Abstract
Immunoassays are antibody-based analytical methods for quantitative/qualitative analysis. Since the principle of immunoassays is based on specific antigen–antibody reaction, the assays have been utilized worldwide for diagnosis, pharmacokinetic studies by drug monitoring, and the quality control of commercially available products. Berson and Yalow were the first to develop an immunoassay, known as radioimmunoassay (RIA), for detecting endogenous plasma insulin [1], a development for which Yalow was awarded the Nobel Prize in Physiology or Medicine in 1977. Even today, after half a century, immunoassays are widely utilized with some modifications from the originally proposed system, e.g., radioisotopes have been replaced with enzymes because of safety concerns regarding the use of radioactivity, which is referred to as enzyme immunoassay/enzyme-linked immunosorbent assay (ELISA). In addition, progress has been made in ELISA with the recent advances in recombinant DNA technology, leading to increase in the range of antibodies, probes, and even systems. This review article describes ELISA and its applications for the detection of plant secondary metabolites.
Keywords: Antibodies, Enzyme-linked immunosorbent assay (ELISA), Hapten, Plant secondary metabolites
Introduction
Since the development of radioimmunoassay (RIA) in 1960, there has been a rapid increase in immunoassay techniques using radioactive labels [1]. However, radioactive labels have been gradually replaced with enzyme labels because of safety concerns associated with radioactivity since the study by Avrameas in 1969, who coupled antigens or antibodies and enzymes using glutaraldehyde [2]. Currently, ELISA has a higher number of immunoassays compared to RIA.
Plant secondary metabolites are plant-produced organic compounds that play an important role in the defense of plants against herbivores, pests, and pathogens, as well as in their adaptation to the environment, although they are not directly involved in the growth and development of organisms [3, 4]. Because of their diverse functions, there has been a dramatic increase in their demand in pharmaceuticals, cosmetics, and pesticides, as well as in food additives [5]. Quality control of these commercial products containing secondary metabolites is crucial as the quality directly affects their potential activity. In addition, Cragg and Newman recently reported that 34% of the currently used drugs originate from natural products [6]. Meanwhile, simple, selective, and sensitive analytical techniques are also required in pharmacodynamic studies for monitoring effective concentration, side effects, and metabolism, leading to a better quality of life for patients. Thus far, various analytical methods have been developed for such purposes, mainly based on high-performance liquid chromatography (HPLC). However, ELISA exhibits several advantages over such techniques because of its simplicity, selectivity, and sensitivity.
The basic facts about ELISA and its practical use for measuring plant secondary metabolites are described in this review.
General principle of ELISA
ELISA is based on the concept of antigen–antibody reactions, representing the chemical interaction between antibodies produced by the B cells of leukocytes and antigens. This specific immune response plays an important role in protecting the body from invaders such as pathogens and toxins. Hence, by exploiting this reaction, ELISA permits the highly sensitive and selective quantitative/qualitative analysis of antigens, including proteins, peptides, nucleic acids, hormones, herbicides, and plant secondary metabolites. To detect these molecules, an antigen or antibody is labeled using enzymes, the so-called enzyme immunoassay, in which alkaline phosphatase (ALP) [7], horseradish peroxidase (HRP) [8], and β-galactosidase [9–11] are commonly used. The antigen in the fluid phase is immobilized on a solid phase, such as a microtiter plate constituting rigid polystyrene, polyvinyl chloride, and polypropylene. Subsequently, the antigen is allowed to react with a specific antibody, which is detected by an enzyme-labeled secondary antibody. The development of color using a chromogenic substrate corresponds to the presence of the antigen. For instance, ALP hydrolyzes p-nitrophenyl phosphate to produce p-nitrophenol, which can be detected at 405 nm (yellow color), and HRP catalyzes the conversion of chromogenic substrates, e.g., 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, 3,3′,5,5′-tetramethylbenzidine, and o-phenylenediamine into colored products. By using chemiluminescent substrates such as chloro-5-substituted adamantyl-1,2-dioxetane phosphate and luminol for ALP and HRP, respectively, and fluorogenic substrates such as 4-methylumbelliferyl galactoside and nitrophenyl galactoside for β-galactosidase, even more sensitive detection can be achieved. These enzyme–substrate reactions are typically completed within 30–60 min, and the reaction stops with the addition of an appropriate solution, e.g., sodium hydroxide, hydrochloric acid, sulfuric acid, sodium carbonate, and sodium azide, for individual reactions [12, 13]. Finally, colored or fluorescent products are detected using a microtiter plate reader.
Advantages and disadvantages of ELISA
Advantages and disadvantages of ELISA are summarized in Table 1. ELISA exhibits the following advantages: (i) Simple procedure. (ii) High specificity and sensitivity, because of an antigen–antibody reaction. (iii) High efficiency, as simultaneous analyses can be performed without complicated sample pre-treatment. (iv) Generally safe and eco-friendly, because radioactive substances and large amounts of organic solvents are not required. (v) Cost-effective assay, as low-cost reagents are used. However, ELISA exhibits the following disadvantages: (i) Labor-intensive and expensive to prepare antibody because it is a sophisticated technique, and expensive culture cell media are required to obtain a specific antibody. (ii) High possibility of false positive or negative results because of insufficient blocking of the surface of microtiter plate immobilized with antigen. (iii) Antibody instability because an antibody is a protein that requires refrigerated transport and storage.
Table 1.
