Abstract
Objective
Interferon Regulatory Factor 6 (IRF6) is critical for craniofacial development, epidermal differentiation, and tissue repair. IRF6 mutations cause Van der Woude Syndrome (VWS) and Popliteal Pterygium Syndrome. Individuals with VWS exhibit craniofacial anomalies, including cleft lip and palate and lip pits. Furthermore, they have an increased risk for wound-healing complications following surgical repair when compared with patients with nonsyndromic cleft lip and palate (NSCLP). However, nothing is known about the skin of these patients. The objective was to characterize the skin of patients with VWS. We hypothesize that IRF6 is required for proper skin homeostasis in humans.
Design
Discarded tissue from a hip was collected during surgical alveolar bone graft. Samples from children with VWS harboring IRF6 mutations (n = 2) were compared with samples from children with NSCLP (n = 7). Histology was assessed following hematoxylin and eosin staining. The expressions of Proliferating Cell Nuclear Antigen, IRF6, P63, and Keratin 10 were determined by immunofluorescence. Keratinocytes were isolated and their proliferation potential was assessed by colony-forming efficiency assay.
Results
Hip skin from children with VWS showed a thicker epidermis when compared with that from children with NSCLP. Proliferating Cell Nuclear Antigen staining revealed an increase in proliferation in syndromic tissues when compared with controls. However, P63 and Keratin 10 expression were similar between groups. Finally, keratinocytes from VWS showed increased long-term proliferation when compared with NSCLP.
Conclusions
These results support, in vivo and in vitro, a previously described role for IRF6 in epidermal proliferation in humans. They further demonstrate a critical function for IRF6 in cutaneous homeostasis.
Keywords: IRF6, skin, proliferation, keratinocytes
Interferon Regulatory Factor 6 (IRF6) belongs to the interferon regulatory factor family of transcription factors. There are nine members in the interferon regulatory factor family, most of which are involved in mediating the interferon response following viral infection as well as in the innate immune response (Taniguchi et al., 2001). IRF6, however, functions as a tumor suppressor and plays a role in wound healing, keratinocyte migration, and epidermal differentiation and is most known for being required in craniofacial development (Ingraham et al., 2006; Jones et al., 2010; Botti et al., 2011; Biggs et al., 2012, 2014).
Mice deficient for irf6 exhibit abnormal skin, limb, and craniofacial morphogenesis (Ingraham et al., 2006). The major cutaneous histological feature of the irf6-deficient mice shows a lack of stratified epidermis. The epidermis is predominantly composed of epithelial cells called keratinocytes and can be further divided into basal and suprabasal layers. The differentiation process from the basal layer up to the surface of the skin is accompanied by changes in key proteins that can be used as characteristic markers of the differentiation process (Biggs et al., 2015). For example, Keratin 14 and Proliferating Cell Nuclear Antigen (PCNA) are both expressed in keratinocytes of the basal layer (Furukawa et al., 1992), whereas Irf6 and Keratin 10 (K10) are both detected in the suprabasal layers (Biggs et al., 2012). Protein 63 (P63) is mainly present in the basal layer, but is also detected in the spinous layer (lower suprabasal layer) of human tissue (Leonard et al., 2011) and part of a feedback loop with IRF6 (Moretti et al., 2010).
The epidermis of Irf6-deficient embryos is thicker when compared with wild-type embryos. Furthermore, the suprabasal keratinocytes fail to stop proliferating and never terminally differentiate, leading to the absence of the stratum corneum, the outermost layer of the epidermis responsible for the cutaneous barrier function. These results, along with our studies with keratinocytes in vitro (Biggs et al., 2012), demonstrated that IRF6 plays a significant role in regulating proliferation and differentiation of keratinocytes.
