Abstract
Osteocytes are terminally differentiated bone cells, derived from osteoblasts, which are vital for the regulation of bone formation and resorption. ECM stiffness and cell seeding density have been shown to regulate osteoblast differentiation, but the precise cues that initiate osteoblast–osteocyte differentiation are not yet understood. In this study, we cultured MC3T3-E1 cells on (A) substrates of different chemical compositions and stiffnesses, as well as, (B) substrates of identical chemical composition but different stiffnesses. The effect of cell separation was investigated by seeding cells at different densities on each substrate. Cells were evaluated for morphology, alkaline phosphatase (ALP), matrix mineralisation, osteoblast specific genes (Type 1 collagen, Osteoblast specific factor (OSF-2)), and osteocyte specific proteins (dentin matrix protein 1 (DMP-1), sclerostin (Sost)). We found that osteocyte differentiation (confirmed by dendritic morphology, mineralisation, reduced ALP, Col type 1 and OSF-2 and increased DMP-1 and Sost expression) was significantly increased on soft collagen based substrates, at low seeding densities compared to cells on stiffer substrates or those plated at high seeding density. We propose that the physical nature of the ECM and the necessity for cells to establish a communication network contribute substantially to a concerted shift toward an osteocyte-like phenotype by osteoblasts in vitro.
Keywords: Bone, Osteoblast, Osteocyte, Cellular differentiation, Extracellular mechanical, environment
1. Introduction
Osteocytes make up over 90% of cells in mature bone (Boukhechba et al., 2009). They play a vital role in skeletal health by acting as mechanosensors that monitor the mechanical environment within bone tissue and signalling to osteoblasts and osteoclasts to remodel the tissue so that bone strength is maintained throughout life (Klein-Nulend et al., 1995; Lanyon, 1993; Jee, 2001). Osteocytes are formed when osteoblasts undergo a dramatic phenotypic transition as they become embedded within newly deposited bone matrix. During this transition their morphology is altered from cuboidal to the dendritic shape associated with osteocytes, which is defined by a rounded cell body, a large nucleus, and long cell processes that extend from the cell body. These processes contact neighbouring cells both within and on the surface of the bone (Marotti et al., 1996; Palazzini et al., 1998; Jee, 2001), and thereby form functional syncitia by establishing a gap junctional communication at these contact points (Donahue, 1998; Palazzini et al., 1998). The gene expression pattern of the cells also undergoes a dramatic change. Expression of the osteoblast marker enzyme alkaline phosphatase (ALP) is greatly reduced (Jee, 2001; Nakano et al., 2004), along with a reduction in type I collagen (ColT1), bone morphogenetic protein 2 (BMP-2) and osteoblast specific factor 2 (OSF-2, also known as periostin) expression (Igarashi et al., 2002; Santos et al., 2011; Wilde et al., 2003). Expression of osteocalcin (Weinreb et al., 1990; Boivin et al., 1990) and E11 (Zhang et al., 2006) is increased, while osteocyte specific markers such as PHEX (Westbroek et al., 2002), Sclerostin (Sost) (Poole et al., 2005) and dentin matrix protein 1 (DMP-1) (Feng et al., 2003; Rios et al., 2005) are either induced de novo or dramatically upregulated.
Type 1 collagen and OSF-2 are early stage osteocyte markers, which are downregulated as osteoblasts begin to develop into osteocytes in vitro (Kato et al., 1997, 2001). DMP-1 is upregulated as the cells begin to extend processes and mineralise their surrounding matrix, while Sost is a late stage osteocyte marker necessary for the regulation of mineralisation (Atkins et al., 2009). The highly selective expression of molecules such as (ColT1) and OSF-2 in osteoblasts, and Sost and DMP-1 in osteocytes has led to their use as phenotypic markers in many studies (Kato et al., 2001; Gu et al., 2006; Gooi et al., 2010; Kramer et al., 2010; Krishnan et al., 2010). However, there remains very little understanding of the cues that control the phenotypic shift from osteoblasts to osteocytes.
Among the possible stimuli for the phenotypic shift from osteoblast to osteocyte are changes in the stiffness of the cell’s extracellular matrix (ECM). ECM stiffness has been shown to strongly regulate a variety of cell behaviours such as migration, proliferation, and differentiation in both osteogenic and non-osteogenic cells (Pelham and Wang, 1997; Khatiwala et al., 2006b; Hsiong et al., 2008). It has been shown that MSC differentiation along with different phenotypic lineages (i.e. adipogenic, myogenic, osteogenic) is dependent in part on substrate stiffness (Engler et al., 2006). In particular MSCs were shown to differentiate into osteoblast-like cells when cultured on collagen coated polyacrylamide substrates, with stiffnesses in the range of 25–40 kPa, whereas cells take on the characteristics of neurons or myoblasts when cultured on substrates with stiffness values similar to those of brain (≈1 kPa) and muscle tissue (≈11 kPa), respectively.
The separation distance of the cells, as controlled by cell seeding density, can also influence the differentiation of osteogenic cells during in vitro cell culture experiments. Cell seeding density plays a role in regulating osteoblast proliferation and matrix mineralisation in three-dimensional constructs in vitro (Holy et al., 2000; Xiao et al., 2006), while the seeding density of human bone marrow stromal cells is an important factor for the development of cell matrix constructs for bone tissue engineering (Lode et al., 2008). Osteogenic differentiation of MSCs in two dimensional culture, as examined by ALP and BMP-2 expression, has also been shown to be increased when cells are cultured at a low seeding density (3× 104 cells/cm2) (Kim et al., 2009). To date, however, research into the effects of both ECM stiffness and cell separation on osteogenic differentiation has focussed on osteoblast differentiation rather than the osteoblast–osteocyte transition. Osteocyte differentiation is necessary for mature bone tissue formation and as such an understanding of this stage of the differentiation pathway is crucial for the development of future tissue engineering strategies for bone.
In this study, we test the hypothesis that osteocyte differentiation is regulated by both ECM stiffness and intercellular separation. To address this we employ in vitro culture of MC3T3-E1 cells to compare cellular differentiation on substrates of different chemical compositions and stiffnesses, as well as on substrates of identical chemical composition but different stiffnesses. The effect of intercellular separation is investigated through the culture of cells at varied initial seeding densities on each of the chosen substrates. Osteocyte differentiation is then examined by quantifying cellular morphology, ALP activity and matrix mineralisation, as well as by the expression of the osteogenic genes ColT1, OSF-2, DMP-1 and Sost.
2. Materials and methods
Experiments were designed to test whether MC3T3-E1 (a pre-osteoblast cell line) cells would undergo phenotypic changes associated with osteoblast–osteocyte transition in vivo, under specific experimental conditions that investigated the role of ECM composition, ECM stiffness and cell seeding density. Morphological changes in MC3T3-E1 cells were assessed, paying particular attention to the development of dendritic cells. ALP activity was quantified using a colorimetric assay, mineralisation was assessed using an alizarin red/cetylpyridinium chloride assay while the expression of Col Type I, OSF-2, DMP-1 and Sost gene’s was quantified by RT-PCR.
