Abstract
cGMP is a second messenger widely used in the nervous system and other tissues. One of the major effectors for cGMP is the serine/threonine protein kinase, cGMP-dependent protein kinase (PKG), which catalyzes the phosphorylation of a variety of proteins including ion channels. Previously, it has been shown that the cGMP-PKG signaling pathway inhibits Ca2+ currents in rat vestibular hair cells and chromaffin cells. This current allegedly flow through voltage-gated CaV1.3L-type Ca2+ channels, and is important for controlling vestibular hair cell sensory function and catecholamine secretion, respectively. Here, we show that native L-type channels in the insulin-secreting RIN-m5F cell line, and recombinant CaV1.3 channels heterologously expressed in HEK-293 cells, are regulatory targets of the cGMP-PKG signaling cascade. Our results indicate that the CaVα1 ion-conducting subunit of the CaV1.3 channels is highly expressed in RIN-m5F cells and that the application of 8-Br-cGMP, a membrane-permeable analogue of cGMP, significantly inhibits Ca2+ macroscopic currents and impair insulin release stimulated with high K+. In addition, KT-5823, a specific inhibitor of PKG, prevents the current inhibition generated by 8-Br-cGMP in the heterologous expression system. Interestingly, mutating the putative phosphorylation sites to residues resistant to phosphorylation showed that the relevant PKG sites for CaV1.3 L-type channel regulation centers on two amino acid residues, Ser793 and Ser860, located in the intracellular loop connecting the II and III repeats of the CaVα1 pore-forming subunit of the channel. These findings unveil a novel mechanism for how the cGMP-PKG signaling pathway may regulate CaV1.3 channels and contribute to regulate insulin secretion.
Keywords: L-type channels, Cav channels, PKG, cGMP, Insulin, Rin-m5F cells
1. Introduction
Voltage-gated Ca2+ (CaV) channels are Ca2+-conducting proteins in the cell membrane that in response to a depolarization undergo a conformational change from a non-conducting state to a transiently high Ca2+ permeable state [1–3]. This allows extracellular Ca2+ to rapidly enter the cytoplasm where it serves as a second messenger to trigger a myriad of cellular responses including neurotransmitter and hormone release, muscle contraction and gene expression, among many others [1–3]. Therefore it comes as no surprise that malfunction of CaV channels resulting from mutation, altered expression or regulation may lead to disease [3,4].
Multiple subtypes of Ca2+ channel currents have been defined by physiological and pharmacological criteria. In some cell types, Ca2+ currents are characterized by their high voltage of activation, large conductance, and specific inhibition by antagonist drugs including dihydropyridines [1,3]. These currents that also show slow voltage-dependent inactivation are named L-type (long-lasting), are present in endocrine cells where they initiate the hormone release process, and in neurons where they are important in the integration of the synaptic input and the regulation of gene expression [1,3].
L-type CaV channels are multisubunit complexes formed by one of four distinct ion-conducting CaVα1 subunits, now called CaV1.1 to CaV1.4, that co-assemble with ancillary CaVβ, CaVα2δ, and in some cases CaVγ subunits [1–5]. The CaVα1 subunit comprises four homologous repeats each consisting of six transmembrane helices and a pore lining domain. These repeated domains are linked via cytoplasmic loops and flanked by intracellular N- and C-termini [1–5]. CaV1.1 and CaV1.4 channels are predominantly expressed in skeletal muscle, pituitary cells and the retina, whereas CaV1.2 and CaV1.3 are broadly distributed throughout the central nervous system, endocrine cells, cardiac myocytes and sinoatrial node cells [3,7]. These channels are substrates for phosphorylation by different protein kinases, which is believed to be of substantial physiological importance, mediating the effects of several hormones and intracellular messengers [6–8].
In particular, L-type channels are regulated by a mechanism originated in the NO-cGMP-PKG signaling pathway. In rat vestibular hair cells it has been reported that NO donors inhibit the macroscopic Ca2+ that flows presumably through L-type of the CaV1.3 class in a voltage-independent manner [9]. The membrane-permeant cGMP analogue 8-Br-cGMP mimicked the inhibitory action of NO donors and KT-5823, a selective inhibitor of cGMP-dependent protein kinase (PKG), prevented the inhibition of the current caused by NO donors and 8-Br-cGMP [9], supporting the idea that NO inhibits the Ca2+ current by the activation of the cGMP-signaling pathway.
These data agree with results obtained in chromaffin cells where application of the membrane-permeable cGMP analogue 8-pCPT-cGMP significantly decreased L-type channel activity and disfavored catecholamine release [10–12]. Interestingly, L-type channels in these cells can be also modulated by the cAMP-PKA signaling cascade but in an opposite direction (i.e., upregulation). Both PKG and PKA appear to affect CaV1.3, as well as CaV1.2 channels, to the same extent both under basal conditions and in response to maximal stimulation [12]. Analogously, experimental evidence supports the idea that PKA may phosphorylate CaV1.3 channel pore-forming α1 subunit at serine residues 1743 and 1816 [13], but nothing is known regarding the phosphorylation sites of PKG.
Using patch-clamp electrophysiology, here we report for the first time that these currents are directly targeted and inhibited by the cGMP-PKG signaling cascade in insulin-secreting RIN-m5F cells. Furthermore, insulin secretion by these cells may be also affected by 8-Br-cGMP and KT-5823 application. Subsequent work using a heterologous expression system and site-directed mutagenesis identified two serine residues (Ser793 and Ser860) in the intracellular loop connecting the II and III repeats of the CaV1.3α1 pore-forming subunit of the channel whose phosphorylation leads to down-regulation of the channel complex.
2. Materials and methods
2.1. Cell culture and transfection
The rat insulin-producing RIN-m5F cells (CRL-11605; ATCC) were grown as monolayers in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum, 110 mg/L sodium pyruvate, 2 mM L-glutamine, 100 units/L penicillin, and 100 μg/L streptomycin. Likewise, HEK-293 cells (CRL-1573; ATCC) were grown also as monolayers in DMEM-high glucose medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum, 110 mg/L sodium pyruvate, 2 mM L-glutamine, 100 U/L penicillin, and 100 μg/L streptomycin. Cell cultures were maintained at 37 °C in 5% CO2, and 95% air humidified atmosphere.