In humans, mutations in IRF6 cause two orofacial clefting syndromes, Van derWoude Syndrome (VWS) and Popliteal Pterygium Syndrome (PPS) (Kondo et al., 2002). In addition to cleft lip and palate, lip pits on lower lips and webbing behind the knees characterize patients with VWS and PPS, respectively. Interestingly, individuals with VWS (haploinsufficient for IRF6) have increased wound-healing complications following surgical repair when compared with individuals with isolated cleft (nonsyndromic cleft lip and palate [NSCLP], no mutation in IRF6; Jones et al., 2010). These results led us to hypothesize that skin from individuals with VWS may be altered when compared with the skin from individuals with NSCLP. However, no study has been conducted to systematically test this hypothesis.
In the current work, we compared the proliferative characteristics of keratinocytes from individuals with VWS with those from individuals with NSCLP. Our results show that the rate of proliferation of keratinocytes was increased in the skin from individuals with VWS when compared with NSCLP, both in vivo and in vitro.
Methods
Informed consent for each child was obtained according to approved institutional review board protocols from the University of Iowa. Discarded skin from the hip of patients with cleft lip and palate (NSCLP and VWS) was collected following alveolar bone grafting surgery. The same surgeon performed the procedures for all of the children. Samples were obtained from eight children with NSCLP and two children with VWS.
A portion of the tissue was fixed in 4% paraformaldehyde and embedded in paraffin. Each paraffin block was then sectioned, and 7-μm thick sections were placed on microscope slides. For histological examination, the slides were stained with hematoxylin and eosin. The thickness of the epidermis was measured between the rete ridges at four different sites per section using Image J (National Institutes of Health, Bethesda, MD). Two to three sections per sample were analyzed. Protein expression was detected using immunofluorescence as previously described (Biggs et al., 2014). Primary antibodies used were: rabbit polyclonal against human IRF6 (Bailey et al., 2005), rabbit polyclonal against PCNA (Biomeda, Foster City, CA), rabbit polyclonal against mouse K10 (Covance, Emerville, CA), and mouse monoclonal against human P63 (clone 4A4; Santa Cruz, SantaMonica, CA). They were detected by the use of secondary antibodies: goat anti rabbit alexa 568 (Molecular Probe, Life Technology, Grand Island, NY) and goat anti mouse FITC (Sigma, St. Louis, MO). DAPI was used to label the nuclear deoxyribonucleic acid. All slides were visualized with a Nikon E800 Eclipse (Nikon, Tokyo, Japan) and photomicrographs acquired with a SPOT RT Slider Diagnostic Instruments CCD camera using Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI). Immunofluorescent images were acquired in black and white, pseudocolorized, and merged.
We quantified the proliferating cells in the epidermis. The number of cells present in the basal layer was counted and recorded using DAPI as a marker for nuclear DNA. PCNA-positive cells in the basal layer were counted and recorded. The percentage of proliferating cells in the basal layer was calculated as the ratio of PCNA+:DAPI+.
The remaining discarded tissue was used to extract keratinocytes from the epidermis as previously described (Michel et al., 1996). Briefly, the tissue was washed with phosphate-buffered saline with 1% penicillin-streptomycin, cut in 2-mm wide strips, and incubated in thermolysin 0.5 mg/ml overnight at 4°C (Germain et al., 1993). The following day, the epidermis was peeled from the dermis and placed in 0.25% trypsin–ethylenediaminetetraacetic acid for 30 minutes at 37°C. Keratinocytes were then grown with a previously irradiated NIH3T3 feeder layer as previously described (Rheinwald and Green, 1975). Colony forming efficiency (CFE) assay was assessed 12 days following the plating of 500 keratinocytes per 60-mm dishes as previously described (Rheinwald and Green, 1975).
Saliva was obtained from the affected children, and DNA was extracted per standard protocols. Genotyping for the genetic variants rs642961 and rs2235371 was performed using TaqMan assays and the primers previously described (Rahimov et al., 2008; Beaty et al., 2010). Genotyping was completed using TaqMan SNP Genotyping Assays (Applied BioSystems/Life Technologies, Foster City, CA) and detected using the Applied BioSystems Prism 7900 HT. Sanger sequencing of exonic regions of the IRF6 gene was performed for children diagnosed with VWS. All sequencing was performed by Functional Biosciences, Inc. (Madison, WI).
