Abstract
Background:
Studies have shown that zinc and selenium deficiency is common in nonalcoholic fatty liver disease (NAFLD). However, the effects of zinc and selenium co-supplementation before and/or after disease progression on NAFLD are not clear enough. The aim of this study was to compare the effects of zinc and selenium co-supplementation before and/or after disease progression on NAFLD prognosis.
Materials and Methods:
Forty male Sprague–Dawley rats (197±4 g) were randomly assigned to 4 dietary groups: normal-fat diet (NFD; receiving 9% of calories as fat), high-fat diet (HFD; receiving 82% of calories as fat), supplementation before disease progression (S+HFD), and supplementation after disease progression (HFD+S). The diets were implemented over a 20-week period in all the groups. Biochemical and histologic parameters were compared between the 4 groups, and between-group comparisons were also carried out.
Results:
There were significant differences in the average food dietary intake (P<0.001), weight (P<0.001), fasting blood sugar (P=0.005), triglyceride (P<0.001), total cholesterol (P<0.001), low-density lipoprotein cholesterol (P=0.002), high-density lipoprotein cholesterol (P=0.001), alanine aminotransferase (P<0.001), and aspartate aminotransferase (P<0.001) between the 4 dietary groups. Serum triglyceride and total cholesterol were significantly lower in the HFD+S Group than in the S+HFD Group (P<0.001 and P=0.003, respectively). Fat accumulation was significantly reduced in the HFD+S Group (P<0.001).
Conclusion:
Zinc and selenium co-supplementation after disease progression improved biochemical and histologic parameters in an experimental model of NAFLD.
Keywords: Fatty liver, Trigelycerids, Lipid profile, Selenium, Zink, Cholestrol
What’s Known
Molecular and cellular studies have shown that zinc and selenium supplementation can be protective in rodent models of type 2 diabetes by stimulating insulin secretion and lowering serum glucose and insulin levels.
Zinc and selenium deficiency is common in nonalcoholic fatty liver disease.
What’s New
Nonalcoholic fatty liver disease is an inflammatory disorder, leading to mineral deficiency in the liver and blood.
Zinc and selenium cosupplementation can reverse the disease progression by improving serum biochemical parameters (e.g., lipid profile and liver enzyme tests) and fat granule accumulation and size in the liver.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common metabolic disease and afflicts 20%–30% of people in Western countries and 15% of people in Asia.1,2 NAFLD is defined as triglyceride (TG) accumulation exceeding 5% of the liver weight.3 Disease progression has a direct link with visceral obesity, glucose intolerance, and dyslipidemia.4 The pathogenesis of NAFLD is unknown; be that as it may, a mechanism that may be involved is liver injury mediated by endotoxins/cytokines, oxidative stress, and hyperinsulinemia.3 Zinc is an important micronutrient in that it plays a key role in the metabolism of macronutrients,5 as well as the synthesis, storage, release, and actions of insulin.6 Molecular and cellular studies have shown that zinc and selenium supplementation can be protective in rodent models of type 2 diabetes by stimulating insulin secretion and lowering serum glucose and insulin levels.7,8 According to previous studies, zinc and selenium deficiency is common in cirrhosis and can affect nitrogen metabolism.9,10
The relationship between zinc/selenium intake and chronic fatty liver disease is complex as zinc/selenium affects the normal functioning of the liver and the liver plays a central role in zinc/selenium hemostasis; accordingly, the deficiencies of these minerals damage liver function and compromise the recovery and restoration of liver tissues.11
To our knowledge, no human or animal studies have been performed to study the effects of zinc supplementation in combination with selenium, before and after disease progression, on the prognosis of NAFLD. So, the aim of this study was to compare the effects of zinc supplementation with selenium before and/or after disease progression on the prognosis of NAFLD based on serum glucose, lipid profile, hepatic enzymes, and histology in an experimental model of Sprague–Dawley rats.