After splitting, HEK-293 cells were seeded at 60% confluence and one day after were transfected using the Lipofectamine Plus reagent (Invitrogen) with 1.0 μg of each plasmid cDNA encoding L-type channel pore-forming subunit CaV1.3α1 (GenBankTM accession number AF370009), cloned into the pcDNA6 vector (Invitrogen), and auxiliary subunits CaVβ3 (M88751), and CaVα2β-1 (M86621) cloned into the pcDNA3 vector (Invitrogen). For electrophysiological recording, 0.6 μg of a plasmid encoding the green fluorescent protein (Green-Lantern; Invitrogen) was used to identify transfected cells.
2.2. RNA extraction, reverse transcription and PCR amplification
Total RNA was extracted from RIN-m5F cells by using TRI-zol (Invitrogen). Reverse transcription was performed using 1 μg of total RNA by the SuperScript III first strand system for RT-PCR (Invitrogen). The sequences of the primers used for PKG amplification were 5′-AAGATTCTCATGCTCAAGGA-3′ and 5′-CAGCTCCAAGTTCTTCATGA-3′, forward and reverse, respectively. β-actin, used as a control, was amplified using a sense primer 5′-AAGATGACCCAGATCATGTT-3′ and an antisense primer 5′-GAGTACTTGCGCTCAGGAGG-3′(Suppl Table S1). End-point PCR was carried out in 50 μl (total volume) containing 5 μl cDNA, 1× PCR buffer, 200 μM each deoxynucleotide triphosphate, 200 nM each primer, 1.5 mM MgCl2, and 2.5 units of Taq DNA polymerase on a thermal cycler for 30 cycles. Denaturation, annealing and elongation were carried out at 94 °C for 45 s, 55 °C for 30 s, and 72 °C for 60 s, respectively. PCRs were performed using Taq DNA polymerase (Invitrogen) with a 200 nM concentration of each primer.
2.3. RNA extraction, and quantitative PCR
Total RNA was isolated from dry pellets of RIN-m5F cells stored at −80 °C using the Ambion RNaqueous4PCR kit (Applied Biosciences) according to the manufacturer’s recommendations. Quantity and quality of total RNA was assessed using a spectrophotometer. cDNA was synthesized from 2 μg total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Amplification efficiency was determined by reducing the template amount in the cDNA synthesis reaction to 200, 20 and 2 ng and calculated using the formula, Ex = (−1/slope) −10, where Ex is the amplification efficiency. The quantitative polymerase chain reaction was performed using a StepOne Plus PCR System and gene-specific FAM- and VIC-labeled Taqman gene expression assays (Applied Biosystems, see Suppl. Table S2) using a total reaction volume of 20 μl, and the equivalent of 50 ng total RNA (0.5 μl) transcribed to cDNA per well. Rat β-actin was used as endogenous control. In order to obtain standard curves, genomic sequences of 200 bp length N- and C-terminal of the Taqman probe were amplified using standard PCR protocols, cloned into a pUC18 cloning vector (Fermentas) and verified by sequencing. We performed quantitative gene expression analysis and data was exported and plotted in Prism Software (GraphPad Inc). Statistical significance was determined using analysis of variance (ANOVA) with Student-Newman-Keuls post-hoc test.
2.4. Electrophysiology
Electrophysiological recordings were performed according to the whole cell variant of the patch clamp technique [14] at room temperature (~22 °C). The bath solution contained (in mM): 10 or 5 BaCl2 (for RIN-m5F and HEK-293, respectively), 125 TEA-Cl, 10 HEPES, and 10 glucose (pH 7.3). Patch pipettes were filled with a solution containing (in mM): 120 CsCl, 10 HEPES, 10 EGTA, 5 MgCl2, 4 ATP, and 0.1 GTP (pH 7.3). The osmolarity was adjusted to ~280 mOsm/L and 300 mOsm/L for the internal and external solutions, respectively. Recordings were performed using an Axopatch 200 B amplifier (Molecular Devices) and filtered at 2 kHz (internal four-pole Bessel filter). Currents were acquired at a frequency of 5.71 kHz by means of a DigiData 1320A digitizer (Molecular Devices) and analyzed using the pCLAMP (Molecular Devices) and SigmaPlot (Systat) software applications. Linear capacitative currents were canceled using the amplifier and by subtraction using a P/4 protocol. Membrane capacitance (Cm) was determined as described previously and used to normalize currents [15]. Series resistance values were typically 2–10 MΩ, and recordings where the voltage error (Ver = Imax × Rs) was >5 mV (at its maximal value) were not subjected to further analysis. Currents were recorded by applying 140 ms pulses, between −70 and 50 mV in the case of RIN-m5F cells and from −70 to 30 mV in the case of HEK-293 cells, in 5-mV increments from a holding potential (Vh) of −80 mV. Current-voltage (I–V) relationships were generated from the peak current obtained during the pulses and were fitted with an equation of the form I = (Vh − Vrev)/[(1 + exp (V0.5 − Vh)/k]) where, I is the peak current density (in pA/pF) at a given Vh, V0.5 is the voltage at which half of the channels are activated, Vrev is the reversal potential, and k is the slope factor [16].
2.5. Insulin secretion
RIN-m5F cells were washed with PBS and preincubated with Krebs-Ringer buffer consisting of (in mM) 25 HEPES, 115 NaCl, 24 NaHCO3, 5 KCl, 1 MgCl2, 2.5 mM CaCl2 and 0.1% BSA for 5 min at 37°C in 5% CO2, and 95% air humidified atmosphere. The preincubation buffer was removed and replaced with Krebs-Ringer buffer containing 40 mM KCl for 10 min at 37 °C. Insulin secretion was assessed by the enzyme-linked immunosorbent assay (ELISA) using the rat insulin ELISA kit (Alpco) according to the manufacturer’s recommendations.
2.6. Site directed mutagenesis
The cDNA encoding the L-type channel pore-forming CaV1.3α1 subunit cloned into the pcDNA6 expression vector was used as a template. The point mutations were introduced with ~40-mer synthetic oligonucleotides using the Quik-Change XL-mutagenesis kit (Stratagene). Initially, single amino acid mutations changing separately two serine residues to alanine (Ser793Ala and Ser860Ala) were created. Next, a double phosphorylation mutant (DPM) was generated by mutating the Ser793Ala cDNA using the Ser860Ala primers. cDNAs of all mutant channel CaV1.3α1 subunit were sequenced on an automated sequencer (ABIPrism310; Suppl Fig. S1C).
2.7. In vitro phosphorylation assay
The loop connecting the II and III repeats of the CaV1.3 WT or S793A/S860A double mutant constructs were incubated with 150 U of bovine lung PKG, Iα (Millipore) and 1 mM cGMP in kinase reaction buffer (20 mM Tris-HCl, pH 7.5, 1 mM MgCl2) in a final volume of 30 μL for 30 min at 37 °C. The reaction was stopped by adding 6 μL (6X) SDS sample buffer, and subsequently boiled for 5 min at 96 °C. Samples were separated by 15% SDS-PAGE and gel bands transferred to nitrocellulose membranes for Western blotting using a mouse anti-phosphoserine monoclonal antibody (Sigma–Aldrich; dilution 1:500).