Advantages and disadvantages of ELISA
| Advantages | Disadvantages |
|---|---|
| Simple procedure | Labor-intensive and expensive to prepare antibody |
| Easy to perform with simple procedure | Sophisticated techniques and expensive culture media are required |
| High specificity and sensitivity | High possibility of false positive/negative |
| ELISA is based on antigen–antibody reaction | Insufficient blocking of immobilized antigen results in false results |
| High efficiency | Antibody instability |
| Simultaneous analysis can be performed without complicated sample pre-treatment | Refrigerated transport and storage are required as an antibody is a protein |
| Generally safe and eco-friendly | |
| Radioactive substances and large amounts of organic solvent are not required | |
| Cost-effective assay | |
| Reagents are relatively low cost |
Types of ELISA
Direct ELISA
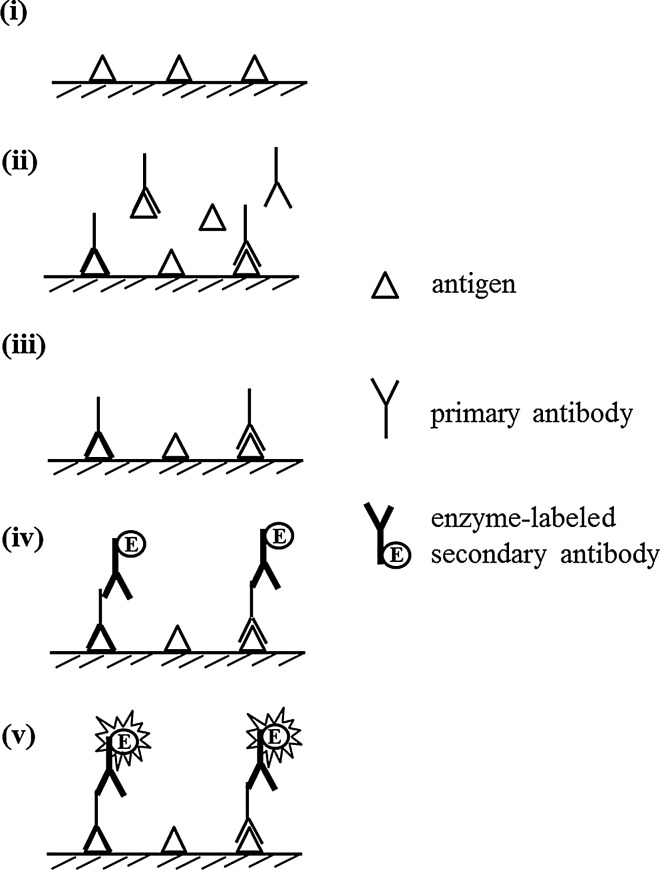
In 1971, Engvall and Perlmann [14] and Van Weemen and Schuurs [15] were the first to develop direct ELISA (Fig. 1), which was the base style for other types of ELISA. Primarily, an antigen or an antibody is immobilized on the surface of microtiter plate. After the surface is blocked with other proteins (e.g., albumin, gelatin, casein, and skimmed-milk [13]) to avoid the non-specific adsorption of other proteins, the corresponding enzyme-labeled antibody or antigen is allowed to react with the immobilized targets, followed by color development with appropriate substrates. With an increasing amount of targets, the signal increases. Direct ELISA is suitable for the qualitative analysis of macromolecules.
Fig. 1.
Direct ELISA to detect antigen (a) and antibody (b). (i) Attach antigen/antibody to solid phase. (ii) Incubate with enzyme-labeled antibody/antigen. (iii) Wash unbound enzyme-labeled antibody/antigen out. (iv) Develop color with substrate
Competitive ELISA
In 1973, Belanger developed competitive ELISA (Fig. 2) to detect rat α-fetoprotein, which involved the development of indirect ELISA and sandwich ELISA [16]. The key event of competitive ELISA is the competitive reaction between targets (antigen or antibody) in the sample and enzyme-labeled targets (antigen or antibody) against corresponding immobilized antibody or antigen. To detect the antigen in competitive ELISA, an enzyme-labeled antigen is used to compete with the target antigens against the immobilized antibody (Fig. 2b). Hence, the higher the amount of antigen in the sample, the lower the amount of enzyme-labeled antigen that binds to the antibody. That is, with an increasing amount of target antigen, the signal decreases. In this case, competitive ELISA is suitable for measuring macromolecules only because a labeling enzyme is required to measure the antigen. If the antigen is a low molecular weight compound (e.g., hapten), resultant hapten–enzyme conjugates are not recognized by the immobilized antibody, leading to failure of the analysis. To detect the antibody, the antigen is immobilized, and the competition between the antibody in the sample and enzyme-labeled antibody is observed (Fig. 2a). In this case, both macromolecules and hapten can be detected when hapten is exposed on the surface of the microtiter plate.
Fig. 2.
Competitive ELISA to detect antigen (a) and antibody (b). (i) Attach antigen/antibody to solid phase. (ii) Incubate antibody/antigen with enzyme-labeled antibody/antigen. (iii) Wash unbound enzyme-labeled antibody/antigen out. (iv) Develop color with substrate
Furthermore, detectable targets (antigen or antibody) can be changed depending on the competitors. When free antigen is used as competitor instead of unlabeled antibody in Fig. 2a, competitive reaction between free antigen and immobilized antigen against enzyme-labeled antibody can be observed, enabling the detection of free antigen (macromolecules and hapten) in the sample in this competitive system, and vice versa when free antibody is used instead of unlabeled antigen in Fig. 2b.
Direct and competitive ELISA methods are simple because only one antibody is required. However, the labeling step is required for each of the ELISA methods, possibly leading to inactivation of the antibody (Table 2).
Table 2.
Characteristics of various types of ELISA
| Direct ELISA | Competitive ELISA | Indirect ELISA | Indirect competitive ELISA | Sandwich ELISA | |
|---|---|---|---|---|---|
| Advantage | Simple because only one antibody is used | Higher sensitivity and versatility than direct methods owing to usage of PAb that recognizes different epitopes of primary antibody | High specificity as two antibodies possessing different epitopes are used | ||
| Disadvantage | Labeling antibody is necessary for each ELISA, which may result in inactivation of antibody | Nonspecific signal is induced through cross-reactivity of secondary antibody | To prepare two different antibodies is labor-intensive and expensive | ||
| Target | Macromolecules | Macromolecules (Hapten) | Macromolecules | Macromolecules (Hapten) | Generally macromolecules |
| Signal (as target antigen increase) | Increase | Decrease | Increase | Decrease | Increase |
Indirect ELISA
Indirect ELISA systems have been developed on the basis of direct ELISA to evaluate the presence of antibody in antisera (Fig. 3) [17, 18]. The key step of this system is the two-binding process of the primary antibody and enzyme-labeled secondary antibody. That is, the target antigen is indirectly detected by the secondary antibody, which is labeled with the enzyme, or the so-called indirect ELISA. The antigen is primarily immobilized on the surface of the microtiter plate, which blocks the surface with blocking proteins as mentioned above. The primary antibody (in antisera) binding to the immobilized antigen is then allowed to react with the enzyme-labeled secondary antibody, followed by the development of color. The signal increases with an increasing amount of the immobilized target antigen. Indirect ELISA is suitable for measuring macromolecules. With the use of antisera as the primary antibody, the presence of a disease-associated antibody in the antisera can be evaluated; thus, indirect ELISA is effectively used to diagnose endocrine diseases [19, 20].
Fig. 3.