Materials and Methods
Animals, Diets, Interventions, and Experimental Design
The experimental protocol was approved by the Iran National Science Foundation, Tehran, Iran (protocol #91056877). This research conforms to the Institutional and National Guide for the Care and Use of Laboratory Animals. Forty male Sprague–Dawley rats (197±4 g) were obtained from the Razi Vaccine and Serum Research Institute, Tehran, Iran. The rats were housed at 21–23°C and controlled humidity (50±5%) under a 12-hour artificial light cycle (7 am to 7 pm). All the animals had ad libitum access to water and the AIN93M diet 1 week before the commencement of the study for adaptation. Then, the rats were randomly assigned to 4 different groups (figure 1). The ingredients of the AIN93M diet are depicted in table 1.
Figure 1.
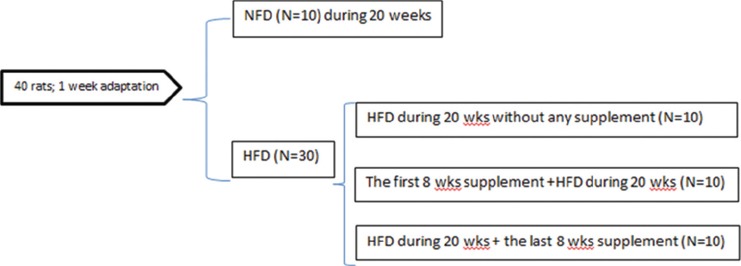
Schematic overview of the experimental study design. The high-fat diet (HFD) group was assigned to 3 groups: 1 group received the high-fat diet over a 20-week period without any supplementation; 1 group received zinc and selenium co-supplements in the 1st 8 weeks; and 1 group received zinc and selenium co-supplements in the last 8 weeks of the study.
Table 1.
Composition of the experimental diets (per 1 kg) during the study period (AIN93M diet)
| Diets nutrients | NFD | HFD |
|---|---|---|
| Casein (g/kg) | 140 | 180 |
| Cornstarch (g/kg) | 630 | 50 |
| Sucrose (g/kg) | 100 | 10 |
| Soy oil (g/kg) | 40 | 21 |
| SFA1 (g/kg) | - | 483 |
| Fiber (g/kg) | 50 | 50 |
| Mineral mix (g/kg) | 35 | 35 |
| Vitamin mix (g/kg) | 10 | 10 |
| L-cys2 (g/kg) | 1.8 | 1.8 |
| Choline bitartrate (g/kg) | 2.5 | 2.5 |
| tert-Butylhydroquinone (g/kg) | 0.008 | 0.008 |
| Energy (kcal/g) | 3.8 | 5.5 |
| As carbohydrate (%) | 76 | 5 |
| As fat (%) | 9 | 82 |
| As protein (%) | 15 | 13 |
NFD: Normal AIN93M diet; HFD: High-fat AIN93M diet;
SFA, Saturated fatty acid as lard;
L-cys, L- cysteine
The rats on high-fat diet were supplemented with zinc sulfate (ZnSO4) and sodium selenite in the 1st 8 weeks and/or in the last 8 weeks of the study. Supplementation was done with ZnSO4 (15 mg/kg)10 and sodium selenite (2.5 mg/kg)12 solutions in distilled water daily by intragastric administration. The animals were weighed on a calibrated balance scale (Marte Scale, EK-3000i, USA) to the nearest 0.1 g weekly for the determination of weight changes.
The animals were thereafter sacrificed after 20 weeks of intervention using 80 mg/kg of ketamine and 10 mg/kg of xylazine. Fasting blood samples were collected in a tube with EDTA, centrifuged at 1000 g for 20 minutes, and plasma was separated and stored at -70°C until the biochemical analyses.
Biochemical Parameters Measurement
The blood levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and TG were measured photometrically in an automatic analyzer (Cobas Integra 400, Roche, Mannheim, Germany). Fasting blood glucose was measured via an enzymatic method (Pars Azmoon Co. kit, Tehran, Iran) using a Liasys AutoAnalyzer. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured via the spectrophotometry method (Pars Azmoon Co. Kit, Tehran, Iran). The selenium and zinc concentrations of the serum and liver were measured using an atomic absorption spectrophotometer (Perkin Elmer 2100B, Germany).
