Abstract
Breast cancer is a multifactorial disease. Benign breast disease (BBD) is one of the most important risk factors for breast cancer. The etiology of BBD is unknown. It is divided into nonproliferative and proliferative diseases. The selection of studies will be based on titles, abstract screening, inclusion and exclusion criteria, and quality assessment. Previous studies have shown that all types of BBD increase the risk of breast cancer, but the risk degree is different for each one. Accurate risk estimation of breast cancer in each category can be very important for proper clinical management. This systematic review and meta-analysis will be conducted on observational studies (traditional case control, nested case control, case cohort, and cohort) published in the Web of Science (ISI), PubMed (MEDLINE), Scopus, Google Scholar, and the key journals of this field such as Breast Cancer Research and Treatment and Cancer Research from January 2000 to June 2015. Reference lists and gray literature will be reviewed too. All the initial retrievals will be performed by 2 researchers independently. The data extraction form will consist of general information concerning the studies, study eligibility, method, risk of bias assessment, and results—including odds ratios, risk ratios, rate ratios, and hazard ratios. The PRISMA and MOOSE guidelines will be used to report our findings. Registration Details: PROSPERO-42016035243
Keywords: Fibrocystic breast disease, Mammary dysplasia, Breast neoplasms, Risk factors, Systematic review
What’s Known
Past studies have shown that 3 categories of benign breast disease) BBD) constitute important risk factors for breast cancer.
There are only a few metaanalyses on the degree of risk. Latest meta-analysis was published in 2015 on data from 1930–2007.
What’s New
Our study will encompass studies from 2000–2015 and provide fresh evidence on the degree of risk in BBD categories
In light of our results, we can recommend the use of different screening programs based on the histological classification of BBD, which could improve patients' health.
Introduction
According to the report of the World Health Organization (WHO), breast cancer is the most common cancer in women worldwide,1-3 such that it accounts for 16% of all cancers in women.1 In the recent decades, the incidence of breast cancer has increased in both developed and developing countries, primarily because of advances in diagnostic methods.4
Breast cancer is a multifactorial disease.4,5 Benign breast disease (BBD) is one of the most important risk factors for breast cancer.6-11 Breast cancer is more frequent in women with BBD than in the general population.9 Previous studies have indicated that 90% of breast lesions are benign.12 The etiology of BBD is unknown.1,9
Genetic predisposition and environmental elements such as diet, alcohol, and physical activity may convert these lesions into cancer via unknown mechanisms. Studies have suggested that some clinical factors such as menopausal status, family history of breast cancer, and age at the diagnosis of BBD also may change the risk of developing BBD to that of breast cancer.12
Pathologically, BBD is divided into nonproliferative disease, proliferative disease without atypia, and proliferative disease with atypia.7,11-13
According to the findings of previous studies, all 3 subtypes of BBD increase the risk of breast cancer, but the risk degree is different in each of them.6,14,15 The risk is higher in proliferative disease, especially atypical proliferative lesions.6,16 Although only a few studies have been previously conducted on the relationship between nonproliferative lesions and subsequent breast cancer,6,8,17 the existing evidence shows that the cancer risk for nonproliferative lesions is lower than that of the other subtypes of BBD.6
Over the recent years, mammography screening programs have been widely used to detect malignant and benign breast diseases, but there are no specific guidelines or protocols to follow-up the subtypes of BBD separately—except for proliferative lesions with atypia.14 The researchers of the present study think that the reason may be insufficient evidence to support a particular follow-up based on the pathological categories of BBD. Thus, it is necessary to carry out more research, systematic reviews, and meta-analyses. Decision-makers in this field need a regular combination of available scientific information. Today, it is easy for a person to be up to date in scientific knowledge because of the burst of information in such a vast array of literature. Review articles and meta-analyses, which summarize the knowledge in a scientific field, eliminate this need.18 We hope that the findings of the current research will provide more relevant evidence and that we will be able to recommend the use of different screening programs for the subtypes of BBD based on our results with a view to improving patients’ health. Early diagnosis and proper management of disease can prevent the complications of cancer and lessen the economic burden on patients and their families.
