Abstract
Background
Several disease-modifying drugs have shown promising effects on cognitive impairment in multiple sclerosis (MS). Alemtuzumab, a humanized monoclonal antibody, is effective in controlling disease activity, however, has not been evaluated for its effects on cognition in detail so far.
Objective
To explore the influence of alemtuzumab on cognitive impairment in active relapsing–remitting MS (RRMS) as well as possible clinical and neuroimaging predictors of cognitive changes during the first year of therapy.
Methods
Extensive neuropsychological assessment was administered to 21 patients with active RRMS at baseline and again after the second treatment with alemtuzumab (mean time span: 15.05 months). Clinical and routine structural neuroimaging markers were explored for their capacity to predict individual courses of cognitive change.
Results
Overall cognitive functioning remained stable or improved during the observational period of alemtuzumab treatment on average. Scores on two neuropsychological tests of processing speed significantly improved and clinically relevant individual gains of processing speed were seen in the majority of patients. Linear regression models showed that clinical and routine neuroimaging measures of disease activity could not fully account for these cognitive changes.
Conclusion
Results suggest that alemtuzumab treatment in active RRMS stabilizes overall cognitive functioning and furthermore positively affects cognitive processing speed. Changes in processing speed were independent from clinical and structural neuroimaging parameters of disease activity and may thus represent an underrated and independent outcome measure to evaluate treatment effects.
Keywords: multiple sclerosis, immunomodulation, alemtuzumab, neuropsychology, cognition, neurocognitive disorders, magnetic resonance imaging
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system, often affecting young adults (1). While cognitive impairment can be present in 40–70% of MS patients (2–4), it is not reflected sufficiently by the expanded disability status scale [EDSS (5)], the standard clinical measure for MS-related disability. Cognitive dysfunctions have a high impact on quality of life, may predict unemployment, and can negatively influence social activities (6). In relapsing–remitting MS (RRMS), deteriorations in information processing speed are regarded to be the primary cognitive deficit and are often accompanied by verbal memory and visuospatial deficits as well as executive dysfunctions (7, 8).
Disease-modifying drugs (DMDs) have been shown to slow disease progression and advances in physical disability (9). Nevertheless, cognition has been evaluated in detail in only a few DMDs so far. Regarding first-line treatments, interferon beta-1a has shown promising effects on processing speed and other cognitive domains (10–12). In line with their stronger effect on conventional clinical and paraclinical outcome measures, monoclonal antibody treatments (e.g., natalizumab and daclizumab-HYP) may even surpass first-line treatments regarding positive effects on cognition, particularly processing speed (13–16).
Alemtuzumab, a highly potent monoclonal antibody treatment, is approved in Europe since 2013 for patients with active RRMS. In randomized controlled trials, alemtuzumab improved relapse rate and disability and also slowed brain volume loss (17–19). In spite of these positive effects on disease activity, no study has so far explored the influence of alemtuzumab on cognition in detail. With the current longitudinal observational study, we sought to investigate the influence of alemtuzumab on a range of cognitive domains assessed with an extensive neuropsychological test battery. With regard to previously established positive effects of potent second-line treatments on cognition, we hypothesized that alemtuzumab may have a stabilizing or even positive effect on cognition, particularly for processing speed as the mainly impaired cognitive domain in MS (7).
Although previous studies showed the potential of DMDs to positively affect cognition, underlying factors that drive this cognitive change have not been clearly identified. We thus aimed to also explore predictors for cognitive changes under alemtuzumab treatment. For this purpose, clinical (EDSS, number of relapses, disease duration, and general intelligence at baseline) as well as routine structural neuroimaging markers [T2 lesion load, gadolinium-enhancing lesions (GELs)] at baseline were analyzed for their potential to predict individual cognitive outcomes.
Materials and Methods
Participants
Patients were recruited at the Department of Neurology at the University Hospital of Muenster between November 2014 and May 2016. All patients were diagnosed with MS according to the revised 2010 McDonald criteria (20) and were eligible for alemtuzumab treatment according to national guidelines and the recent alemtuzumab summary of product characteristics (SMPC). The treatment decision toward alemtuzumab was made independently of this study. The interval between prior treatment and alemtuzumab was according to national guidelines. None of the patients reported critical side-effects under his/her previous DMT and alemtuzumab treatment was initiated due to high or ongoing disease activity in all cases except one (here, treatment was switched from natalizumab because of increased risk for development of progressive multifocal leukoencephalopathy). As part of the clinical routine and according to national guidelines and the SMPC, all patients underwent clinical examination including determination of neurological status and disability as well as MRI of the brain and extensive laboratory examination. Eligible patients were then screened for participation in this study and gave informed consent upon enrollment. Patients with a history of psychiatric disorders, recent relapses (time span to last relapse >1 month) or neurological conditions other than MS were excluded. Clinical data including MRI imaging data were extracted from the medical charts of the patients. Ethical approval was given by local authorities (2014-398-f-S). Patients underwent baseline neuropsychological assessment at the screening visit prior to first alemtuzumab infusion. Follow-up assessment took place at the first visit after the second course of alemtuzumab [mean time span in months: 15.05 (SD = 2.06)].
Procedure and Materials
The extensive neuropsychological test battery covered the domains of verbal learning, verbal and visual memory, attentional span, processing speed, visuoconstruction, and executive functions. Additionally, a measure of premorbid intelligence and questionnaires for depressive symptoms and fatigue were included. For details regarding the test protocol and cognitive domains, see Table 1.
Table 1.