Results
To determine the effect of IRF6 mutations on keratinocyte proliferation in humans, we collected discarded skin from the hips of children undergoing alveolar bone graft surgery. We carefully controlled for the same anatomical site (the hip), the age of the children (8 to 9 years old), and the surgical procedure. All of these parameters were identical between our children with VWS (carrying a heterozygous mutation in the IRF6 gene) and children with NSCLP (Table 1). Furthermore, we genotyped all participants for two genetic variants that have been previously reported to be associated with cleft lip and palate (rs642961 and rs2235371) and found a similar distribution between children with VWS and children with NSCLP. Because of these stringent parameters, we identified two children with VWS and eight children with NSCLP (who will serve as the control group) to carry on our study.
TABLE 1.
Characteristics of Included Children With Cleft Lip and Palate
| Patient | Cleft Type† | Lip Pits | Genotype at rs642961 | Genotype at rs2235371 | IRF6 Mutation |
|---|---|---|---|---|---|
| 1* | BCL+A | Yes | GG | GG | R250Q (de Lima et al., 2009) |
| 2 | UCL+A | Yes | nd | GA | R412X (de Lima et al., 2009) |
| 3 | UCLP+A | No | GG | nd | |
| 4 | CLP | No | GG | GG | |
| 5 | BCLP+A | No | GG | GG | |
| 6 | UCLP | No | GG | GA | |
| 7 | BCLP+A | No | GG | GG | |
| 8 | BCLP+A | No | GG | nd | |
| 9 | UCLP+A | No | GG | GG |
This child is the same as individual no. 14 in our previous study (Jones et al., 2010).
CP= cleft palate only; CLP = cleft lip/palate; CL = cleft lip only; A = alveolus; U = unilateral; B = bilateral; nd = not determined.
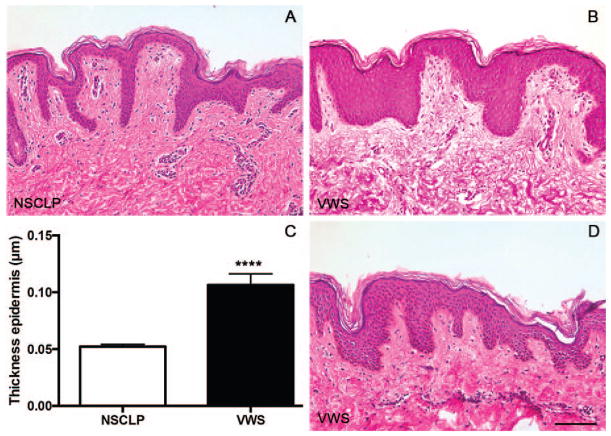
By examining the histological features of the skin from children with NSCLP, we observed a rather thin epidermis composed of 5 to 10 layers of keratinocytes that was well organized with formed deep rete ridges (Fig. 1A). However, cutaneous hip biopsies from children with VWS exhibited a thick and compact epidermis and lacked the deep rete ridges (Fig. 1B and 1D). We measured the actual thickness of the epidermis and found that children with VWS had an epidermis two times as thick as the epidermis of children with NSCLP (Fig. 1C). The dermis of all of the biopsies appeared similar between the two groups. Collectively, these data suggest that IRF6 mutations alter the epidermal thickness in human skin.
FIGURE 1.
The epidermis of children with Van der Woude syndrome (VWS) is thicker when compared with the epidermis of children with nonsyndromic cleft lip and palate (NSCLP). A, B, D: Hematoxylin and eosin staining of skin from the hips of children with NSCLP (A) or VWS (B, D). C: Thickness of the epidermis (NSCLP, n = 6; VWS, n = 2). ****P < .0001 after unpaired t test. Scale bar = 100 μm.