We found 2 meta-analyses related to our review.9,19 The results of the newest meta-analysis, published in 2015, confirmed that BBD elevates later risk of breast cancer; that study was performed on articles which included data from 1930 to 2007.9 We found no other relevant study in this field apart from the aforementioned one. Most of the studies before 2000 did not have enough information about the degree of breast cancer risk associated with the subtypes of BBD.20 On the other hand, the diagnosis of BBD in different studies has been based on different histological criteria; accordingly, the current study will be conducted on all studies from 2000 to 2015. We need information on the subtypes of BBD because an accurate risk estimation of breast cancer for each category is crucial for the improvement of BBD clinical management.1
In the present systematic review and meta-analysis, we will seek to answer the questions whether the subtypes of BBD are associated with an elevated risk of later breast cancer and how much is the risk degree of each subgroup of BBD.
Objectives
To estimate breast cancer risk associated with proliferative disease with/without atypia versus nonproliferative disease
To estimate the age-adjusted risk of breast cancer associated with proliferative disease with/without atypia versus nonproliferative disease
To estimate the menopause-adjusted risk of breast cancer associated with proliferative disease with/without atypia versus nonproliferative disease
To estimate the family history-adjusted risk of breast cancer associated with proliferative disease with/without atypia versus nonproliferative disease.
Methods
Type of Studies
We will enter all studies in our systematic review and meta-analysis which have clearly defined breast cancer risk in women with proliferative disease with/without atypia versus nonproliferative disease. These studies encompass both retrospective and prospective investigations with each of the following designs: Traditional case-control, nested case-control, and cohort.
Type of Participants
The participants of the selected studies will be women with a biopsy confirmation of the subtypes of BBD (nonproliferative, proliferative without atypia, and proliferative with atypia). The women will be of any age, and there will be no restriction in terms of menopause status, family history of breast cancer in the person or a first-degree relative, race or ethnicity, and parity.
Inclusion and Exclusion Criteria
Inclusion
Studies included in the present review will be those that have reported breast cancer risk associated with all pathologic categories of BBD, including nonproliferative and proliferative diseases with/without atypia. We will select the eligible studies from January 2000 to June 2015. All studies in the English language will be included in our review. The main effect size measures reported in the studies will include odds ratios, risk ratios, rate ratios, and hazard ratios.
Exclusion
All studies which have no clear pathologic category, are nonstratified, and have reported only the breast cancer risk associated with BBD will be excluded. As regards studies whose abstracts are available only, if it is possible, we will purchase the articles; otherwise, we will exclude them from our review.
Type of Outcome Measures
Primary outcomes
We will estimate the relative risk of breast cancer in individuals with proliferative disease with/without atypia versus nonproliferative breast disease.
Secondary outcomes
The relative risk of breast cancer in patients with a confirmed pathologic diagnosis of BBD will be estimated based on subgroups of age, menopause status, and family history of breast cancer in a first-degree relative.
Search Strategy and Information Sources
Strategy
In order to achieve the aims of the present study, we will conduct electronic search in the following databases: Web of Science (ISI), PubMed (MEDLINE), Scopus, Google Scholar, and the key journals of this field such as Breast Cancer Research and Treatment and Cancer Research. Our search will be restricted to published studies in the English language from January 2000 to June 2015. All the initial retrievals will be performed by 2 researchers independently. Gray literature will also be reviewed.