Indirect ELISA to analyze antibody. (i) Attach antigen to solid phase. (ii) Incubate with primary antibody. (iii) Wash unbound primary antibody out. (iv) Incubate with enzyme-labeled secondary antibody. (v) Develop color with substrate
Indirect competitive ELISA
Indirect competitive ELISA (icELISA) involves the combination of indirect ELISA and competitive ELISA (Fig. 4). The target antigen is immobilized on a solid phase of the microtiter plate and is blocked. Subsequently, free target antigen and antibody are allowed to incubate and there is a competition between the immobilized antigen and free antigen against antibodies. The primary antibody that binds to the immobilized antigen is detected by the enzyme-labeled secondary antibody. Similar to the case in competitive ELISA, in icELISA, the signal decreases with increasing amount of the free antigen. icELISA can be applied for measuring both the macromolecules and hapten when hapten is exposed on the surface of the microtiter plate.
Fig. 4.
Indirect competitive ELISA to detect antigen. (i) Attach antigen to solid phase. (ii) Incubate free target antigen with primary antibody. (iii) Wash unbound free target antigen and primary antibody out. (iv) Incubate with enzyme-labeled secondary antibody. (v) Develop color with substrate
The use of enzyme-labeled secondary antibodies in indirect methods (e.g., indirect ELISA and icELISA) exhibit advantages over direct methods (direct and competitive ELISA) with respect to sensitivity and versatility [16]. Polyclonal antibody is a type of enzyme-labeled secondary antibody that recognizes different epitopes of the primary antibody, leading to increased sensitivity as compared to direct methods. In addition, a universal secondary antibody can be used if the original animal species of the primary antibody are unified. Thus, the secondary antibody is commercially available, leading to high versatility. Indirect ELISA clearly exhibits disadvantages with respect to the secondary antibody, i.e., the cross-reaction of the secondary antibody should be considered (Table 2).
Sandwich ELISA
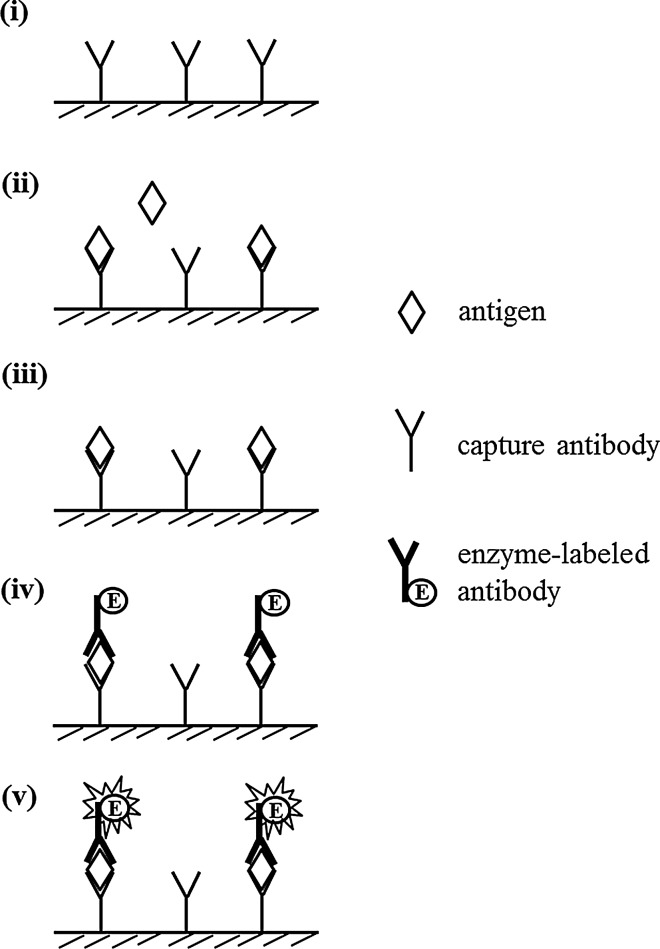
In this system, the target antigen is detected via anchoring between two antibodies, which recognize different epitopes, or the so-called sandwich system (Fig. 5) [16]. Sandwich ELISA starts from the immobilization of an antibody, called a capture antibody, on the microtiter plate. After blocking the plate surface to avoid non-specific adsorption of other proteins, the antigen in the sample is allowed to react with the immobilized capture antibody, and the antigen bound to the capture antibody is then sandwiched with an enzyme-labeled antibody for color development. This direct system can be modified to the indirect system by using primary and enzyme-labeled secondary antibodies. The signal increases with increasing amount of antigen. As two antibodies containing different epitopes are required against the target antigen, sandwich ELISA is generally suitable for measuring macromolecules with some exceptions. Ciguatoxins, which are produced in the marine dinoflagellate Gambierdiscus toxicus, are the major causative toxins of ciguatera seafood poisoning. Ciguatoxins are structurally classified as ladder-like polyethers with a molecular weight of 1111 Da. Oguri et al. divided these polyethers into two parts and prepared different monoclonal antibodies (MAbs) to individually recognize each part for constructing a sandwich ELISA system to measure ciguatoxins [21]. More recently, Boscolo et al. reported a sandwich ELISA method for marine biotoxins, e.g., palytoxins with a molecular weight of 2680 Da [22]. The sandwich was formed by using two antibodies obtained from the same antigen with different antibodies, i.e., MAb and PAb, which were used as the capture and primary antibodies, respectively.
Fig. 5.
Sandwich ELISA for specific detection of antigen. (i) Attach capture antibody to solid phase. (ii) Incubate with target antigen. (iii) Wash unbound target out. (iv) Incubate with enzyme-labeled antibody. (v) Develop color with substrate
A highly specific assay can be obtained via a sandwich system because of the use of two antibodies. However, it is an expensive and labor-intensive process to prepare two antibodies. In addition, one more step is required in the sandwich system because immobilization is necessary for capture antibody, which increases the assay time (Table 2).
Open sandwich ELISA (OS-ELISA)
Advances in DNA technology have enabled the development of unique and interesting immunoassays based on the interaction of variable regions of heavy (VH) and light (VL) chains, which are binding regions for antigens [23]. In the presence of an antigen, the interaction between VH and VL regions is enhanced to form a ternary complex. In the aforementioned report, OS-ELISA started from coating of a solid-phase microtiter plate with streptavidin. After blocking, the VL region conjugated with biotin was allowed to react with streptavidin to immobilize the VL region. In the next process, the phage-displayed VH region was incubated with hen egg lysozyme, which was used as an antigen. Finally, the phage-displayed VH regions forming a ternary complex were detected by the HRP-labeled antibody to develop color. The obtained signal increases with increasing amount of antigen. Currently, this OS-ELISA has been modified to be more easy and effective, and several studies on OS-ELISA for measuring both the macromolecule and hapten have been reported [24–27].