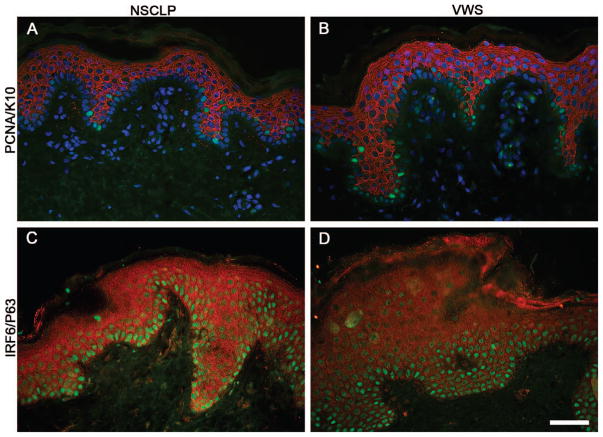
To further investigate the epidermis in children with VWS, we examined the expression of PCNA, a marker of proliferating cells. PCNA was detected in the basal layer of the epidermis (K10-negative cells) in skin from children with NSCLP and VWS (Fig. 2A and 2B). However, we observed a twofold increase in the percentage of PCNA-positive cells in the basal layer of VWS when compared with NSCLP (Table 2). K10, a marker of epidermal differentiation, was restricted to suprabasal cells in both groups (Fig. 2A and 2B), suggesting the initiation of epidermal differentiation is initially proper. P63 is typically restricted to the basal layer of the epidermis in the mouse (Biggs et al., 2012). However, in human skin it has been previously reported to extend its expression up to the first suprabasal layer of keratinocytes (Furukawa et al., 1992). Our results are consistent with these findings in both groups (Fig. 2C and 2D). Although children with VWS carry a mutation in IRF6, they are heterozygotes for the mutation, suggesting a reduction in the level of IRF6 expression. As shown in Fig. 2C, we detected IRF6 throughout the epidermis of the skin from children with NSCLP. The level of expression appeared to be decreased in the epidermis of children with VWS when compared with those from NSCLP (Fig. 2D), yet immunofluorescent staining is hardly amenable to quantitative studies. Overall, our results demonstrate a role for IRF6 in regulating the proliferation of keratinocytes in vivo, with no apparent effect on markers of early differentiation.
FIGURE 2.
Increased proliferation in the epidermis of Van der Woude syndrome (VWS) when compared with nonsyndromic cleft lip and palate (NSCLP). A, B: Immunodetection of Proliferating Cell Nuclear Antigen (green) and Keratin 10 (red) in cutaneous sections from children with NSCLP (A) or with VWS (B). (C, D) Immunodetection of Protein 63 (green) and Interferon Regulatory Factor 6 (red) in cutaneous sections from children with NSCLP (C) or with VWS (D). DAPI labels nuclear deoxyribonucleic acid (A, B). Scale bar = 50 μm.
TABLE 2.
Percentage of Proliferating Cells in Epidermis From Children With NSCLP and VWS†
| Patient-Clinical Phenotype | Percentage of PCNA+ Cells‡ | Average | P* |
|---|---|---|---|
| 1-VWS | 12.87 | ||
| 2-VWS | 10.23 | ||
| Average VWS | 11.55 | ||
| 3-NSCLP | 5.89 | ||
| 4-NSCLP | 4.44 | ||
| 5-NSCLP | 5.56 | ||
| 6-NSCLP | 5.19 | ||
| 7-NSCLP | 3.96 | ||
| 8-NSCLP | 8.62 | ||
| 9-NSCLP | 2.5 | ||
| Average NSCLP | 5.16 | .004 |
NSCLP = nonsyndromic cleft lip and palate; VWS = Van der Woude syndrome; PCNA = Proliferating Cell Nuclear Antigen.
Data are means of two to four technical replicates.
P < .05 is considered significant after Student’s t test between the 2 groups.