2.8. Chemicals and drugs
8-Br-cGMP (Cat. # B1381), SNP (Cat. # 71778), CPTIO (Cat. # C221), and H-89 (Cat. # B1427) were obtained from Sigma-Aldrich, prepared as stock in distilled water, aliquoted and stored at −20 °C. KT5823 (Cat. # K1388) and Calyculin A (Cat. # C5552) were prepared as stock in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the bath recording solution was <0.05% (vol/vol). All other chemicals were of reagent grade and obtained from different commercial sources.
3. Results
3.1. The cGMP-PKG signaling pathway regulates native L-type CaV channels and insulin release in RIN-m5F cells
It has been reported that L-type CaV channels can be regulated by the NO-cGMP-PKG pathway in chromaffin cells from mouse and bovine [7,9,12], and in hair cells of the inner ear from rat and frog [11,17]. Likewise, there is growing evidence for the presence of this signaling cascade in pancreatic β-cells, though there are only a few reports concerning the action mechanism of NO in these cells [18–20].
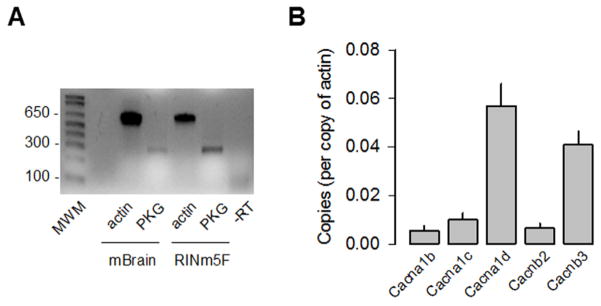
Therefore, we first investigated the functional consequences of the cGMP-PKG pathway activation on the regulation of L-type CaV channel activity in the insulin-secreting cell line RIN-m5F. To this end, end-point RT-PCR and real-time quantitative PCR were used as methods to analyze the expression of PKG and different subunits that compose the L-type CaV channel complexes in the RIN-m5F cells. By using specific primers (see Suppl Table S2), conspicuous RNA signals for PKG were consistently observed in both the RIN-m5F cells and the whole mouse brain used as control tissue (Fig. 1A). In addition, given that RIN-m5F cells co-express CaV1.2 and CaV1.3 L-type as well as non-L-type (CaV2.2) channels [21], we performed mRNA copy number analysis for the three pore-forming subunits of these channels, as well as two genes encoding the CaVβ auxiliary subunits. The copy number of β-actin mRNA was used as normalization control. As can be seen in Fig. 1B, CaV1.3α1 and CaVβ3 were the predominant subunits in the RIN-m5F cells and were expressed about five- to nine-fold higher compared to the other corresponding subunits analyzed. Interestingly, the CaV1.2 transcripts accounted only ~20% of the L-type channels expressed in these cells.
Fig. 1.
Expression of PKG and CaV channel subunits in RIN-m5F cells. A) RT-PCR analysis of PKG cDNA expression in RIN-m5F cells. Total RNA from mouse whole brain was used as a positive control. Actin was used as an internal control. Primer sequences are indicated in Supplemental Table S1. B) Levels of different CaV channel subunit mRNAs expressed in RIN-m5F cells estimated from gene quantitative RT-PCR data and expressed as a number of copies of β-actin mRNA expression.
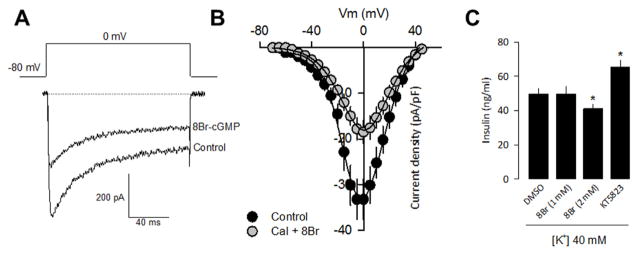
To assess the potential involvement of PKG in the regulation of L-type CaV channel activity, we next analyzed the effect of 8-Br-cGMP, a membrane-permeable analogue of cGMP, on the whole-cell currents in RIN-m5F cells incubated at room temperature (~22 °C) for 10 min before electrophysiological recordings. To enhance the effects of PKG activation, this study monitored whole cell currents in the presence of 10 mM Ba2+ as charge carrier, and Calyculin A, an inhibitor of protein phosphatases, which by itself do not modify Ca2+ current density. In these conditions, a robust voltage-dependent inward current was observed, and according to the results in the preceding section they may be carried mostly by CaV1.3/CaVβ3 channels. The currents decayed with a slow time course (τ= 31.1 ± 2.3 ms) and the average current density-voltage relationships (peak current amplitude normalized by Cm reached a maximum at about 0 mV (Fig. 2A and B). Notably, the application of 8-Br-cGMP significantly decreased the whole cell current in RIN-m5F cells to about 55% of its control value at 0 mV (n = 12–14 cells). We next investigated the physiological impact of the activation of PKG by 8-Br-cGMP on L-type CaV channel activity by assessing insulin secretion from RIN-m5F cells. Interestingly, release of insulin triggered by Ca2+ influx in response to high K+-induced membrane depolarization (40 mM K+; 10 min) was significantly reduced (~20%) in cells treated with 8-Br-cGMP at a 2 mM concentration. Conversely, application of the specific PKG antagonist KT-5823 increased (~30%) insulin release triggered by depolarization with high K+ (Fig. 2C). Altogether, these data stresses the importance of L-type CaV channel regulation by cGMP-PKG signaling pathway for fine tuning insulin release.
Fig. 2.
PKG inhibit L-type CaV channels in RIN-m5F cells and contributes to determine insulin secretion. A) Representative traces of Ba2+ currents (IBa) through Ca2+ channels in RIN-m5F cells in the control condition and after 8-Br-cGMP (1 mM) application. B) Average current density-voltage relationships for IBa recorded from RIN-m5F cells in the absence and presence of 8-Br-cGMP (1 mM). C) High K+-induced insulin secretion from RIN-m5F cells in the absence and the presence of 8-Br-cGMP as indicated. RIN-m5F cells were incubated with KRB buffer containing 40 mm KCl. Insulin content in the supernatants was measured by ELISA. The mean ± S.E. of four independent experiments is shown.