We will find unpublished studies by searching Google Scholar. If those studies are eligible, we will include them in our review. In PubMed, first we will find equivalent words for BBD based on MeSH terms. Then, we will create our appropriate syntaxes. The main syntax will be “Breast Cancer”[tiab] AND (“Benign Breast Disease”[tiab] OR “Nonproliferative Breast Disease” OR “Proliferative Breast Disease” OR “Mammary Dysplasia”[tiab] OR “Mastopathy”[tiab] OR “Breast Fibrocystic Changes”[tiab] OR “Microglandular Adenosis*”[tiab] OR “Chronic Cystic Mastitis”[tiab]). Proportionate syntax for the Web of Science (ISI) will be “Breast Cancer” AND “Benign Breast Disease” OR “Nonproliferative Breast Disease” OR “Proliferative Breast Disease” OR “Mammary Dysplasia” OR “Mastopathy”. Our syntax in Scopus will be “Breast Cancer” AND “Benign Breast Disease” OR “ Nonproliferative Breast Disease” OR “Proliferative Breast Disease” OR “Mammary Dysplasia” OR “Mastopathy” OR “Breast Fibrocystic Changes” OR “Microglandular Adenosis*” OR “Chronic Cystic”. Proportionate syntax for Google Scholar will be “Breast Cancer” + “Benign Breast Disease” + “Proliferative Breast Disease” + “Mastopathy”. Our syntax in specialized journals (“Breast Cancer Research and Treatment” and “Cancer Research”) will be “Breast Cancer” AND “Benign Breast disease” OR “Nonproliferative Breast Disease” OR “Proliferative Breast Disease”.
In the final step of the search, we will check the list references of the included articles as the last source of our search. Finally, after finding all relevant studies, we will select the main studies according to the following steps.
Study Selection
The selection of the main studies for inclusion in our review will be done in 2 stages. In the first stage, assessment will be conducted based on title/abstract screening and duplicates. Studies will be excluded from our study if there is no access to the full text of the study, if there are no primary data according to the aims of our study, and if there is no report of the effect size measures. After removing the irrelevant articles by providing reasons, in the second stage, we will perform full text assessment according to the inclusion and exclusion criteria. Articles will be excluded if they are nonstratified as regards BBD, if they contain no clear pathologic categories of BBD, if they contain unknown histology, and if they do not report the effect size measures. Both stages will be performed by 2 reviewers independently, and any disagreement between the reviewers will be resolved by discussion; otherwise, the opinion of a 3rd reviewer will be sought. If there is some unclear information, we will contact the corresponding author(s) of the studies. Figure 1 summarizes the process of literature selection in the present systematic review and meta-analysis.
Figure 1.
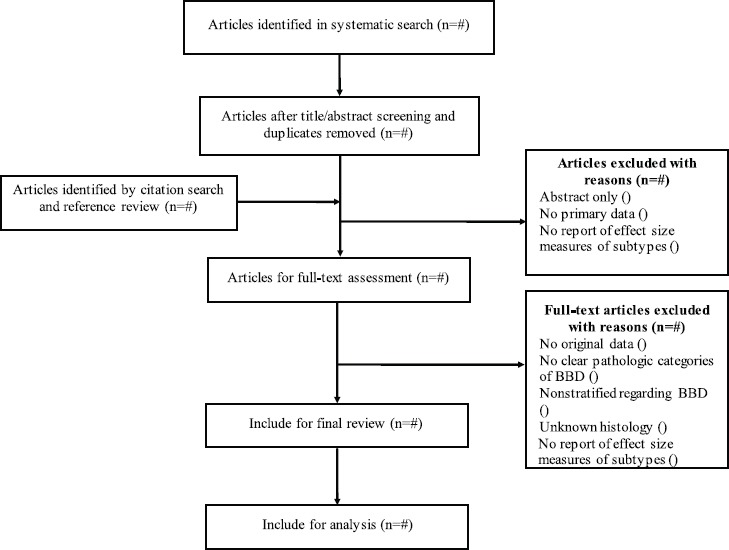
Flow diagram of the systematic search and selection process of articles.
Data Extraction
After removing the irrelevant articles and determining the main studies, we will conduct the last review to complete data extraction for each of the final articles. The data extraction form (Appendix I) consists of general information on the studies, study eligibility, method, risk of bias, quality assessment, and results.