Types of antibody
In ELISA, any antibody can be used. In the first report of immunoassay developed by Berson and Yalow, PAb present in the antisera of immunized guinea pigs was used to detect human insulin [1]. However, issues related to the specificity of different batches were particularly concerning until the development of the MAb technology by Köhler and Milstein in 1975 [28]. In 1984, together with Niels Kaj Jerne, they were awarded the Nobel Prize in Physiology or Medicine. The emergence of MAb has helped in overcoming the issues with PAb. Since then, advances in DNA technology have enabled the production of recombinant antibodies, which include single-chain variable fragment (scFv) antibody, bispecific Bis-scFv, fragment antigen-binding (Fab) antibody, bispecific Fab2, trispecific Fab3, bivalent minibody, and multibody (diabody, triabody, and tetrabody) [29]. All of the aforementioned recombinant antibodies can be applied to ELISA, although the corresponding secondary antibodies need to be prepared.
ELISA for plant secondary metabolites
Specificity of antibody against hapten
Most of the useful plant secondary metabolites are low molecular weight compounds (i.e., hapten) with immense structural diversity, which are generally classified on the basis of their biosynthesis pathway [30, 31]. Hence, ELISA used for their analysis is the competitive type (competitive ELISA or icELISA) using MAb or PAb. When MAb is compared with PAb against hapten, MAb tends to exhibit higher specificity because PAb recognizes several epitopes, while MAb recognizes only one epitope. In addition, hybridoma cells secreting MAb exhibiting desirable characteristics can be screened. Pongkitwitoon et al. have prepared PAb against bioactive isoflavonoids, daidzin (DZ), by immunizing rabbits with DZ–bovine serum albumin (BSA) conjugates to develop icELISA [32]. By comparing the cross-reactivity (CR), which is the factor of specificity calculated by the ratio of IC50 for DZ to that for the test compounds, of PAb with that of MAb obtained from the same DZ–BSA conjugates [33], the specificity of MAb to DZ was greater than that of PAb (Table 3).
Table 3.
Chemical structures of representative isoflavonoids and cross-reactivities (CRs) of PAb [32], MAb [33] produced from DZ–BSA conjugates prepared by NaIO4 oxidation method, and MAb [39] produced from DZ–cBSA conjugates obtained by Mannich reaction
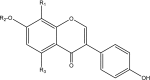
| Isoflavonoids | R1 | R2 | R3 |
|---|---|---|---|
| Daidzin (DZ) | H | Glc– | H |
| Daidzein | H | H | H |
| Genistin | H | Glc– | OH |
| Genistein | H | H | OH |
| Puerarin | Glc– | H | H |
Apart from the types of antibodies, the design of hapten-carrier proteins considerably affects the specificity of the resultant antibody. The sodium periodate (NaIO4) oxidation method is the typical method for preparing the hapten-carrier protein conjugates for glycosides, which involves the oxidative cleavage of vicinal 1,2-diols of the sugar moieties to form imides with the amino group of lysine residues in the carrier proteins. Therefore, several anti-glycoside antibodies are prepared by the conjugates obtained from the NaIO4 oxidation method, which include paeoniflorin [34], solamargine [35], bacopaside I [36], saikosaponin a [37], liquiritin [38], and DZ [32, 33]. However, they tend to exhibit broad CR, especially with compounds containing similar aglycone parts. To obtain an MAb specific to DZ, Yusakul et al. recently designed hapten-carrier protein conjugates using the Mannich reaction, leading to the production of highly specific MAb to DZ (Table 3) [39]. Kitisripanya et al. investigated the effect of difference between the conjugates prepared via the NaIO4 oxidation method and the Mannich reaction on the specificity of the resultant PAb against miroestrol, which is a strong estrogenic compound produced in Pueraria candollei [40]. The PAb obtained from the hapten conjugate derived from the Mannich reaction exhibits higher specificity to miroesterol than that of the PAb obtained via the NaIO4 oxidation method, suggesting that the Mannich reaction is an important reaction for obtaining specific anti-hapten antibodies.
In addition to the method to prepare hapten-carrier protein conjugates, the number of hapten molecules bound to carrier proteins affects the specificity of antibodies. The hapten numbers are typically evaluated via matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF–MS) using sinapinic acid as the matrix [41]. The relationship between the number of hapten molecules and antibody specificity has been investigated using a mercaptopropionic acid derivative of atrazine: high antibody titers with moderate antibody specificity are induced from 15–30 hapten molecules per carrier protein, while a lower number of hapten molecules exhibits a slower immune response with higher specificity [42]. This observation was also supported by the PAb against miroesterol reported by Kitisripanya et al. [40]. Recently, MAbs against the Cephalotaxus alkaloid harringtonine and pyrrolizidine alkaloid monocrotaline have been independently produced from their BSA conjugates using the NaIO4- and N,N′-carbonyldiimidazole-mediated methods, respectively, for their determination in plants. Both of the resultant MAbs exhibit extremely high specificity to both targets with high sensitivity, although only two hapten molecules are bound to BSA [43, 44].
Utilization of antibody in icELISA depending on specificity
Antibodies exhibiting broad CR sometimes act as a useful and effective tool for recognizing a bioactive skeleton or a group of bioactive compounds because of the simultaneous determination by icELISA using the antibodies. Ginsenosides are the major compounds produced in ginseng and are classified into two groups according to their structure: 20(S)-protopanaxadiol and 20(S)-protopanaxatriol [45]. As they are considered as active compounds that exert various pharmacological activities of ginseng, such as tonic, immunomodulatory, antimutagenic, and anti-aging activities, they are focused as a target for quantitative/qualitative analysis in ELISA. With respect to 20(S)-protopanaxadiol, MAbs against G-Rb1 [46] and Rg3 [47] have been produced, while those against G-Re [48], G-Rg1 [49], and Rh1 and Rg2 [50] have been produced as representatives of 20(S)-protopanaxatriol for their specific determination. Interestingly, Morinaga et al. have produced MAb against G-Re exhibiting broad CR with G-Rd (76.2%) and G-Rg1 (70.9%) in addition to G-Re (100%) itself, enabling the development of icELISA for the simultaneous determination of the total ginsenosides in plant samples (Table 4) [51, 52].
Table 4.