3.2. The cGMP-PKG signaling pathway regulates recombinant L-type CaV1.3 channels
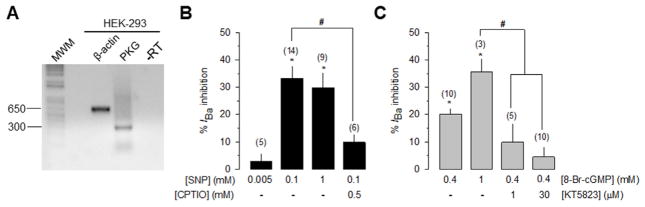
We next investigated the regulation of CaV1.3 channels by the NO-cGMP-PKG pathway using recombinant channels in the HEK-293 cell line, a heterologous expression system that does not express endogenous CaV channels [15,16]. Hence, the CaV1.3α1 channel pore-forming subunit together with the CaVβ3 and the CaVα2δ-1 auxiliary subunits were transiently co-transfected in HEK-293 cells and electrophysiological recordings were performed 48 h after transfection. In an initial series of experiments, we confirmed the endogenous expression of PKG in the HEK-293 cells by RT-PCR experiments (Fig. 3A). Next, we evaluated the action of the nitric oxide (NO) donor sodium nitroprusside (SNP), as well as 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (CPTIO), a specific scavenger of NO, on the whole cell Ba2+ currents through recombinant channels heterologously expressed in the HEK-293 cell line. The application of 100 μM SNP significantly decreased current amplitude through recombinant CaV1.3 channels in HEK-293 cells (~33 ± 5%; n = 14 cells; P < 0.01) with no significant effects on the current activation or inactivation rates (Fig. 3B). A similar decrease in current amplitude was observed after the application of 1 mM SNP. The inhibitory action of SNP (100 μM) on the currents was significantly reduced (to ~10 ± 3%; n = 6 cells; compared with the ~33% caused by SNP alone; P < 0.01) in the presence of CPTIO (500 μM). These results suggest that the currents through CaV1.3/CaVβ3/CaVα2δ-1 channels heterologously expressed in HEK-293 cells may be modulated by NO.
Fig. 3.
The NO-cGMP-PKG pathway inhibited recombinant L-type CaV 1.3 channel activity. A) RT-PCR analysis of PKG cDNA expression in the HEK-293 cell line. Actin was used as an internal control. Primer sequences are indicated in Supplemental Table S1. B) Bar chart of the percentage inhibition ± S.E. of IBa measured with test pulses to −30 mV at different concentrations of SNP alone, or after 500 μM CPTIO application. The number of recorded cells is indicated in parenthesis. C) Comparison of the of the percentage inhibition ± S.E. of IBa measured with test pulses to −30 mV at different concentrations of 8-Br-cGMP alone or in combination of KT-5823, as indicated.
In order to investigate whether the current inhibition produced by NO was mediated by an increase in the levels of cGMP and the activation of PKG, we next carried out a series of experiments in the presence of 8-Br-cGMP and KT-5823, a specific inhibitor of PKG. As can be seen in Fig. 3C, the application of 0.4 and 1 mM of 8-Br-cGMP significantly decreased current amplitude through recombinant CaV1.3 channels in HEK-293 cells (~20 and ~35%, respectively; n = 3–10 cells; P < 0.01) with no significant effects on the current activation or inactivation rates. Interestingly, co-application of 0.4 mM 8-Br-cGMP and KT-5823 (1 or 30 μM) significantly prevented the inhibitory action of 8-Br-cGMP (Fig. 3C). These results suggest that the effects induced by 8-Br-cGMP on the CaV1.3/CaVβ3/CaVα2δ-1 channel currents could be mediated by the activation of PKG.
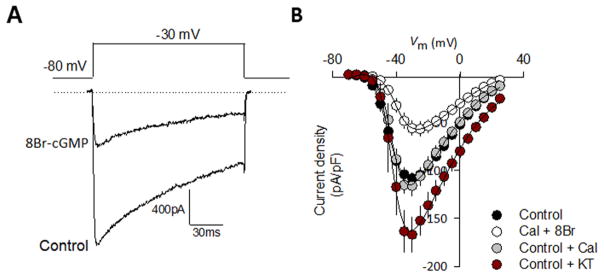
Superimposed traces in Fig. 4A show that 8-Br-cGMP at a concentration of 1 mM significantly decreased the whole cell current after ~10 min of incubation (to ~52% of the control value at −30 mV; n = 22 cells; P < 0.01), mimicking to some extent the action of SNP. Boltzmann fitting shown in the I–V relationships (Fig. 4B) indicated that the V0.5 of current activation was slightly shifted (by <5 mV) in the presence of 8-Br-cGMP and changes in the slope parameter were also not statistically significant. On the other hand, the inhibitory effect of 8-Br-cGMP on the currents was fully prevented by the use of the PKG inhibitor KT-5823 (10 μM), indicating that the inhibition produced by 8-Br-cGMP is mediated by the activation of the PKG.
Fig. 4.
PKG activation modifies current density through recombinant L-type CaV 1.3 channels. A) Representative superimposed current traces recorded in HEK-293 cells expressing CaV 1.3/CaV α2 δ-1/CaV β3 channels in the absence and presence of 8-Br-cGMP (1 mM). Currents were evoked by 140-ms depolarizing pulses from a Vh of −80 to −30 mV. B) Average current density-voltage relationships for IBa recorded from HEK-293 cells expressing CaV 1.3/CaV α2 δ-1/CaV β3 channels in the absence and presence of 8-Br-cGMP (1 mM), Calyculin (10 nM) and KT-5823 (10 μM), as indicated.
3.3. Identification of the sites of cGMP-dependent phosphorylation in the CaV1.3α 1 subunit
Having shown that CaV1.3 channel modulation by NO implies the participation of PKG, we next searched for the presence of the consensus sequence for PKG phosphorylation in the pore-forming CaVα1 channel subunit sequence using the database publicly available at the URL http://gps.biocuckoo.org/. We identified several sites in the CaV1.3α1 sequence as potential PKG substrates with high score, including Thr455, Ser697, Ser793, Ser860 and Ser1581 (Suppl Fig. S1A). The first site was located in the I–II loop of the CaVα1 subunit, other three were present in the II–III loop, and one more in the C-terminal of the protein. However, serine residues 793 and 860 were identified as the main sites of PKG phosphorylation because they were conserved among species and exhibited the highest scores (Suppl Fig. S1B).
