Abstract
Hypoxia through transcription factor HIF1α plays a critical role in cancer development. In prostate cancer, HIF1α interplays with androgen receptor (AR) to contribute to the progression of this disease to its lethal form—castration-resistant prostate cancer (CRPC). Hypoxia upregulates several epigenetic factors including histone demethylase KDM3A which is a critical co-factor of HIF1α. However, how histone demethylases regulate hypoxia signaling is not fully understood. Here, we report that histone demethylase PHF8 plays an essential role in hypoxia signaling. Knockdown or knockout of PHF8 by RNAi or CRISPR-Cas9 system reduced the activation of HIF1α and the induction of HIF1α target genes including KDM3A. Mechanistically, PHF8 regulates hypoxia inducible genes mainly through sustaining the level of trimethylated histone 3 lysine 4 (H3K4me3), an active mark in transcriptional regulation. The positive role of PHF8 in hypoxia signaling extended to hypoxia-induced neuroendocrine differentiation (NED), wherein PHF8 cooperates with KDM3A to regulate the expression of NED genes. Moreover, we discovered that the role of PHF8 in hypoxia signaling is associated with the presence of full-length AR in CRPC cells. Collectively, our study identified PHF8 as a novel epigenetic factor in hypoxia signaling, and the underlying regulatory mechanisms likely apply to general cancer development involving HIF1α. Therefore, targeting PHF8 can potentially be a novel therapeutic strategy in cancer therapy.
Keywords: PHF8, HIF1α, KDM3A, H3K4me3, Hypoxia, Prostate cancer
1. Introduction
Hypoxia plays a critical role in tumor angiogenesis, apoptotic resistance, metabolic switch from oxidation to glycolysis, and genomic instability, thereby contributing to cancer development [1,2]. Hypoxia is evident in prostate tumors, increasing with clinical stage, patient age, and is associated with a lower biochemical free relapse rate [3–5]. Moreover, it has been suggested that radiotherapy-resistant hypoxic tumor cells govern the overall responsiveness of prostate cancer to current therapies [3]. Crosstalk between hypoxia and androgen receptor (AR) signaling via the key transcription factors hypoxia-inducible factor 1-alpha (HIF1α) and hypoxia-inducible factor 2-alpha (HIF2α; also known as EPAS1) [6] has been extensively studied: Androgens induce HIF1α activation through the PI3K/AKT pathway [7]; hypoxia and AR synergistically regulate the expression of prostate-specific antigen (PSA) [8,9]; hypoxia enhances the transcriptional activity of AR at low androgen level and contributes to androgen-independent growth of LNCaP cells [10]. Additionally, HIF1α can form a ternary complex with AR and β-catenin at the androgen response elements (AREs) of AR target genes [11]. Consistent with these findings, synergistic targeting of AR and HIF1α has been shown to inhibit castration-resistant prostate cancer (CRPC) cells [9].
Histone demethylases are involved in the transcriptional output of the AR and hypoxia signaling pathways, and thus contribute to prostate cancer development [12]. For example, the histone demethylase KDM3A serves as a transcriptional coactivator of HIF1α and AR in regulation of their target genes [13]. KDM3A is a JmjC domain-containing histone demethylase. Since the members of this family require oxygen (O2) as a co-factor [14], their O2 thresholds dictate their demethylation activities, and hence their functions under hypoxia [15]. For example, KDM3A and Jumonji Domain Containing 2B (JMJD2B) are active at 1% O2 in HeLa cells [16], and KDM3A is active under 0.5% O2 in LNCaP cells whereas Jumonji Domain-Containing Protein 2A (JMJD2A) and Jumonji/ARID Domain-Containing Protein 1B (JARID1B) lose their demethylation activities under these conditions [13]. The enhancement of the activities of particular histone demethylases by hypoxia results in a feed-forward loop that ensures that they contribute to transcriptional regulation under hypoxia [15]. This regulatory mechanism compensates for the partial loss of their demethylation activities, and those of other histone demethylases under hypoxia. However, how histone demethylases regulate hypoxia signaling is not fully understood.
We recently reported that PHF8 (PHD finger protein 8) is dynamically regulated during the neuroendocrine differentiation (NED) that occurs in prostate cancer, and that the c-MYC-miR-22 axis contributes to the regulation of PHF8 in the context of androgen depletion and IL-6 treatment [17]. However, the c-MYC-miR-22-PHF8 axis is decoupled under hypoxia (1% O2, 6 day treatment), with c-MYC downregulated but PHF8 post-transcriptionally upregulated. Recently, it was reported that in the context of hypoxia HIF1α and HIF2α transcriptionally upregulate PHF8, which interacts with AR and enhances its transcriptional activity [18]. In the current study, we report PHF8 plays a critical role in hypoxia signaling as it: positively regulates KDM3A which is a critical coactivator of HIF1α; indirectly sustains H3K4me3 levels on select hypoxia-inducible genes; and is required for full activation of HIF1α through various mechanisms. In the context of prostate cancer, PHF8 appears to execute its regulatory function during hypoxia signaling in AR-positive prostate cancer cells.
2. Materials and methods
2.1. Cell culture
RWPE1, LNCaP, Phoenix A, and 293T cells were obtained from ATCC. LNCaP-Abl (passage 75), 22Rv1, DU145 and PC3 cells were kind gifts from Dr. Zoran Cullig (University of Innsbruck, Austria), Dr. Michael Henry (University of Iowa), and Dr. Frederick Domann (University of Iowa), respectively. RWPE-1 cells were maintained in Keratinocyte-SFM media supplemented with 5 ng/ml epidermal growth factor (EGF) and 0.05 ng/ml bovine pituitary extract (BPE; Life Technologies). LNCaP and 22Rv1 cells were maintained in RPMI 1640 medium. DU145, Phoenix A and 293T cells were maintained in high glucose DMEM (Life Technologies). PC3 cells were maintained in DMEM-F12 (Life Technologies). All three media contained L-glutamine, 10% Fetal Bovine Serum (FBS), 100 units/ml Penicillin, 100 μg/ml Streptomycin, 1 mM sodium pyruvate and 15 μg/ml Plasmocin™ prophylaxis (InvivoGen). LNCaP-Abl cells were cultured in RPMI medium complemented with 10% charcoal stripped FBS (CS-FBS). A humidified hypoxia chamber calibrated to 1% O2 using a 95%N2/5%CO2 gas mixture (Coy Labs) was used for hypoxia treatment. Cells were seeded at a density of 142 cells/mm2 and 38 cells/mm2 for short (< 96 h) and long (6 days) hypoxia treatments, respectively. Proteasome inhibitors MG132 and MG115 (Sigma) were applied to a final concentration of 25 μM.
2.2. Plasmids and stable cell lines
pOZ-N-Flag-HA-PHF8-WT and F279S vectors were described previously [19]. pcDNA3-wild-type and -H1190Y KDM3A were kindly provided by Dr. Yi Zhang (Harvard Medical School). They were subcloned into the XhoI and NotI sites of pOZ-N-Flag-HA vector. Control and PHF8 shRNAs and siRNAs were described previously [17]. The sequence to shPHF8_2 was GGCCTAGAAATGCCAACTTCA. This latter PHF8 shRNA and the two shRNA (1 and 2) sequences for KDM3A [20] were cloned into the AgeI and EcoRI sites of a TetON-pLKO-puro vector, which was a gift from Dmitri Wiederschain (Addgene plasmid # 21915) [21]. Retrovirus and lentivirus packaging of the pOZ and TetON-pLKO-puro vectors, infection, and stable selection were performed as described previously [17]. Doxycycline at 1 μg/ml and 0.5 μg/ml was applied to induce the expression of target shRNAs for experiments lasting < 72 h and 6 days, respectively.
2.3. PHF8 knockout by CRISPR-Cas9 system
Two pairs of sgRNAs (pair one: CACCGATCAGCGAAAGGCGC AGAAC and AAACGTTCTGCGCCTTTCGCTGATC; pair two: CACCGT GGCATTTGTTGGGCGGATC and AAACGATCCGCCCAACAAATGCCAC) targeting the coding region of the JmjC domain located in exon eight of PHF8 were synthesized according to CRISPR design (http://crispr.mit.edu/) and cloned into the pSpCas9(BB)-2A-GFP vector (Addgene plasmid ID: 48138) using the protocol described previously [22]. 293T cells were transfected with the sequence-verified plasmid DNA using lipofectamine 2000. Two days after transient transfection, the GFP-positive cells were sorted by flow cytometry (BD Biosciences, BD FACS Aria II) and plated in 96-well plates. The individual colonies were collected for genotyping and sequence-based verification.