2.1. Experimental design: substrate stiffness and seeding density experiments
Preliminary studies investigated the effect of a range of different substrates and seeding densities on MC3T3-E1 morphology. The results of these studies were used to select the experimental conditions outlined here. MC3T3-E1 cells were plated at seeding densities of 103 or 104 cells/cm2 and cultured on: (a) NaOH neutralised collagen (Col), (b) NaOH neutralised collagen cross-linked with N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDAC) at 2.5 mM (ColEDAC1), (c) NaOH neutralised collagen crosslinked with EDAC at 12.5 mM (ColEDAC2), (d) acetic acid neutralised collagen (ColAA), (e) thin matrigel (Mat-Thin), (d) thick matrigel (MatThick) and (f) uncoated tissue culture plastic (TC plastic). Cells were allowed to grow on all substrates for 1, 4, 9, 14 or 21 days, respectively. Triplicate wells were analysed for each condition to quantify cell morphology, ALP expression, ECM mineralisation and gene expression. MLO-Y4 cells were cultured for 4 days on acetic acid neutralised collagen (ColAA) as a positive control (Kato et al., 1997).
2.2. Cell culture
Two bone cell lines were used in this study. MC3T3-E1 cells are a murine derived osteoblast cell line, which can express high amounts of ALP, type 1 collagen and OSF-2 (Hurley et al., 1993; Oshima et al., 2002). The cells are capable of differentiating into osteocytes and mineralising their surrounding matrix (Sudo et al., 1983). They are considered to be a good model of primary osteoblasts (Quarles et al., 1992). For these studies MC3T3-E1 cells were maintained in Alpha Modified Eagle’s Medium (α-MEM) supplemented with 10% foetal bovine serum, 100 U/mL penicillin streptomycin and 100 μg/mL L-glutamine (all Sigma Aldrich) prior to all experiments.
MLO-Y4 cells are a murine derived cell line which share numerous characteristics with primary osteocytes such as high production of osteocalcin and E11 and low expression of alkaline phosphatase and OSF-2, as well as the extension of numerous dendritic processes per cell (Kato et al., 1997; Bonewald, 1999). MLO-Y4 cells were cultured at 5× 103 cells/cm2 on type 1 collagen at 0.15 mg/mL in acetic acid and cultured in Dulbecco’s MEM supplemented with 5% foetal bovine serum, 5% foetal calf serum (Sigma Aldrich), 100 U/mL penicillin streptomycin and 100 μg/mL L-glutamine as recommended by Kato et al. (1997).
2.3. Preparation of ECM substrates
Type 1 rat tail collagen (Life Technologies) was neutralised with NaOH (Sigma Aldrich) at 18.4 μM/mg collagen, and diluted with 10% phosphate buffered saline (PBS) (Sigma Aldrich) and 68% distilled H2O. The mixture was then pipetted in 150 μL volumes onto 13 mm diameter coverslips (Sarstedt) and incubated for 30 min at 37 °C, before being double rinsed with sterile PBS. This resulted in the formation of a soft, thick, gel like coating on the coverslips (Col). To produce substrates of different mechanical stiffnesses but identical ligand density, substrates were cross-linked with EDAC (Sigma Aldrich) by incubating at 19 μM/mg collagen (ColEDAC1) or 95 μM/mg collagen (ColEDAC2) EDAC for 3.5 h at room temperature as described previously (Haugh et al., 2011). Substrates were then rinsed with PBS and incubated in fresh PBS for 3 h at room temperature to remove any remaining EDAC before being washed twice with sterile distilled H2O.
Type 1 rat tail collagen was also neutralised with acetic acid (Sigma Aldrich) at 2.34 μM/mg collagen and incubated for 1 h at room temperature to create a thin collagen coating (ColAA) of identical ligand density to the NaOH neutralised collagen substrates described above. This mixture was pipetted onto 13 mm diameter coverslips in 150 μL volumes and allowed to incubate at room temperature for 60 min. Excess liquid was then removed and substrates were double rinsed with sterile PBS prior to cell plating.
Matrigel (Sigma Aldrich) was diluted 1:3 in α-MEM and plated on 13 mm coverslips in volumes of 200 μL. The cover-slips were then incubated for either 30 min at 37 °C or 1 min at room temperature to create thick (1.5 mm—MatThick) and thin (40 μm—MatThin) substrates. Again substrates were washed twice with sterile PBS prior to cell plating.
2.4. Substrate stiffness measurement by atomic force microscopy (AFM)
Substrate stiffness measurements were conducted using an Agilent 5500 Atomic Force Microscope (AFM). A pyrex-nitride pyramidal tip of face angle 30° was used. Force–distance curves were obtained from each of the following substrates: Col, ColEDAC1, ColEDAC2, ColAA and TC. These curves were then used to calculate the material stiffness according to the following equation:
| (1) |
where E is Young’s Modulus, v is Poisson’s ratio, α is the face angle of the tip, δ is the penetration depth of the tip and F is the force measured at the AFM control. F is calculated through the following equation:
| (2) |
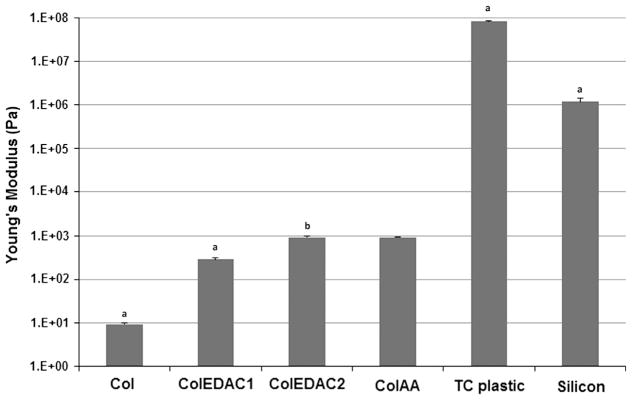
where k is the spring constant of the cantilever and x is the measured piezo movement. The spring constant, k, was calculated by finding the resonant frequency of the cantilever during testing (52 N/m). Using this equation substrate stiffness was measured five times at three different locations on each substrate and the values were averaged to generate a stiffness value for each measured substrate. The accuracy of the AFM methods used in this work was confirmed by testing a silicon sample of known stiffness of 1 MPa (shown in Fig. 2).
Fig. 2.
Substrate stiffness measurements by atomic force microscopy. Error bars indicate standard deviation of repeat measurements. (a) Statistical difference from all other values. (b) Statistical difference from Col, ColEDAC1, TC plastic and silicon.
2.5. Morphological analysis of cell phenotype
Cultures were fixed using 4% paraformaldehyde (Fluka) in piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (Sigma Aldrich) after 1, 4, 9 or 14 days of culture. Cells were permeabilised with Triton-X100 (Sigma Aldrich), diluted to 0.05% in PBS, and then incubated in phalloidin-TRITC according to the manufacturer’s protocol (Life technologies) to stain the actin cytoskeleton. Cells were then counterstained with DAPI dilactate (Sigma Aldrich) and rinsed with PBS prior to being mounted in DPX mounting media (Sigma Aldrich) for imaging.