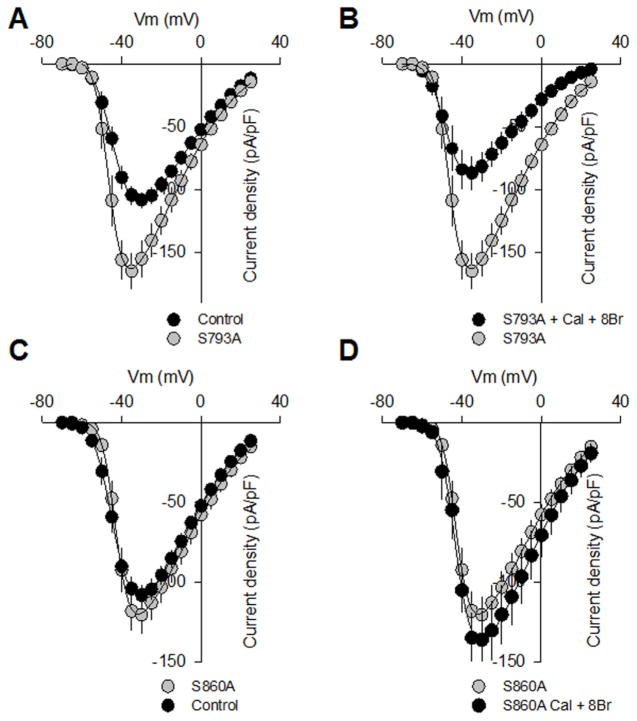
To determine whether these sites were indeed phosphorylated by PKG, we next generated two individual and one double mutation in which these serine residues were substituted with alanines by site-directed mutagenesis, and then we tested them in whole cell patch-clamp recordings. First, a construct encoding the full-length CaV1.3α1 along with the auxiliary subunits (CaVβ3/CaVα2δ-1) cDNAs were transiently transfected into HEK-293 cells. It is worth mentioning that the recombinant channels showed satisfactory levels of expression and generated typical current waveforms expected from L-type CaV1.3 channels. Unexpectedly, current density was larger in cells transfected with CaV1.3 channels harboring the Ser793 mutation at most of the voltages tested (Fig. 5A), but no differences in current kinetics were evident. The corresponding I–V relationship showed a significant ~1.5-fold increase in current density in the control, however in cells treated with 8-Br-cGMP (Fig. 5B) there was a decrease similar to that observed for the wild-type channels.
Fig. 5.
Mutation of two serine residues (S763 and S860) to delete PKG-mediated phosphorylation sites in the CaV 1.3α1 subunit alters channel functional expression. A) Average current density-voltage relationships for IBa recorded from HEK-293 cells expressing the wild-type CaV 1.3 channel and its mutant variant (Ser793Ala) in the control condition and after incubation with 1 mM 8- Br-cGMP plus 10 nM Calyculin A (B). IBa density was calculated at a series of test pulses applied from a Vh of −80 mV in 5 mV steps between −70 and 30 mV. C) Average current density-voltage relationships for IBa recorded from HEK-293 cells expressing the wild-type CaV 1.3 channel and its mutant variant (Ser860Ala) in the control condition and after incubation with 1 mM 8- Br-cGMP plus 10 nM Calyculin A (D).
Given that PKG, PKA and PKC phosphorylation sites are distinct, these data suggest that the cGMP-PKG pathway may be already active at basal resting conditions, and down-regulate the channels by phosphorylating the CaV1.3α1 at position Ser793. However, it has been also recognized that the existence of distinct phosphorylation sites for PKG and PKA does not rule out the possibility that they may mutually interfere on channel gating [7]. For this reason, we next decided to test the actions of the PKA blocker H-89 on the whole-cell currents through wild-type channels. It is acknowledged that the cGMP-PKG-mediated down-regulation of CaV1.3 L-type channels may exert an opposite action to the cAMP/PKA-mediated up-regulation. In this scenario, we hypothesized that if serine residue at position 793 was a regulatory target of PKA, then currents through wild-type channels were going to be increased when PKA activity was blocked by H-89. However, the application of H-89 (100 nM) significantly decreased current density (~22 ± 6% at −30 mV; n = 5, P = 0.05; Suppl. Fig. S2B) through wild-type channels, indicating that indeed PKA phosphorylate different serine sites of the CaV1.3α1 subunit. On the other hand, the second and most convincing evidence in favor that the CaV1.3 channels are phosphorylated at rest by the cGMP-PKG pathway comes from experiments using the PKG blocker KT-5823. As can be seen in Fig. 4B, application of KT-5823 alone causes a significant increase in current density through wild-type CaV1.3 channels which is similar to the up-regulation observed for the Ser793Ala channel currents (Fig. 5A and B).
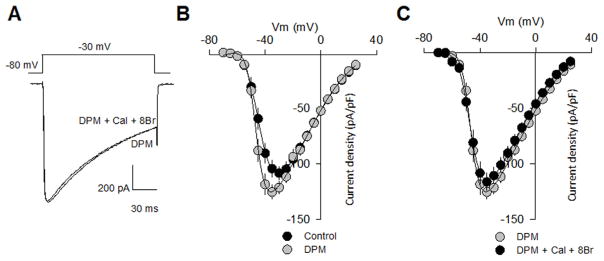
In sharp contrast, substitution of serine 860 by alanine produced mutant CaV1.3 channels refractory to modulation by PKG (Fig. 5C and D), supporting the unique importance of this amino acid residue in the CaV1.3α1 channel subunit as a direct regulatory target of PKG. Last, as observed in currents recorded through Ser860Ala mutant CaV1.3 channels, the magnitude of the currents through CaV1.3 harboring the double phosphorylation mutation was not different from that observed with the wild type channels, both in the absence and presence of 8-Br-cGMP (Fig. 6). As in all cases, no differences in voltage dependence or current kinetics were also apparent. These data are consistent with the results of an in vitro phosphorylation assay in which a HIS fusion protein containing the region of interest (the loop connecting the II and III repeats in CaV1.3α1) was generated using the pRSET A expression vector (Invitrogen), and then expressed in BL21 cells. Western blot analysis using an anti-phosphoserine monoclonal antibody showed that PKG phosphorylated the wild-type II–III loop of the CaV1.3α1 HIS fusion protein, whereas the double phosphorylation mutant construct displayed negligible levels of phosphorylation (Suppl. Fig. S2C).
Fig. 6.