Neuropsychological tests and cognitive domains measured.
| Cognitive domain | Neuropsychological test | Outcome measures |
|---|---|---|
| Verbal learning | Rey auditory verbal learning test (RAVLT) | RAVLT 1–5 total words learned |
| Verbal memory | Rey auditory verbal learning test (RAVLT) | RAVLT 6 recall after interference; RAVLT 7 delayed recall after 30 min.; RAVLT recognition 8 |
| Attentional span | Digit span forwards | No. of digits memorized |
| Visual memory | Rey complex figure test (RCFT) | RCFT immediate recall |
| Visuoconstruction | Rey complex figure test (RCFT) | RCFT copy |
| Executive functions | Verbal fluency S-words and animals; Trail Making Test B (TMT-B); digit span backwards | S-words named during 1 min resp.; seconds needed for Trail B; no. of digits memorized |
| Processing speed | Trail Making Test A (TMT-A); Symbol Digit Modalities Test (SDMT); Rey complex figure test (RCFT) | Seconds needed for Trail A No. of correct responses in 90 s (written) Time to copy in seconds |
| Intelligence | Standard Progressive Matrices (SPM) | No. of correct responses |
Test administration was conducted in accordance with the manuals in a quiet setting by an experienced neuropsychologist. Mean administration time of the test battery was 1.5 h. In order to avoid practice effects, alternative forms of neuropsychological tests were used for the follow-up testing whenever practice effects could be expected [Rey Auditory Verbal Learning Test (21, 22) (RAVLT), Rey Complex Figure Test (23) (RCFT)]. Test raw scores were standardized and transformed into normative percentile ranks controlling for age, sex, and education. Patients were classified as cognitively impaired when they scored beneath the fifth percentile rank in two or more cognitive measures.
Statistical Analysis
Statistical analyses were conducted with SPSS Statistics 24 (IBM, New York, NY, USA); figures were created using Prism7 (GraphPad Software, CA, USA) and Microsoft Excel. Prior to employing statistical tests, test data were visually examined for skewness and outliers and checked for normality using the Kolmogorov–Smirnov test. Wilcoxon signed-rank tests were used to compare baseline and follow-up raw test scores and median percentile ranks. Alpha-levels were corrected for multiple comparisons using the Bonferroni-method (14 comparisons, corrected α = 0.0036). Median percentile ranks across all cognitive tests were computed for each patient at baseline and follow-up as a measure of general cognitive functioning. To evaluate individual courses of cognitive change, the number of patients with clinically significant impairment in two or more cognitive tests at follow-up was compared to baseline and tested for significance using McNemar’s tests. In order to explore predictors of this individual cognitive change (operationalized by subtracting the number of impaired tests at baseline from the number of impaired tests at follow-up), multiple regression models were employed. Commonly used clinical baseline parameters (EDSS, number of T2 lesions, number of GELs, relapse rate, disease duration as well as general intelligence) were added to this regression model as potential predictors. In a second model, the baseline-to-follow-up differences in EDSS, T2 lesion load, GELs, and relapse rate were also investigated as potential predictors of cognitive change.
Results
Demographic and Clinical Data
Demographic and clinical characteristics at baseline and follow-up are summarized in Table 2. Twenty-one patients (10 females, mean age 32.38 with a range of 18–50 years) were included in the study. At baseline, the mean EDSS was 2.12 (SD = 1.34). MRI showed that patients had a mean of 33.71 (SD = 23.28) T2 lesions and 4.67 (SD = 7.39) GELs [mean proportion of GEL/T2 lesions: 0.11 (SD = 0.14)]. Self-reported depressive symptoms on Beck’s Depression Inventory II (29) were mild with an average score of 9.62 [SD = 6.63; BDI-II; cut-off >18.5 for depression in MS patients (30)]. The mean fatigue score as assessed by the Wuerzburger Fatigue Inventory for MS (31) (WEIMuS; cut-off >32) was 27.72 (SD = 13.6) indicating mild self-reported fatigue in our sample.
Table 2.
Means of demographic and clinical data at baseline and follow-up with p-values of Wilcoxon signed-rank tests.
| Demographic data | Range | ||||
|---|---|---|---|---|---|
| Female, n (%) | 10 (50.00) | N/A | |||
| Education in yearsa (SD) | 11.48 (1.25) | 9–13 | |||
| Age in years (SD) | 32.38 (8.72) | 18–50 | |||
| Follow-up period in months (SD) | 15.05 (2.06) | 12–19 | |||
| Disease duration in years (SD) | 4.98 (5.44) | 0–21.3 | |||
| Clinical data | Baseline | Range | Follow-up | Range | p |
| EDSS (SD) | 2.12 (1.34) | 0–4.5 | 1.88 (1.53) | 0–5 | 0.410 |
| No. of relapses (SD) | 2.76 (1.97) | 0–6 | 2.90 (2.14) | 0–7 | 0.083 |
| Mean relapse frequencyd (SD) | 1.38 (0.99) | 0–3 | 0.14 (0.36) | 0–1 | <0.001 |
| No. prior treatments (SD) | 1.81 (1.69) | 0–7 | N/A | N/A | |
| Prior treatment | N/A | N/A | N/A | ||
| Naïve | 6 | ||||
| Beta-interferon | 3 | ||||
| Glatiramer acetate | 2 | ||||
| Dimethyl fumarate | 3 | ||||
| Fingolimod | 4 | ||||
| Natalizumab | 3 | ||||
| Months between treatmentse (SD) | 3.67 (4.55) | 0–18 | N/A | N/A | |
| No. T2 lesions | 33.71 (23.28) | 7–80 | 34.38 (24.06) | 7–81 | 0.034 |
| No. GELs | 4.67 (7.39) | 0–25 | 0.19 (0.40) | 0–1 | 0.002 |
| GEL/T2 | 0.11 (0.14) | 0–0.4 | 0.01 (0.02) | 0–0.1 | 0.003 |
| No. patients with TPO antibodiesb | 5 | N/A | 8 | N/A | 0.250 |
| BDI-II raw score (SD)c | 9.61 (6.63) | 0–20 | 9.52 (11.04) | 0–46 | 0.099 |
| WEIMuS raw score (SD) | 27.72 (13.6) | 3–44 | 23.29 (19.47) | 0–56 | 0.052 |
Clinical data of patients with RRMS and Alemtuzumab treatment (N = 21).
