Abstract
We studied cultured hippocampal neurons from embryonic wildtype, major histocompatibility complex class I (MHCI) heavy chain-deficient (KbDb−/−) and NSE-Db (which have elevated neuronal MHCI expression) C57BL/6 mice. KbDb−/− neurons displayed slower neuritogenesis and establishment of polarity, while NSE-Db neurons had faster neurite outgrowth, more primary neurites, and tended to have accelerated polarization. Additional studies with ϐ2M−/− neurons, exogenous ϐ2M, and a self-MHCI monomer suggest that free heavy chain cis interactions with other surface molecules can promote neuritogenesis while tripartite MHCI interactions with classical MHCI receptors can inhibit axon outgrowth. Together with the results of others, MHCI appears to differentially modulate neuritogenesis and synaptogenesis.
Keywords: major histocompatibility complex class I (MHCI), neurodevelopment, ϐ2M, MHCI-deficient, neurite, free heavy chain, neurite, axon, soluble MHC
1. Introduction
Classical major histocompatibility complex class I (MHCI) molecules play a central role in orchestrating the function of the innate and adaptive immune systems. MHCI is tripartite, consisting of a membrane-bound heavy chain that is non-covalently associated with ϐ2-microglobulin (ϐ2M) and a small peptide of 8–10 amino acids from a cytosolic antigen. On the cell surface, MHCI is screened by CD8+ T cells to identify cells expressing foreign antigens and by natural killer (NK) cells to identify infected or transformed cells (reviewed in (Lanier, 2005; Natarajan et al., 1999)).
Recently, neuronal MHCI has been shown to also play a key role in neuronal synaptic refinement in the postnatal CNS. Mice lacking MHCI have deficiencies in pruning inappropriate synaptic connections and have abnormal synaptic transmission (Glynn et al., 2011; Goddard et al., 2007; Huh et al., 2000). Neuronal MHCI expression levels are inversely correlated with synaptic density (Glynn et al., 2011; Goddard et al., 2007). Transgenic NSE-Db C57BL/6 mice that have elevated levels of MHCI on their neurons (by virtue of a neuron specific enolase promoter driving the expression of a self-Db heavy chain) have abnormal retinothalamic projections and reduced expression of synaptic markers in some regions of their hippocampus (Rall et al., 1995; Wu et al., 2011a).
Little is known about how MHCI exerts its neurobiological activity. Some neurons express the classical MHCI receptors Ly49 and PirB (Syken et al., 2006; Zohar et al., 2008). Ly49 and PirB receptors can bind to MHCI on the same cell surface in cis, or MHCI on another cell surface in trans (e.g., (Back et al., 2009; Chalifour et al., 2009; Held and Mariuzza, 2008; Masuda et al., 2007)). PirB-deficient mice, however, do not display the neurological aberrations that have been noted in MHCI-deficient mice (Syken et al., 2006). MHCI can also dissociate after reaching the cell surface, forming a pool of free heavy chains (Arosa et al., 2007; Rock et al., 1991; Schell et al., 2002; Schnabl et al., 1990) which have been termed “open conformers” (while tripartite MHCI is referred to as “closed conformers”, reviewed in (Arosa et al., 2007)). These MHCI open conformers can associate in cis with other free heavy chains forming homodimers. The open conformers can also bind in cis to insulin receptors, epidermal growth factor (EGF) receptors, IL-2 receptor, transferrin receptor, CD8αϐ and others (perhaps including insulin-like growth factor (IGF)) receptors), forming heterodimers that can modulate cellular function and growth (Arosa et al., 2007; Capps et al., 1993; Due et al., 1986; Fehlmann et al., 1985; Jelonek et al., 1998; Olsson et al., 1994; Phillips et al., 1986; Ramalingam et al., 1997; Santos et al., 2004).