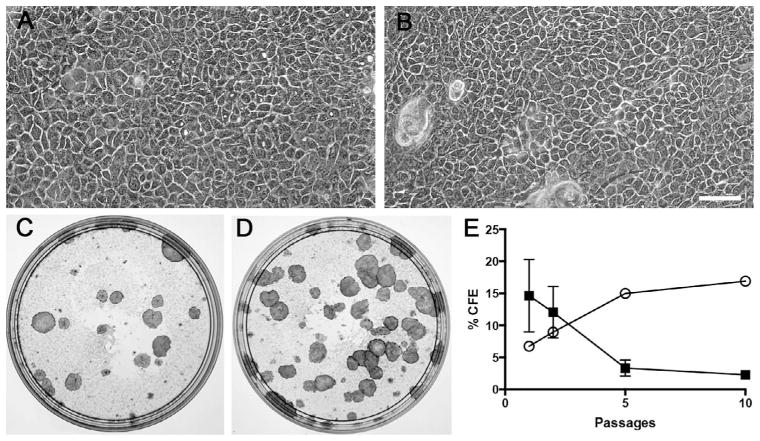
We previously demonstrated that IRF6 was required for in vitro proliferation (Biggs et al., 2012). Using murine keratinocytes, we showed that cells from Irf6-deficient embryos exhibited increased CFE, a standard indicator of keratinocyte proliferation and lifespan (Rheinwald and Green, 1975) as well as long-term proliferation (Biggs et al., 2012). To determine if IRF6 also had an effect on human cell proliferation in vitro, we isolated keratinocytes from the hip skin of patient 1 (VWS) and patients 3 to 9 (NSCLP). Keratinocytes from both groups exhibited similar morphology (Fig. 3A and 3B). After the first subculture, CFE ranged from 0.6% to 38.2% (average of 13.67%) in cells from children with NSCLP, whereas it was 6% in keratinocytes from VWS. The serial subculture of keratinocytes from children with NSCLP showed that of seven primary lines established, only six were successfully subcultured twice, four subcultured five times, and one 10 times. During that time, the CFE decreased from 13.6% to 2.3%. On the other hand, cells from the child with VWS were not only successfully subcultured 10 times but also the CFE increased from 6% to 17%. Cells were not carried further than 10 subcultures. Together, these data are anecdotal evidence that human mutations in IRF6 affect the keratinocyte proliferation in vitro.
FIGURE 3.
Increased long-term proliferative potential in keratinocytes from Van der Woude syndrome (VWS) when compared with nonsyndromic cleft lip and palate (NSCLP). A, B: Phase contrast micrographs of keratinocytes obtained from the skin of children with NSCLP (A) or VWS (B). C to E: Macrographs of colony forming efficiency (CFE) assay performed with keratinocytes from children with NSCLP (C) or VWS (D). E: Average of CFE over 10 culture passages (solid square, NSCLP, n=7 at passage 1 [P1], n=6 at P2, n=5 at P5, n=1 at P10; open circle, VWS, n=1). Data are means ± SEM. Scale bar = 100 μm.
Discussion
In the present study, we report that the epidermis of children with VWS baring mutations in IRF6 is thicker when compared with the epidermis of children with NSCLP carrying no IRF6 variants. We suggest that this phenotype is a result of increased keratinocyte proliferation in a cell-autonomous fashion. Our data are consistent with a previously described role for IRF6 in epidermal proliferation, and further support in humans is provided of a critical role for IRF6 in epidermal homeostasis.
Having access to mice heterozygotes and homozygotenull for Irf6, we previously evaluated epidermal thickness and proliferation in their skin. The results, from published and unpublished work, demonstrate that only mice deficient for Irf6 exhibit a cutaneous phenotype, with an increased thickness of the epidermis and ectopic proliferation in the first few suprabasal keratinocytes (Ingraham et al., 2006). Skin from heterozygous mice was indistinguishable from wild type (Canady, Biggs, and Dunnwald, personal communication). Although the heterozygous mouse may be the closest in vivo model for VWS because they both harbor one unaffected IRF6 allele, its epidermis is far from being similar to the human epidermis. Particularly, the thickness of the murine suprabasal layers is limited to two to three cells thick, whereas it is 8 to 10 cell layers in humans. Because of such a thin tissue, it may require more than 50% reduction in Irf6 to observe an effect on the murine epidermis. Of note, no living human has ever been reported with homozygous IRF6 mutations (likely because of the severity of the phenotype incompatible with life), therefore the comparison with Irf6-deficient mice is not possible.