2.4. Transfections, western blotting, antibodies and RT-PCR
Transfection of siRNA duplexes targeting PHF8 and western blotting were conducted as previously described [17]. The antibodies against KDM3A, PHF8, Chromogranin A/CgA, γTubulin, HIF1α, β-Actin, ENO2 and HA have been described previously [17]. In addition, antibodies against the following proteins were utilized: H3K27me2 (39245) from Active Motif; H3K9me2 (#1220) and H3 (#1791) from Abcam; H3K4me3 (#07-473), and WDR5 (#07-706) from Millipore; and WDR5 (#A302-429A) from Bethyl Labs. Secondary antibodies were anti-mouse- or anti-rabbit-conjugated with horse radish peroxidase (BioRad). Western blotting intensities were quantified using the Adobe Photoshop luminosity function. RT-PCR and relevant primers have been described previously [17]. Supplementary Table 1 shows the additional PCR primers used.
2.5. Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) followed by PCR was performed as previously described [19,23]. Briefly, cells were fixed with methanol-free 1% formaldehyde (Thermo Fisher), quenched with 0.125 M glycine, and then lysed with ChIP lysis buffer (50 mM HEPES, pH 7.9, 140 mM NaCl, 1 mM EDTA, 10% Glycerol, 0.5% NP-40, 0.25% Triton-X 100). The cell pellets were washed with ChIP wash buffer (10 mM Tris-HCl, pH 8.1, 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA) before being resuspended in ChIP shearing buffer (10 mM Tris-HCl, pH 8.1, 1 mM EDTA, 0.1% SDS). Sonication was performed using the QSonica® Q700 sonicator at 25% amplification; 30 s ON/30 s OFF with 5 min of elapse time (Qsonica Inc). Triton-X and NaCl were then added directly to the sheared chromatin to a final concentration of 1% and 150 mM, respectively. The chromatin suspension was normalized to 1 μg/ml using A280 spectrometry before being pre-cleared using control IgG (GenScript) and Protein A/G agarose beads (GenScript Inc). These A/G beads were beforehand blocked using sperm DNA and BSA (New England BioLabs). Immunoprecipitation (IP) was performed on 1 mg lysate (or 500 μg for modified histones) with the indicated antibodies. Protein-antibody-bead complexes were collected by centrifugation and washed three times consecutively in ChIP low-salt wash buffer (20 mM HEPES, pH 7.9, 2 mM EDTA, 0.1% SDS, 1% Triton X-100 150 mM NaCl), ChIP high-salt wash buffer with 500 mM NaCl, ChIP LiCl2 buffer (100 mM Tris-HCl, pH 7.5, 0.5 M LiCl, 1% NP-40, 1% sodium deoxycholate) and ChIP TE buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA). The bound protein-antibody-bead complexes and the input DNA were eluted from the beads using ChIP elution buffer (1 M Tris-HCl, pH 8.0, 0.5 M EDTA, 1% SDS) before being reverse crosslinked in the same buffer at 65 °C overnight. The eluted complexes were then digested with RNase and proteinase K according to the manufacturer’s instructions (RPI Inc.), and the DNA was extracted using phenol-chloroform-isoamyl alcohol (Ambion). DNA was subsequently precipitated using a standard ethanol precipitation technique and dissolved in water before subjected to PCR. See Supplementary Table 2 for the list of ChIP-PCR primers.
2.6. Statistics
All gene expression data were organized and analyzed as previously described [17]. Two-way ANOVA and the Student’s t-test were used for multiple and paired comparisons, respectively. A p value < 0.05 was considered statistically significant.
3. Results
3.1. PHF8 knockout by the CRISPR-Cas9 system attenuates hypoxia signaling in 293T cells
We recently reported that treatment by hypoxia (1% O2, 6 days) but not by androgen deprivation or IL-6, led to post-transcriptional upregulation of PHF8 in LNCaP cells [17]. A recent study showed that hypoxia (1% O2, 24 h) upregulated the levels of PHF8 protein, with marginal upregulation of its mRNA levels in LNCaP cells, and that PHF8 protein maintains its demethylation activity under hypoxia condition [18]. These findings prompted us to question whether PHF8 plays a role in hypoxia signaling.
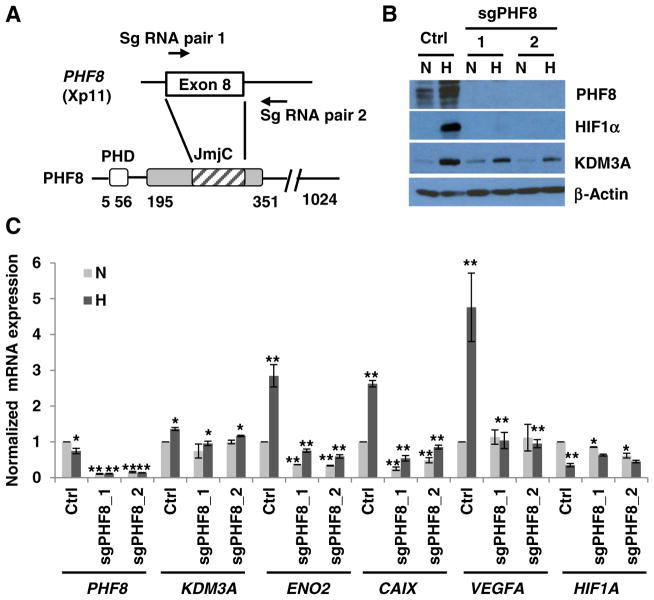
First, we employed the CRISPR-Cas9 system to knock out PHF8 in 293T cells. The small guide RNAs (sgRNAs) target exon 8, which encodes amino acids (aa) 262–315 of the JmjC domain and abolished PHF8 expression (Fig. 1A and B). PHF8 knockout inhibited the hypoxic (1% O2, 24 h) activation of HIF1α protein, attenuated the upregulation of KDM3A protein (Fig. 1B), impaired the induction of the hypoxia-inducible genes including VEGFA, ENO2, and CAIX, and marginally attenuated the induction of KDM3A at mRNA level (Fig. 1C). Notably, the basal levels of most of these genes are downregulated in PHF8 knockout cells: ENO2 and CAIX are downregulated by 50%, KDM3A and HIF1A are slightly downregulated (Fig. 1C). Interestingly, hypoxia downregulated the mRNA level of HIF1A, which is consistent with a previous report [24]. Moreover, despite the impaired hypoxia induced stabilization of HIF1α protein, the upregulation of KDM3A is only attenuated by PHF8 knockout. This phenomenon prompted us to ask if HIF1α is activated at early time points. Indeed, there are slight HIF1α activations from 2 to 12 h by hypoxia treatment (Supplementary Fig. 1), implicating a partial activation of hypoxia signaling even when PHF8 is knocked out.
Fig. 1.
PHF8 knockout by the CRISPR-Cas9 system in 293T cells reduces hypoxia responses. A. Schematic illustration of the strategy for knocking out PHF8. Small guide RNAs (Sg RNA) were designed to truncate exon 8 and part of intron 8. PHD: plant homeodomain; JmJC: Jumonji C domain. B and C. Control (Ctrl) or PHF8 knockout 293T cells (sgPHF8) were incubated under normoxia (N) or hypoxia (H; 1% O2) for 24 h. The protein and RNA levels of the indicated genes were assayed by western blotting and RT-PCR, respectively. In the control cells, gene expression under hypoxia vs. normoxia was compared; in the knockout cells, gene expression was compared to that in the respective control cells (hypoxia or normoxia). The standard deviation (S.D.) was obtained from at least three independent experiments. The Student’s t-test was used to calculate significance. *: p < 0.05; **: p < 0.01.
3.2. PHF8 positively regulates HIF1α and hypoxia-inducible genes in LNCaP cells
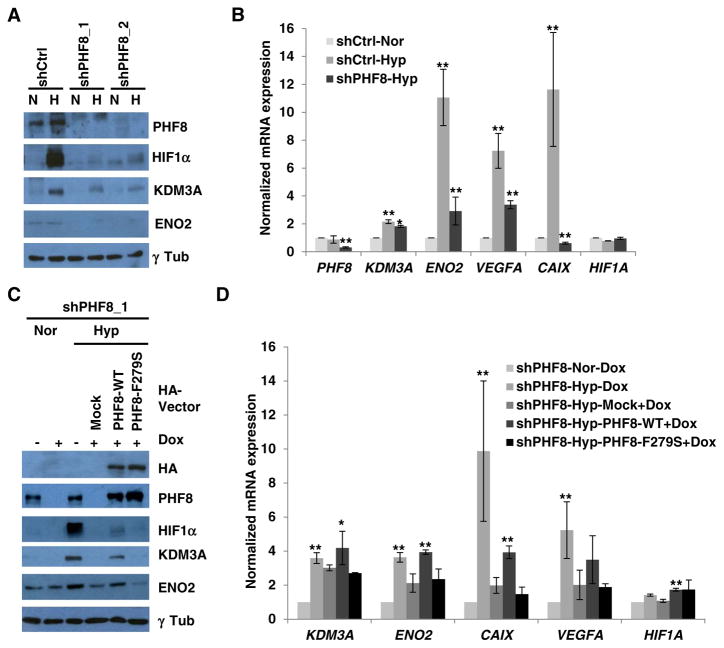
We next asked if PHF8 plays a role in hypoxia signaling in prostate cancer cells, as we and others reported that hypoxia upregulates PHF8 in LNCaP cells [17,18]. A time course of hypoxia (1% O2) treatment in LNCaP cells revealed that the PHF8 protein, but not the mRNA, is slightly upregulated after 24 h, continuing through 6 days (Supplementary Fig. 2A and B). This is consistent with the published data on the upregulation of PHF8 protein [17,18], but not RNA level [17]. We also found that ENO2 was strongly upregulated at later time points (Supplementary Fig. 2A). PHF8 knockdown in LNCaP cells by two independent shRNAs attenuated hypoxic induction of HIF1α, KDM3A and ENO2 proteins (Fig. 2A), and significantly lowered the induction of ENO2, VEGFA, and CAIX at mRNA level (Fig. 2B). However, the mRNA levels of HIF1A and KDM3A were either not affected or only marginally attenuated by PHF8 depletion respectively (Fig. 2B), which is consistent with that observed in 293T cells (Fig. 1B and C).