Phosphorylation at sites S763 and S860 of CaV 1.3α1 subunit influences channel functional expression. A) Representative traces of Ba2+ currents recorded from HEK-293 cells expressing DPM mutant channels in absence and presence of 8-Br-cGMP (1 mM) plus 10 nM Calyculin A. B) Average current density-voltage relationships for IBa from HEK-293 cells expressing the wild-type CaV 1.3 channel and its double phosphorylation mutant (DPM) variant in the control condition and after incubation with 1 mM 8-Br-cGMP plus 10 nM Calyculin A (C). IBa density was calculated at a series of test pulses applied from a Vh of −80 mV in 5 mV steps between −70 and 30 mV.
4. Discussion
L-type CaV channels are regulatory targets for a range of second messengers in different tissues. These modulatory effects are generally mediated through G protein signaling cascades linked to protein kinases [3,6,7,22–24]. In this context, functional studies in rat vestibular hair cells and chromaffin cells have documented an inhibitory effect of PKG on L-type CaV1.3 channels [7,11,12]. These observations raised the possibility that CaV1.3 channel activity in other cell lines and tissues, for example insulin-secreting cells, may be also inhibited by PKG. In this report, we present electrophysiological observations supporting the view that a membrane-permeable analogue of cGMP may effectively modulate L-type CaV channels through classical PKG signaling in the clonal RIN-m5F cell line. Consistent with this idea, blockade of PKG using a specific inhibitor attenuated the ability of 8-Br-cGMP to inhibit L-type CaV channels in the RIN-m5F cells. These results are the first to suggest that L-type channels in insulin-secreting cells are directly targeted by cGMP signaling through PKG (Fig. 7).
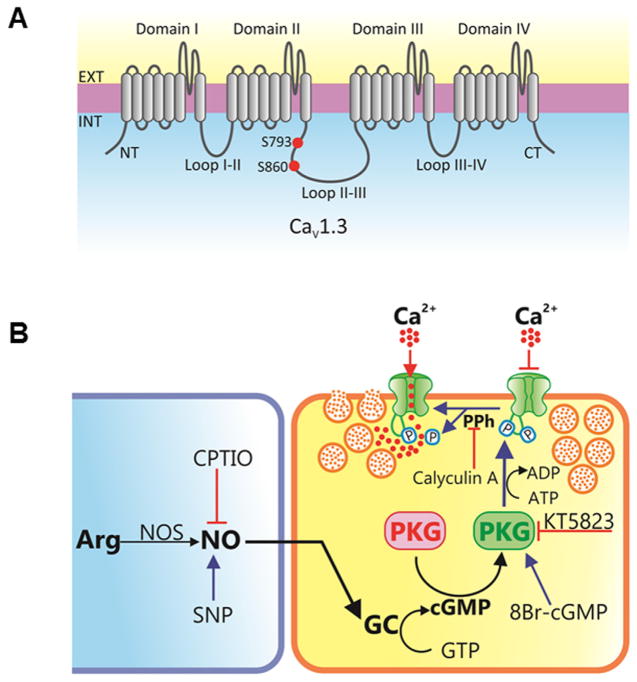
Fig. 7.
Schematic overview of the mechanisms involved in PKG-induced regulation of L-type CaV channel activity and insulin secretion. A) The L-type channel complex is composed of the pore-forming CaV α1 and auxiliary subunits (CaV α2 δ, CaV β, and CaV γ). The pore-forming CaV α1 subunits consist of four transmembrane domains (I–IV) and the linker joining II and III encompasses the putative PKG phosphorylation sites (S793 and S860). B) NO is synthesized by the enzyme NO synthase (NOS) through the oxidation of L-arginine to NO and L-citrulline, with the assistance of cofactors. NO endogenously produced by NOS or released from exogenously applied NO donors (sodium nitroprusside, SNP) activates NO-sensitive guanylate cyclase (GC) leading to increased synthesis of cGMP which activates PKG. The protein kinase catalyzes the phosphorylation of the L-type CaV channels which cause a decrease in Ca2+ influx through the cell membrane resulting in reduced release of insulin in RIN-m5F cells. The inhibitory action of PKG is blocked by the selective inhibitor KT-5823 and terminated by protein phosphatases (PPh). Calyculin A, is a serine/threonine phosphatase inhibitor.
Given that in clonal insulin-secreting cell lines and pancreatic islets from different species, L-type CaV channels are coupled to glucose-stimulated insulin secretion [25], it was expected that PKG-mediated channel inhibition may also affect hormone release. The present study reveals that indeed this regulation significantly reduced insulin secretion triggered by Ca2+ influx in response to high K+-induced membrane depolarization, demonstrating the importance of L-type channel regulation by cGMP-PKG cascade for fine tuning hormone release. Here it is worth mentioning that insulin secretion in mice depends mainly on the activity of CaV1.2 channels, but in rats and humans it has been shown that it relies mainly on the activity of the CaV1.3 channels [25–27].
Previous studies have also documented the regulation of L-type CaV1.3 channels by post-translational modifications including phosphorylation by different protein kinases. For example, it has been observed that protein kinase C (PKC) phosphorylates CaV1.3α1 channel subunit at serine residue 81 located at the N-terminal of the protein and that this phosphorylation decreases Ca2+ current amplitude in tsA201 cells expressing recombinant channels [28]. On the other hand, phosphorylation of CaV1.3 channels by cAMP-dependent protein kinase (PKA) results in an increase in current density through native and recombinant channels [12,29,30].
Interestingly, Carbone and colleagues (2012) determined in chromaffin cells that L-type CaV1.3 and CaV1.2 channels are regulated with the same effectiveness, but with opposite effects, by PKA and PKG phosphorylation. Hence, phosphorylation by PKA causes an increase in Ca2+ currents, whereas PKG phosphorylation results in a decrease in Ca2+ current amplitude. These authors reported that the effects of these protein kinases on channel activity are independent and may also participate in the regulation of catecholamine release. However, the specific sites of such phosphorylation events were not established [7,9,12].
In agreement with these previous reports, in the present work we observed a down-regulation of CaV1.3 channel activity (specifically of the CaV1.342A isoform) by PKG in HEK-293 cells, and that this regulation is mediated by phosphorylation in two serine residues located at position 793 and 860 in the intracellular loop that connects the II and III repeated domains of the pore-forming CaVα1 subunit of the channel complex (Fig. 6). These might actually be phosphorylated in the cellular context, as no apparent changes in the electrophysiological properties of the mutant channels were observed.