Histological Assessment
The hematoxylin and eosin (H & D) stain method was used for histological analysis and evaluated by a pathologist in a blinded manner. Parts of hepatic lobes were removed from each animal and were fixed in a 10% formaldehyde buffer. Then, the samples were dehydrated in ascending grades of alcohol and embedded in paraffin. Sections at 5-Mm thickness were taken and stained with hematoxylin and eosin. Images were captured at 400× magnification and were analyzed using the Image-Portlab software. According to the H & D method, fat accumulation was classified as stage 0 (<5% of fat accumulation), stage 1 (5%–33% of fat accumulation), stage 2 (34%–66% of fat accumulation), and stage 3 (>66% of fat accumulation in the liver cells).13
Statistical Analysis
All the data are expressed as mean ± standard deviation (SD). The level of significance was set at a P<0.05. The statistical analyses were performed with IBM SPSS Statistics software (version 16; IBM Corp.). Normal distribution in the variables was checked using the Kolmogorov–Smirnov test. The continuous variables were compared between the groups using the ANOVA, followed by the post-hoc test to compare the between-group differences.
Results
Serum Biochemical Parameters Measurements
Our results showed that there were significant differences in the average of dietary intake, weight, and all the biochemical parameters between the 4 groups (P<0.01). Although dietary intake (g/d) was significantly higher in the NFD Group than in the HFD Group (P<0.001), the rats in the HFD Group significantly received more calories than those in the NFD Group (P<0.001). Fasting blood sugar (P=0.01), TG (P<0.001), TC (P<0.001), LDL-C (P=0.001), ALT (P<0.001), and AST (P=0.002) were significantly higher in the HFD Group than in the NFD Group. The serum and liver levels of zinc and selenium were significantly higher in the NFD Group than in the HFD Group (P<0.001 in all comparisons).
Co-supplementation with zinc and selenium after disease progression (HFD+S) significantly decreased the serum levels of fasting blood sugar (P=0.01), TG (P<0.001), TC (P=0.001), LDL-C (P<0.001), ALT (P<0.001), and AST (P=0.002). Nonetheless, before disease co-supplementation (S+HFD) decreased only the serum levels of TG (P=0.04), ALT (P<0.001), and AST (P<0.001) compared with the HFD Group. The serum TG and TC levels in the HFD+S Group were significantly lower than those in the S+HFD Group (P<0.001 and P=0.003, respectively). The serum and liver levels of zinc and selenium were significantly higher in the supplemented groups (S+HFD and HFD+S) than in the HFD Group (P<0.001). Serum zinc and selenium, as well as liver zinc levels, were significantly higher in the S+HFD Group than in the HFD+S Group (P<0.001 in all comparisons), while the liver level of selenium in the HFD+S Group was significantly higher than that in the S+HFD Group (P<0.001) (table 2).
Table 2.
Effects of 8 weeks’ zinc and selenium co-supplementation on the body measurements and serum biochemical parameters of the rats with fatty liver induced by 12 weeks of a high-fat diet
| Groups variables | n=10 | P value† | |||
|---|---|---|---|---|---|
| NFD | HFD | S+HFD | HFD+S | ||
| Weight (g) | |||||