Fig. 2.
The histone demethylase activity of PHF8 is required for its activation of HIF1α and its upregulation of target genes. A and B. LNCaP cell lines stably expressing a doxycycline-inducible control (Ctrl) or PHF8 shRNAs were incubated under normoxia (N or Nor) or hypoxia (H or Hyp;1% O2) for 24 h. The protein and RNA levels of the indicated genes were quantified by western blotting and RT-PCR, respectively. For RT-PCR, gene expression in the control RNAi cells under hypoxia was compared to that in cells under normoxia. In the case of the PHF8 RNAi cells, gene expression under hypoxia was compared to that in the corresponding control. C and D. pOZ-empty (Mock), -wild-type (WT) or -mutant (F279S) PHF8 were stably expressed in the LNCaP-PHF8shRNA_1 cell line. The cells were treated as in A and B, with doxycycline treatment as indicated (Dox), and gene expression was assessed accordingly. For RT-PCR, in the PHF8 RNAi cells, gene expression under hypoxia was compared to that under normoxia. In the rescue experiments, gene expression in cells expressing wild-type or mutant PHF8 was compared with that in mock-treated cells. The S.D. was obtained from at least three independent experiments. The Student’s t-test was used to calculate significance. *: p < 0.05; **: p < 0.01.
The fact that PHF8 knockdown by shRNAs in LNCaP cells did not affect HIF1A mRNA when compared with the impaired activation of HIF1α protein suggests that PHF8 indirectly regulates HIF1α protein. Thus, we tested if PHF8 indirectly regulates HIF1α through proteasome-mediated degradation pathway by applying proteasome inhibitors MG132 and MG115 to LNCaP cells. Both inhibitors did not restore HIF1α in LNCaP cells with PHF8 knockdown (Supplementary Fig. 3). Meanwhile, both inhibitors slightly restored PHF8 protein under normoxia condition, implying that hypoxia may stabilize PHF8 through inhibiting its proteasome-mediated degradation or sustaining the deubiquitinase that counteracts with the ubiquitination-mediated degradation of PHF8. Beyond, we observed an interesting phenomenon whereby the proteasome inhibitors did not restore HIF1α protein under normoxia, which is consistent with a previous finding that the proteasome-mediated degradation pathway is not the major regulatory mechanism for HIF1α in LNCaP cells [25].
Asensio-Juan et al. reported a microarray study in LNCaP cells in which PHF8 was knocked down by siRNAs, and HIF1A was one of the downregulated genes [26]. We then knocked down PHF8 with siRNAs in LNCaP cells and found attenuated induction of HIF1α and KDM3A proteins (Supplementary Fig. 4A). Importantly, we replicated the published data that HIF1A mRNA was downregulated by siRNA mediated PHF8 knockdown (Supplementary Fig. 4B). Notably, the LNCaP-PHF8shRNA cells that had undergone puromycin selection and doxycycline induction may change some of their properties leading to a discrepancy between the effects of siRNA- vs. shRNA-mediated knockdown on the transcriptional regulation of HIF1A by PHF8. Notwithstanding these differences, the downregulation of HIF1α at protein level is consistent in LNCaP cells with PHF8 knockdown by either shRNA or siRNA.
To determine if PHF8 demethylase activity is required under hypoxia, we performed a rescue experiment in LNCaP-PHF8shRNA cells. Expression of exogenous wild-type PHF8 (Fig. 2C), not the catalytically lethal mutant F279S [17,19], slightly restored HIF1α, KDM3A and ENO2 proteins (Fig. 2C). This pattern was also observed at the mRNA level of ENO2 and CAIX, marginally on KDM3A, but not that of VEGFA and HIF1A (Fig. 2D), implicating that the transcriptional regulatory roles of PHF8 are stronger on ENO2 and CAIX under this experimental condition. These data support that PHF8 executes its roles in hypoxia signaling through its demethylation activity.
3.3. PHF8 is essential for maintaining H3K4me3 levels on hypoxia-inducible genes
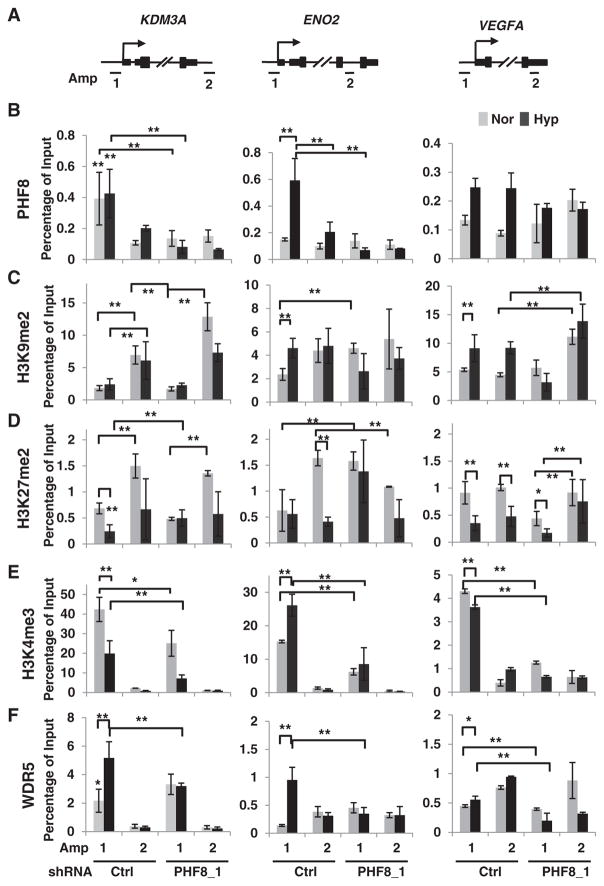
Consistent with the partial activation of HIF1α in 293T cells, we observed the same in LNCaP cells: HIF1α is partially activated from 2 to 12 h in PHF8 knockdown cells (Supplementary Fig. 1). These results demonstrate that hypoxia signaling appears to be partially activated even when PHF8 is knocked down. This phenomenon prompted us to investigate how PHF8 targets specific hypoxia-inducible genes, and how it regulates histone modifications on these genes. Analysis of PHF8 ChIP-seq (chromatin immunoprecipitation followed by deep sequencing) data obtained from K562 and H1 human embryonic stem cells in the Encyclopedia of DNA Elements (ENCODE) [27] shows that PHF8 binding signals at the proximal promoter and/or transcription start sites (TSS) regions are strong at HIF1A, VEGFA, moderate at KDM3A and weak at ENO2 in both cell lines (Supplementary Fig. 5). Concordantly, PHF8 binding sites overlap with H3K4me3 on these genes, as PHF8 binds to H3K4me3 through its PHD domain [19]. We compared H3K4me3 signals from LNCaP cells obtained from ENCODE to mirror PHF8 bindings in LNCaP cells (Supplementary Fig. 5). Accordingly, we designed amplicon 1 for PHF8 bind sites and amplicon 2 for the control region around the 3′ UTR (untranslated region) (Fig. 3A). We will use TSS (amplicon 1) and 3′ UTR (amplicon 2) to simplify the data interpretation in the following sessions. ChIP with beads only (Mock) at least on the KDM3A and ENO2 genes was used to initially validate the specificities of the antibodies used in this study (Supplementary Fig. 6).
Fig. 3.