An interesting finding of our work is that PKG seems to affect CaV1.3 differentially depending on the phosphorylation site. As mentioned earlier, alanine substitution of CaVα1.3 serine at position 860 fully prevented the PKG-mediated inhibition consistent with the premise that phosphorylation at this residue site may be responsible for the decrease in CaV1.3 current amplitude after exposure to 8-Br-cGMP and Calyculin A. As mentioned earlier, Calyculin A by itself does not modify current density (Suppl Fig. S2A). Interestingly however, current density in basal conditions was larger in cells transfected with the Ser793Ala mutant channels, which represented an indication that the cGMP-PKG pathway may be active at basal resting conditions, in agreement with previous reports [12], and down-regulate the channels by phosphorylating the CaV1.3α1 subunit at this serine residue. In support of this interpretation, application of the PKG blocker KT-5823 caused an increase in current density through wild-type CaV1.3 channels similar to the up-regulation observed in the case of the Ser793Ala mutant channels. In this scenario, a concerted action of the two phosphorylation sites might have a cumulative effect when both sites are phosphorylated to produce maximal values of L-type currents inhibition.
It should be mentioned here that previous studies have shown differential effects on several CaV channels by the NO-cGMP-PKG signaling pathway. These reports suggest a down-regulation of the N-type CaV channels in human neuroblastoma cells IMR32 [31], and in dorsal root ganglia neurons [32]. Likewise, it has been observed that NO donors decrease Ca2+ currents through T-type channels in rat cerebral arterial smooth muscle cells [33], and P/Q-type CaV channels in rat cortical neurons [34].
However, in cortical neurons the activation of the NO-cGMP-PKG cascade had no effect on currents through N- and L-type channels [34]. In addition, it has been reported also that activation of the NO signaling cascade actually enhances L- and P/Q-type current in mouse neurons of the medial nucleus of the trapezoid body [35]. Though the reason for these discrepancies are presently unknown, they may lie in the fact that some NO effects are mediated via cGMP, but some others depend on the ability NO to generate free radicals or produce peroxynitrite leading to protein S-nitrosylation or nitrotyrosination [36]. Indeed, redox-modulation of L-type channels has been reported in cardiac myocytes, hippocampal neurons and vestibular hair cells [11,37,38]. However, in our experiments this may not be the case because the inhibitory effect of the NO donor SNP was almost fully blocked by the NO scavenger CPTIO. In addition, it should be further noted that CaV1.3 channels are associated with CaVβ and CaVα2δ auxiliary subunits which may also be subject to post-translational modifications. In line with this, previous studies have shown that the regulation of L-type CaV1.2 channels by PKG depends also on phosphorylation of the CaVβ2 auxiliary subunit [25]. This finding could help explaining the variations observed regarding the regulation of L-type CaV channels by PKG.
In all, the results obtained in the present work show that the repertoire of PKG down-regulation of L-type Ca2+ channels may extend to CaV1.3 channels in both a heterologous system and in a cellular model that natively expresses these channels, and also show additional evidence of the functional effect this regulation may have on hormone secretion. By using a site-directed mutagenesis approach we have pinpointed the residues within the CaV1.3α1 pore-forming subunit, which are modulated by the cGMP-PKG signaling pathway. Likewise, given the low-threshold of activation and slow rate of inactivation of CaV1.3 channels, they could carry sufficient inward current at subthreshold potentials to activate K+ channels which contribute to determine the membrane resting potential, as well as the action potential shape and frequency during spontaneous firing or sustained depolarizations [39,40]. Therefore, the findings described here also suggest that PKG phosphorylation could play important roles in the regulation of numerous cellular processes.
Supplementary Material
Acknowledgments
This work was supported by funds from Conacyt (grant 221660) to RF; R00MH099405 to AA. We thank Mercedes Urban for expert technical assistance. Doctoral fellowship from Conacyt to PD is gratefully acknowledged. Additional support was provided by the Dr. John P. and Therese Mulcahy Endowed Professorship in Ophthalmology to SK.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ceca.2017.05.008.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Gandini MA, Sandoval A, Felix R. Patch-clamp recording of voltage-sensitive Ca2+ channels. Cold Spring Harb Protoc. 2014;2014:325–329. doi: 10.1101/pdb.top066092. References. [DOI] [PubMed] [Google Scholar]
- 2.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felix R. Calcium channelopathies. Neuromol Med. 2006;8:307–318. doi: 10.1385/NMM:8:3:307. [DOI] [PubMed] [Google Scholar]
- 5.Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol. 2016;594:5369–5390. doi: 10.1113/JP272262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felix R. Molecular regulation of voltage-gated Ca2+ channels. J Recept Signal Transduct Res. 2005;25:57–71. doi: 10.1081/rrs-200068102. [DOI] [PubMed] [Google Scholar]
- 7.Vandael DH, Mahapatra S, Calorio C, Marcantoni A, Carbone E. CaV 1.3 and CaV 1.2 channels of adrenal chromaffin cells: emerging views on cAMP/cGMP-mediated phosphorylation and role in pacemaking. Biochim Biophys Acta. 2013;1828:1608–1618. doi: 10.1016/j.bbamem.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Nafzger S, Rougier JS. Calcium/calmodulin-dependent serine protein kinase CASK modulates the L-type calcium current. Cell Calcium. 2017;61:10–21. doi: 10.1016/j.ceca.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Carabelli V, D’Ascenzo M, Carbone E, Grassi C. Nitric oxide inhibits neuroendocrine CaV 1 L-channel gating via cGMP-dependent protein kinase in cell-attached patches of bovine chromaffin cells. J Physiol. 2002;541:351–366. doi: 10.1113/jphysiol.2002.017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz PM, Rodriguez-Pascual F, Koesling D, Torres M, Förstermann U. Functional coupling of nitric oxide synthase and soluble guanylyl cyclase in controlling catecholamine secretion from bovine chromaffin cells. Neuroscience. 1998;82:255–265. doi: 10.1016/s0306-4522(97)00274-1. [DOI] [PubMed] [Google Scholar]