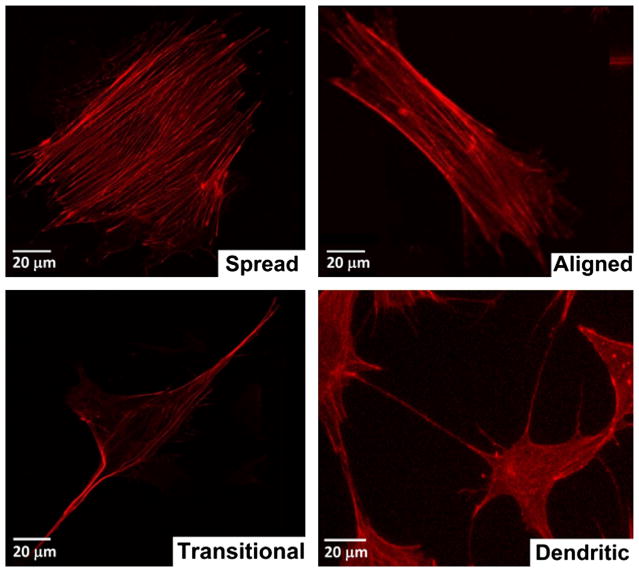
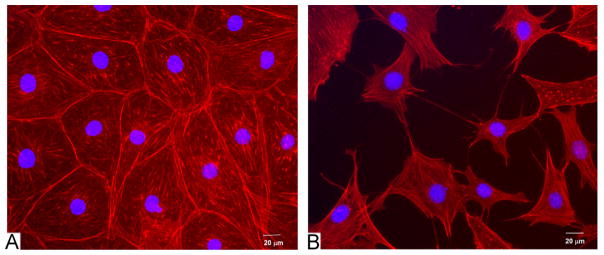
Images were taken using an Olympus IX50 inverted fluorescence microscope at different locations on the coverslips at 10 × magnification, giving a 0.62 cm2 image area. In total 15 images were taken at each timepoint, for each condition (five on each replicate coverslip). Cell processes were defined as cellular features located at the cell membrane that had a cross-sectional diameter of not more than 1 μm and extended for a distance of at least 5 μm. Using this classification cell morphologies were quantified according as follows: (1) spread cells had no cell processes and exhibited a spread morpology, (2) aligned cells had no cell processes but exhibited preferential alignment in a particular direction (defined by a short axis to long axis ratio of less than 0.5), (3) dendritic cells exhibited the small cell body and long thin cell processes associated with osteocytes, and (4) transitional cells showed a spread morphology with processes extending from the cell body. Fig. 1 shows an example of each assigned morphological description. MLO-Y4 cells cultured for 4 days on acetic acid neutralised collagen were analysed according to the same classification system and used as a positive control.
Fig. 1.
Examples of each morphology used to perform morphological analysis of the cells on each substrate. All cells were stained with rhodamine–phallodin.
2.6. Quantification of cell number
Cell number was quantified through a Hoechst 33258 assay kit according to the manufacturer’s protocol (Abcam). Hoechst fluorescently labels double-stranded DNA which can then be detected using a plate reader (Perkin Elmer 2030).
2.7. Quantification of ALP activity
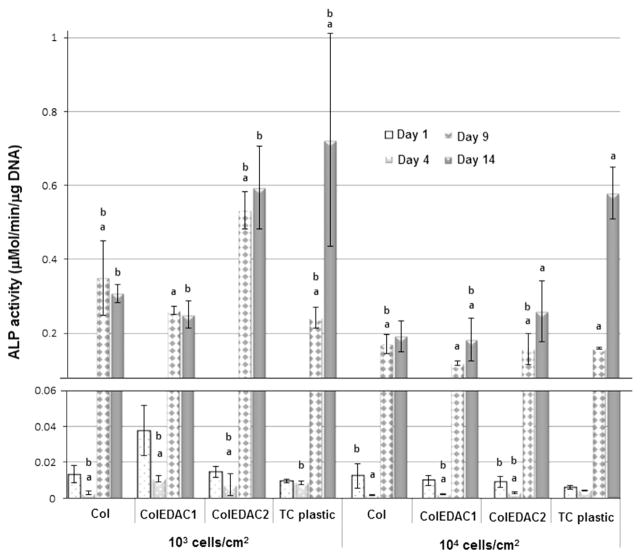
ALP activity was analysed in cells plated on Col, ColEDAC1, ColEDAC2, and TC plastic. Col and ColEDAC1 were chosen as they had the highest percentage of dendritic cells by morphological analysis. ColEDAC2 was chosen as it is chemically identical to ColEDAC1. This allowed for the effect of substrate stiffness to be examined independent of chemical differences in the substrates. TC plastic was used as a control substrate. Intracellular ALP activity was measured using a colorimetric ALP assay (Sigma Aldrich) as described previously (Birmingham et al., 2012), at all four timepoints, for all conditions. Briefly, cells were lysed by freezing at −80 °C, thawing at room temperature and mechanically scraped from the substrate. The ALP assay uses p-nitrophenyl phosphate (pNPP), which changes its emission wavelength when dephosphorylated by ALP. The change in emission wavelength was measured at 540 nm on a plate reader (Perkin Elmer 2030). Results were then normalised to cell number as determined through the Hoescht assay outlined above. Cell culture media from the same substrates (Col, ColEDAC1, ColEDAC2, and TC plastic) were also analysed to quantify extracellular ALP production. Media were changed 24 h before cell culture media were harvested and analysed using the p-nitrophenyl phosphate assay described above. Blank media were used as a background control, to ensure that any phosphate or calcium in the media (140 mg/L sodium phosphate and 200 mg/L calcium chloride in α-MEM) did not influence the overall differences between groups.
These experiments sought to analyse changes in ALP production over time as an indicator of osteoblast to osteocyte transition. It is known that immature MC3T3-E1 cells initially produce low levels of ALP, but as these cells differentiate into mature osteoblasts they upregulate ALP production and finally as they differentiate into osteocytes they decrease ALP production (Mikuni-Takagaki et al., 1995).
2.8. Mineralisation
Mineralisation of the extracellular matrix by MC3T3-E1 cells was analysed after 4, 9 and 14 days using an alizarin red/cetylpyridinium chloride assay (Sigma Aldrich), as described previously (Birmingham et al., 2012; Brennan et al., 2012; Stanford et al., 1995), on specific substrates (Col, ColEDAC1, ColEDAC2, TC plastic). Media were removed and cells were rinsed twice in PBS before being incubated with a 2% alizarin red solution (Sigma Aldrich) for 20 min on an orbital rocker. The solution was removed and the substrates were washed three times in deionised H2O to remove any unbound alizarin red. Cells were then incubated with 10% cetylpyridinium chloride solution (Sigma Aldrich) on an orbital rocker for 20 min at room temperature to disintegrate bound alizarin red. The absorbance of these samples at 562 nm was measured using a plate reader (Perkin Elmer 2030) to determine calcium deposition. MC3T3-E1 media were used as a control to ensure reported levels of calcium were due to cellular activity rather than media components (200 mg/L calcium chloride in α-MEM).
2.9. Gene expression
Expression of osteoblast specific (Type 1 collagen, OSF-2) and osteocyte specific (DMP-1, Sost) gene’s was analysed by RT-PCR on specific substrates (Col, ColEDAC1, ColEDAC2) after 14 and 21 days of culture. These substrates were chosen based on the results of the morphological studies, as cells cultured on Col and ColEDAC1 at 103 cells/cm2 had the highest levels of dendrite formation indicative of osteocyte differentiation, whereas cells cultured on ColEDAC2 and/or plated at 104 cells/cm2 were predominantly spread indicative of osteoblast differentiation.
Cells were lysed using 1 mL TRI reagent before phase separation by the introduction of 200 μL chloroform. RNA was precipitated using 70% ethanol and then washed using an ENZA RNA isolation kit according to the manufacturer’s protocol (Omega Bio-tek) and dissolved in 30 μL of RNAse free water (Qiagen). The quality of the RNA was measured using a Nanodrop spectrometer (Thermo-scientific) before being converted to cDNA using a cDNA synthesis kit (Omega Biosciences) and Gene Amp 9700 A Thermal cycler (Applied Biosystems).