Studies of MHCI’s biological activity have focused on its role in modulating postnatal synaptogenesis. Because MHCI is expressed on embryonic neurons well before postnatal synaptic pruning (e.g., (Corriveau et al., 1998; Escande-Beillard et al., 2010; Zohar et al., 2008)), MHCI may influence earlier aspects of neurodevelopment. To examine whether MHCI plays a role in neuronal development we studied the development of cultured hippocampal neurons from C57BL/6 mice with normal (wildtype), reduced (KbDb−/− and ϐ2M−/−), or elevated (NSE-Db mice) MHCI levels on their neurons. We describe how neuronal MHCI levels can modulate neuronal development and provide evidence suggesting that MHCI open conformer cis interactions with other cell surface proteins promote neuritogenesis while interactions with closed MHCI inhibit embryonic axon outgrowth.
2. Materials and Methods
2.1 Animals
C57BL/6 and C57BL/6 ϐ2M-deficient (ϐ2M−/−) mice were purchased from the Jackson Laboratory. Heavy chain-deficient C57BL/6 KbDb−/− mice were purchased from Taconic Farms. C57BL/6 NSE-Db mice were generated by backcrossing of NSE-Db mice (Rall et al., 1995) with C57BL/6 mice for 10 generations and then breeding them to homozygosity for the transgene (Wu et al., 2011a). All animal studies were approved by the UCLA Animal Use and Care Committee.
2.2 Hippocampal neuronal culture
Breeder female mice were checked daily for the formation of a vaginal plug and the day of plug detection was designated embryonic day 0 (E0). Hippocampi were dissected from E15 brains, treated with papain/DNase solution in Leibovitz’s L15-media (Invitrogen) and mechanically dissociated (as previously described (Bilousova et al., 2006)). After washing, cells were plated on poly-DL-ornithine and laminin coated glass coverslips (70,000 cells per cover slip) and grown in Neurobasal medium with B27 supplement, 25 μM glutamate and 2% penicillin-streptomycin (Invitrogen) in a 37°C incubator with 5% CO2. Each experiment included cultures of wildtype C57BL/6 and one to two other genotypes that were prepared side-by-side for direct comparison. To some wildtype cultures exogenous human ϐ2M (3 μM, Serotec), a self-MHCI monomer (Db/H13a, 200 pM), or a control nonself MHCI monomer (Dk/polyoma protein MT 389–397, 200 pM) were added immediately after plating, as previously described (Escande-Beillard et al., 2010; Wu et al., 2011b).
2.3 Immunocytochemistry
Hippocampal neurons were grown for 1 or 2 days in vitro and then fixed in 4% paraformaldehyde in PBS, pH 7.4 for 30 minutes at room temperature. After washing with PBS, cells were permeabilized with 0.1% Triton X-100, blocked in 5% chicken serum or 1% bovine serum albumin and incubated overnight at 4°C with rabbit anti-ϐIII-tubulin (2μg/ml, clone TUJ1 1-15-79, Covance) and with rhodamine phalloidin (1:50, Invitrogen) for 1 day in vitro neurons, or with mouse anti-MAP2 (a dendritic marker, 2μg/ml, clone HM-2; Sigma-Aldrich) for 2 day in vitro neurons. Other cultures were co-stained with anti-MHCI antibody (OX-18, 10 μg/ml, BD Bioscience). After washing with PBS containing 0.05% Tween-20 (PBST), the cells were incubated with Alexa 488-conjugated chicken anti-rabbit IgG (4 μg/ml, Invitrogen) only, or in combination with Alexa 594-conjugated chicken anti-mouse IgG (4 μg/ml, Invitrogen), for 1 hour at room temperature. After washing with PBST, the cells were mounted on glass slides with Vectorshield media containing DAPI (Vector Laboratories). Fluorescent images were captured using an inverted microscope (Axiovert 200, Zeiss) with 20x objective lenses (Plan-Apochromat), a CCD camera (International Power Sources) and MetaMorph Software (version 6.2; Universal Imaging Corp).