N, sample size; EDSS, Expanded Disability Status Scale; RRMS, relapsing–remitting multiple sclerosis; GEL, gadolinium-enhancing lesions; TPO, thyroid peroxidase antibody; BDI-II, Beck’s Depression Inventory-II (cut-off: 18.5); WEIMuS, Würzburger Fatigue Inventory for Multiple Sclerosis (cut-off: 32); N/A, Not applicable.
aEducation levels were grouped into three levels by years of general schooling, job trainings as well as academic training.
bCompared using McNemar’s test.
cAvailable from N = 13 at baseline, N = 21 at follow-up.
dMean relapse frequency of patients per year.
eInterval between most recent prior treatment and alemtuzumab administration (months).
Clinical and Cognitive Outcome
During the treatment phase, EDSS improved from 2.12 (SD = 1.34) to 1.88 (SD = 1.53; p = 0.410). Three patients each had one relapse within the study period (hypesthesia, dysesthesia, sensorimotor symptoms). Mean relapse frequency per person/year decreased significantly from 1.38 (SD = 0.99) to 0.14 (SD = 0.36) at follow-up (p < 0.001). T2 lesion load increased to 34.38 (SD = 24.06; p = 0.034). The number of GELs significantly decreased from 4.67 (SD = 7.39) to 0.19 (SD = 0.40; p = 0.002) and also the proportion of GEL/T2 lesions significantly decreased (p = 0.003). No significant differences from baseline to follow-up were seen for mean depressive symptoms (p = 0.099) or fatigue (p = 0.052).
Overall Cognitive Change
The median percentile rank of 35.24 (SD = 22.42) at baseline improved to 42.96 (SD = 19.78) at follow-up (p = 0.086), indicating a statistical trend toward overall cognitive improvement.
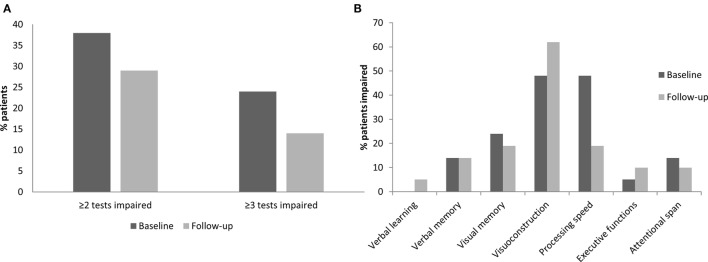
In order to evaluate individual cognitive change, the number of patients with impaired test performance (cut-off point for impairment: <5th percentile rank) was also compared between baseline and follow-up (Figure 1A). At baseline, 8/21 patients (38%) were impaired in two or more neuropsychological tests and 5/21 patients (24%) were impaired in three or more tests. At follow-up, the number of patients impaired on two or more tests had decreased to 6/21 patients (29%) and the number of patients impaired in three or more tests decreased to 3/21 (14%). Using McNemar’s test for paired samples, these changes failed to reach statistical significance (all ps > 0.05).
Figure 1.
Cognitive change in overall cognition and in specific neuropsychological domains over the observational period of 15 months. (A) Percentage of patients with impairment in ≥2 tests and impairment in ≥3 tests at baseline and follow-up. (B) Percentage of patients impaired in each neuropsychological domain at baseline and follow-up.
Cognitive Change in Specific Domains
Figure 1B depicts the proportion of patients with cognitive impairment at baseline and follow-up in the different neuropsychological domains. At baseline, processing speed and visuoconstruction were the domains with the highest number of impaired patients; furthermore, patients showed impairments in visual and verbal memory, attentional span, and executive functions. At follow-up, a lower proportion of patients was impaired in the domain of processing speed [Symbol Digit Modalities Test (27) (SDMT), RCFT copy time, and Trail Making Test part A (24) (TMT-A)] when compared to baseline. This reduction was shown to be statistically significant by means of McNemar’s test (p = 0.031). No other domain showed significant changes between baseline and follow-up, and most importantly, no significant decrease in performance was seen under alemtuzumab.
Cognitive Change in Single Processing Speed Tests
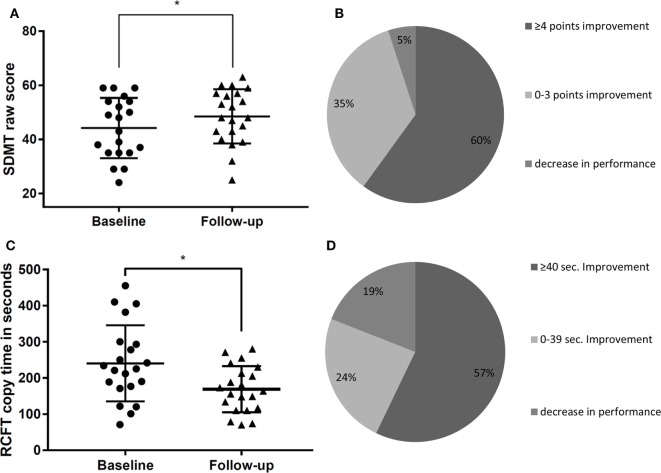
Table 3 shows mean cognitive test results at baseline and follow-up testing grouped by neuropsychological domain. Consistent with our finding of a decrement of impaired patients in the processing speed domain, there was also a significant improvement in two single tests of processing speed. Mean patients’ scores on the written SDMT significantly improved from baseline to follow-up (p < 0.001; Figure 2A). This improvement can be regarded as clinically relevant in 12/21 patients, according to Morrow et al. (32), who defined an individually relevant improvement on the SDMT as four points according to the finding that a 4-point decrease on the SDMT was detectable in patients who advanced from employment to work disability within 3 years (Figure 2B). Similar to the SDMT, patients on average needed significantly less time on the RCFT time to copy condition during follow-up testing than during baseline testing (p = 0.002; Figure 2C). Improvement of the RCFT copy time was clinically relevant (as defined by an improvement of at least 40 s; Figure 2D) in 12/21 patients. Although no other test comparison between baseline and follow-up reached statistical significance, almost all mean test scores improved or remained stable over the course of observation (Table 3).