RT-PCR was performed on a Step-One plus analyser (Applied Biosystems) using Taqman probes (Applied Biosystems) for Type 1 collagen (Mm00801666_g1) and OSF-2 (Mm00450111_m1) on the cells harvested after 14 days of culture and DMP-1 (Mm00803833_g1) and Sost (Mm04208528_m1) on cells harvested after 21 days of culture. RT-PCR data were analysed using the 2−ΔΔCt method (Livak and Schmittgen, 2001), with GAPDH (Mm03302249_g1) as a housekeeping gene and all reactions were conducted in biological and technical triplicates.
2.10. Statistical analysis
All experiments were conducted in biological triplicate (N=3). Cell morphologies were compared to the positive control (MLO-Y4 cells cultured on ColAA) and a negative control (MC3T3-E1 cells cultured on TC plastic). Statistical significance of the effects of substrate stiffness and seeding density on cell morphology was determined by using a Welch’s t-test to find the p-values of the difference between each mean. Pierce’s criterion was used to determine statistical outliers in ALP and mineralisation data, while Student’s t-test was used to determine statistical difference in ALP, mineralisation and gene expression experiments.
3. Results
3.1. AFM mechanical properties
TC plastic had a stiffness of 80.1±4.8 MPa, while silicon, tested as a control of known stiffness, was measured as 1.18±0.2 MPa. Col was the softest substrate with a stiffness of 9.7±0.3 Pa. As expected substrate stiffness increased as a function of the concentration of crosslinking agent. ColEDAC1 and ColEDAC2 had stiffnesses of 286±22.1 Pa and 957±12.0 Pa, respectively. ColAA had a similar stiffness to ColEDAC2 at 921±9.6 Pa. All substrates had significantly different stiffnesses from one another with the exception of the ColEDAC2 and ColAA substrates, which did not show a significant difference (p<0.05). These results are summarised in Fig. 2. Matrigel is liquid at room temperature and so the stiffness of these two substrate could not be measured.
3.2. Morphological analysis of cell phenotype
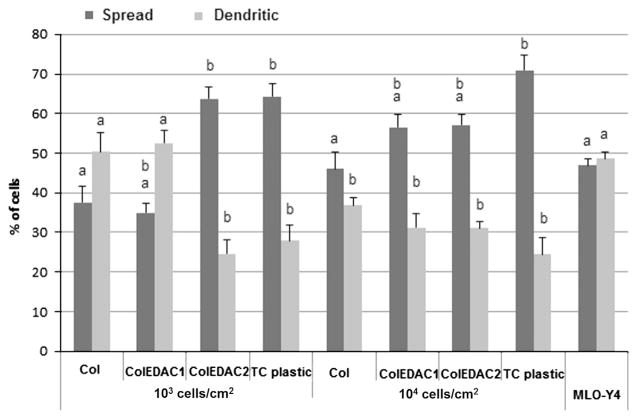
After 14 days, 70.1% of MC3T3-E1 cells cultured on the TC plastic control were classed as spread with 24.5% extending cell processes. Cells cultured on the stiff (ColEDAC2) or intermediate (ColEDAC1) stiffness collagen based substrates at high initial seeding density showed a similar morphological pattern with approximately 56% of cells classed as spread and 31% exhibiting cell processes at both culture conditions. A lower percentage of spread cells (46.0%) was observed on the softest substrate at the higher seeding density of 104 cells/cm2, while 36.8% of cells on these substrates extended cell processes. Cells cultured at the lower seeding density of 103 cells/cm2 on ColEDAC2 or TC plastic also predominantly exhibited a spread morphology with over 63% of cells being classed as spread and less than 28% extending cell processes under both culture conditions. In contrast, cells cultured on the softest (Col) or intermediate stiffness (ColEDAC1) substrates at 103 cells/cm2 were predominantly osteocyte-like with over 50% extending cell processes under both culture conditions, while less than 37% of the cells cultured under these conditions were classed as spread. About 47% of the MLO-Y4 control cells were classed as spread while 48.6% extended cell processes. Sample images of MC3T3-E1 cells on Col and TC plastic are shown in Fig. 5. MLO-Y4 cells were cultured for 4 days on ColAA as a positive control with 50.3±2.7% of these cells classed as dendritic, see Figs. 3 and 4.
Fig. 5.
Extracellular ALP activity over time of MC3T3’s on: Col, ColEDAC1, ColEDAC2, TC plastic, measured from media extracted from wells upon harvest. Error bars indicate standard deviation of repeat wells. (a) Statistical difference from the previous timepoint of same condition (p<0.01). (b) Statistical difference from control (MC3T3’s on tissue culture plastic at 104 cells/cm2) and same timepoint (p<0.01).
Fig. 3.
Percentage of cells displaying the dendritic morphology typical of osteocytes after 14 days of culture on each substrate at an initial seeding density of either 103 cells/cm2 or 104 cells/cm2. Error bars indicate standard deviation of repeat wells. (a) Statistical difference with negative control (MC3T3’s on TC plastic at 104 cells/cm2, p<0.01). (b) Statistical difference with positive control (MLO-Y4’s on ColAA at 104 cells/cm2, p<0.01).
Fig. 4.
Sample morphologies from (A) TC plastic at 104 cells/cm2 for 14 days and (B) Col at 103 cells/cm2 for 14 days.
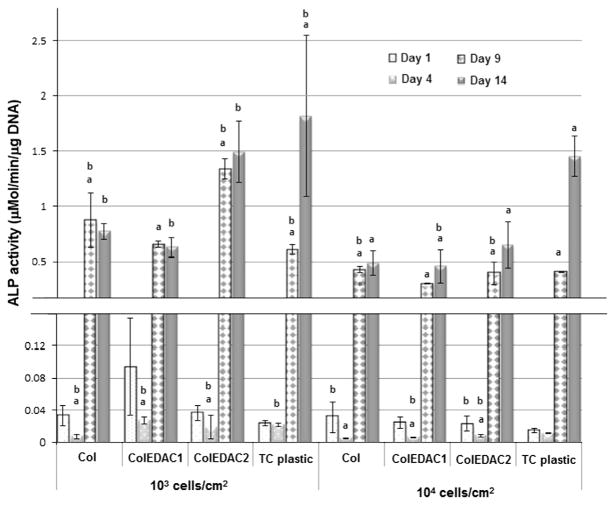
3.3. ALP activity of cells
Both intracellular and extracellular alkaline phosphatase activity increased continuously in MC3T3-E1 cells cultured on TC plastic and ColEDAC2 for the duration of culture for both seeding densities, see Figs. 5 and 6. All cells cultured at the higher seeding density of 104 cells/cm2 on all substrates also showed an increase in ALP activity for the duration of culture. Similarly ALP expression increased significantly up to 9 days of culture in MC3T3-E1 cells cultured at a low seeding density (103 cells/cm2) on the two softest substrates (Col and ColEDAC1). Expression in cells on these two substrates at the lower seeding density of 103 cells/cm2 was then downregulated after 14 days of culture (although this change was not statistically significant).
Fig. 6.