PHF8 is essential for maintaining H3K4me3 levels on hypoxia-inducible genes. A. Schematic illustration of PCR amplicons for probing the proximal promoter region (Amp 1) or non-specific control loci (Amp 2) of the indicated genes. B. LNCaP cells stably expressing doxycycline-inducible control (Ctrl) or PHF8 shRNAs were cultured under normoxia (Nor) or hypoxia (Hyp; 1% O2) for 12 h. The specific ChIP antibodies are indicated by row. The ChIP-PCR data are interpreted as percentage of 1% input DNA, with the values between amplicons compared as follows: 1) in control RNAi cells, Amp 1 versus Amp 2 under normoxia and under hypoxia; 2) in PHF8 RNAi cells, Amp 1 versus Amp 2 under normoxia and under hypoxia; 3) Amp1 between control and PHF8 RNAi cells under normoxia and under hypoxia, the same amplicon 2.
The S.D. was obtained from at least three independent experiments. For calculation of significance, two-way ANOVA was used in the case of multiple comparisons, and the Student’s t-test was used in the case of paired comparison(s). *: p < 0.05; **: p < 0.01.
In LNCaP cells under normoxia, the enrichment of PHF8 is strong at the TSS of KDM3A, weak at VEGFA, and is nearly not present at ENO2 (Fig. 3B). PHF8 knockdown significantly reduced its enrichment at KDM3A, but not at the TSS of VEGFA and ENO2 (Fig. 3B), suggesting that PHF8 does not bind to VEGFA and ENO2. Notably, under hypoxia (1% O2, 12 h), PHF8 binding remained prominent at the TSS of KDM3A, occurred at that of ENO2, but did not occur at that of VEGFA (Fig. 3B). These results indicated that PHF8-mediated regulation of KDM3A and ENO2 is direct, but that of VEGFA is indirect.
We next examined how PHF8 acts on these genes by assessing levels of H3K9me2 and H3K27me2, which are established PHF8 demethylation substrates [19,28], and H3K4me3, for which PHF8 binding has been well characterized [19]. For the case of H3K9me2, it is enriched at the 3′ UTRs of KDM3A and ENO2 under normoxia condition; PHF8 knockdown elevated H3K9me2 at the 3′ UTRs of KDM3A, VEGFA and at the TSS of ENO2 under normoxia (Fig. 3C). Although, the elevated H3K9me2 at the TSS of ENO2 seems to reflect the demethylation activity of PHF8, PHF8 ChIP showed no binding at that region. Thus, all these increased H3K9me2 levels appear to be indirect effect of PHF8 knockdown. Hypoxia elevated H3K9me2 levels at the TSS of ENO2 and VEGFA and at the 3′ UTR of VEGFA (Fig. 3C). PHF8 knockdown blocked the elevation of H3K9me2 at the TSS of VEGFA. Again, this effect is indirect as PHF8 does not bind to VEGFA. The elevated H3K9me2 at the TSS of ENO2 does not support the demethylation activity of PHF8 as hypoxia-induced the enrichment of PHF8 at this locus. Collectively, these data do not support the role of PHF8 in regulation of H3K9me2 under either normoxia or hypoxia conditions at the indicated genes.
In the case of H3K27me2, it is enriched at the 3′ UTRs of KDM3A and ENO2 under normoxia (Fig. 3D). Under normoxia, knockdown of PHF8 upregulated H3K27me2 at the TSS of ENO2 and downregulated H3K27me2 at the 3′ UTR of ENO2, respectively (Fig. 3D). As PHF8 does not bind ENO2, these effects are indirect. Hypoxia had a general negative effect on H3K27me2: downregulation at the TSS of KDM3A, VEGFA, and at the 3′ UTR of ENO2 and VEGFA (Fig. 3D). However, PHF8 knockdown impaired the downregulation of H3K27me2 at the TSS of KDM3A and increased H3K27me2 at the TSS of ENO2 by hypoxia (Fig. 3D). PHF8 knockdown appears to attenuate the elevation of this mark the 3′ UTR of KDM3A under hypoxia. These results suggest that PHF8 demethylates H3K27me2 at least at the TSS of KDM3A under hypoxia condition.
It was reported that HIF1α tends to bind to its target genes with the presence of H3K4me3 and basal expression [29]. We [19] and others [30] previously observed that in HeLa cells loss of PHF8 function led to a reduction of H3K4me3 levels on PHF8 target genes. Given that PHF8 binds to H3K4me3 through its PHD domain [19], we hypothesized that this interaction also plays a critical role in maintaining the levels of this modification. H3K4me3 ChIP on LNCaP cells under normoxia revealed its occupancy at the TSS of all three genes (Fig. 3E). Of note, the order of H3K4me3 in terms of ranking at the TSS for the three target genes was KDM3A > ENO2 > VEGFA. In the context of hypoxia, H3K4me3 increased on ENO2, which could theoretically account for the increased binding of PHF8. Hypoxia-induced downregulation of H3K4me3 on KDM3A seems to argue the unchanged PHF8 occupancy, however, the higher levels of H3K4me3 even under hypoxia condition seem to be enough to sustain PHF8. Importantly, PHF8 knockdown led to a decrease in H3K4me3 levels at the TSS of all the genes under both normoxia and hypoxia, even though PHF8 does not bind to VEGFA (Fig. 3E). These data strongly support our hypothesis that PHF8 is important in maintaining H3K4me3 levels. The special case on VEGFA implies that PHF8 may act on a component (s) of the H3K4me3 methyltransferase complex.
PHF8 has been reported to interact with components of the H3K4me3 methyltransferase complex, including SETA1 [30] and WDR5 [31], and PHF8 knockdown impairs the recruitment of SETA1 [30]. Thus, we tested whether the role of PHF8 in maintaining H3K4me3 levels applies to the hypoxia-inducible genes in LNCaP cells. We found that hypoxia led to an increase in the enrichment of WDR5 on KDM3A and ENO2 (Fig. 3F), and PHF8 knockdown impaired this enhancement under hypoxia (Fig. 3F). In the case of VEGFA, WDR5 was not enriched at its TSS, and thus may not be responsible for the decrease of H3K4me3 in PHF8 knockdown cells. These results support our hypothesis that PHF8 interacts with WDR5 and its associated methyltransferase complex to maintain H3K4me3, at least on the two genes tested.
To further examine if the role of PHF8 in regulating H3K4me3 under hypoxia condition is conserved, we carried ChIP for PHF8 and H3K4me3 in 293T cells in which PHF8 was knocked out. We focused on KDM3A, ENO2 and HIF1A as PHF8 transcriptionally regulates these genes in these cells. The PHF8 binding sites on HIF1A were determine from ENCODE data (Supplementary Fig. 5). Importantly, the enrichments of PHF8 were found at the TSS of KDM3A and HIF1A under both normoxia and hypoxia conditions, and those were induced at the TSS of ENO2 by hypoxia (Supplementary Fig. 7B). H3K4me3 was enriched at the TSS of these genes with the highest level at HIF1A and was enhanced at the TSS of ENO2 by hypoxia (Supplementary Fig. 7B). Importantly, PHF8 knockout reduced H3K4me3 levels at the TSS of all the three genes under hypoxia condition in addition to that on HIF1A under normoxia condition (Supplementary Fig. 7B). Collectively, the role of PHF8 in maintaining the levels of H3K4me3 is conserved in both LNCaP and 293T cells.
3.4. PHF8 co-operates with KDM3A to regulate select markers of NED
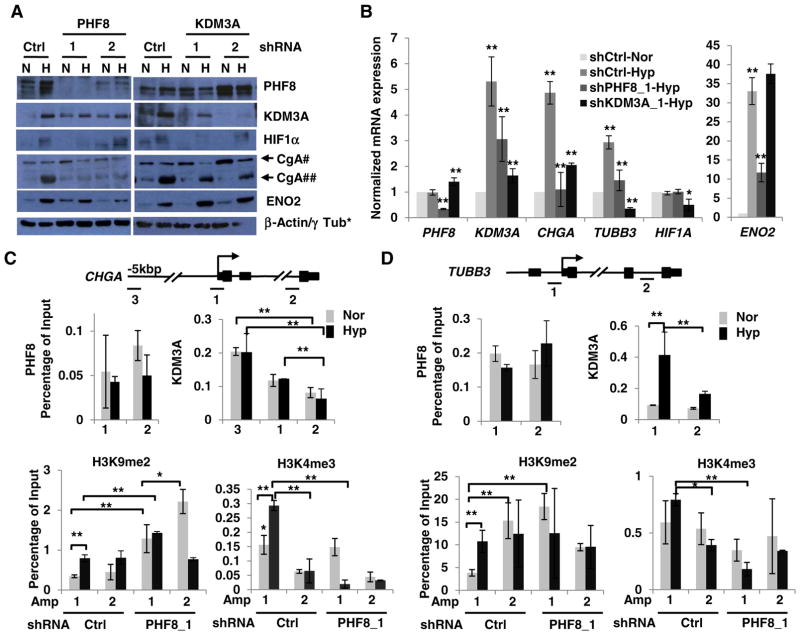
Prostate cancer cells subjected to hypoxia for 3 or 7 days exhibit features of NED including neurite lengthening and the upregulation of neuronal markers such as ENO2, β-Tubulin III, CgA and ASH-1 [32–34]. We recently reproduced the hypoxic (1% O2 for 6 days) induction of ENO2 and CgA in LNCaP cells, but we saw only partial neurite lengthening [17]. As both PHF8 and KDM3A are upregulated by 6-day hypoxia treatment, we sought to determine if they play roles in hypoxia-induced NED genes. Indeed, PHF8 knockdown reduced the hypoxic induction of ENO2, CgA and KDM3A at both the protein and mRNA levels as well as the mRNA level of TUBB3 (Fig. 4A and B). KDM3A knockdown attenuated the induction of CgA but not ENO2 at both protein and mRNA levels and of TUBB3 at mRNA level (Fig. 4A and B). Notably, KDM3A knockdown also led to reduced activation of HIF1α at both protein and mRNA levels. These data suggest that PHF8 and KDM3A play regulatory roles on select genes that characterize hypoxia-induced NED in LNCaP cells.