Table 3.
Mean and SD of neuropsychological raw values at baseline and follow-up.
| Cognitive domain | Cognitive measure | Mean baseline (SD) | Range baseline | Mean follow-up (SD) | Range follow-up | p-Value |
|---|---|---|---|---|---|---|
| Verbal learning | RAVLT 1–5 | 57.10 (9.22) | 40–72 | 58.29 (11.29) | 30–75 | 0.069 |
| Verbal memory | RAVLT 6 | 11.70 (3.11) | 5–15 | 11.33 (3.81) | 1–15 | 0.917 |
| RAVLT 7 | 11.60 (3.60) | 2–15 | 12.33 (3.00) | 6–15 | 0.229 | |
| RAVLT recog. | 14.40 (1.05) | 11–15 | 14.33 (1.49) | 9–15 | 0.364 | |
| Attentional span | Digit span forwards | 7.48 (2.56) | 2–12 | 7.52 (2.04) | 3–12 | 0.930 |
| Processing speed | RCFT time | 240.38 (105.34) | 71–455 | 168.95 (63.89) | 70–280 | 0.002 |
| SDMT | 44.20 (11.15) | 24–59 | 48.52 (10.05) | 25–63 | <0.001 | |
| TMT-A | 27.95 (9.88) | 13–44 | 27.80 (9.25) | 16–50 | 0.662 | |
| Visuoconstruction | RCFT copy | 31.62 (4.23) | 18–36 | 30.43 (3.18) | 21.5–33 | 0.071 |
| Visual memory | RCFT recall | 20.12 (6.67) | 8.5–31 | 21.69 (6.38) | 7–34.5 | 0.590 |
| Executive functions | Digit span BW | 6.95 (2.33) | 4–11 | 7.48 (3.49) | 3–19 | 0.977 |
| Trail Making Test B (TMT-B) | 66.85 (23.44) | 34–120 | 64.69 (19.00) | 29–107 | 0.641 | |
| Phon. fluency | 13.81 (5.23) | 7–29 | 13.90 (5.22) | 3–26 | 0.610 | |
| Sem. fluency | 22.95 (5.58) | 10–37 | 24.14 (6.65) | 11–40 | 0.256 | |
Wilcoxon signed-rank tests comparing the baseline cognitive function with cognitive function at follow-up in N = 21 RRMS patients with Alemtuzumab treatment. Cognitive tests in bold show a significant performance increase at level α = 0.036 (Bonferroni correction for number of comparisons).
RAVLT, Rey auditory verbal learning test; digit span: digit span forward; RCFT, Rey Complex Figure Test; TMT-A, Trail Making Test part A; SDMT, Symbol Digit Modalities Test (written); Digit Span BW, Digit span backwards; TMT-B, Trail Making Test part B; Phon. Fluency, Phonematic verbal fluency with letter S (1 min.); Sem. fluency, Semantic verbal fluency with animals (1 min).
Figure 2.
Cognitive change in processing speed tasks [Symbol Digit Modalities Test (SDMT), Rey Complex Figure Test (RCFT) copy time] at baseline and follow-up. (A) Individual scores of patients at baseline and follow-up on the SDMT, *Significance on alpha-level 0.0036. (B) Proportion of patients who showed a clinically significant change of ≥4 points, 0–3 points, or did not improve on the SDMT. (C) Individual scores of patients at baseline and follow-up on the RCFT copy time measure, *Significance on alpha-level 0.0036. (D) Proportion of patients who improved by ≥40 s, 0–39 s, or did not improve on the RCFT copy time measure.
Predictors of Cognitive Change
Baseline Predictors of Cognitive Change
In order to test whether cognitive change could be predicted by clinical routine parameters at baseline, EDSS, number of T2 lesions, number of GELs, number of relapses, disease duration, and general intelligence were analyzed for their influence on the course of cognition by means of a multiple regression model. The dependent variable was the difference between the number of impaired tests at baseline and follow-up. The proportion of explained variance in this model was R2 = 0.27. None of the included predictors reached statistical significance (all ps > 0.05).
Clinical Change as Predictor of Cognitive Change
In order to explore whether individual cognitive change from baseline to follow-up was fully dependent on changes in clinical parameters, baseline-to-follow-up difference scores of EDSS, T2 lesion load, number of GEL, and relapse rate were again explored as potential predictors using a multiple regression model. The proportion of explained variance of the model was R2 = 0.10. None of the entered variables were significant predictors of cognitive change (ps > 0.05).