General information: Code of the article, title of the article, reference number, reviewers’ initials, publication details, first author, journal’s title, year of publication, volume, and first page
Study eligibility: Name of the country, total study period, participants, study setting, inclusion criteria (in the study), exclusion criteria (in the study), total population at the start of the study, age of the study population, and type of the outcome measures
Method: Aims of the study, study design (traditional case-control, nested case-control, and cohort), and ethical approval obtained for the study
Risk of bias assessment: Quality assessment through a modified form of quality assessment (STROBE: Special checklist for observational studies) (table 1)
Table 1.
Quality assessment form (modified form of STROBE)
| Items | Yes=1 | No=0 | Unclear=0 | |
|---|---|---|---|---|
| Abstract | 1. Clearly define the study design and the main results of the study | |||
| Objectives | 2. State specific objectives, including any prespecified hypotheses | |||
| Study design | 3. Present the key elements of the study design early in the paper | |||
| Setting | 4. Describe the setting | |||
| 5. Introduce the locations | ||||
| 6. Include the periods of recruitment | ||||
| 7. Present case definition/exposure | ||||
| Participants | 8. Give the eligibility criteria | |||
| 9. List the sources and methods of the selection of the participants | ||||
| 10. Describe the methods of follow-up/Give the rationale for the choice of cases and controls | ||||
| 11. For matched studies, give the matching criteria and the number of exposed and unexposed/controls per case | ||||
| Variables | 12. Clearly define all the outcomes | |||
| 13. Clearly define the potential confounders and effect modifiers | ||||
| Data sources/measurement | 14. Give the sources of data and details of the methods of assessment | |||
| Bias | 15. Address the potential sources of bias | |||
| Study size | 16. Explain how the study size was arrived at | |||
| Statistical methods | 17. Describe all the statistical methods | |||
| 18. Include methods used to control for confounding | ||||
| 19. If applicable, explain how loss to follow-up/matching of cases and controls was addressed | ||||
| 20. Describe any sensitivity analyses | ||||
| Total | ||||
Results of studies: Odds ratios, risk ratios, rate ratios, and hazard ratios
Risk of Bias Assessment
The checklist of the modified form of STROBE will be filled for each study by the 2 reviewers independently. To determine the eligibility of the articles, we will use the sum score of quality items. All studies with any score will be included in the review, and finally subgroup analysis will be performed on low-quality and high-quality studies, if it is applicable, and the cutoff score will be 15.
Missing Data
If there is any unclear information, we will contact the corresponding author(s) of the studies. We will report all the missing data if we cannot find them.
Heterogeneity Assessment
The heterogeneity of the studies will be assayed using the χ2 test of homogeneity (significant at P<0.1), and I2 statistic will be performed on the quantitative data. In cases where heterogeneity between studies is extensive in a way that pooling analysis is not possible, we will report only a narrative presentation. When there is heterogeneity between the studies (I2 >50%), a Mantel–Haenszel random-effects model will be used to pool the data in a meta-analysis. To determine the factors that are associated with breast cancer, we will use meta-regression. We will include age at biopsy, family history of breast cancer, and menopause status in the meta-regression models.
Report of Biases Assessment
Publication bias will be assessed using the Begg plot and test and the Egger plot and test in addition to the Funnel plots.
Data Synthesis
We will estimate the risk of breast cancer according to the subtypes of benign breast disease (proliferative disease with/without atypia vs. nonproliferative disease). The following subgroups will be analyzed, if possible.
Subgroup Analysis
We will do subgroup analysis based on age at biopsy, family history of breast cancer, and menopause status, if applicable.
Reporting of the Review
The PRISMA and MOOSE guidelines will be used to report our systematic review and meta-analysis protocol. A Flow diagram will be drawn to depict the systematic search and the selection process of the articles. The list of the excluded studies and the reason for that will be reported too. The qualitative data of the studies will be described in the text.
Acknowledgment
The authors appreciate the unsparing cooperation of the Research Vice-Chancellorship of Guilan University of Medical Sciences.
Conflict of Interest: None declared.