Fig. 4.
PHF8 and KDM3A differentially regulate select NED markers and PHF8 regulates H3K9me2 and H3K4me3. A and B. LNCaP cells that stably express doxycycline-inducible Ctrl or PHF8- or KDM3A-shRNAs were cultured under normoxia (N or Nor) or hypoxia (H or Hyp; 1% O2) for six days. Expression of the indicated proteins and mRNAs was quantified by western blotting and RT-PCR, respectively. In B, the expression of genes was compared: 1) between normoxia and hypoxia in the control RNAi cells; 2) between the PHF8 RNAi or KDM3 RNAi and the control RNAi under hypoxia. The S.D. was obtained from at least three independent experiments. The Student’s t-test was used to calculate the significance. *: p < 0.05; **: p < 0.01. *: β-Actin and γ Tub were used as loading controls for PHF8 and KDM3A RNAi experiments, respectively. #: precursor CgA, ##: cleaved CgA. C and D. LNCaP-ctrl or PHF8 shRNA cells were treated as in A and Band subjected to ChIP assay with the indicated antibodies. PCR amplicons for CHGA and TUBB3 are illustrated above C and D, respectively. Amp 3: enhancer; Amp 1: proximal promoter/transcription start site; Amp 2: non-specific control loci. The ChIP-PCR data were interpreted as percentage of input DNA (1%) and the comparisons were done as described in Fig. 3. The S.D. was obtained from at least three independent experiments. Significance was calculated using two-way ANOVA in the case of multiple comparisons, and the Student’s t-test in the case of paired comparisons. *: p < 0.05; **: p < 0.01.
To determine how PHF8 and KDM3A regulate the select NED genes, we carried out ChIP experiments for CHGA and TUBB3. ENCODE data [27] were used to predict PHF8 occupancy on these genes (Supplementary Fig. 8). We also included the binding profiles of other transcription factors as reference; one was REST, which is known to repress CHGA [35]. We found that REST is enriched about 10 kb and 5 kb upstream of the CHGA TSS and in the first exon. Given that REST and KDM3A are known to regulate enhancers [35,36], we also tested an amplicon 3 for a potential enhancer on CHGA (Fig. 4C). Notably, PHF8 is not enriched at the TSS of CHGA and TUBB3 under either normoxia or hypoxia (Fig. 4C and D). In contrast, KDM3A is significantly enriched at the potential enhancer region of CHGA compared with its moderate enrichment at the TSS especially under hypoxia (Fig. 4C). KDM3A is also enriched at the TSS of TUBB3 under hypoxia (Fig. 4D). PHF8 knockdown significantly increased H3K9me2 levels at the CHGA TSS under both normoxia and hypoxia as well as in the control region under normoxia (Fig. 4C). PHF8 knockdown led to an increase of H3K9me2 at the TSS of TUBB3 under normoxia (Fig. 4D), implicating that PHF8 contributes to the basal expression of TUBB3. H3K4me3 is enriched at the TSS of both CHGA and TUBB3 under hypoxia, and the enrichment is significant reduced by PHF8 knockdown (Fig. 4C and D). The phenomenon that PHF8 does not bind to CHGA, and TUBB3, or VEGFA (Fig. 3B), but, PHF8 knockdown led to a decrease of H3K4me3 levels at the TSS of these genes implying PHF8 regulates global H3K4me3. Indeed, PHF8 knockdown in LNCaP cells lowered H3K4me3 levels in both normoxia and hypoxia (Supplementary Fig. 9).
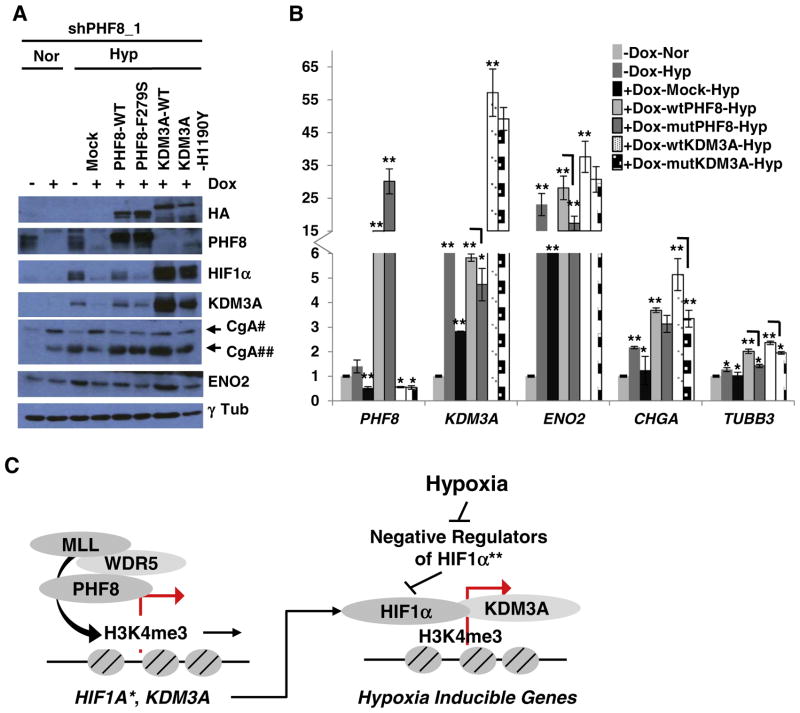
Based on the findings on H3K9me2, i.e. PHF8 does not bind CHGA or TUBB3, but, it regulates H3K9me2 at their TSS, we hypothesized that PHF8 indirectly regulates H3K9me2 through KDM3A. To test this hypothesis, we carried out rescue experiments, overexpressing either wild-type or enzymatically lethal mutant PHF8 or KDM3A in LNCaP-PHF8shRNA cells. Wild type PHF8 partially restored HIF1α, KDM3A, and ENO2, while mutant PHF8 (F279S) also showed a slight rescue of KDM3A (Fig. 5A). The restoration of KDM3A, ENO2 and TUBB3 mRNAs by wild-type PHF8 is stronger than that by mutant PHF8. However, both wild-type and F279S PHF8 restored CgA at both the protein and mRNA levels (Fig. 5A and B); indicating this requires a histone demethylase-independent function of PHF8. Notably, wild-type KDM3A effciently restored expression of HIF1α, ENO2 and CgA, but not that of PHF8. We speculate that the restoration of HIF1α can resume hypoxia signaling. Indeed, wild-type KDM3A restored the expression of CgA and ENO2 at both protein and mRNA levels, in addition to TUBB3 at mRNA level (Fig. 5A and B). The catalytically inactive KDM3A mutant (H1190Y) also partially restored HIF1α and CgA protein levels, as well as ENO2 mRNA levels (Fig. 5A and B), indicating that a demethylase-independent function of KDM3A is required. This could potentially allude to a previously described scaffold function of KDM3A [37].
Fig. 5.
PHF8 acts upstream of KDM3A to regulate specific hypoxia-induced NED markers. A. pOZ-empty (Mock), -wild-type (WT) or -mutant (F279S) PHF8, and -WT or -mutant (H1190Y) KDM3A were stably expressed in LNCaP-PHF8shRNA_1 cells. The cells were treated as in A, and the indicated proteins were quantified by western blotting. #: precursor CgA, ##: cleaved CgA. B. The mRNA levels of the indicated genes were quantified by RT-PCR. Doxycycline (Dox) treatment is indicated. Gene expression was compared as follows: 1) between hypoxia and normoxia in the cells without doxycycline treatment; 2) between mock and PHF8 RNAi cells without doxycycline treatment under hypoxia; 3) between wild-type PHF8 or wild-type KDM3A and mock cells; 4) between mutant and wild-type PHF8; 5) between mutant and wild-type KDM3A. The S.D. was obtained from at least three independent experiments. Statistical significance was calculated by two-way ANOVA in the case of multiple comparisons and by Student’s t-test in the case of paired comparisons. *: p < 0.05; **: p < 0.01. C. Model of the mechanisms whereby PHF8 positively regulates hypoxia signaling. PHF8 plays roles in hypoxia signaling at multiple levels: PHF8 sustains H3K4me3 on hypoxia-inducible genes to prime HIF1α binding; PHF8 positively regulates KDM3A, a critical co-activator of HIF1α; PHF8 transcriptionally regulates HIF1A and post-transcriptionally regulates the activation of HIF1α protein. *: observed in 293T, LNCaP-Abl, 22Rv1 and LNCaP cells (by siRNA only); **: PHF8 may indirectly repress the negative regulators of HIF1α.