Discussion
Outcome under Alemtuzumab
The present study provides a comprehensive and systematic analysis of the early changes in cognition following alemtuzumab treatment in active RRMS. Alemtuzumab had a positive effect on disease activity, and at least stabilizing effects on cognition within the first 15 months of therapy in patients with active MS. During the treatment period, the median percentile rank of all cognitive measures showed a trend toward improvement. Additionally, the proportion of patients impaired in ≥3 tests was reduced from 24% at baseline to 14% at follow-up, even though this result did not reach statistical significance, possibly due to small sample size and low statistical power. Reduction of cognitive impairment was mainly driven by overall gains regarding information processing speed, which improved significantly. Our findings provide further evidence that highly potent DMDs can stabilize and possibly even reduce cognitive impairment in active RRMS. Effect-sizes for cognitive outcome measures from this study cannot be directly compared to those of other DMDs due to broadly varying methodology. Several previous studies have shown that cognitive impairment increased slightly or remained stable (i.e., the percentage of cognitively impaired patients slightly increased from 21.4 to 21.6%) over the course of 2 years under interferon treatment (33–35). Thus, effects on overall cognition under alemtuzumab observed in the present study may be larger and rather comparable with those of natalizumab seen in previous studies, one of which reports a similar reduction in the percentage of cognitively impaired patients from 29 to 19% after 1 year (16, 36).
In line with previous results, patients showed pronounced impairment in specific cognitive domains already at baseline, i.e., in processing speed, verbal and visual memory, visuoconstruction, executive functions, and attentional span. Interestingly, alemtuzumab had a pronounced significant positive effect on information processing speed, the primarily impaired neuropsychological domain in MS (7). At follow-up after 15 months, patients had improved significantly in both the SDMT and the RCFT time to copy condition when compared to baseline underlining the validity of a general and overarching improvement in processing speed tasks during the observational period. Processing speed, as commonly measured by the SDMT or Paced Auditory Serial Addition Task (37) (PASAT-3), also primarily improved under treatment with other DMDs (12, 14–16, 38). It may be concluded that significant improvement in information processing speed is the most essential and driving factor of DMD-related cognitive stabilization or improvement.
While previous studies evaluating cognitive change under DMDs faced the critique of lacking clinical relevance, the changes seen in the SDMT in our sample were clinically relevant in 12/21 patients according to the conventions proposed by Morrow et al. (32). The SDMT is the most frequently employed neuropsychological test for processing speed in MS, has been shown to predict employment status, and correlates with activities of daily living (39, 40). Even though our study sample mainly consisted of young, well-educated adults in employment, it represents a group of patients with active RRMS carrying high risks of cognitive decline. Generally, patients with RRMS show declines in cognitive functioning over the course of the disease, especially during the first 5 years after symptom onset (41, 42). Alemtuzumab, as a potent monoclonal antibody treatment, is administered to patients who show high disease activity despite first-line treatments or initially exhibit a highly active disease course, consequently carrying high lesion load and brain atrophy (43, 44). Previous literature shows that high lesion load in the long run and brain atrophy leaves patients more vulnerable to cognitive decline (43, 45). For patients with active RRMS, improvement in information processing speed, e.g., as measured by the SDMT, may be relevant to quality of life and employment status (39, 46, 47).
Predictors of Cognitive Change
Disease-modifying drugs may stabilize and even improve clinical as well as cognitive outcome measures in MS patients (9–16, 38, 48). Nevertheless, previous studies did not comprehensively analyze which factors at baseline can predict this stabilization and improvement. The present study aimed at exploring standard clinical baseline parameters as potential predictors of cognitive change; however, disease severity, duration of symptoms, number of relapses, T2 lesion load, GELs, and general intelligence at baseline could not predict overall cognitive changes under alemtuzumab treatment during the present short observational period of 15 months. Similarly, cognitive change was shown to be widely independent of clinical improvement during the study period by regression analyses (difference scores between baseline and follow-up of EDSS, relapse rate, T2 lesions, and number of GELs). Thus, the improvement of overall cognition cannot solely be explained by improvements on clinical parameters in our study sample.
Previous results regarding general associations between cognitive impairment and early disease severity markers in MS are contradictory. Disease severity and duration have been shown to only have a weak correlation with cognitive dysfunction in single studies (2). The EDSS may have issues with sensitivity of change and reliability (49). T2 lesion burden has previously shown to be less correlated with cognitive impairment than brain atrophy (50, 51). Volumetric analyses show promising associations with cognitive abilities in MS (52, 53). Particularly, global and deep gray matter atrophy or diffuse damage of gray and white matter seem more closely linked to progression of cognitive impairment in early MS (45, 54). Literature suggests a relationship between brain volume and measures of processing speed, i.e., SDMT and PASAT-3 score (52, 55, 56). For natalizumab, a protective role has been suggested for cortical gray matter volume during a course of 3 years that may be associated with a reduced rate of cognitive deterioration (13, 16). Similarly, the potent anti-inflammatory effect of alemtuzumab positively affects structural brain integrity parameters in patients with active RRMS. Alongside, outcome measures of reduced neuroinflammation, patients treated with alemtuzumab showed slowed brain volume loss (17, 19, 57). These effects on brain imaging parameters may be linked with improvement of cognition especially for processing speed, as this domain is closely associated with global brain atrophy (58). Automated structural volumetric imaging procedures, however, have not been established in clinical routine yet and were not evaluated in this study. The predictors tested in this study might not be suited for prognostic statements of positive or negative cognitive outcome under alemtuzumab. To conclude, cognitive change seems to be independent of the routine clinical parameters assessed under treatment with alemtuzumab and may thus be an underrepresented but potentially important outcome measure for treatment effects in future clinical trials.
Limitations
Some methodological limitations have to be considered when interpreting our results. The present study is limited by a small sample size and may have been underpowered to detect standard clinical and neuroimaging markers that predict cognitive change under alemtuzumab treatment. A second limitation is the absence of randomized control groups. A control group composed of clinically and cognitively matched MS patients receiving an alternative disease-modifying drug could account for non-treatment-related changes in cognition. Because the present monocentric study observed patients with highly active MS only, longitudinal recruitment of a control group showing comparable clinical and cognitive baseline characteristics was not feasible. Due to the high disease activity and lesion load, patients in the present sample were, however, at high risk for cognitive decline, especially within the first years of disease (41–43). Hence, treatment success may be deduced from stabilization of cognition under alemtuzumab without comparison to a control group. Future multicentric RCTs are warranted to confirm our results. Furthermore, future studies with longer observation periods are of interest to explore whether outcomes are maintained or whether further effects can be captured.