Appendix
Appendix I.
Data extraction form
| Review title | |||
|---|---|---|---|
| 1. General information | |||
| Code of the article | |||
| Reference number | |||
| Reviewers’ initials | |||
| Publication details: | |||
| First author | |||
| Journal’s title and year | |||
| Volume and first page | |||
| 2. Study eligibility | |||
| Name of the country | |||
| Total study period | |||
| Participants: | |||
| Study setting (e.g., urban, rural, hospital- based, or population-based) | |||
| Inclusion criteria (in the study) | |||
| Exclusion criteria (in the study) | |||
| Total population at the start of the study | |||
| Age of the study population | |||
| Type of the outcome measures | Odds Ratio/Risk Ratio/Rate Ratio/Hazard Ratio | ||
| Should this study be included in the review? | Yes No Maybe Reasons for No or Maybe: | ||
| 3. Method | |||
| Aims of the study | |||
| Study design | Traditional case control Nested case control Case cohort Cohort | ||
| Ethical approval obtained for the study | |||
| 4. Risk of bias assessment | |||
| Quality scale | Yes=1/No=0/Unclear=0 | ||
| Items | |||
| Abstract | 1. Clearly define the study design and the main results of the study | ||
| Objectives | 2. State specific objectives, including any prespecified hypotheses | ||
| Study design | 3. Present the key elements of the study design early in the paper | ||
| Setting | 4. Describe the setting | ||
| 5. Introduce the locations | |||
| 6. Include the periods of recruitment | |||
| 7. Present case definition/exposure | |||
| Participants | 8. Give the eligibility criteria | ||
| 9. List the sources and methods of the selection of the participants | |||
| 10. Describe the methods of follow-up/Give the rationale for the choice of cases and controls | |||
| 11. For matched studies, give the matching criteria and the number of exposed and unexposed/controls per case | |||
| Variables | 12. Clearly define all the outcomes | ||
| 13. Clearly define the potential confounders and effect modifiers | |||
| Data sources/measurement | 14. Give the sources of data and details of the methods of assessment | ||
| Bias | 15. Address the potential sources of bias | ||
| Study size | 16. Explain how the study size was arrived at | ||
| Statistical methods | 17. Describe all the statistical methods | ||
| 18. Include methods used to control for confounding | |||
| 19. If applicable, explain how loss to follow-up/matching of cases and controls was addressed | |||
| 20. Describe any sensitivity analyses | |||
| Total | |||
| 5. Results: | |||
| Outcome | |||
| Number of cases | |||
| Number of controls | |||
| Number of exposed cases | |||
| Number of unexposed cases | |||
| Crude results (95% CI) | |||
| Adjusted results (95% CI) | |||
| Adjusted for which confounders? | |||
References
- 1.Hornberger J, Chen SC, Li Q, Kakad P, Quay SC. Proliferative epithelial disease identified in nipple aspirate fluid and risk of developing breast cancer:A systematic review. Curr Med Res Opin. 2015;31:253–62. doi: 10.1185/03007995.2014.988209. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Shao N, Zhang YJ, Wu ZH, Li ZB, Ren ZF, et al. Risk assessment of breast cancer in Guangdong, China:A community-based survey. Asian Pac J Cancer Prev. 2012;13:2759–63. doi: 10.7314/APJCP.2012.13.6.2759. [DOI] [PubMed] [Google Scholar]
- 3.Memon ZA, Kanwal N, Sami M, Larik PA, Farooq MZ. Risk of Breast Cancer among young women and importance of early screening. Asian Pac J Cancer Prev. 2015;16:7485–9. doi: 10.7314/APJCP.2015.16.17.7485. [DOI] [PubMed] [Google Scholar]
- 4.Mahouri K, Dehghani Zahedani M, Zare S. Breast cancer risk factors in south of Islamic Republic of Iran:A case-control study. East Mediterr Health J. 2007;13:1265–73. doi: 10.26719/2007.13.6.1265. [DOI] [PubMed] [Google Scholar]