Taken together, PHF8 plays a critical role in maintaining H3K4me3 or the induction of H3K4me3 by hypoxia, thus, priming hypoxia-inducible genes for activation by HIF1α. Moreover, PHF8 also serves as a positive regulator of HIF1α and its co-activator KDM3A. These mechanisms are illustrated in Fig. 5C.
3.5. The regulation of hypoxia signaling by PHF8 is associated with AR status in prostate cancer cells
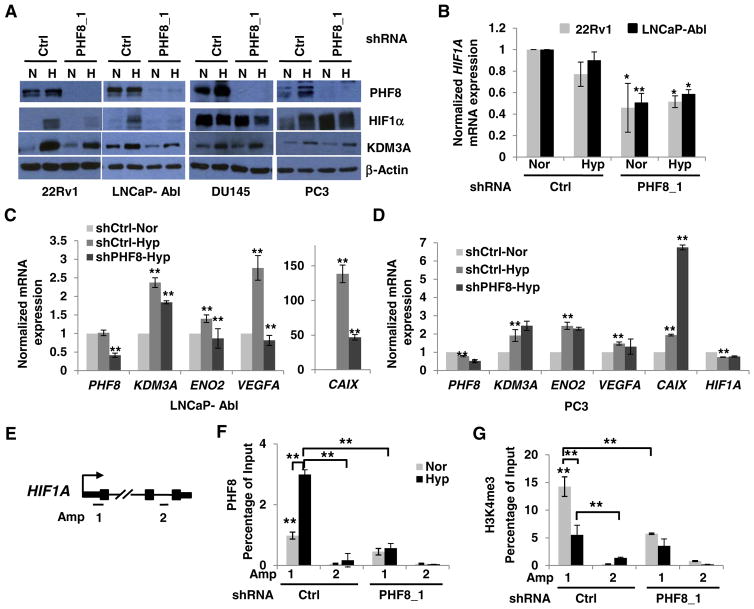
To determine whether the co-activator role of PHF8 in hypoxia signaling is conserved and whether this regulatory function is associated with AR status during PCa disease progression, we utilized 22Rv1, LNCaP-Abl, and DU145, PC3 cell lines. We have demonstrated that LNCaP-Abl cells are a CRPC derivative of LNCaP cells that express full length AR. On the other hand, 22Rv1, but not DU145 or PC3 cells do not express full length AR protein (Supplementary Fig. 10). In 22Rv1 and LNCaP-Abl cells, PHF8 knockdown led to a dramatic reduction of HIF1α activation and attenuated the upregulation of KDM3A protein (Fig. 6A). Importantly, it also led to reduced expression of the HIF1A mRNA in these two cell lines under both normoxia and hypoxia (Fig. 6B). The effects of PHF8 on HIF1α and KDM3A proteins and the HIF1A mRNA were reproduced in LNCaP-Abl cells by siRNA-mediated PHF8 knockdown (Supplementary Fig. 4A and B). In contrast, PHF8 knockdown only slightly reduced HIF1α in DU145 cells and has no effect on HIF1α in PC3 cells under hypoxia. KDM3A, therefore, was not affected in these two cell lines (Fig. 6A). Interestingly, PHF8 knockdown did not affect the already present HIF1α protein in DU145 cells, but upregulated HIF1α protein in PC3 cells, under normoxia. Further analysis showed that PHF8 knockdown attenuated hypoxia-induced expression of the KDM3A, ENO2, VEGFA, and CAIX mRNAs in LNCaP-Abl cells, but, not in PC3 cells (Fig. 6C and D). In contrast, we observed an increase of CAIX at mRNA levels in PC3-PHF8shRNA cells under hypoxia. These data suggest that the co-activator function of PHF8 in hypoxia signaling is positively correlated with the presence of the full-length AR in the cell lines tested.
Fig. 6.
PHF8 regulates HIF1α and hypoxia-inducible genes in CRPC cells positive for full-length AR. A. Doxycycline-inducible control (Ctrl) or PHF8-targeted shRNAs were stably expressed in the indicated cell lines. The cells were induced by doxycycline for 72 h and cultured under normoxia (N or Nor) or hypoxia (H or Hyp; 1% O2) for 24 h. The indicated proteins were quantified by western blotting. B. HIF1A mRNA was assessed by RT-PCR in 22Rv1 and LNCaP-Abl cells. PHF8 RNAi cells were compared to controls undergoing the same treatment. C and D. The mRNA levels of the indicated genes were quantified by RT-PCR. Comparisons: between normoxia and hypoxia in the control RNAi; between PHF8 RNAi and the control RNAi cells under hypoxia. The S.D. was obtained from at least three independent experiments. Significance was calculated using the Student’s t-test. *: p < 0.05; **: p < 0.01. E. Schematic illustration of PCR amplicons to probe the Transcription Start Site (Amp 1) or non-specific control loci (Amp 2) of HIF1A gene. F and G. LNCaP-Abl cells stably expressing doxycycline inducible Ctrl or PHF8 shRNAs were cultured in normoxia (Nor) or 1% O2 hypoxia (Hyp) for 12 h. The ChIP-PCR data are interpreted as percent of 1% input DNA and the comparisons are as described in Fig. 3. The S.D. was obtained from at least three independent experiments. Two way ANOVA for multiple comparisons and Student t-test for paired comparison (s) were used to calculate the significance. *: p < 0.05; **: p < 0.01.
We next carried out ChIP experiments to test if PHF8 binds to HIF1A and regulates H3K4me3 in LNCaP-Abl cells. We found that PHF8 was enriched at the TSS of HIF1A and the enrichment was increased by hypoxia treatment, although H3K4me3 was decreased by hypoxia (Fig. 6F and G). PHF8 knockdown dramatically reduced the basal levels of H3K4me3 at normoxia (Fig. 6F and G), further supporting a role for PHF8 in maintaining H3K4me3 levels at steady state. Collectively, our study revealed that PHF8 plays a critical role in the hypoxia-induced activation and upregulation of HIF1α and KDM3A in both androgen sensitive prostate cancer and CRPC cells. At chromatin level, PHF8 is essential in maintaining H3K4me3 levels (see Model in Fig. 5C). In the context of prostate cancer, the regulatory function of PHF8 in hypoxia is present mostly in the cells of full-length AR, suggesting a novel mechanism whereby PHF8 contributes to the prostate cancer progression including CRPC.
4. Discussion
Hypoxia has numerous effects on tumors and their responsiveness to therapy [1]. Thus, understanding the molecular mechanisms, including any epigenetic factors involved in hypoxia signaling is critical for the development of novel cancer therapies. Histone demethylases represent a major class of epigenetic factors, and play important roles in transcriptional regulation [14]. These enzymes require oxygen as a co-factor; therefore, their demethylation activity can be inhibited under hypoxia. However, a compensatory mechanism that upregulates their expression under hypoxia can maintain, even enhance their functions [15]. The histone demethylase PHF8 contributes to oncogenesis by promoting epithelial to mesenchymal transition (EMT) [23] and cell-cycle progression [17,23,38], and inhibiting apoptosis [26,39]. This study identifies a novel regulatory role for PHF8 during hypoxia, showing that it acts through HIF1α and H3K4me3, shedding light on novel functions of PHF8 in cancer biology.
Using various loss-of-function systems such as shRNA & siRNA-mediated knockdown of PHF8 in LNCaP, LNCaP-Abl cells, and CRISPR-Cas9 system-mediated knockout of PHF8 in 293T cell, we demonstrate that PHF8 transcriptionally regulates HIF1A. These findings concur with a previous independent study which showed that HIF1A levels are reduced following siRNA-mediated knockdown of PHF8 in LNCaP cells [26]. However, the reduction in levels of HIF1A mRNA expression (20–40%) in these cell lines does not fully explain the reduction or absence of HIF1α protein. Proteasome inhibitors did not restore HIF1α protein levels in the cells with PHF8 loss-of-function. Thus, the regulatory mechanisms whereby PHF8 regulates HIF1α still remain to be studied. PHF8 might indirectly regulate HIF1α through its positive regulators. PHF8 knockdown arrests the cell cycle at G0/G1 [17], and could thereby reduce the level of phosphorylated AKT, which is known to be critical for HIF1α stability [40,41]. This potential link may also explain the mechanism underlying the association of PHF8-mediated regulation of HIF1α with AR status in prostate cancer cells, in part because, activated AR signaling can activate HIF1α through autocrine loop involving EGF/PI3K/PKB [7] or directly through transcriptional regulation [42]. Additional supporting evidence is the recently identified HIF/PHF8/AR axis, in which PHF8 regulates AR target genes under hypoxia [18], thus, it is also possible that PHF8 indirectly regulates HIF1A through AR signaling. A systematic approach is still needed to tackle these possible mechanisms. Furthermore, PHF8 may regulate HIF1α through protein synthesis as PHF8 is known to positively regulate ribosome RNAs [31].