Chemical structures of representative ginsenosides and cross-reactivities (CRs) of MAb 4G10 and scFv used for simultaneous determination of total ginsenosides in plant samples by icELISA [51, 52, 62]
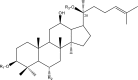
| Ginsenosides | R1 | R2 | R3 |
|---|---|---|---|
| Protopanaxatriol | |||
| G-Re | H | Rha1–2Glc–O– | Glc– |
| G-Rg1 | H | Glc–O– | Glc– |
| Protopanaxadiol | |||
| G-Rb1 | Glc1–2Glc– | H | Glc1–6Glc– |
| G-Rc | Glc1–2Glc– | H | Ara(f)1–6Glc– |
| G-Rd | Glc1–2Glc– | H | Glc– |
Sandwich ELISA
Sandwich ELISA has been widely accepted to exhibit higher specificity and wider working range as compared to the other types of ELISA. However, it is difficult to prepare two antibodies possessing different epitopes, especially for haptens because steric hindrance may disturb the antigen–antibody reaction because of the small size of the haptens. Therefore, true sandwich assays for hapten can be rarely developed, except for tacrolimus [53], angiotensin II [54], and naringin (Nar; which is a major flavonoid glycoside found in citrus fruits) [55]. Among these haptens, Nar is the smallest hapten with a molecular weight of 580 Da and the only plant secondary metabolite. Two hybridoma cell lines secreting different antibodies have been carefully screened, which enabled construction of a sandwich for Nar [55]. Interestingly, specificity to Nar in sandwich ELISA using two MAbs dramatically increases as compared with that in icELISA using a single MAb.
ELISA using a recombinant antibody
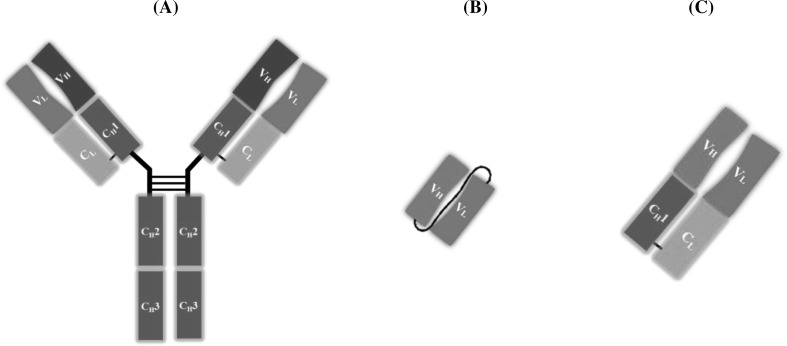
DNA recombinant technology has enabled the production of antibodies in Escherichia coli [56] and other organisms [57–60]; currently, recombinant antibodies (rAbs) have been reported to exhibit several advantages over conventional MAb and PAb in terms of production speed, the ability to modify properties through mutagenesis, and information on antibody–target interaction. Among rAbs, scFv and antigen-binding fragment (Fab) of an antibody are structurally independent units containing antigen-binding sites (Fig. 6). scFv consists of VH and VL chains with a flexible peptide linker of Gly and Ser, where the C-terminus of VH is linked to the N-terminus of VL and vice versa. Thus, their size decreases approximately to one-sixth of the original parental IgG molecule. Fab consists of a two-binding arm containing VH and VL chains, in addition to the constant regions of heavy (CH1) and light (CL) chains. They have become popular as a probe for ELISA because the original affinity and specificity of the original IgG molecule are maintained (Table 4).
Fig. 6.
Schematic diagram of representative antibodies, IgG molecule (a), single chain variable fragment (scFv) antibody (b), and antigen-binding fragment (Fab) (c)
A secondary antibody is required to detect rAbs in icELISA. The Fc region of immunoglobulin (MAb/PAb) is typically used as the epitope of secondary antibody for high versatility, while tags such as poly His-tag, T7-tag, and E-tag are commonly used as epitopes of secondary antibodies for rAb because they can be genetically incorporated into genes without disturbing the tertiary structure and activity of the rAb. Thus far, various scFvs against plant secondary metabolites have been constructed and expressed in E. coli to develop icELISA, including plumbagin [61], G-Re [62], DZ [63], wogonin glucuronide [64], and paclitaxel [65]. Similarly, Fab-based icELISA has been reported for artemisinin, which is produced from traditional Chinese herbal medicines, e.g., Artemisia annua L. and wogonin glucuronide, for their determination [66, 67]. They can be genetically engineered; therefore, fluorescent single-domain antibodies (fluobodies), chimera proteins of a green fluorescent protein (GFP), and an scFv also have been utilized in immunoassays. This combination always results in a 1:1 ratio between the fluorochrome and scFv, which overcomes the disadvantage of direct methods in immunoassays, i.e., deactivation of the antibodies with labeling enzymes. Furthermore, immunoassays using fluobodies enabled skipping of the time-consuming secondary antibody step with high sensitivity. Some studies have focused on these useful fluobodies to develop rapid and sensitive fluorescent-linked immunosorbent assays (FLISA) for plant secondary metabolites, including plumbagin [68] and G-Re [69]. In these reports, the fluobodies fusing scFv at the C-terminus of GFP were found to exhibit better affinity and sensitivity than those fusing at the N-terminus of GFP.
Conclusion
To date, various methods for the quantitative or qualitative analysis of plant secondary metabolites have been developed because a lot of marketed drugs are generated from plant secondary metabolites, such as morphine (analgesic drug), vinblastine (antineoplastic drug), paclitaxel (antineoplastic drug), quinine (antimalarial drug), digitoxin (cardiotonic drug), and so on, and the accurate, sensitive, and selective evaluation of these drugs leads to safe clinical and general usages.
In this review, ELISA has been discussed in detail; it is representative of various analytical methods because of its several advantages over other analytical methods in terms of simplicity, cost efficiency, and selectivity. However, all types of ELISA exhibit more or less advantages and disadvantages. A barrier for further development of ELISA is the preparation of specific antibodies against the target hapten. Even in this advanced era, there are many important plant secondary metabolites for which antibodies are not available. ELISA would be more familiar to us if the antibody or antibody-mimicking probes that are alternatively used in ELISA could be obtained more easily.
Acknowledgement
This work was funded by a Grant-in-Aid for Young Scientists (B) [17K15466] of the Japan Society for the Promotion of Science (JSPS). This work was also supported by the Chulalongkorn visiting professor grants.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Change history
1/5/2018
The article Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites, written by Seiichi Sakamoto, Waraporn Putalun, Sornkanok Vimolmangkang, Waranyoo Phoolcharoen, Yukihiro Shoyama, Hiroyuki Tanaka and Satoshi Morimoto, was originally published electronically on the publisher’s internet portal (currently SpringerLink) on 21 November 2017 without open access.