A neurologically healthy control group could account for practice effects after a similar time span to follow-up testing. Practice effects on neuropsychological tests are an issue, which has to be considered when testing repeatedly. This concern was handled through the administration of alternative test forms. Moreover, the testing at follow-up was conducted at an interval of 15 months after baseline testing, rendering our results robust to many previously reported practice effects. Especially the SDMT has been proven to be a highly sensitive measure, which is not prone to practice effects when retesting after 1 year (59). Regarding the SDMT, it has to be considered that the written form used in the present study may be dependent on motor function. However, none of the included patients reported or showed pronounced motor slowing of the hands in clinical examination.
Implications and Conclusion
In conclusion, the present study is the first to show positive or at least stabilizing early effects of alemtuzumab treatment on cognition. Alemtuzumab stabilized disease progression and improved overall cognitive functioning, specifically processing speed within the observational period of 15 months. Cognitive improvement seems to be partly independent of clinical change under alemtuzumab, further emphasizing the need to test for cognitive impairment in active MS as a potentially relevant and independent marker of disease activity. Research has shown that cognitive changes in MS patients may be present even when the concept of “no evidence of disease activity” is fulfilled on the basis of clinical and neuroimaging data (60).
In order to validate and extend the findings of positive effects of alemtuzumab on cognition in active RRMS, future studies need to examine larger sample sizes, implement randomized control groups, and include a long-term follow-up. Additionally, future research needs to consider more advanced clinical and neuroimaging baseline characteristics (e.g., global atrophy, deep gray matter atrophy, diffuse white matter damage, and functional network connectivity) to better predict cognitive change under DMDs.
Ethics Statement
This study was carried out in accordance with the recommendations of the local ethics committee (Aerztekammer Westfalen-Lippe) with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the local ethics committee (Aerztekammer Westfalen-Lippe).
Author Contributions
ER: drafting the manuscript, analysis and interpretation of data, statistical analyses, acquisition of data, and study coordination. SP and TR: revising manuscript for content and acquisition of data. HL: study concept and acquisition of data. HW: revising manuscript for medical writing. SM: study concept and revising the manuscript. AJ: study concept, revising the manuscript, study supervision, and supervision of statistical analyses.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Nick Fulcher for helpful comments on the manuscript and all the patients for their participation. This work was supported by the German Ministry of Education, Science, Research and Technology (01GI1603D, SM). This work was supported by “Alemtuzumab in autoimmune inflammatory neurodegeneration: mechanisms of action and neuroprotective potential” ALAIN study (2014-000709-10, SM).
Footnotes
Funding. No study-related funding. ER and AJ have no financial disclosures to report. SP received travel reimbursements and lecturing honoraria from Sanofi Genzyme and Biogen. TR received travel expenses and financial research support from Genzyme and Novartis and received honoraria for lecturing from Roche, Merck, Genzyme, Biogen, and Teva. HL received honoraria from Merck Serono GmbH for conducting neuropsychological assessments. HW receives honoraria for acting as a member of Scientific Advisory Boards and as consultant for Biogen, Evgen, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG, Sanofi-Genzyme, as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Sanofi-Genzyme, TEVA, and WebMD Global. HW is acting as a paid consultant for Abbvie, Actelion, Biogen, IGES, Novartis, Roche, Sanofi-Genzyme, and the Swiss Multiple Sclerosis Society. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children’s Foundation, Biogen GmbH, GlaxoSmithKline GmbH, Roche Pharma AG, Sanofi-Genzyme. SM has received honoraria for lecturing, travel expenses for attending meetings and financial research support from Almirall, Bayer Health Care, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, and Teva.
References
- 1.Trapp BD, Ransohoff RM, Fisher E, Rudick RA. Neurodegeneration in multiple sclerosis: relationship to neurological disability. Neuroscience (1999) 5:48–57. 10.1177/107385849900500107 [DOI] [Google Scholar]
- 2.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in mutliple sclerosis. I. Frequency, patterns, and prediction. Neurology (1991) 41:685–91. 10.1212/WNL.41.5.685 [DOI] [PubMed] [Google Scholar]
- 3.DeLuca GC, Yates RL, Beale H, Morrow SA. Cognitive impairment in multiple sclerosis: clinical, radiologic and pathologic insights. Brain Pathol (2015) 25:79–98. 10.1111/bpa.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol (2008) 7:1139–51. 10.1016/S1474-4422(08)70259-X [DOI] [PubMed] [Google Scholar]
- 5.Kurtzke JF. Rating neurologic impairment in multiple sclerosis : an expanded disability status scale (EDSS). Neurology (1983) 33:1444–52. 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 6.Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology (1991) 41:692–6. 10.1212/WNL.41.5.692 [DOI] [PubMed] [Google Scholar]
- 7.Deluca J, Chelune GJ, Tulsky DS, Lengenfelder J, Chiaravalloti ND. Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J Clin Exp Neuropsychol (2004) 26:550–62. 10.1080/13803390490496641 [DOI] [PubMed] [Google Scholar]