Evidence demonstrating the interplay between hypoxia and the dynamics of histone methylation is mounting [15,43]. For example, hypoxia leads to an increase in H3K4me3 by inhibiting their responsible demethylase [44]. Hypoxia also leads to an increase in H3K9me2 by upregulating the methyltransferase G9a [45]. Our data show that PHF8 has distinct effects on H3K9me2 and H3K27me2, and that the outcome for each is gene-dependent. However, PHF8 appears to maintain H3K4me3 levels on even genes that are not bound by PHF8, suggesting that PHF8 may both positively regulate H3K4me3 methyltransferase and indirectly inhibit H3K4me3 demethylase. Genome-wide RNA-seq studies are required to reveal these mechanisms. Regardless of the details however, it is clear that the role of PHF8 in sustaining H3K4me3 is critical for hypoxia signaling, because HIF1α preferentially targets and regulates genes with H3K4me3 [29].
It remains puzzling how hypoxia regulates PHF8. Our data do not support the previously reported mechanism involving transcriptional regulation [18] and other studies show that HIF1α does not bind to the PHF8 promoter [46,47]. It is possible that hypoxia signaling regulates PHF8 through post-translational mechanisms. USP7 counteracts the ubiquitination to stabilize PHF8 in breast cancer cells, in return, PHF8 transcriptionally regulates USP7, forming a regulatory feedback loop [38]. Hypoxia upregulates USP7 in glioma cells [48]. Thus, hypoxia could regulate PHF8 through USP7. Beyond, USP7 deubiquitinates and stabilizes HIF1α, PHF8 could regulate HIF1α through USP7 [49]. These hypotheses need to be tested in prostate cancer cells.
In conclusion, we identified a novel druggable epigenetic factor playing an important role in hypoxia signaling. The underlying mechanisms include that PHF8 positively regulates the hypoxia-induced activation of HIF1α and its co-activator histone demethylase KDM3A; PHF8 maintains the level of H3K4me3, which primes the genes to be regulated by HIF1α. This work sheds light on understanding the epigenetic mechanisms in hypoxia signaling and paves the way for further studies evaluating PHF8 inhibitors in cancer therapy.
Supplementary Material
Acknowledgments
We thank Dr. Brad Amendt, Dr. Weizhou Zhang, and Dr. Michael Henry for valuable discussions; and Dr. Zoran Cullig, Dr. Michael Henry and Dr. Frederick Domann for sharing the LNCaP-Abl, 22Rv1, DU145 and PC3 cell lines. We also thank Dr. Yi Zhang for sharing KDM3A plasmids. This work was supported by Dr. Qi’s laboratory start-up funds from the Department of Anatomy and Cell Biology of the Carver College of Medicine at the University of Iowa, and the following research grants to Dr. Qi: a Carver Trust Young Investigator Award (Grant # 01-224) from the Roy. J. Carver Charitable Trust; an Oberley Seed Grant from Holden Comprehensive Cancer Center (HCCC) (NIH Grant P30 CA086862); an ACS-IRG seed grant (Grant IRG-77-004-34) from the American Cancer Society administered through HCCC, the University of Iowa; and a pilot grant from the Institute for Clinical and Translational Science (ICTS), the University of Iowa (NIH and CTSA grant UL1RR024979). This work was also supported by the Genomics and Flow Cytometry core facilities (NIH Grant P30 CA086862) at the University of Iowa. DL was supported through UI SROP (Summer Research Opportunity Program) at the University of Iowa. We also thank Drs. Christine Blaumueller and Marie Gaine for editorial consultation.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbagrm.2017.07.005.
Footnotes
Transparency document
The http://dx.doi.org/10.1016/j.bbagrm.2017.07.005 associated with this article can be found, in online version.
Authors contributions
PKM and HHQ designed the research. PKM performed most of the experiments. PS generated PHF8 knockout 293T cells. XJ assisted in the hypoxia time course studies. QL, AU, MM, DL and SC helped in various experiments. PKM and HHQ co-wrote the paper. HHQ supervised this study.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 2.Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013;17:30–54. doi: 10.1111/jcmm.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marignol L, Coffey M, Lawler M, Hollywood D. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat Rev. 2008;34:313–327. doi: 10.1016/j.ctrv.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Stewart GD, Ross JA, McLaren DB, Parker CC, Habib FK, Riddick AC. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 2010;105:8–13. doi: 10.1111/j.1464-410X.2009.08921.x. [DOI] [PubMed] [Google Scholar]
- 5.Milosevic M, Warde P, Menard C, Chung P, Toi A, Ishkanian A, McLean M, Pintilie M, Sykes J, Gospodarowicz M, Catton C, Hill RP, Bristow R. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res. 2012;18:2108–2114. doi: 10.1158/1078-0432.CCR-11-2711. [DOI] [PubMed] [Google Scholar]
- 6.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mabjeesh NJ, Willard MT, Frederickson CE, Zhong H, Simons JW. Androgens stimulate hypoxia-inducible factor 1 activation via autocrine loop of tyrosine kinase receptor/phosphatidylinositol 3′-kinase/protein kinase B in prostate cancer cells. Clin Cancer Res. 2003;9:2416–2425. [PubMed] [Google Scholar]
- 8.Horii K, Suzuki Y, Kondo Y, Akimoto M, Nishimura T, Yamabe Y, Sakaue M, Sano T, Kitagawa T, Himeno S, Imura N, Hara S. Androgen-dependent gene expression of prostate-specific antigen is enhanced synergistically by hypoxia in human prostate cancer cells. Mol Cancer Res. 2007;5:383–391. doi: 10.1158/1541-7786.MCR-06-0226. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth KT, McCarthy HO, Devlin A, Ming L, Robson T, McKeown SR, Worthington J. Hypoxia selects for androgen independent LNCaP cells with a more malignant geno- and phenotype. Int J Cancer. 2008;123:760–768. doi: 10.1002/ijc.23418. [DOI] [PubMed] [Google Scholar]
- 10.Mitani T, Yamaji R, Higashimura Y, Harada N, Nakano Y, Inui H. Hypoxia enhances transcriptional activity of androgen receptor through hypoxia-inducible factor-1alpha in a low androgen environment. J Steroid Biochem Mol Biol. 2011;123:58–64. doi: 10.1016/j.jsbmb.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Mitani T, Harada N, Nakano Y, Inui H, Yamaji R. Coordinated action of hypoxia-inducible factor-1alpha and beta-catenin in androgen receptor signaling. J Biol Chem. 2012;287:33594–33606. doi: 10.1074/jbc.M112.388298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrath J, Trojer P. Targeting histone lysine methylation in cancer. Pharmacol Ther. 2015;150:1–22. doi: 10.1016/j.pharmthera.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Lee HY, Yang EG, Park H. Hypoxia enhances the expression of prostate-specific antigen by modifying the quantity and catalytic activity of Jumonji C domain-containing histone demethylases. Carcinogenesis. 2013;34:2706–2715. doi: 10.1093/carcin/bgt256. [DOI] [PubMed] [Google Scholar]
- 14.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock RL, Dunne K, Walport LJ, Flashman E, Kawamura A. Epigenetic regulation by histone demethylases in hypoxia. Epigenomics. 2015:1–21. doi: 10.2217/epi.15.24. [DOI] [PubMed] [Google Scholar]
- 16.Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem. 2008;283:36542–36552. doi: 10.1074/jbc.M804578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maina PK, Shao P, Liu Q, Fazli L, Tyler S, Nasir M, Dong X, Qi HH. c-MYC drives histone demethylase PHF8 during neuroendocrine differentiation and in castration-resistant prostate cancer. Oncotarget. 2016;7:75585–75602. doi: 10.18632/oncotarget.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong D, Liu Q, Liu G, Yuan W, Wang L, Guo Y, Lan W, Zhang D, Dong S, Wang Y, Xiao H, Mu J, Mao C, Wong J, Jiang J. The HIF/PHF8/AR axis promotes prostate cancer progression. Oncogene. 2016;5:e283. doi: 10.1038/oncsis.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi HH, Sarkissian M, Hu GQ, Wang Z, Bhattacharjee A, Gordon DB, Gonzales M, Lan F, Ongusaha PP, Huarte M, Yaghi NK, Lim H, Garcia BA, Brizuela L, Zhao K, Roberts TM, Shi Y. Histone H4K20/H3K9 demethylase PHF8 regulates zebrafish brain and craniofacial development. Nature. 2010;466:503–507. doi: 10.1038/nature09261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramadoss S, Guo G, Wang CY. Lysine demethylase KDM3A regulates breast cancer cell invasion and apoptosis by targeting histone and the non-histone protein p53. Oncogene. 2017;36:47–59. doi: 10.1038/onc.2016.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiederschain D, Wee S, Chen L, Loo A, Yang G, Huang A, Chen Y, Caponigro G, Yao YM, Lengauer C, Sellers WR, Benson JD. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle. 2009;8:498–504. doi: 10.4161/cc.8.3.7701. [DOI] [PubMed] [Google Scholar]