| -beginning | 197±4 | 196±3 | 193±4 | 193±5 | 0.89 |
| -end of studya | 342±6* | 358±14* | 362±6* | 360±5* | <0.001 |
| Liver (g) | 7.5±4 | 7.8±2 | 7.6±0.2 | 7.8±0.2 | 0.92 |
| Average food intake | |||||
| g/da | 13±0.5 | 10±0.4 | 10.1±0.5 | 10.1±0.4 | <0.001 |
| kcal/da | 49.4±1.9 | 55±2.2 | 55.5±2.75 | 55.5±2.2 | <0.001 |
| FBS (mg/dL)a,c | 88.4±24.7 | 167.5±86.7 | 197.8±45.2 | 96.4±30.7 | 0.005 |
| TG (mg/dL)a,b,cd | 59.1±10.7 | 82.6±18.7 | 67.6±7.8 | 41.3±11.4 | <0.001 |
| TC (mg/dL)a,c,d | 75±6.5 | 101.2±12.1 | 91.1±10.8 | 74.9±10.7 | <0.001 |
| LDL-C (mg/dL)a,c | 36.7±5.9 | 52.1±12.2 | 43.4±13.1 | 31.4±14.1 | 0.002 |
| HDL-C (mg/dL) | 26.2±2.9 | 31.9±3.2 | 34.3±3.3 | 35.9±8.7 | 0.001 |
| ALT (IU/l)a,b,c | 71.4±14.3 | 149±13.3 | 95.6±19.7 | 99.3±22.2 | <0.001 |
| AST (IU/L)a,b,c | 115.3±40.3 | 198.7±21.1 | 124.7±11.4 | 141.2±46.2 | <0.001 |
| Serum Zn (µg/dL)a,b,c,d | 78±4.6 | 33±7 | 97±4 | 56±5 | <0.001 |
| Serum Se (µg/dL)a,b,d | 8.8±1.5 | 5.5±2.3 | 12.4±1.3 | 6.4±3.1 | <0.001 |
| Liver Zn (µg/g)a,b,c,d | 101±2.6 | 54.2±7 | 111±1.5 | 73.7±12 | <0.001 |
| Liver Se (µg/g)a,b,c,d | 5.6±1.08a | 2.2±1.07a,b,c | 7.7±1.02b,d | 11.2±1.25c,d | <0.001 |
cholesterol; TG: Triglyceride; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; ALT: Alanine aminotransferase; AST: Aspartate amino transferase; NFD: Normal AIN93M diet throughout the study; HFD: High-fat AIN93M diet throughout the study; S+HFD: Zinc and selenium co-supplementation at the beginning of the study and before disease progression; HFD+S: Zinc and selenium co-supplementation after disease progression;
significant difference between the NFD and HFD groups;
significant difference between the HFD and S+HFD groups;
significant difference between the HFD and HFD+S groups;
significant difference between the S+HFD and HFD+S groups;
P<0.05 as compared with baseline within the groups, values are reported as mean±SD.
One-way ANOVA, followed by post-hoc test, was used. (Significance was considered at a P<0.05.) TC: Total
Histological Analysis
As is shown in figure 2, more and lager fat granules were obtained in the HFD Group as a model for NAFLD progression. Thirty percent of liver cells were accumulated by fat granules in the HFD Group, compared with the NFD Group. The fat accumulation rate in the S+HFD Group and the HFD+S Group was 15.36% and 4.7%, correspondingly. Co-supplementation with zinc and selenium after disease progression (HFD+S) significantly decreased the size and number of fat granules in the liver compared with the HFD Group. Also, fat granules were significantly smaller and fewer in the liver of the HFD+S Group than in the liver of the S+HFD Group (P<0.001) (figure 2).
Figure 2.
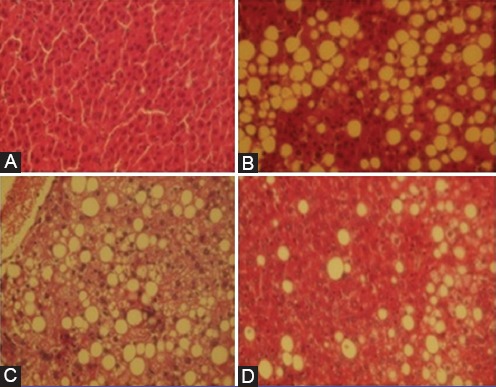
Micrographs show typical hepatic steatosis in the high-fat AIN93M group with a mass of more and larger fat granules. In contrast, fewer and smaller fat granules are observed in the HFD+S Group than in the HFD Group and the S+HFD Group. A: Normal-fat diet (NFD) group, B: High-fat diet (HFD) group, C: Zinc and selenium co-supplementation in the 1st 8 weeks of the study, before disease progression (S+HFD), D: Zinc and selenium co-supplementation in the last 8 weeks of the study, after disease progression (HFD+S).