IRF6 is known to regulate craniofacial development and epidermal proliferation (Ingraham et al., 2006; Biggs et al., 2012). Consequently, patients carrying IRF6 mutations exhibit cleft lip and palate (Kondo et al., 2002). In this study, we show that they also exhibit increased proliferation in the basal layer of their epidermis. Not only did our findings demonstrate the change in proliferation in cutaneous tissue but also in keratinocyte in culture. Particularly, we observed an increase in CFE in cells from a child with VWS when compared with cells from children with NSCLP. This is reminiscent of our findings with murine keratinocytes, which also showed an increase in CFE in Irf6-deficient keratinocytes when compared with wild-type cells (Biggs et al., 2014). Interestingly, the initial proliferation potential (as assessed by the CFE) was not very high in VWS keratinocytes, but increased over several subcultures. This is in contrast with cells from NSCLP that had their initial CFE high and then declined to the point that only one of seven cell lines were still proliferating after 10 passages. We saw a similar phenomenon in murine keratinocytes, where wild-type and Irf6-deficient keratinocytes proliferated at the same rate for the first few days, then Irf6-deficient cells showed increased proliferation when compared to control. These results not only support the overall function for IRF6 as a regulator of epithelial cell proliferation but also demonstrate that this effect is cell autonomous and conserved between humans and mice.
We did not perform a systematic analysis of markers of epidermal differentiation in our samples. However, we examined the expression of K10, P63, and IRF6 and did not detect ectopic expression of these proteins in samples from VWS when compared with NSCLP. Although immunofluorescence is not conductive to quantitative studies, it appears that the level of IRF6 was decreased in the epidermis of children with VWS when compared with samples from children with NSCLP. This would support a functional role for the IRF6 mutations and is consistent with the notion that individuals with IRF6 mutations are haploinsufficient.
VWS is a rare syndrome with an estimated frequency of one in 30,000 live births (Dixon et al., 2011). At the University of Iowa, about two patients per year will undergo cleft repair surgery. It is well known that cellular proliferation is highly variable based on the age of the patient and the anatomical origin of the sample (Rheinwald and Green, 1975; Michel et al., 1996). We realize that our study was performed with only one or two samples from children with VWS, but our control group had eight children that were matched for both age and anatomical site. We collected a few additional samples from children with VWS, but the age and anatomical origin of the sample was variable with no appropriate controls. Given these facts, we believe our results have scientific merit; to our knowledge, they are the first to evaluate keratinocyte behavior in children with VWS. It would be interesting to extend our findings to other discarded tissue from the site of the orofacial defect (collected during primary lip repair) as well as to use the cells to test for other previously described IRF6 functions (such as wound healing or tumor suppression).
Acknowledgments
The authors are grateful for the contribution of all the patients and their families. They acknowledge Jeff Murray, Steve Goudy, and Brian Schutte for the inspiration of these studies, and Jamie L’Heureux, Nichole Nidey, and Jennifer Rigdon for their assistance with this project. Partial financial support was provided by a grant from the National Institute of Health, R37DE08559. KatieHixonwas supported by funds from the Iowa Center for Research for Undergraduates.
Partial financial support was provided by a grant from the National Institutes of Health (R37DE08559).
Footnotes
A portion of this study was presented at the 2013 Biomedical Engineering Society (BMES) Annual Meeting in Seattle, Washington.
Contributor Information
Ms. Katherine Hixon, Department of Pediatrics, The University of Iowa.
Ms. Lindsey Rhea, Department of Pediatrics, The University of Iowa.
Ms. Jennifer Standley, Department of Pediatrics, The University of Iowa.
Mr. Frank J. Canady, Department of Pediatrics, The University of Iowa.
Dr. John W. Canady, Department of Pediatrics, The University of Iowa; Department of Plastic Surgery, The University of Iowa, Iowa City, Iowa.
Dr. Martine Dunnwald, Department of Pediatrics, The University of Iowa.