- 11.Almanza A, Navarrete F, Vega R, Soto E. Modulation of voltage-gated Ca2+ current in vestibular hair cells by nitric oxide. J Neurophysiol. 2007;97:1188–1195. doi: 10.1152/jn.00849.2006. [DOI] [PubMed] [Google Scholar]
- 12.Mahapatra S, Marcantoni A, Zuccotti A, Carabelli V, Carbone E. Equal sensitivity of CaV 1.2 and CaV 1.3 channels to the opposing modulations of PKA and PKG in mouse chromaffin cells. J Physiol. 2012;590:5053–5073. doi: 10.1113/jphysiol.2012.236729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramadan O, Qu Y, Wadgaonkar R, Baroudi G, Karnabi E, Chahine M, Boutjdir M. Phosphorylation of the consensus sites of protein kinase A on alpha1D L-type calcium channel. J Biol Chem. 2009;284:5042–5049. doi: 10.1074/jbc.M809132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 15.Avila G, Sandoval A, Felix R. Intramembrane charge movement associated with endogenous K+ channel activity in HEK-293 cells. Cell Mol Neurobiol. 2004;24:317–330. doi: 10.1023/B:CEMN.0000022765.52109.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, Zamponi GW. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci. 2004;20:1–13. doi: 10.1111/j.1460-9568.2004.03434.x. [DOI] [PubMed] [Google Scholar]
- 17.Lv P, Rodriguez-Contreras A, Kim HJ, Zhu J, Wei D, Choong-Ryoul S, Eastwood E, Mu K, Levic S, Song H, Yevgeniy PY, Smith PJ, Yamoah EN. Release and elementary mechanisms of nitric oxide in hair cells. J Neurophysiol. 2010;103:2494–2505. doi: 10.1152/jn.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tejedo JR, Ramirez R, Cahuana GM, Rincon P, Sobrino F, Bedoya FJ. Evidence for involvement of c-Src in the anti-apoptotic action of nitric oxide in serum-deprived RINm5F cells. Cell Signal. 2001;13:809–817. doi: 10.1016/s0898-6568(01)00206-6. [DOI] [PubMed] [Google Scholar]
- 19.Tejedo JR, Cahuana GM, Ramirez R, Esbert M, Jimenez J, Sobrino F, Bedoya FJ. Nitric oxide triggers the phosphatidylinositol 3-kinase/Akt survival pathway in insulin-producing RINm5F cells by arousing Src to activate insulin receptor substrate-1. Endocrinology. 2004;145:2319–2327. doi: 10.1210/en.2003-1489. [DOI] [PubMed] [Google Scholar]
- 20.Bedoya FJ, Salguero-Aranda C, Cahuana GM, Tapia-Limonchi R, Soria B, Tejedo JR. Regulation of pancreatic beta-cell survival by nitric oxide: clinical relevance. Islets. 2012;4:108–118. doi: 10.4161/isl.19822. [DOI] [PubMed] [Google Scholar]
- 21.Gandini MA, Sandoval A, González-Ramírez R, Mori Y, de Waard M, Felix R. Functional coupling of Rab3-interacting molecule 1 (RIM1) and L-type Ca2+ channels in insulin release. J Biol Chem. 2011;286:15757–15765. doi: 10.1074/jbc.M110.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Zamponi GW. Regulation of voltage gated calcium channels by GPCRs and post-translational modification. Curr Opin Pharmacol. 2016;32:1–8. doi: 10.1016/j.coph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Altier C, Zamponi GW. Signaling complexes of voltage-gated calcium channels and G protein-coupled receptors. J Recept Signal Transduct Res. 2008;28:71–81. doi: 10.1080/10799890801941947. [DOI] [PubMed] [Google Scholar]
- 25.Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- 26.Yang G, Shi Y, Yu J, Li Y, Yu L, Welling A, Hofmann F, Striessnig J, Juntti-Berggren L, Berggren PO, Yang SN. CaV 1.2 and CaV 1.3 channel hyperactivation in mouse islet beta cells exposed to type 1 diabetic serum. Cell Mol Life Sci. 2015;72:1197–1207. doi: 10.1007/s00018-014-1737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuccotti A, Clementi S, Reinbothe T, Torrente A, Vandael DH, Pirone A. Structural and functional differences between L-type calcium channels: crucial issues for future selective targeting. Trends Pharmacol Sci. 2011;32:366–375. doi: 10.1016/j.tips.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Baroudi G, Qu Y, Ramadan O, Chahine M, Boutjdir M. Protein kinase C activation inhibits CaV 1.3 calcium channel at NH2-terminal serine 81 phosphorylation site. Am J Physiol Heart Circ Physiol. 2006;291:H1614–H1622. doi: 10.1152/ajpheart.00095.2006. [DOI] [PubMed] [Google Scholar]
- 29.Qu Y, Baroudi G, Yue Y, El-Sherif N, Boutjdir M. Localization and modulation of α1D (Cav1.3) L-type Ca channel by protein kinase. A Am J Physiol Heart Circ Physiol. 2005;288:H2123–H2130. doi: 10.1152/ajpheart.01023.2004. [DOI] [PubMed] [Google Scholar]
- 30.Marshall MR, Clark JP, 3rd, Westenbroek R, Yu FH, Scheuer T, Catterall WA. Functional roles of a C-terminal signaling complex of CaV 1 channels and A-kinase anchoring protein 15 in brain neurons. J Biol Chem. 2011;286:12627–12639. doi: 10.1074/jbc.M110.175257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Ascenzo M, Martinotti G, Azzena GB, Grassi C. cGMP/protein kinase G-dependent inhibition of N-type Ca2+ channels induced by nitric oxide in human neuroblastoma IMR32 cells. J Neurosci. 2002;22:7485–7492. doi: 10.1523/JNEUROSCI.22-17-07485.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca2+ channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001;86:304–311. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]
- 33.Harraz OF, Brett SE, Welsh DG. Nitric oxide suppresses vascular voltage-gated T-type Ca2+ channels through cGMP/PKG signaling. Am J Physiol Heart Circ Physiol. 2014;306:H279–H285. doi: 10.1152/ajpheart.00743.2013. [DOI] [PubMed] [Google Scholar]
- 34.Petzold GC, Scheibe F, Braun JS, Freyer D, Priller J, Dirnagl U, Dreier JP. Nitric oxide modulates calcium entry through P/Q-type calcium channels and N-methyl-D-aspartate receptors in rat cortical neurons. Brain Res. 2005;1063:9–14. doi: 10.1016/j.brainres.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 35.Tozer AJ, Forsythe ID, Steinert JR. Nitric oxide signalling augments neuronal voltage-gated L-type (CaV 1) and P/Q-type (CaV 2.1) channels in the mouse medial nucleus of the trapezoid body. PLoS One. 2012;7:e32256. doi: 10.1371/journal.pone.0032256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tjong YW, Jian K, Li M, Chen M, Gao TM, Fung ML. Elevated endogenous nitric oxide increases Ca2+ flux via L-type Ca2+ channels by S-nitrosylation in rat hippocampal neurons during severe hypoxia and in vitro ischemia. Free Radic Biol Med. 2007;42:52–63. doi: 10.1016/j.freeradbiomed.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- 40.Vandael DH, Marcantoni A, Carbone E. CaV 1.3 channels as key regulators of neuron-like firings and catecholamine release in chromaffin cells. Curr Mol Pharmacol. 2015;8:149–161. doi: 10.2174/1874467208666150507105443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.