- 5.Kotsopoulos J, Chen WY, Gates MA, Tworoger SS, Hankinson SE, Rosner BA. Risk factors for ductal and lobular breast cancer:Results from the nurses' health study. Breast Cancer Res. 2010;12:R106. doi: 10.1186/bcr2790. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castells X, Domingo L, Corominas JM, Tora-Rocamora I, Quintana MJ, Bare M, et al. Breast cancer risk after diagnosis by screening mammography of nonproliferative or proliferative benign breast disease:A study from a population-based screening program. Breast Cancer Res Treat. 2015;149:237–44. doi: 10.1007/s10549-014-3208-z. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamimi RM, Rosner B, Colditz GA. Evaluation of a breast cancer risk prediction model expanded to include category of prior benign breast disease lesion. Cancer. 2010;116:4944–53. doi: 10.1002/cncr.25386. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tice JA, O'Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst. 2013;105:1043–9. doi: 10.1093/jnci/djt124. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyrstad SW, Yan Y, Fowler AM, Colditz GA. Breast cancer risk associated with benign breast disease:Systematic review and meta-analysis. Breast Cancer Res Treat. 2015;149:569–75. doi: 10.1007/s10549-014-3254-6. [DOI] [PubMed] [Google Scholar]
- 10.Schnitt SJ. Benign breast disease and breast cancer risk:Morphology and beyond. Am J Surg Pathol. 2003;27:836–41. doi: 10.1097/00000478-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Cote ML, Ruterbusch JJ, Alosh B, Bandyopadhyay S, Kim E, Albashiti B, et al. Benign breast disease and the risk of subsequent breast cancer in African American women. Cancer Prev Res (Phila) 2012;5:1375–80. doi: 10.1158/1940-6207.CAPR-12-0175. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabat GC, Jones JG, Olson N, Negassa A, Duggan C, Ginsberg M, et al. A multi-center prospective cohort study of benign breast disease and risk of subsequent breast cancer. Cancer Causes Control. 2010;21:821–8. doi: 10.1007/s10552-010-9508-7. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worsham MJ, Raju U, Lu M, Kapke A, Cheng J, Wolman SR. Multiplicity of benign breast lesions is a risk factor for progression to breast cancer. Clin Cancer Res. 2007;13:5474–9. doi: 10.1158/1078-0432.CCR-07-0928. [DOI] [PubMed] [Google Scholar]
- 14.Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. Magnitude and laterality of breast cancer risk according to histologic type of atypical hyperplasia:Results from the Nurses' Health Study. Cancer. 2007;109:180–7. doi: 10.1002/cncr.22408. [DOI] [PubMed] [Google Scholar]
- 15.Worsham MJ, Abrams J, Raju U, Kapke A, Lu M, Cheng J, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J. 2007;13:115–21. doi: 10.1111/j.1524-4741.2007.00388.x. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aroner SA, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Rosner BA, et al. Radial scars and subsequent breast cancer risk:Results from the Nurses' Health Studies. Breast Cancer Res Treat. 2013;139:277–85. doi: 10.1007/s10549-013-2535-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Costantino JP, Tan-Chiu E, Wickerham DL, Paik S, Wolmark N. Lower-category benign breast disease and the risk of invasive breast cancer. J Natl Cancer Inst. 2004;96:616–20. doi: 10.1093/jnci/djhs105. [DOI] [PubMed] [Google Scholar]
- 18.Dickersin K. Systematic reviews in epidemiology:Why are we so far behind? Int J Epidemiol. 2002;31:6–12. doi: 10.1093/ije/31.1.6. [DOI] [PubMed] [Google Scholar]
- 19.Zhou WB, Xue DQ, Liu XA, Ding Q, Wang S. The influence of family history and histological stratification on breast cancer risk in women with benign breast disease:A meta-analysis. J Cancer Res Clin Oncol. 2011;137:1053–60. doi: 10.1007/s00432-011-0979-z. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–37. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]