Contributor Information
Seiichi Sakamoto, Phone: +81 92 642 6581, Email: s.sakamoto@phar.kyushu-u.ac.jp.
Hiroyuki Tanaka, Phone: +81 92 642 6581, Email: htanaka@phar.kyushu-u.ac.jp.
References
- 1.Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Immunochemistry. 1969;6:43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- 3.Bennett RN, Wallsgrove RM. Secondary metabolites in plant defence mechanisms. New Phytol. 1999;127:617–633. doi: 10.1111/j.1469-8137.1994.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 4.Fürstenberg-Hägg J, Zagrobelny M, Bak S. Plant defense against insect herbivores. Int J Mol Sci. 2013;14:10242–10297. doi: 10.3390/ijms140510242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgaud F, Gravot A, Milesi S, Gontier E. Production of plant secondary metabolites: a historical perspective. Plant Sci. 2001;161:839–851. doi: 10.1016/S0168-9452(01)00490-3. [DOI] [Google Scholar]
- 6.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avrameas S, Ternynck T, Guesdon JL. Coupling of enzymes to antibodies and antigens. Scand J Immunol. 1978;8:7–23. doi: 10.1111/j.1365-3083.1978.tb03880.x. [DOI] [Google Scholar]
- 8.Nakane PK, Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974;22:1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- 9.Craven GR, Steers E, Anfinsen CB. Purification, composition and molecular weight of the β-galactosidase of Escherichia coli K12. J Biol Chem. 1965;240:2468–2477. [PubMed] [Google Scholar]
- 10.Dray F, Andrieu JM, Renaud F. Enzyme immunoassay of progesterone at the pictogram level using β-galactosidase as label. Biochim Biophys Acta. 1975;403:131–138. doi: 10.1016/0005-2744(75)90016-9. [DOI] [PubMed] [Google Scholar]
- 11.Comoglio S, Celada F. An immuno-enzymatic assay of cortisol using E. coli β-galactosidase as label. J Immunol Methods. 1976;10:161–170. doi: 10.1016/0022-1759(76)90167-8. [DOI] [PubMed] [Google Scholar]
- 12.Hitchcock CHS, Bailey FJ, Crimes AA, Dean DAG, Davis PJ. Determination of soya proteins in food using an enzyme-linked immunosorbent assay procedure. J Sci Food Agric. 1981;32:157–165. doi: 10.1002/jsfa.2740320211. [DOI] [Google Scholar]
- 13.Crowther JR. Stages in ELISA. Methods Mol Biol. 2009;516:43–78. doi: 10.1007/978-1-60327-254-4_3. [DOI] [PubMed] [Google Scholar]
- 14.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA) Quant Assay immunoglobul G Immunochem. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 15.Van Weemen BK, Schuurs AH. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971;15:232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- 16.Belanger L, Sylvestre C, Dufour D. Enzyme-linked immunoassay for alpha-fetoprotein by competitive and sandwich procedures. Clin Chim Acta. 1973;48:15–18. doi: 10.1016/0009-8981(73)90211-8. [DOI] [PubMed] [Google Scholar]
- 17.Lindström P, Wager O. IgG autoantibody to human serum albumin studied by the ELISA-technique. Scand J Immunol. 1978;7:419–425. doi: 10.1111/j.1365-3083.1978.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 18.Slightom EL. The analysis of drugs in blood, bile, and tissue with an indirect homogeneous enzyme immunoassay. J Forensic Sci. 1978;23:292–303. doi: 10.1520/JFS10760J. [DOI] [PubMed] [Google Scholar]
- 19.Nierwenhuijzen Kruseman AC. Application of ELISA for assessment of antiserum immunoreactivity in endocrine immunocytochemical studies. J Clin Pathol. 1983;36:406–410. doi: 10.1136/jcp.36.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matyjaszek-Matuszek B, Pyzik A, Nowakowski A, Jarosz MJ. Diagnostic methods of TSH in thyroid screening tests. Ann Agric Environ Med. 2013;20:731–735. [PubMed] [Google Scholar]
- 21.Oguri H, Hirama M, Tsumuraya T, Fujii I, Maruyama M, Uehara H, Nagumo Y. Synthesis-based approach toward direct sandwich immunoassay for ciguatoxin CTX3C. J Am Chem Soc. 2003;125:7608–7612. doi: 10.1021/ja034990a. [DOI] [PubMed] [Google Scholar]
- 22.Boscolo S, Pelin M, De Bortoli M, Fontanive G, Barreras A, Berti F, Sosa S, Chaloin O, Bianco A, Yasumoto T, Prato M, Poli M, Tubaro A. Sandwich ELISA assay for the quantitation of palytoxin and its analogs in natural samples. Environ Sci Technol. 2013;47:2034–2042. doi: 10.1021/es304222t. [DOI] [PubMed] [Google Scholar]
- 23.Ueda H, Tsumoto K, Kubota K, Suzuki E, Nagamune T, Nishimura H, Schueler PA, Winter G, Kumagai I, Mohoney WC. Open sandwich ELISA: a novel immunoassay based on the interchain interaction of antibody variable region. Nat Biotechnol. 1996;14:1714–1718. doi: 10.1038/nbt1296-1714. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Munakata Y, Morita K, Shinoda T, Ueda H. Sensitive detection of estrogenic mycotoxin zearalenone by open sandwich immunoassay. Anal Sci. 2007;23:65–70. doi: 10.2116/analsci.23.65. [DOI] [PubMed] [Google Scholar]
- 25.Ihara M, Suzuki T, Kobayashi N, Goto J, Ueda H. Open-sandwich enzyme immunoassay for one-step noncompetitive detection of corticosteroid 11-deoxycortisol. Anal Chem. 2009;81:8298–8304. doi: 10.1021/ac900700a. [DOI] [PubMed] [Google Scholar]
- 26.Islam KN, Ihara M, Dong J, Kasagi N, Mori T, Ueda H. Direct construction of an open-sandwich enzyme immunoassay for one-step noncompetitive detection of thyroid hormone T4. Anal Chem. 2011;83:1008–1014. doi: 10.1021/ac102801r. [DOI] [PubMed] [Google Scholar]