Intracellular ALP activity over time of MC3T3’s on: Col, ColEDAC1, ColEDAC2, TC plastic. Error bars indicate standard deviation of repeat wells. * Statistical outlier. (a) Statistical difference from the previous timepoint of same condition (p<0.01). (b) Statistical difference from control (MC3T3’s on tissue culture plastic at 104 cells/cm2) and same timepoint (p<0.01).
3.4. Mineralisation
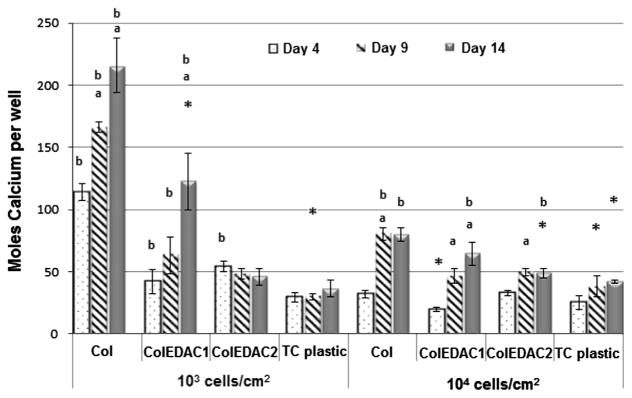
The results of the mineralisation of the ECM over time caused by MC3T3’s cultured on the different substrates are presented in Fig. 7. No increase in mineral production over time by MC3T3-E1 cells on tissue culture plastic at either 103 or 104 cells/cm2 was observed. Similarly, on the stiffer collagen substrate (ColEDAC2) with a seeding density of 103 cells/cm2, no significant change in mineralisation over time was observed. However, a significant increase was observed from day 4 to day 9 on this substrate with a seeding density of 104 cells/cm2. The highest levels of mineralisation were observed on Col and ColEDAC1 when cells were cultured at 103 cells/cm2. An increase in mineralisation over time was also observed in these culture conditions. An increase in mineralisation over time was also observed on ColEDAC1 with a seeding density of 104 cells/cm2. However, levels of mineralisation under this condition were significantly lower than in either Col or ColEDAC1 with a seeding density of 103 cells/cm2.
Fig. 7.
Mineralisation of ECM over time caused by MC3T3’s cultured on: Col, ColEDAC1, ColEDAC2, TC. Error bars indicate standard deviation of repeat wells. * Outlier from statistical data. (a) Statistical difference from the previous timepoint (p<0.05). (b) Statistical difference from control (MC3T3’s on tissue culture plastic at 104 cells/cm2) at same timepoint (p<0.05).
3.5. Gene expression
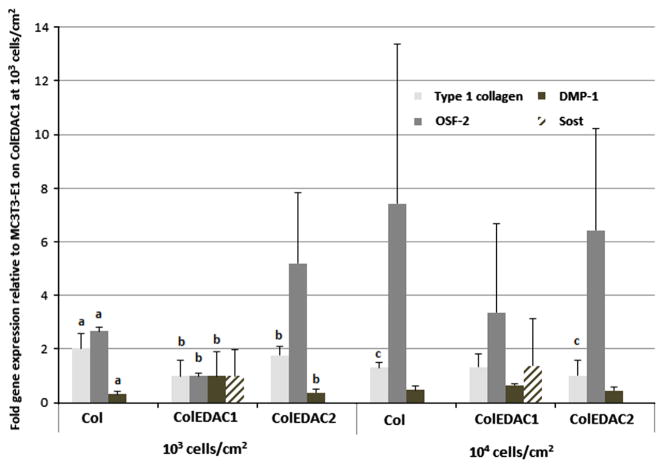
The results of the gene expression of MC3T3-E1 cells are shown in Fig. 8. MC3T3-E1 cells cultured on ColEDAC1 at 103 cells/cm2 were chosen as the control from which to measure the relative expression of all genes examined. Type 1 collagen expression was lowest in cells cultured on ColEDAC1 at the lower seeding density. Low levels of type 1 collagen expression were also observed in cells cultured on Col and ColEDAC2 at the higher seeding density of 104 cells/cm2, see Fig. 8. OSF-2 was lowest in cells cultured on ColEDAC1 at 103 cells/cm2, with a low level of expression also observed in cells cultured on the softest substrate (Col) at 103 cells/cm2. DMP-1 expression after 21 days of culture was highest in cells cultured on ColEDAC1 at the 103 cells/cm28, while Sost was detected in trace amounts in cells cultured on ColEDAC1 at both seeding densities, but not in cells cultured on any other substrates.
Fig. 8.
Gene expression in MC3T3’s cultured on: Col, ColEDAC1, ColEDAC2 for 14 (type 1 collagen, OSF-2) or 21 (DMP-1, Sost) days. Error bars indicate standard deviation. (a) Statistical difference from Col at the same seeding density. (b) Statistical difference from ColEDAC1 at the same seeding density. (c) Statistical difference from 103 cells/cm2 seeding density on the same substrate (p<0.05).
4. Discussion
The results of this study show that MC3T3-E1 cells progress along the osteogenic lineage to become osteocyte-like cells when cultured on soft collagen substrates at low seeding density (103 cells/cm2), as indicated by phenotypic changes associated with early osteocytic differentiation (dendritic morphology, reduction in ALP expression, ECM mineralisation). Furthermore, downregulation of the osteoblast specific genes Col type 1 and OSF-2 as well as increased expression of DMP-1, a gene upregulated during osteoblast to osteocyte differentiation, and the late stage osteocyte marker Sost can be induced in MC3T3-E1 cells through culture on collagen based substrates of approximately 286 Pa at an initial seeding density of 103 cells/cm2. In contrast, cells cultured on stiffer collagen based substrates, or at a high initial cell seeding density proliferated and displayed the spread morphology, continually high ALP expression, low levels of ECM mineralisation, and high levels of Type 1 collagen and OSF-2 expression associated with the osteoblast phenotype. As a whole these findings show that the mechanical and compositional properties of the ECM, as well as the necessity for the cells to establish a communication network, contribute greatly to osteocyte differentiation.
The use of the cell lines MLO-Y4 and MC3T3-E1 is a possible limitation to this study. However, both cell lines have been shown to be excellent representatives of primary osteocytes and osteoblasts respectively (Sudo et al., 1983; Kato et al., 1997; Quarles et al., 1992; Bonewald, 1999). MLO-Y4 cells exhibit low levels of ALP activity, which does not change over time (Kato et al., 1997). Primary osteoblasts/osteocytes were not used because of difficulties in cell isolation and characterisation (Hentunen, 2010) and the necessity for large cell numbers to satisfy the numerous experimental conditions and timepoints in our study design. Furthermore the expression of Sost and DMP-1 is downregulated in primary osteocytes when cultured on non-mineralised collagen or fibronectin matrices (Yang et al., 2009), and for this reason they were deemed inappropriate for our collagen-based substrate experiments.
It should be noted that these cells did not express significant levels of Sost, which is a late stage osteocyte marker, involved in the regulation of bone formation. However, Sost expression has only previously been induced in vitro in the MC3T3-E1 cell line through the addition of osteogenic growth factors (Mattinzoli et al., 2012; Uchihashi et al., 2013), and in vivo it is not expressed until after mineralisation has begun (Poole et al., 2005). Our results show trace amounts of Sost expression in cells cultured on the ColEDAC1 substrate, but not on any other substrate. Furthermore, increased DMP-1 expression has not previously been reported without the addition of osteogenic growth factors or direct application of mechanical strain (Krishnan et al., 2010; Mattinzoli et al., 2012). The results presented here show a significant increase in DMP-1 expression and trace amounts of Sost when cells are cultured on soft collagen based substrates (286 Pa) at low initial seeding density (103 cells/cm2). These findings highlight the importance of substrate mechanical properties even in the absence of commonly used osteogenic factors.