Discussion
Recently a published meta-analysis14 and a review15 reported controversial results regarding the treatment of NAFLD. Various pharmacologic approaches focusing on weight reduction and reversal of insulin resistance were mentioned, either by lifestyle intervention alone or in combination with pharmacotherapy. However, besides sustained weight loss, no other single intervention has exhibited explicit statistically significant efficacy in the treatment of NAFLD. This highlights the need for novel approaches to the prevention and treatment of NAFLD. To our knowledge, the present study is the 1st animal research to compare the effects of zinc and selenium supplementation, before and/or after NAFLD progression, on serum glucose, lipid profile, hepatic enzymes, and fat accumulation. By feeding rats a high-fat diet (containing 82% of calories as fat), we induced marked hepatic lipid accumulation as assessed both by serum biochemical analysis and by histopathologic analysis. Our results demonstrated that a combination of zinc and selenium supplementation after fatty liver disease progression had better effects than before disease progression on serum glucose, lipid profile, and hepatic fat accumulation. Previous studies have shown that serum and liver zinc concentrations were significantly reduced in patients with alcoholic steatosis, hepatitis, and cirrhosis.16 Animal studies have demonstrated that dietary zinc supplementation attenuates alcohol-induced liver injury,17,18 suggesting the importance of zinc in the progression of alcoholic liver disease. While the link between zinc and selenium supplementation before and after NAFLD progression has not been determined, increasing evidence suggests that zinc plays a critical role in the regulation of hepatic lipid metabolism.19
In our study, zinc and selenium co-supplementation decreased serum lipid profile, as well as hepatic fat accumulation, after disease progression, while it had no more effects before disease creation because infection and inflammation significantly reduce the blood levels of zinc in chronic liver diseas.9 Stress hormones and pro-inflammatory markers such as tumor necrosis factor-α lead to change in zinc metabolism.20 On the other hand, blood selenium levels decrease in chronic liver disease.10 Thus, supplementation after disease progression exerts more significant effects due to the depletion of micronutrients.
Hyperglycemia increases the glycation of lipoproteins, including LDL and HDL, associated with the elevation of TG levels in the blood through increased synthesis from glucose and impaired lipid metabolism.21
In a human study in the U.K., researchers showed that higher serum selenium levels were associated with increased total and non-HDL-C levels, but not with HDL-C. These findings raise additional concern about the potential adverse lipid profile levels of high selenium status.22 Our study showed that zinc and selenium co-supplementation led to a reduction in disease progression, but it had no effect before disease progression. These results may be due to depletion in zinc and selenium resulting from fatty liver disease.
To the best of our knowledge, the current study is the 1st animal investigation to assess the effect of zinc supplementation with selenium on the prognosis of fatty liver disease, before and after disease progression. In our study, supplementation after disease progression improved serum glucose, lipid profile, and hepatic enzymes and histology. We created stage 1 of fatty liver with a high-fat diet. This is an animal model and, therefore, the effects of the diets cannot be directly transposed to humans. Rather, this study provides indications vis-à-vis the possible effects of such dietary manipulations on NAFLD progression and may, as such, suggest future insights for research into the underlying mechanisms.
Conclusion
NAFLD is an inflammatory disorder and leads to mineral deficiency in the liver and blood. Zinc and selenium co-supplementation after disease progression can reverse the progression by improving serum biochemical parameters (e.g. lipid profile and liver enzyme tests) and fat granule accumulation and size in the liver.
Acknowledgment
We express our gratitude to our colleagues in the Laboratory Animal Reproduction Research Center at Iran University of Medical Sciences, Tehran, Iran. The present article was financially supported by Iran National Science Foundation, Tehran, Iran (grant #91056877).
Conflict of Interest: None declared.
