hip and knee. J Bone Joint Surg Br. 2012;94(7):974–81. doi: 10.1302/0301-620X.94B7.28219. [DOI] [PubMed] [Google Scholar]
- 8.Papakostidou I, et al. Factors affecting the quality of life after total knee arthroplasties: a prospective study. BMC Musculoskelet Disord. 2012;13:116. doi: 10.1186/1471-2474-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Egmond JC, Verburg H, Mathijssen NM. The first 6 weeks of recovery after total knee arthroplasty with fast track. Acta Orthop. 2015;86(6):708–13. doi: 10.3109/17453674.2015.1081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakel BA, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebo-controlled trial. Pain. 2014;155(12):2599–611. doi: 10.1016/j.pain.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain. 1994;58(3):387–92. doi: 10.1016/0304-3959(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 12.Brander VA, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003;(416):27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 13.Noiseux NO, et al. Preoperative predictors of pain following total knee arthroplasty. J Arthroplasty. 2014;29(7):1383–7. doi: 10.1016/j.arth.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins NJ, et al. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S208–28. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins NJ, Roos EM. Patient-reported outcomes for total hip and knee arthroplasty: commonly used instruments and attributes of a “good” measure. Clin Geriatr Med. 2012;28(3):367–94. doi: 10.1016/j.cger.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Giesinger JM, et al. WOMAC, EQ-5D and Knee Society Score Thresholds for Treatment Success After Total Knee Arthroplasty. J Arthroplasty. 2015;30(12):2154–8. doi: 10.1016/j.arth.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 19.Elwood LS, Wolitzky-Taylor K, Olatunji BO. Measurement of anxious traits: a contemporary review and synthesis. Anxiety Stress Coping. 2012;25(6):647–66. doi: 10.1080/10615806.2011.582949. [DOI] [PubMed] [Google Scholar]
- 20.Feeney SL. The relationship between pain and negative affect in older adults: anxiety as a predictor of pain. J Anxiety Disord. 2004;18(6):733–44. doi: 10.1016/j.janxdis.2001.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Rinaldi P, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694–8. doi: 10.1034/j.1600-0579.2003.00216.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoyl MT, et al. Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc. 1999;47(7):873–8. doi: 10.1111/j.1532-5415.1999.tb03848.x. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan MJL, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assessment. 1995;7:524–32. [Google Scholar]
- 24.Burns LC, et al. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. J Pain Res. 2015;8:21–32. doi: 10.2147/JPR.S64730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnin MP, Basiglini L, Archbold HA. What are the factors of residual pain after uncomplicated TKA? Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1411–7. doi: 10.1007/s00167-011-1549-2. [DOI] [PubMed] [Google Scholar]
- 26.Hinrichs-Rocker A, et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - a systematic review. Eur J Pain. 2009;13(7):719–30. doi: 10.1016/j.ejpain.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Vina ER, Hannon MJ, Kwoh CK. Improvement following total knee replacement surgery: Exploring preoperative symptoms and change in preoperative symptoms. Semin Arthritis Rheum. 2016;45(5):547–55. doi: 10.1016/j.semarthrit.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh JA, Lewallen DG. Depression in primary TKA and higher medical comorbidities in revision TKA are associated with suboptimal subjective improvement in knee function. BMC Musculoskelet Disord. 2014;15:127. doi: 10.1186/1471-2474-15-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brander V, et al. Pain and depression influence outcome 5 years after knee replacement surgery. Clin Orthop Relat Res. 2007;464:21–6. doi: 10.1097/BLO.0b013e318126c032. [DOI] [PubMed] [Google Scholar]
- 30.Lungu E, Vendittoli PA, Desmeules F. Preoperative Determinants of Patient-reported Pain and Physical Function Levels Following Total Knee Arthroplasty: A Systematic Review. Open Orthop J. 2016;10:213–31. doi: 10.2174/1874325001610010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kauppila AM, et al. Outcomes of primary total knee arthroplasty: the impact of patient-relevant factors on self-reported function and quality of life. Disabil Rehabil. 2011;33(17–18):1659–67. doi: 10.3109/09638288.2010.543749. [DOI] [PubMed] [Google Scholar]
- 32.Edwards RR, et al. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307–11. doi: 10.1155/2009/273783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen KK, et al. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. 2015;156(1):55–61. doi: 10.1016/j.pain.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 34.Wylde V, et al. Central sensitization as a determinant of patients’ benefit from total hip and knee replacement. Eur J Pain. 2017;21(2):357–365. doi: 10.1002/ejp.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamath AF, et al. Ethnic and gender differences in the functional disparities after primary total knee arthroplasty. Clin Orthop Relat Res. 2010;468(12):3355–61. doi: 10.1007/s11999-010-1461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