- 22.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao P, Liu Q, Maina PK, Cui J, Bair TB, Li T, Umesalma S, Zhang W, Qi HH. Histone demethylase PHF8 promotes epithelial to mesenchymal transition and breast tumorigenesis. Nucleic Acids Res. 2017;45:1687–1702. doi: 10.1093/nar/gkw1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavadas MA, Mesnieres M, Crifo B, Manresa MC, Selfridge AC, Scholz CC, Cummins EP, Cheong A, Taylor CT. REST mediates resolution of HIF-dependent gene expression in prolonged hypoxia. Sci Rep. 2015;5:17851. doi: 10.1038/srep17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong JY, Zhou JR, Gao C, Feldman L, Sytkowski AJ. Human selenium binding protein-1 (hSP56) is a negative regulator of HIF-1alpha and suppresses the malignant characteristics of prostate cancer cells. BMB Rep. 2014;47:411–416. doi: 10.5483/BMBRep.2014.47.7.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjorkman M, Ostling P, Harma V, Virtanen J, Mpindi JP, Rantala J, Mirtti T, Vesterinen T, Lundin M, Sankila A, Rannikko A, Kaivanto E, Kohonen P, Kallioniemi O, Nees M. Systematic knockdown of epigenetic enzymes identifies a novel histone demethylase PHF8 overexpressed in prostate cancer with an impact on cell proliferation, migration and invasion. Oncogene. 2012;31:3444–3456. doi: 10.1038/onc.2011.512. [DOI] [PubMed] [Google Scholar]
- 27.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yatim A, Benne C, Sobhian B, Laurent-Chabalier S, Deas O, Judde JG, Lelievre JD, Levy Y, Benkirane M. NOTCH1 nuclear interactome reveals key regulators of its transcriptional activity and oncogenic function. Mol Cell. 2012;48:445–458. doi: 10.1016/j.molcel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia X, Kung AL. Preferential binding of HIF-1 to transcriptionally active loci determines cell-type specific response to hypoxia. Genome Biol. 2009;10:R113. doi: 10.1186/gb-2009-10-10-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Tanasa B, Tyurina OV, Zhou TY, Gassmann R, Liu WT, Ohgi KA, Benner C, Garcia-Bassets I, Aggarwal AK, Desai A, Dorrestein PC, Glass CK, Rosenfeld MG. PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature. 2010;466:508–512. doi: 10.1038/nature09272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng W, Yonezawa M, Ye J, Jenuwein T, Grummt I. PHF8 activates transcription of rRNA genes through H3K4me3 binding and H3K9me1/2 demethylation. Nat Struct Mol Biol. 2010;17:445–450. doi: 10.1038/nsmb.1778. [DOI] [PubMed] [Google Scholar]
- 32.Liang H, Studach L, Hullinger RL, Xie J, Andrisani OM. Down-regulation of RE-1 silencing transcription factor (REST) in advanced prostate cancer by hypoxia-induced miR-106b~25. Exp Cell Res. 2014;320:188–199. doi: 10.1016/j.yexcr.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danza G, Di Serio C, Rosati F, Lonetto G, Sturli N, Kacer D, Pennella A, Ventimiglia G, Barucci R, Piscazzi A, Prudovsky I, Landriscina M, Marchionni N, Tarantini F. Notch signaling modulates hypoxia-induced neuroendocrine differentiation of human prostate cancer cells. Mol Cancer Res. 2012;10:230–238. doi: 10.1158/1541-7786.MCR-11-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin TP, Chang YT, Lee SY, Campbell M, Wang TC, Shen SH, Chung HJ, Chang YH, Chiu AW, Pan CC, Lin CH, Chu CY, Kung HJ, Cheng CY, Chang PC. REST reduction is essential for hypoxia-induced neuroendocrine differentiation of prostate cancer cells by activating autophagy signaling. Oncotarget. 2016;7:26137–26151. doi: 10.18632/oncotarget.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svensson C, Ceder J, Iglesias-Gato D, Chuan YC, Pang ST, Bjartell A, Martinez RM, Bott L, Helczynski L, Ulmert D, Wang Y, Niu Y, Collins C, Flores-Morales A. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res. 2014;42:999–1015. doi: 10.1093/nar/gkt921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mimura I, Nangaku M, Kanki Y, Tsutsumi S, Inoue T, Kohro T, Yamamoto S, Fujita T, Shimamura T, Suehiro J, Taguchi A, Kobayashi M, Tanimura K, Inagaki T, Tanaka T, Hamakubo T, Sakai J, Aburatani H, Kodama T, Wada Y. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol Cell Biol. 2012;32:3018–3032. doi: 10.1128/MCB.06643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe Y, Rozqie R, Matsumura Y, Kawamura T, Nakaki R, Tsurutani Y, Tanimura-Inagaki K, Shiono A, Magoori K, Nakamura K, Ogi S, Kajimura S, Kimura H, Tanaka T, Fukami K, Osborne TF, Kodama T, Aburatani H, Inagaki T, Sakai J. JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis. Nat Commun. 2015;6:7052. doi: 10.1038/ncomms8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q, Ma S, Song N, Li X, Liu L, Yang S, Ding X, Shan L, Zhou X, Su D, Wang Y, Zhang Q, Liu X, Yu N, Zhang K, Shang Y, Yao Z, Shi L. Stabilization of histone demethylase PHF8 by USP7 promotes breast carcinogenesis. J Clin Invest. 2016;126:2205–2220. doi: 10.1172/JCI85747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun X, Qiu JJ, Zhu S, Cao B, Sun L, Li S, Li P, Zhang S, Dong S. Oncogenic features of PHF8 histone demethylase in esophageal squamous cell carcinoma. PLoS One. 2013;8:e77353. doi: 10.1371/journal.pone.0077353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 41.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 42.Tao L, Qiu J, Jiang M, Song W, Yeh S, Yu H, Zang L, Xia S, Chang C. Infiltrating T cells promote bladder cancer progression via increasing IL1→androgen receptor→HIF1alpha→VEGFa signals. Mol Cancer Ther. 2016;15:1943–1951. doi: 10.1158/1535-7163.MCT-15-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chervona Y, Costa M. The control of histone methylation and gene expression by oxidative stress, hypoxia, and metals. Free Radic Biol Med. 2012;53:1041–1047. doi: 10.1016/j.freeradbiomed.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou X, Sun H, Chen H, Zavadil J, Kluz T, Arita A, Costa M. Hypoxia induces trimethylated H3 lysine 4 by inhibition of JARID1A demethylase. Cancer Res. 2010;70:4214–4221. doi: 10.1158/0008-5472.CAN-09-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, Yan Y, Davidson TL, Shinkai Y, Costa M. Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res. 2006;66:9009–9016. doi: 10.1158/0008-5472.CAN-06-0101. [DOI] [PubMed] [Google Scholar]
- 46.Xia X, Lemieux ME, Li W, Carroll JS, Brown M, Liu XS, Kung AL. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci U S A. 2009;106:4260–4265. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krieg AJ, Rankin EB, Chan D, Razorenova O, Fernandez S, Giaccia AJ. Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene expression and tumor growth. Mol Cell Biol. 2010;30:344–353. doi: 10.1128/MCB.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danilovskyi SV, Minchenko DO, Moliavko OS, Kovalevska OV, Karbovskyi LL, Minchenko OH. ERN1 knockdown modifies the hypoxic regulation of TP53, MDM2, USP7 and PERP gene expressions in U87 glioma cells. Ukr Biochem J. 2014;86:90–102. [PubMed] [Google Scholar]
- 49.Wu HT, Kuo YC, Hung JJ, Huang CH, Chen WY, Chou TY, Chen Y, Chen YJ, Chen YJ, Cheng WC, Teng SC, Wu KJ. K63-polyubiquitinated HAUSP deubiquitinates HIF-1alpha and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat Commun. 2016;7:13644. doi: 10.1038/ncomms13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.