Osteocyte differentiation has been induced in MC3T3-E1 cells through treatment with fibroblast growth factor 2 (FGF-2) (Gupta et al., 2010), or by culture of the cells on collagen based substrates in the presence of osteogenic factors (Uchihashi et al., 2013). Cell migration, focal adhesion formation as well as calcium deposition by MC3T3-E1 cells have been shown to depend on substrate stiffness (Khatiwala et al., 2006a). Furthermore, mineralisation by embryonic stem cells has been shown to increase on stiffer substrates (Evans et al., 2009), while perhaps most interestingly, culture of pluripotent mesenchymal cells on a relatively rigid ECM substrate (40 kPa) favored osteoblastic differentiation, whereas culture on a more flexible substrate (1 kPa) led to a neural differentiation pathway (Engler et al., 2006). However, previous studies have only examined stem cell/osteoprogenitor to osteoblast differentiation and no study has monitored osteocyte differentiation as a function of ECM stiffness. In the current study we show for the first time the importance of ECM stiffness for regulating pre-osteoblast to early osteocyte transition. While osteoblast differentiation has previously been shown to be enhanced on stiffer substrates (20–40 kPa (Engler et al., 2006; Kong et al., 2005)), our studies suggest that early osteocyte differentiation is in fact governed by softer substrates (<300 Pa). In vivo osteocytes are formed when certain osteoblasts become embedded in newly formed osteoid, which is a soft, non-mineralised collagen matrix (Franz-Odendaal et al., 2006; Dallas and Bonewald, 2010), and simultaneously undergo the morphological changes associated with osteocyte differentiation (Doty, 1981). The cells begin to downregulate certain osteoblast specific genes, such as Type 1 collagen and OSF-2, while upregulation occurs in DMP-1 (Yang et al., 2004; Rios et al., 2005), and the osteocyte specific gene Sost (Yang et al., 2009; Winkler et al., 2003). It is interesting to note from the results of our study that the cells which displayed the traits of osteocyte differentiation were cultured on soft collagen based substrates. We propose that this substrate provides a similar extracellular mechanical environment to osteoid (Engler et al., 2006), and that this mechanical environment is necessary for osteoblast to osteocyte transition in vivo. However, the precise mechanical properties of osteoid are unknown, as the tissue represents a thin layer approximately 350 nm deep (Engler et al., 2006), and as such there are significant challenges with obtaining samples for ex vivo mechanical testing. Applying methods such as nanoindentation to assess mechanical properties in situ, would be inappropriate as the stiffness of a thin sample will be affected by the surrounding mineralised bone tissue (Oliver and Pharr, 1992).
Previous studies have established the importance of seeding density for differentiation of BMSCs into osteoblasts; ALP activity in rat bone marrow stromal cells was higher when cells were seeded at a lower initial seeding density of 3× 104 per cm2, compared to 14.9× 104 per cm2 (Kim et al., 2009). Bone formation on implanted HA scaffolds, plated with goat bone marrow cells, was shown to be increased when cells were plated at higher seeding densities up to 47.8× 106 cells/cm3 in osteogenic media for 7 days prior to implantation (Wilson et al., 2002). MG-63 osteosarcoma cells cultured at a low initial seeding densities in 3D collagen scaffolds showed significantly higher rates of ALP expression than those at higher seeding densities by 2 days (Bitar et al., 2007). The results presented here show similar changes in ALP expression over time due to initial cell seeding density, albeit that these previous studies were performed with different cell phenotypes, and also substrate stiffness and seeding densities were an order of magnitude larger than those used in this study.
It has already been widely hypothesised that osteocytes extend processes to facilitate intercellular communication (Donahue, 1998; Palazzini et al., 1998). It is intriguing to speculate that the combined effect of substrate stiffness and cell seeding density observed here is driven by the need for osteocytes to establish a communication network. The osteocyte network is akin to that of neurites and it has previously been reported that neurite formation increases on substrates in the range of 250–500 Pa (Georges et al., 2006; Estell, 2012). Therefore, we propose that the formation of dendrites is driven by ECM stiffness and intercellular separation across a range of phenotypes. Osteoblastic cells on stiffer substrates find it easier to proliferate allowing the formation of gap junctions between cell bodies without the need to establish cell processes (Yamaguchi et al., 1994; Yellowley et al., 2000). However, cells on softer substrates might be prevented from proliferating sufficiently due to the mechanics of the substrate, and after a time might resort to extending processes to establish a communication network with neighbouring cells.
Several candidate molecules are known to be involved in the osteoblast–osteocyte transition, in particular small GTPases including Rac, Rho and CDC42, which govern cellular spreading, migration and the extension of cellular processes (Huveneers and Danen, 2009; Tanaka-Kamioka et al., 1998; Kamioka et al., 2007; McNamara et al., (2009)). Loss of ALP activity, as observed in this study, involves not only a change in gene expression but also loss or inactivation of pre-existing enzyme in osteoblasts. Such losses could occur through shedding of membrane-derived vesicles or by enzymatic cleavage of the enzyme itself (Dean et al., 1996; Xie, 1995). Finally, diffusible signals may also play important roles in the osteoblast–osteocyte transition; it has recently been demonstrated that sustained stimulation of osteoblastic cells with the oncostatin-M, a member of the IL6 family, could induce a broad range of gene expression changes consistent with osteocyte formation in cultured osteoblasts (Brounais et al., 2008).
5. Conclusion
These experiments represent the first investigation into the effects of both substrate stiffness and cell seeding density on osteoblast–osteocyte transition and shed light on the external cues (substrate stiffness and intercellular separation) necessary for osteocyte differentiation. For the first time, pre-osteoblast MC3T3 cells have been induced to undergo osteocyte differentiation, without the addition of growth factors or application of mechanical loading, when cultured on soft collagen based substrates provided intercellular separation necessitates process formation. Furthermore, for the cells to differentiate further along the osteogenic pathway, to the level of a early osteocyte, an optimal stiffness of approximately 286 Pa must be used. We propose that this simulates the in vivo environment of the cells, where osteoblasts develop on osteoid, a soft collagen based matrix, and subsequently differentiate when they become embedded within this matrix. Knowing how the mechanical environment affects osteocyte development is a vital step in the recreation of viable bone tissue for implantation.
Acknowledgments
The MLO-Y4 cell line was received as a kind gift from Professor Lynda Bonewald (School of Dentistry, University of Missouri, Kansas City, MO). The authors would like to acknowledge funding from the European Research Council (ERC), Grant no.: 258992 (BONEMECHBIO), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institute of Health (NIH), Grant nos.: AR041210 and AR057139, and the NUI Galway College of Engineering and Informatics Research Fellowship.