2.4 Image analysis
Data were collected from at least 3 independent cultures of each genotype. From each culture 20 images were randomly taken from at least 2 cover slips per a time point and at least 100 cells per group were analyzed. Images were imported to Photoshop and each neuron fully seen in the image was manually traced. The length of neurites, the number of primary neurites and the number of branch points were measured in a blinded manner. Only protrusions longer than the cell diameter were considered neurites and measured. A neuron was considered polarized if it had more than one neurite and one of the neurites was more than two times longer than the others (Dotti et al., 1988). Neurons with two axons (based on the lack of MAP-2 immunostaining) were rarely seen in any cultures regardless of genotype and were excluded from analysis. We analyzed total neurite length per cell (by summation of the lengths of all primary neurites and their branches), average neurite length of unpolarized neurons, and average axonal and dendritic length of polarized neurons. Neuron cell density was determined by counting ϐIII-tubulin-positive cells on each image. Data from independent experiments were combined based on the one-way ANOVA test. Differences among groups were analyzed by one-way ANOVA, followed by post-hoc Bonferroni correction and the difference between two groups was analyzed by Student’s t-test. Differences were considered significant at p<0.05.
3. Results
3.1 Classical MHCI expression promotes neurite growth
C57BL/6 mice have two loci, H-2K and H-2D, which express b haplotype heavy chains. KbDb−/− mice express no heavy chains without affecting either ϐ2M or nonclassical MHCI (Ib) protein expression (Vugmeyster et al., 1998). Embryonic NSE-Db mouse neurons have increased levels of MHCI on their surface compared to that of wildtype mice, as demonstrated by in vivo and in vitro functional assays, as well as immunostaining (Joseph et al., 2011; Rall et al., 1995). We immunostained E15 hippocampal neurons from wildtype, NSE-Db and KbDb−/− C57BL/6 mice side-by-side with an antibody that preferentially stains classical MHCI heavy chains (Fig. 1). These studies confirmed greatly reduced MHCI immunoreactivity on KbDb−/− mouse hippocampal neurons and elevated MHCI expression in NSE-Db mouse neurons, consistent with previous studies of these mice (Joseph et al., 2011; McConnell et al., 2009; Rall et al., 1995).
Figure 1. Immunostaining of MHCI.
Anti-MHCI staining of wildtype, KbDb−/− and NSE-Db 1 DIV hippocampal neurons. Red=anti-MHCI (OX-18), green=anti-ϐIII-tubulin. There is slight staining of KbDb−/− neurons probably due to OX-18 recognition of MHCI Ib molecules. Scale bar = 20 μm.
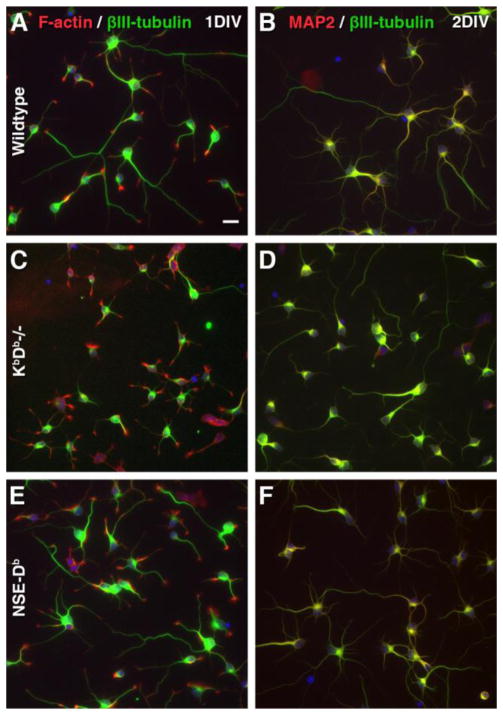