References
- Bailey CM, Kahalkahali-Ellis Z, Kondo S, Margaryan NV, Seftor REB, Wheaton WW, Amir S, Pins MR, Schutte BC, Hendrix MJC. Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6. Identification of a novel serpin partnership. J Biol Chem. 2005;280:34210–34217. doi: 10.1074/jbc.M503523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty TH, Murray JC, Marazita ML, Munger RG, Ruczinski I, Hetmanski JB, Liang KY, Wu T, Murray T, Fallin MD, et al. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat Genet. 2010;42:525–531. doi: 10.1038/ng.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs LC, Goudy SL, Dunnwald M. Palatogenesis and cutaneous repair: a two-headed coin. Dev Dyn. 2015;244:289–310. doi: 10.1002/dvdy.24224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs LC, Naridze R, Demali KA, Lusche DF, Kuhl S, Soll DR, Schutte BC, Dunnwald M. Interferon Regulatory Factor 6 regulates keratinocyte migration. J Cell Sci. 2014;127:2840–2848. doi: 10.1242/jcs.139246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs LC, Rhea L, Schutte BC, Dunnwald M. Interferon regulatory factor 6 is necessary, but not sufficient, for keratinocyte differentiation. J Invest Dermatol. 2012;132:50–58. doi: 10.1038/jid.2011.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botti E, Spallone G, Moretti F, Marinari B, Pinetti V, Galanti S, De Meo PD, De Nicola F, Ganci F, Castrignano T, et al. Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas. Proc Natl Acad Sci U S A. 2011;108:13710–13715. doi: 10.1073/pnas.1110931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima RL, Hoper SA, Ghassibe M, Cooper ME, Rorick NK, Kondo S, Katz L, Marazita ML, Compton J, Bale S, et al. Prevalence and nonrandom distribution of exonic mutations in interferon regulatory factor 6 in 307 families with Van der Woude syndrome and 37 families with popliteal pterygium syndrome. Genet Med. 2009;11:241–247. doi: 10.1097/GIM.0b013e318197a49a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MJ, Marazita ML, Beaty TH, Murray JC. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12:167–178. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa F, Imamura S, Fujita M, Kinoshita K, Yoshitake K, Brown WR, Norris DA. Immunohistochemical localization of proliferating cell nuclear antigen/cyclin in human skin. Arch Dermatol Res. 1992;284:86–91. doi: 10.1007/BF00373375. [DOI] [PubMed] [Google Scholar]
- Germain L, Rouabhia M, Guignard R, Carrier L, Bouvard V, Auger FA. Improvement of human keratinocyte isolation and culture using thermolysin. Burns. 1993;2:99–104. doi: 10.1016/0305-4179(93)90028-7. [DOI] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–1340. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Canady JW, Brookes JT, Wehby GL, L’Heureux J, Schutte BC, Murray JC, Dunnwald M. Wound complications after cleft repair in children with Van der Woude syndrome. J Craniofac Surg. 2010;21:1350–1353. doi: 10.1097/SCS.0b013e3181ec6aad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Schutte BC, Richardson RJ, Bjork BC, Knight AS, Watanabe Y, Howard E, Ferreira de Lima RLL, Daack-Hirsch S, Sander A, et al. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–289. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MK, Kommagani R, Payal V, Mayo LD, Shamma HN, Kadakia MP. DeltaNp63alpha regulates keratinocyte proliferation by controlling PTEN expression and localization. Cell Death Differ. 2011;18:1924–1933. doi: 10.1038/cdd.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Török N, Godbout M-J, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- Moretti F, Marinari B, Lo Iacono N, Botti E, Giunta A, Spallone G, Garaffo G, Vernersson-Lindahl E, Merlo G, Mills AA, et al. A regulatory feedback loop involving p63 and IRF6 links the pathogenesis of 2 genetically different human ectodermal dysplasias. J Clin Invest. 2010;120:1570–1577. doi: 10.1172/JCI40267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimov F, Marazita ML, Visel A, Cooper ME, Hitchler MJ, Rubini M, Domann FE, Govil M, Christensen K, Bille C, et al. Disruption of an AP-2alpha binding site in an IRF6 enhancer is associated with cleft lip. Nat Genet. 2008;40:1341–1347. doi: 10.1038/ng.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]