References
- Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by γ-carboxylation-dependent and -independent mechanisms. American Journal of Physiology— Cell Physiology. 2009;297:C1358–C1367. doi: 10.1152/ajpcell.00216.2009. [DOI] [PubMed] [Google Scholar]
- Birmingham E, Niebur GL, Mchugh PE, Shaw G, Barry FP, Mcnamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. European Cells and Materials. 2012;23:13–27. doi: 10.22203/ecm.v023a02. [DOI] [PubMed] [Google Scholar]
- Bitar M, Brown RA, Salih V, Kidane AG, Knowles JC, Nazhat SN. Effect of cell density on osteoblastic differentiation and matrix degradation of biomimetic dense collagen scaffolds. Biomacromolecules. 2007;9:129–135. doi: 10.1021/bm701112w. [DOI] [PubMed] [Google Scholar]
- Boivin G, Morel G, Lian JB, Anthoine-Terrier C, Dubois PM, Meunier PJ. Localization of endogenous osteocalcin in neonatal rat bone and its absence in articular cartilage: Effect of warfarin treatment. Virchows Archives. 1990;417:505–512. doi: 10.1007/BF01625731. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. Journal of Bone and Mineral Metabolism. 1999;17:61–65. doi: 10.1007/s007740050066. [DOI] [PubMed] [Google Scholar]
- Boukhechba F, Balaguer T, Michiels JF, Ackermann K, Quincey D, Bouler JM, Pyerin W, CARLE GF, Rochet N. Human primary osteocyte differentiation in a 3D culture system. Journal of Bone and Mineral Research. 2009;24:1927–1935. doi: 10.1359/jbmr.090517. [DOI] [PubMed] [Google Scholar]
- Brennan O, O’Brien FJ, Mcnamara LM. Estrogen plus estrogen receptor antagonists alter mineral production by osteoblasts in vitro. Hormone and Metabolic Research. 2012;44:154. doi: 10.1055/s-0031-1291358. [DOI] [PubMed] [Google Scholar]
- Brounais B, Chipoy C, Mori K, Charrier C, Battaglia S, Pilet P, Richards CD, HEYMANN D, Rédini F, Blanchard F. Oncostatin M induces bone loss and sensitizes rat osteosarcoma to the antitumor effect of Midostaurin in vivo. Clinical Cancer Research. 2008;14:5400–5409. doi: 10.1158/1078-0432.CCR-07-4781. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Annals of the New York Academy of Sciences. 2010;1192:437–443. doi: 10.1111/j.1749-6632.2009.05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D, Boyan B, Muniz O, Howell D, Schwartz Z. Vitamin D metabolites regulate matrix vesicle metalloproteinase content in a cell maturation-dependent manner. Calcified Tissue International. 1996;59:109–116. doi: 10.1007/s002239900096. [DOI] [PubMed] [Google Scholar]
- Donahue HJ. Gap junctional intercellular communication in bone: a cellular basis for mechanostat set point. Calcified Tissue International. 1998:62. doi: 10.1007/s002239900398. [DOI] [PubMed] [Google Scholar]
- Doty S. Morphological evidence of gap junctions between bone cells. Calcified Tissue International. 1981;33:509–512. doi: 10.1007/BF02409482. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Estell EG. Biological Engineering. University of Maine; 2012. Substrate Stiffness and Adhesivity Influence Neuron Axonal Growth. [Google Scholar]
- Evans ND, Minelli C, Gentleman E, Lapointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM. Substrate stiffness affects early differentiation events in embryonic stem cells. European Cells and Materials. 2009;18:1–14. doi: 10.22203/ecm.v018a01. [DOI] [PubMed] [Google Scholar]
- Feng H, Lu YE, Tsutsui Xie, Kunieda Castranio, Bonewald Scott, Mishina The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. Journal of Dental Research. 2003;82:776–780. doi: 10.1177/154405910308201003. [DOI] [PubMed] [Google Scholar]
- Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Developmental Dynamics. 2006;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- Georges M, Meaney Sawyer, Janmey Matrices with compliance comparable to that of brain tissue select neuronal over glial growth n mixed cortical cultures. Biophysical Journal. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi JH, Pompolo S, Karsdal MA, Kulkarni NH, Kalajzic I, Mcahren SHM, Han B, Onyia JE, Ho PWM, Gillespie MT, Walsh NC, Chia LY, Quinn JMW, Martin TJ, Sims NA. Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone. 2010;46:1486–1497. doi: 10.1016/j.bone.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Gu G, Nars M, Hentunen T, Metsikkö K, Väänänen H. Isolated primary osteocytes express functional gap junctions in vitro. Cell and Tissue Research. 2006;323:263–271. doi: 10.1007/s00441-005-0066-3. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Yoo DJ, Hebert C, Niger C, Stains JP. Induction of an osteocyte-like phenotype by fibroblast growth factor-2. Biochemical and Biophysical Research Communications. 2010;402:258–264. doi: 10.1016/j.bbrc.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh MG, Murphy CM, Mckiernan RC, Altenbuchner C, O’Brien FJ. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Engineering Part A. 2011;17:1201–1208. doi: 10.1089/ten.TEA.2010.0590. [DOI] [PubMed] [Google Scholar]
- Hentunen T. MLO-Y4 Cell Line as a Tool to Study the Regulatory Functions of Osteocytes. RSCI; Dublin: 2010. [Google Scholar]
- Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. Journal of Biomedical Materials Research. 2000;51:376–382. doi: 10.1002/1097-4636(20000905)51:3<376::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hsiong SX, Carampin P, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. Journal of Biomedical Materials Research Part A. 2008;87A:145–156. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- Hurley MM, Abreu C, Harrison JR, Lichtler AC, Raisz LG, Kream BE. Basic fibroblast growth factor inhibits type I collagen gene expression in osteoblastic MC3T3-E1 cells. Journal of Biological Chemistry. 1993;268:5588–5593. [PubMed] [Google Scholar]
- Huveneers S, Danen EHJ. Adhesion signaling—crosstalk between integrins, Src and Rho. Journal of Cell Science. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Kamiya N, Ito K, Takagi M. In situ localization and in vitro expression of osteoblast/osteocyte factor 45 mRNA during bone cell differentiation. The Histochemical Journal. 2002;34:255–263. doi: 10.1023/a:1021745614872. [DOI] [PubMed] [Google Scholar]
- Jee WSS. Integrated bone tissue physiology: anatomy and physiology. In: Cowin SC, editor. Bone Mechanics Handbook. 2. CRC Press, LLC; Boca Raton: 2001. [Google Scholar]
- Kamioka H, Ishihara Y, Ris H, Murshid SA, Sugawara Y, Takano-Yamamoto T, Lim SS. Primary cultures of chick osteocytes retain functional gap junctions between osteocytes and between osteocytes and osteoblasts. Microscopy and Microanalysis. 2007;13:108–117. doi: 10.1017/S143192760707016X. [DOI] [PubMed] [Google Scholar]
- Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF. Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. Journal of Bone and Mineral Research. 2001;16:1622–1633. doi: 10.1359/jbmr.2001.16.9.1622. [DOI] [PubMed] [Google Scholar]
- Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. Journal of Bone and Mineral Research. 1997;12:2014–2023. doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- Khatiwala CB, Peyton SR, Putnam AJ. Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. American Journal of Physiology—Cell Physiology. 2006a;290:C1640–C1650. doi: 10.1152/ajpcell.00455.2005. [DOI] [PubMed] [Google Scholar]
- Khatiwala CB, Peyton SR, Putnam AJ. Osteogenic differentiation of Mc3t3-E1 cells regulated by substrate stiffness requires mapk activation. Proceedings of the Sixth Annual Meeting of the American Institute of Chemical Engineers, Cell Adhesion and Migration; San Francisco, CA. 2006b. p. 338a. [Google Scholar]