References
- 1.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis:a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Marsh S, Hu J, Feng W, Wu C. The Pathogenesis of Nonalcoholic Fatty Liver Disease:Interplay between Diet, Gut Microbiota, and Genetic Background. Gastroenterol Res Pract. 2016;2016:2–73. doi: 10.1155/2016/2862173. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–13. doi: 10.2337/db11-0832. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marceau P, Biron S, Hould FS, Marceau S, Simard S, Thung SN, et al. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999;84:1513–7. doi: 10.1210/jcem.84.5.5661. [DOI] [PubMed] [Google Scholar]
- 5.Song Y, Wang J, Li XK, Cai L. Zinc and the diabetic heart. Biometals. 2005;18:325–32. doi: 10.1007/s10534-005-3689-7. [DOI] [PubMed] [Google Scholar]
- 6.Simon SF, Taylor CG. Dietary zinc supplementation attenuates hyperglycemia in db/db mice. Exp Biol Med (Maywood) 2001;226:43–51. doi: 10.1177/153537020122600107. [DOI] [PubMed] [Google Scholar]
- 7.Begin-Heick N, Dalpe-Scott M, Rowe J, Heick HM. Zinc supplementation attenuates insulin secretory activity in pancreatic islets of the ob/ob mouse. Diabetes. 1985;34:179–84. doi: 10.2337/diab.34.2.179. [DOI] [PubMed] [Google Scholar]
- 8.Meerarani P, Reiterer G, Toborek M, Hennig B. Zinc modulates PPARgamma signaling and activation of porcine endothelial cells. J Nutr. 2003;133:3058–64. doi: 10.1093/jn/133.10.3058. [DOI] [PubMed] [Google Scholar]
- 9.McClain CJ, Antonow DR, Cohen DA, Shedlofsky SI. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res. 1986;10:582–9. doi: 10.1111/j.1530-0277.1986.tb05149.x. [DOI] [PubMed] [Google Scholar]
- 10.Ko WS, Guo CH, Yeh MS, Lin LY, Hsu GS, Chen PC, et al. Blood micronutrient, oxidative stress, and viral load in patients with chronic hepatitis C. World J Gastroenterol. 2005;11:4697–702. doi: 10.3748/wjg.v11.i30.4697. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamoulis I, Kouraklis G, Theocharis S. Zinc and the liver:an active interaction. Dig Dis Sci. 2007;52:1595–612. doi: 10.1007/s10620-006-9462-0. [DOI] [PubMed] [Google Scholar]
- 12.da Rocha JT, Speranca A, Nogueira CW, Zeni G. Hypolipidaemic activity of orally administered diphenyl diselenide in Triton WR-1339-induced hyperlipidaemia in mice. J Pharm Pharmacol. 2009;61:1673–9. doi: 10.1211/jpp/61.12.0013. [DOI] [PubMed] [Google Scholar]
- 13.Petts G, Lloyd K, Goldin R. Fatty liver disease. Diagn Histopathol. 2014;20:102–8. doi: 10.1016/j.mpdhp.2014.01.008. [DOI] [Google Scholar]
- 14.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 15.Schuppan D, Gorrell MD, Klein T, Mark M, Afdhal NH. The challenge of developing novel pharmacological therapies for non-alcoholic steatohepatitis. Liver Int. 2010;30:795–808. doi: 10.1111/j.1478-3231.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Moreno F, Gonzalez-Reimers E, Santolaria-Fernandez F, Galindo-Martin L, Hernandez-Torres O, Batista-Lopez N, et al. Zinc, copper, manganese, and iron in chronic alcoholic liver disease. Alcohol. 1997;14:39–44. doi: 10.1016/s0741-8329(96)00103-6. [DOI] [PubMed] [Google Scholar]
- 17.Kang X, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation enhances hepatic regeneration by preserving hepatocyte nuclear factor-4alpha in mice subjected to long-term ethanol administration. Am J Pathol. 2008;172:916–25. doi: 10.2353/ajpath.2008.070631. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ. Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term ethanol exposure. Exp Biol Med (Maywood) 2008;233:540–8. doi: 10.3181/0710-RM-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadhim HM, Ismail SH, Hussein KI, Bakir IH, Sahib AS, Khalaf BH, et al. Effects of melatonin and zinc on lipid profile and renal function in type 2 diabetic patients poorly controlled with metformin. J Pineal Res. 2006;41:189–93. doi: 10.1111/j.1600-079X.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 20.Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol. 1997;272:E952–6. doi: 10.1152/ajpendo.1997.272.6.E952. [DOI] [PubMed] [Google Scholar]
- 21.Laakso M, Lehto S. Epidemiology of risk factors for cardiovascular disease in diabetes and impaired glucose tolerance. Atherosclerosis. 1998;137(Suppl):S65–73. doi: 10.1016/S0021-9150(97)00314-6. [DOI] [PubMed] [Google Scholar]
- 22.Stranges S, Laclaustra M, Ji C, Cappuccio FP, Navas-Acien A, Ordovas JM, et al. Higher selenium status is associated with adverse blood lipid profile in British adults. J Nutr. 2010;140:81–7. doi: 10.3945/jn.109.111252. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