- 27.Hara Y, Dong J, Ueda H. Open-sandwich immunoassay for sensitive and broad-range detection of a shellfish toxin gonyautoxin. Anal Chim Acta. 2013;793:107–113. doi: 10.1016/j.aca.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 29.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 30.Hadacek F, Bachmann G. Low-molecular-weight metabolite systems chemistry. Front Environ Sci. 2015;3:12. doi: 10.3389/fenvs.2015.00012. [DOI] [Google Scholar]
- 31.Harborne JB. Classes and functions of secondary products. In: Walton NJ, Brown DE, editors. Chemicals from plants, perspectives secondary plant products. London: Imperial College Press; 1999. pp. 1–25. [Google Scholar]
- 32.Pongkitwitoon B, Sakamoto S, Tanaka H, Tsuchihashi R, Kinjo J, Morimoto S, Putalun W. Enzyme-linked immunosorbent assay for total isoflavonoids in Pueraria candollei using anti-puerarin and anti-daidzin polyclonal antibodies. Planta Med. 2010;76:831–836. doi: 10.1055/s-0029-1240725. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto S, Yusakul G, Pongkitwitoon B, Paudel MK, Tanaka H, Morimoto S. Simultaneous determination of soy isoflavone glycosides, daidzin and genistin by monoclonal antibody-based highly sensitive indirect competitive enzyme-linked immunosorbent assay. Food Chem. 2015;169:127–133. doi: 10.1016/j.foodchem.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Lu Z, Morinaga O, Tanaka H, Shoyama Y. A quantitative ELISA using monoclonal antibody to survey paeoniflorin and albiflorin in crude drugs and traditional Chinese herbal medicines. Biol Pharm Bull. 2003;26:862–866. doi: 10.1248/bpb.26.862. [DOI] [PubMed] [Google Scholar]
- 35.Ishiyama M, Shoyama Y, Murakami H, Shinohara H. Production of monoclonal antibodies and development of an ELISA for solamargine. Cytotechnology. 1995;18:153–158. doi: 10.1007/BF00767762. [DOI] [PubMed] [Google Scholar]
- 36.Phrompittayarat W, Putalun W, Tanaka H, Jetiyanon K, Wittaya-Areekul S, Ingkaninan K. Determination of pseudojujubogenin glycosides from Brahmi based on immunoassay using a monoclonal antibody against bacopaside I. Phytochem Anal. 2007;18:411–418. doi: 10.1002/pca.996. [DOI] [PubMed] [Google Scholar]
- 37.Zhu S, Shimokawa S, Shoyama Y, Tanaka H. A novel analytical ELISA-based methodology for pharmacologically active saikosaponins. Fitoterapia. 2006;77:100–108. doi: 10.1016/j.fitote.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Fujii S, Morinaga O, Uto T, Nomura S, Shoyama Y. Development of a monoclonal antibody-based immunochemical assay for liquiritin and its application to the quality control of licorice products. J Agric Food Chem. 2014;62:3377–3383. doi: 10.1021/jf404731z. [DOI] [PubMed] [Google Scholar]
- 39.Yusakul G, Sakamoto S, Juengwatanatrakul T, Putalun W, Tanaka H, Morimoto S. Preparation and application of a monoclonal antibody against the isoflavone glycoside daidzin using a Mannich reaction-derived hapten conjugate. Phytochem Anal. 2016;27:81–88. doi: 10.1002/pca.2604. [DOI] [PubMed] [Google Scholar]
- 40.Kitisripanya T, Jutathis K, Inyai C, Komaikul J, Udomsin O, Yusakul G, Tanaka H, Putalun W. Anti-miroestrol polyclonal antibodies: a comparison of immunogen preparations used to obtain desired antibody properties. J Nat Med. 2016;70:296–299. doi: 10.1007/s11418-015-0949-x. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka H, Xuan LJ, Morimoto S, Shoyama Y, Isobe R, Nojima K. Direct determination of naturally occurring biologically active compound–serum albumin conjugate by matrix-assisted laser desorption/ionization mass spectrometry. J Spectrosc. 2001;15:1–18. doi: 10.1155/2001/231032. [DOI] [Google Scholar]
- 42.Singh KV, Kaur J, Varshney GC, Raje M, Suri CR. Synthesis and characterization of hapten-protein conjugates for antibody production against small molecules. Bioconjug Chem. 2004;15:168–173. doi: 10.1021/bc034158v. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto S, Yusakul G, Tsuneura Y, Putalun W, Usui K, Miyamoto T, Tanaka H, Morimoto S. Sodium periodate-mediated conjugation of harringtonine enabling the production of a highly specific monoclonal antibody, and the development of a sensitive quantitative analysis method. Analyst. 2017;142:1140–1148. doi: 10.1039/C6AN02751B. [DOI] [PubMed] [Google Scholar]
- 44.Sakamoto S, Nagamitsu R, Yusakul G, Miyamoto T, Tanaka H, Morimoto S. Ultrasensitive immunoassay for monocrotaline using monoclonal antibody produced by N,N′-carbonyldiimidazole mediated hapten-carrier protein conjugates. Talanta. 2017;168:67–72. doi: 10.1016/j.talanta.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 45.Leung KW, Wong AST. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka H, Fukuda N, Shoyama Y. Formation of monoclonal antibody against a major ginseng component, ginsenoside Rb1 and its characterization. Cytotechnology. 1999;29:115–120. doi: 10.1023/A:1008068718392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joo EJ, Ha YW, Shin H, Son SH, Kim YS. Generation and characterization of monoclonal antibody to ginsenoside Rg3. Biol Pharm Bull. 2009;32:548–552. doi: 10.1248/bpb.32.548. [DOI] [PubMed] [Google Scholar]
- 48.Morinaga O, Tanaka H, Shoyama Y. Detection and quantification of ginsenoside Re in ginseng samples by a chromatographic immunostaining method using monoclonal antibody against ginsenoside Re. J Chromatogr B. 2006;830:100–104. doi: 10.1016/j.jchromb.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 49.Fukuda N, Tanaka H, Shoyama Y. Formation of monoclonal antibody against a major ginseng component, ginsenoside Rg1 and its characterization. Monoclonal antibody for a ginseng saponin. Cytotechnology. 2000;34:197–204. doi: 10.1023/A:1008162703957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qu H, Wang Y, Shan W, Zhang Y, Feng H, Sai J, Wang Q, Zhao Y. Development of ELISA for detection of Rh1 and Rg2 and potential method of immunoaffinity chromatography for separation of epimers. J Chromatogr B. 2015;985:197–205. doi: 10.1016/j.jchromb.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 51.Morinaga O, Tanaka H, Shoyama Y. Enzyme-linked immunosorbent assay for the determination of total ginsenosides in ginseng. Anal Lett. 2006;39:287–296. doi: 10.1080/00032710500476979. [DOI] [Google Scholar]