- Kim K, Dean D, Mikos AG, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly (propylene fumarate) disks. Biomacromolecules. 2009;10:1810–1817. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Nulend J, Der Plas VAN, Semeins A, Ajubi C, Frangos N, Nijweide J, Burger EP. Sensitivity of osteocytes to biomechanical stress in vitro. The FASEB Journal. 1995;9:441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Molecular and Cellular biology. 2010;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Dhurjati R, Vogler E, Mastro A. Osteogenesis in vitro: from pre-osteoblasts to osteocytes. In Vitro Cellular & Developmental Biology—Animal. 2010;46:28–35. doi: 10.1007/s11626-009-9238-x. [DOI] [PubMed] [Google Scholar]
- Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcified Tissue International. 1993:53. doi: 10.1007/BF01673415. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lode A, Bernhardt A, GELINSKY M. Cultivation of human bone marrow stromal cells on three-dimensional scaffolds of mineralized collagen: influence of seeding density on colonization, proliferation and osteogenic differentiation. Journal of Tissue Engineering and Regenerative Medicine. 2008;2:400–407. doi: 10.1002/term.110. [DOI] [PubMed] [Google Scholar]
- Marotti G, Palazzini S, Palumbo C, Ferretti M. Ultrastructural evidence of the existence of a dendritic network throughout the cells of the osteogenic lineage: the novel concept of wiring- and volume-transmission in bone. Bone. 1996;19(Suppl 3):151S. [Google Scholar]
- Mattinzoli D, Corbelli PMA, Ikehata M, Zennaro C, Armelloni S, Li M, Giardino L, Rastaldi MP. A novel model of in vitro osteocytogenesis induced by retinoic acid treatment. European Cells and Materials. 2012;24:403–425. doi: 10.22203/ecm.v024a29. [DOI] [PubMed] [Google Scholar]
- McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2009;292:355–363. doi: 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni-Takagaki Y, Kakai Y, Satoyoshi M, Kawano E, Suzuki Y, Kawase T, Saito S. Matrix mineralization and the differentiation of osteocyte-like cells in culture. Journal of Bone and Mineral Research. 1995;10:231–242. doi: 10.1002/jbmr.5650100209. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Beertsen W, Vandenbos T, Kawamoto T, Oda K, Takano Y. Site-specific localization of two distinct phosphatases along the osteoblast plasma membrane: tissue non-specific alkaline phosphatase and plasma membrane calcium ATPase. Bone. 2004;35:1077–1085. doi: 10.1016/j.bone.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. Journal of Materials Research. 1992;7:1564–1583. [Google Scholar]
- Oshima A, Tanabe H, Yan T, Lowe GN, Glackin CA, Kudo A. A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I family, is regulated by the bHLH transcription factor, twist. Journal of Cellular Biochemistry. 2002;86:792–804. doi: 10.1002/jcb.10272. [DOI] [PubMed] [Google Scholar]
- Palazzini S, Palumbo C, Ferretti M, Marotti G. Stromal cell structure and relationships in perimedullary spaces of chick embryo shaft bones. Anatomy and Embryology. 1998;197:349–357. doi: 10.1007/s004290050145. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole KES, Bezooijen VAN, Loveridge RL, Hamersma N, Papapoulos H, Löwik SE, Reeve J, CW Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. The FASEB Journal. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. Journal of Bone and Mineral Research. 1992;7:683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- Rios HF, Dusevich LYV, Eick D, Bonewald LF, Feng JQ. DMP1 is essential for osteocyte formation and function. Journal of Musculoskeletal & Neuronal Interactions. 2005;5:325–327. [PubMed] [Google Scholar]
- Santos A, Bakker A, Willems H, Bravenboer N, Bronckers A, Klein-Nulend J. Mechanical loading stimulates BMP7, but not BMP2, production by osteocytes. Calcified Tissue International. 2011;89:318–326. doi: 10.1007/s00223-011-9521-1. [DOI] [PubMed] [Google Scholar]
- Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 10601 BSP) Journal of Biological Chemistry. 1995;270:9420–9428. doi: 10.1074/jbc.270.16.9420. [DOI] [PubMed] [Google Scholar]
- Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. The Journal of Cell Biology. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Kamioka K, Kamioka H, Ris H, Lim SS. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. Journal of Bone and Mineral Research. 1998;13:1555–1568. doi: 10.1359/jbmr.1998.13.10.1555. [DOI] [PubMed] [Google Scholar]
- Uchihashi K, Aoki S, Matsunobu A, Toda S. Osteoblast migration into type I collagen gel and differentiation to osteocyte-like cells within a self-produced mineralized matrix: a novel system for analyzing differentiation from osteoblast to osteocyte. Bone. 2013;52:102–110. doi: 10.1016/j.bone.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Weinreb M, Shinar D, Rodan GA. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. Journal of Bone and Mineral Research. 1990;5:831–842. doi: 10.1002/jbmr.5650050806. [DOI] [PubMed] [Google Scholar]
- Westbroek I, de Rooij KE, Nijweide PJ. Osteocyte-specific monoclonal antibody MAb OB7.3 is directed against phex protein. Journal of Bone and Mineral Research. 2002;17:845–853. doi: 10.1359/jbmr.2002.17.5.845. [DOI] [PubMed] [Google Scholar]
- Wilde J, Yokozeki M, Terai K, Kudo A, Moriyama K. The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell and Tissue Research. 2003;312:345–351. doi: 10.1007/s00441-002-0664-2. [DOI] [PubMed] [Google Scholar]
- Wilson CE, Dhert WJA, Blitterswijk Van, Verbout AJ, CA, de Bruijn JD. Evaluating 3D bone tissue engineered constructs with different seeding densities using the alamarBlue™ assay and the effect on in vivo bone formation. Journal of Materials Science: Materials in Medicine. 2002;13:1265–1269. doi: 10.1023/a:1021139415528. [DOI] [PubMed] [Google Scholar]
- Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. Journal of Biological Chemistry. 2006;281:30884–30895. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L. Streptolysin-O induces release of glycosylphosphatidylinositol-anchored alkaline phosphatase from ROS cells by vesiculation independently of phospholipase action. Biochemical Journal. 1995;305:529–537. doi: 10.1042/bj3050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi DT, Ma D, Lee A, Huang J, Gruber HE. Isolation and characterization of gap junctions in the osteoblastic MC3T3-E1 cell line. Journal of Bone and Mineral Research. 1994;9:791–803. doi: 10.1002/jbmr.5650090605. [DOI] [PubMed] [Google Scholar]
- Yang IK, LUY, Guo D, Harris MA, Gluhak-Heinrich J, Bonewald LF, Feng JQ, Rowe DW, Harris SE. In vitro and in vivo study on osteocyte-specific mechanical signaling pathways. Journal of Musculoskeletal and Neuronal Interactions. 2004;4:386–387. [PubMed] [Google Scholar]
- Yang MAH, Heinrich Jelica Gluhak, Dayong Guo, Bonewald Lynda F, Stephen, Harris E. Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO-Y4 osteocyte cell model. Bone. 2009;44:32–45. doi: 10.1016/j.bone.2008.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. Journal of Bone and Mineral Research. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- Zhang K, BAC, Ye L, Kotha S, Dallas M, Lu Y, ZHAO S, Harris M, Harris SE, Feng JQ, Bonewald LF. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Molecular and Cellular biology. 2006;26:845–853. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]