- 8.Nocentini U, Pasqualetti P, Bonavita S, Buccafusca M, De Caro MF, Farina D, et al. Cognitive dysfunction in patients with relapsing-remitting multiple sclerosis. Mult Scler (2006) 12:77–87. 10.1191/135248506ms1227oa [DOI] [PubMed] [Google Scholar]
- 9.Torkildsen O, Myhr KM, Bø L. Disease-modifying treatments for multiple sclerosis – a review of approved medications. Eur J Neurol (2016) 23:18–27. 10.1111/ene.12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer JS, Priore RL, Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, et al. Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Ann Neurol (2000) 48:885–92. [DOI] [PubMed] [Google Scholar]
- 11.Barak Y, Achiron A. Effect of interferon-beta-1b on cognitive functions in multiple sclerosis. Eur J Neurol (2002) 47:11–4. 10.1159/000047940 [DOI] [PubMed] [Google Scholar]
- 12.Penner IK, Stemper B, Calabrese P, Freedman MS, Polman CH, Edan G, et al. Effects of interferon beta-1b on cognitive performance in patients with a first event suggestive of multiple sclerosis. Mult Scler (2012) 18:1466–71. 10.1177/1352458512442438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portaccio E, Stromillo ML, Goretti B, Hakiki B, Giorgio A, Rossi F, et al. Natalizumab may reduce cognitive changes and brain atrophy rate in relapsing-remitting multiple sclerosis: a prospective, non-randomized pilot study. Eur J Neurol (2013) 20:986–90. 10.1111/j.1468-1331.2012.03882.x [DOI] [PubMed] [Google Scholar]
- 14.Benedict RHB, Cohan S, Lynch SG, Riester K, Wang P, Castro-Borrero W, et al. Improved cognitive outcomes in patients with relapsing–remitting multiple sclerosis treated with daclizumab beta: results from the DECIDE study. Mult Scler (2017). 10.1177/1352458517707345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstock-Guttman B, Galetta SL, Giovannoni G, Havrdova E, Hutchinson M, Kappos L, et al. Additional efficacy endpoints from pivotal natalizumab trials in relapsing-remitting MS. J Neurol (2012) 259:898–905. 10.1007/s00415-011-6275-7 [DOI] [PubMed] [Google Scholar]
- 16.Mattioli F, Stampatori C, Bellomi F, Scarpazza C, Capra R. Natalizumab significantly improves cognitive impairment over three years in MS: pattern of disability progression and preliminary MRI findings. PLoS One (2015) 10(7):e0131803. 10.1371/journal.pone.0131803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet (2012) 380:1819–28. 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]
- 18.CAMMS223 Trial Investigators. Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med (2008) 359:1786–801. 10.1056/NEJMoa0802670 [DOI] [PubMed] [Google Scholar]
- 19.Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet (2012) 380:1829–39. 10.1016/S0140-6736(12)61768-1 [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol (2011) 69:292–302. 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmstädter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest. Ein praktikables und differenziertes Instrumentarium zur Prüfung der verbalen Gedächtnisleistungen. Schweiz Arch Neurol Psychiatr (1990) 141:21–30. [PubMed] [Google Scholar]
- 22.Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch Psychol (1941) 28:21. [Google Scholar]
- 23.Meyers J, Meyers K. Rey Complex Figure Test and Recognition Trial: Professional Manual. Odessa: Psychological Assessment Resources; (1995). [Google Scholar]
- 24.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson: Neuropsychology Press; (1985). [Google Scholar]
- 25.Wechsler D. Wechsler Memory Scale – Revised Edition. Manual. New York: The Psychological Corporation; (1987). [Google Scholar]
- 26.Aschenbrenner A, Tucha O, Lange K. Regensburger Wortflüssigkeits-Test (RWT). Göttingen: Hogrefe; (2001). [Google Scholar]
- 27.Smith A. Symbol Digit Modalities Test (SDMT). Los Angeles, CA: Western Psychological Services; (1982). [Google Scholar]
- 28.Raven J. Progressive Matrices: A Perceptual Test of Intelligence. Individual Form. Oxford: Oxford Psychologists Press Ltd; (1938). [Google Scholar]
- 29.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry (1961) 4:561–71. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 30.Sacco R, Santangelo G, Stamenova S, Bisecco A, Bonavita S, Lavorgna L, et al. Psychometric properties and validity of Beck depression inventory II in multiple sclerosis. Eur J Neurol (2016) 23:744–50. 10.1111/ene.12932 [DOI] [PubMed] [Google Scholar]
- 31.Flachenecker P, König H, Meissner H, Müller GRP. Fatigue in multiple sclerosis: validation of the WEIMuS scale (“Würzburger Erschöpfungs-Inventar bei Multipler Sklerose”). Neurol und Rehabil (2008) 14:299–306. 10.1007/s00115-005-1990-x [DOI] [Google Scholar]
- 32.Morrow SA, Drake A, Zivadinov R, Munschauer F, Weinstock-Guttman B, Benedict RH. Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. Clin Neuropsychol (2010) 24:1131–45. 10.1080/13854046.2010.511272 [DOI] [PubMed] [Google Scholar]
- 33.Patti F, Amato MP, Bastianello S, Caniatti L, Di Monte E, Lijoi F, et al. Subcutaneous interferon beta-1a has a positive effect on cognitive performance in mildly disabled patients with relapsing-remitting multiple sclerosis: 2-year results from the COGIMUS study. Ther Adv Neurol Disord (2009) 2:67–77. 10.1177/1756285608101379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patti F, Amato MP, Bastianello S, Caniatti L, Di Monte E, Ferrazza P, et al. Effects of immunomodulatory treatment with subcutaneous interferon beta-1 a on cognitive decline in mildly disabled patients with relapsing–remitting multiple sclerosis. Mult Scler (2010) 16:68–77. 10.1177/1352458509350309 [DOI] [PubMed] [Google Scholar]