- 52.Sritularak B, Morinaga O, Yuan CS, Shoyama Y, Tanaka H. Quantitative analysis of ginsenosides Rb1, Rg1, and Re in American ginseng berry and flower samples by ELISA using monoclonal antibodies. J Nat Med. 2009;63:360–363. doi: 10.1007/s11418-009-0332-x. [DOI] [PubMed] [Google Scholar]
- 53.Wei TQ, Zheng YF, Dubowy M, Sharma M. Sandwich assay for tacrolimus using 2 antitacrolimus antibodies. Clin Chem. 2014;60:621–630. doi: 10.1373/clinchem.2013.214023. [DOI] [PubMed] [Google Scholar]
- 54.Towbin H, Motz J, Oroszlan P, Zingel O. Sandwich immunoassay for the hapten angiotensin II. A novel assay principle based on antibodies against immune complexes. J Immunol Methods. 1995;181:167–176. doi: 10.1016/0022-1759(94)00343-U. [DOI] [PubMed] [Google Scholar]
- 55.Qu H, Wang X, Qu B, Kong H, Zhang Y, Shan W, Cheng J, Wang Q, Zhao Y. Sandwich enzyme-linked immunosorbent assay for naringin. Anal Chim Acta. 2016;903:149–155. doi: 10.1016/j.aca.2015.09.058. [DOI] [PubMed] [Google Scholar]
- 56.Better M, Chang CP, Robinson RR, Horwitz AH. Escherichia coli secretion of an active chimeric antibody fragment. Science. 1988;240:1041–1043. doi: 10.1126/science.3285471. [DOI] [PubMed] [Google Scholar]
- 57.Jost CR, Kurucz I, Jacobus CM, Titus JA, George AJT, Segal DM. Mammalian expression and secretion of functional single-chain Fv molecules. J Biol Chem. 1994;269:26267–26273. [PubMed] [Google Scholar]
- 58.Bei R, Schlom J, Kashmiri SV. Baculovirus expression of a functional single-chain immunoglobulin and its IL-2 fusion protein. J Immunol Methods. 1995;186:245–255. doi: 10.1016/0022-1759(95)00149-5. [DOI] [PubMed] [Google Scholar]
- 59.Davis GT, Bedzyk WD, Voss EW, Jacobs TW. Single chain antibody (SCA) encoding genes: one-step construction and expression in eukaryotic cells. Biotechnology. 1991;9:165–169. doi: 10.1038/nbt0291-165. [DOI] [PubMed] [Google Scholar]
- 60.Whitelam GC, Cockburn W, Owen MR. Antibody production in transgenic plants. Biochem Soc Trans. 1994;22:940–945. doi: 10.1042/bst0220940. [DOI] [PubMed] [Google Scholar]
- 61.Sakamoto S, Taura F, Putalun W, Pongkitwitoon B, Tsuchihashi R, Morimoto S, Kinjo J, Shoyama Y, Tanaka H. Construction and expression of specificity-improved single-chain variable fragments against the bioactive naphthoquinone, plumbagin. Biol Pharm Bull. 2009;32:434–439. doi: 10.1248/bpb.32.434. [DOI] [PubMed] [Google Scholar]
- 62.Pongkitwitoon B, Sakamoto S, Morinaga O, Juengwatanatrakul T, Shoyama Y, Tanaka H, Morimoto S. Single-chain variable fragment antibody against ginsenoside Re as an effective tool for the determination of ginsenosides in various ginsengs. J Nat Med. 2011;65:24–30. doi: 10.1007/s11418-010-0446-1. [DOI] [PubMed] [Google Scholar]
- 63.Yusakul G, Sakamoto S, Pongkitwitoon B, Tanaka H, Morimoto S. Effect of linker length between variable domains of single chain variable fragment antibody against daidzin on its reactivity. Biosci Biotechnol Biochem. 2016;80:1306–1312. doi: 10.1080/09168451.2016.1156482. [DOI] [PubMed] [Google Scholar]
- 64.Paudel MK, Sakamoto S, Huy LV, Tanaka H, Miyamoto T, Morimoto S. The effect of varying the peptide linker length in a single chain variable fragment antibody against wogonin glucuronide. J Biotechnol. 2017;251:47–52. doi: 10.1016/j.jbiotec.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Yusakul G, Sakamoto S, Tanaka H, Morimoto S. Efficient expression of single chain variable fragment antibody against paclitaxel using Bombyx mori nucleopolyhedrovirus bacmid DNA system and its characterizations. J Nat Med. 2016;70:592–601. doi: 10.1007/s11418-016-0981-5. [DOI] [PubMed] [Google Scholar]
- 66.Paudel MK, Takei A, Sakoda J, Juengwatanatrakul T, Sasaki-Tabata K, Putalun W, Shoyama Y, Tanaka H, Morimoto S. Preparation of a single-chain variable fragment and a recombinant antigen-binding fragment against the anti-malarial drugs, artemisinin and artesunate, and their application in an ELISA. Anal Chem. 2012;84:2002–2008. doi: 10.1021/ac203131f. [DOI] [PubMed] [Google Scholar]
- 67.Paudel MK, Sakamoto S, Tanaka H, Morimoto S. An overview and comparison of a recombinant antigen-binding fragment and an antigen-binding fragment from a monoclonal antibody against wogonin glucuronide. J Nat Med. 2017;71:703–710. doi: 10.1007/s11418-017-1100-y. [DOI] [PubMed] [Google Scholar]
- 68.Sakamoto S, Taura F, Pongkitwitoon B, Putalun W, Tsuchihashi R, Kinjo J, Tanaka H, Morimoto S. Development of sensitivity-improved fluorescence-linked immunosorbent assay using a fluorescent single-domain antibody against the bioactive naphthoquinone, plumbagin. Anal Bioanal Chem. 2010;396:2955–2963. doi: 10.1007/s00216-010-3535-9. [DOI] [PubMed] [Google Scholar]
- 69.Sakamoto S, Tanizaki Y, Pongkitwitoon B, Tanaka H, Morimoto S. A chimera of green fluorescent protein with single chain variable fragment antibody against ginsenosides for fluorescence-linked immunosorbent assay. Protein Expr Purif. 2011;77:124–130. doi: 10.1016/j.pep.2011.01.010. [DOI] [PubMed] [Google Scholar]