- 35.Lacy M, Hauser M, Pliskin N, Assuras S, Valentine MO, Reder A. The effects of long-term interferon-beta-1b treatment on cognitive functioning in multiple sclerosis: a 16-year longitudinal study. Mult Scler (2013) 19:1765–72. 10.1177/1352458513485981 [DOI] [PubMed] [Google Scholar]
- 36.Iaffaldano P, Viterbo RG, Paolicelli D, Lucchese G, Portaccio E, Goretti B, et al. Impact of Natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis : a prospective, open-label, two years observational study. PLoS One (2012) 7:1–7. 10.1371/journal.pone.0035843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gronwall D. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills (1977) 44:367–73. 10.2466/pms.1977.44.2.367 [DOI] [PubMed] [Google Scholar]
- 38.Kappos L, Radue EW, Chin P, Ritter S, Tomic D, Lublin F. Onset of clinical and MRI efficacy occurs early after fingolimod treatment initiation in relapsing multiple sclerosis. J Neurol (2016) 263:354–60. 10.1007/s00415-015-7978-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler (2017) 23:721–33. 10.1177/1352458517690821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strober LB, Christodoulou C, Benedict RH, Westervelt HJ, Melville P, Scherl WF, et al. Unemployment in multiple sclerosis: the contribution of personality and disease. Mult Scler (2012) 18:647–53. 10.1177/1352458511426735 [DOI] [PubMed] [Google Scholar]
- 41.Reuter F, Zaaraoui W, Crespy L, Faivre A, Rico A, Malikova I, et al. Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. J Neurol Neurosurg Psychiatry (2011) 82:1157–9. 10.1136/jnnp.2010.213744 [DOI] [PubMed] [Google Scholar]
- 42.Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci (2006) 245:41–6. 10.1016/j.jns.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 43.Dubey D, Cano C, Stuve O. Intractable and highly active relapsing multiple sclerosis – role of alemtuzumab. Neuropsychiatr Dis Treat (2015) 11:2405–14. 10.2147/NDT.S90473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fazekas F. Fingolimod in the treatment algorithm of relapsing remitting multiple sclerosis: a statement of the Central and East European (CEE) MS Expert Group. Wien Med Wochenschr (2012) 162:354–66. 10.1007/s10354-012-0123-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filippi M, Rocca MA, Benedict RH, DeLuca J, Geurts JJ, Rombouts SA, et al. The contribution of MRI in assessing cognitive impairment in multiple sclerosis. Neurology (2010) 75:2121–8. 10.1212/WNL.0b013e318200d768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker-Collo S. Quality of life in multiple sclerosis: does information-processing speed have an independent effect? Arch Clin Neuropsychol (2006) 21:167–74. 10.1016/j.acn.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 47.Strober L, Chiaravalloti N, Moore N, DeLuca J. Unemployment in multiple sclerosis (MS): utility of the MS Functional Composite and cognitive testing. Mult Scler (2014) 20:112–5. 10.1177/1352458513488235 [DOI] [PubMed] [Google Scholar]
- 48.Comi G. Effects of disease modifying treatments on cognitive dysfunction in multiple sclerosis. Neurol Sci (2010) 31:261–4. 10.1007/s10072-010-0436-9 [DOI] [PubMed] [Google Scholar]
- 49.Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol (2014) 14:58. 10.1186/1471-2377-14-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis. Arch Neurol (2016) 61:226–30. 10.1001/archneur.61.2.226 [DOI] [PubMed] [Google Scholar]
- 51.Christodoulou C, Krupp LB, Liang Z, Huang W, Melville P, Roque C, et al. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology (2003) 60:1793–8. 10.1212/01.WNL.0000072264.75989.B8 [DOI] [PubMed] [Google Scholar]
- 52.Amato MP, Portaccio E, Goretti B, Zipoli V, Battaglini M, Bartolozzi ML, et al. Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol (2007) 64:1157. 10.1001/archneur.64.8.1157 [DOI] [PubMed] [Google Scholar]
- 53.Zivadinov R, Sepcic J, Nasuelli D, De Masi R, Bragadin LM, Tommasi MA, et al. A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry (2001) 70:773–80. 10.1136/jnnp.70.6.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deppe M, Krämer J, Tenberge JG, Marinell J, Schwindt W, Deppe K, et al. Early silent microstructural degeneration and atrophy of the thalamocortical network in multiple sclerosis. Hum Brain Mapp (2016) 37:1866–79. 10.1002/hbm.23144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vollmer T, Huynh L, Kelley C, et al. Relationship between brain volume loss and cognitive outcomes among patients with multiple sclerosis: a systematic literature review. (2016) 37(2):165–79. 10.1007/s10072-015-2400-1 [DOI] [PubMed] [Google Scholar]
- 56.Batista S, Zivadinov R, Hoogs M, Bergsland N, Heininen-Brown M, Dwyer MG, et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol (2012) 259:139–46. 10.1007/s00415-011-6147-1 [DOI] [PubMed] [Google Scholar]
- 57.Arnold DL, Fisher E, Brinar VV, Cohen JA, Coles AJ, Giovannoni G, et al. Superior MRI outcomes with alemtuzumab compared with subcutaneous interferon β-1a in MS. Neurology (2016) 87:1464–72. 10.1212/WNL.0000000000003169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deloire MS, Ruet A, Hamel D, Bonnet M, Dousset V, Brochet B. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology (2011) 76:1161–7. 10.1212/WNL.0b013e318212a8be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glanz BI, Healy BC, Hviid LE, Chitnis T, Weiner HL. Cognitive deterioration in patients with early multiple sclerosis: a 5-year study. J Neurol Neurosurg Psychiatry (2012) 83:38–43. 10.1136/jnnp.2010.237834 [DOI] [PubMed] [Google Scholar]
- 60.Damasceno A, Damasceno BP, Cendes F. No evidence of disease activity in multiple sclerosis: implications on cognition and brain atrophy. Mult Scler (2016) 22:64–72. 10.1177/1352458515604383 [DOI] [PubMed] [Google Scholar]