To evaluate the biological effects of different levels of MHCI expression on the early stages of neuronal development, we studied the morphology of cultured primary E15 hippocampal neurons obtained from wildtype, KbDb−/− and NSE-Db mice after 24 hours (i.e., one day in vitro (1 DIV)) and 48 hours (2 DIV) in culture. In this widely used culture model (Craig and Banker, 1994), the vast majority cells are neurons since there are few glia in the E15 mouse hippocampus and because the culture conditions inhibit the proliferation of glia (e.g., see Fig. 1). Previous studies have shown that after 6–12 hours in culture, most embryonic hippocampal neurons have extended short neurites of about equal length that are termed “minor neurites” (Craig and Banker, 1994). These unpolarized neurons are referred to as “stage II neurons”. Between 24-and 48 hours in culture one of the minor neurites elongates rapidly to form a single axon. These polarized neurons are referred to as “stage III” neurons (Craig and Banker, 1994). Consistent with past observations, we found that after 24 hours in culture, most hippocampal neurons from wildtype, KbDb−/− and NSE-Db mice had formed several neurites, and some of the neurons displayed morphological features of polarization (Fig. 2A, C, E). Neurite outgrowth and polarization continued to progress and were more extensive in 48 hour hippocampal cultures from all three mouse strains. Neurons with polarized morphology lacked dendritic marker MAP-2 immunoreactivity in their longest neurites, confirming biochemically their axonal specification (Fig. 2B, D, F). Hippocampal cultures from all three mouse strains had a similar cell density at both 1 DIV and 2 DIV, indicating that MHCI-deficiency, or elevated MHCI expression, did not affect neuronal survival (Fig. S1A).
Figure 2. Representative images of hippocampal cultures.
C57BL/6 (WT) (A, B), KbDb – deficient (C, D) and NSE-Db - transgenic (E, F) primary hippocampal cultures in 1 DIV (A, C, E) and 2 DIV (B, D, F). Neurons were visualized by fluorescent labeling with anti-βIII-tubulin antibodies (green) and either F-actin binding phalloidin (red; A, C, E) or anti-MAP2 antibodies (red; B, D, F). Scale bar = 20 μm.
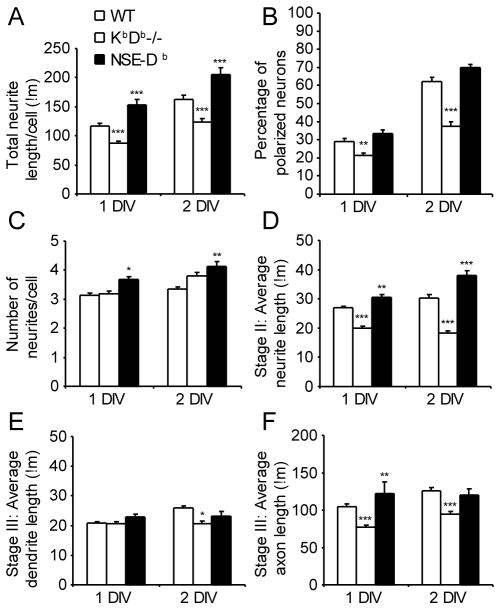
Quantitative analysis of neurite outgrowth in cultures revealed that the level of MHCI expression significantly affected multiple parameters of neurite growth in 1 DIV and 2 DIV cultures. First, at 1 DIV, the average total length of KbDb−/− neurites was significantly less than that of wild type neurons (Fig. 3A). Moreover, KbDb−/− neurons had significant slower establishment of axon-dendritic polarity relative to neurons from wildtype and NSE-Db mice at both 1 DIV and 2 DIV (Fig. 3B). In contrast, NSE-Db neurons had significantly greater total neurite outgrowth than wildtype neurons in both 1 DIV and 2 DIV cultures (Fig 3A). The average number of primary neurites was also significantly increased on NSE-Db transgenic neurons in comparison with wildtype at both 1 DIV and 2 DIV (Fig. 3C). Finally, on average, more NSE-Db neurons had established axon-dendritic polarity at both 1 DIV and 2 DIV than wildtype neurons, although this was not statistically significant (p=0.08 for both, Fig. 3B).
Figure 3. MHCI heavy chain promotes neuritogenesis and accelerates polarization of hippocampal neurons in vitro.
Quantitative analyses of mean neurite outgrowth and axon-dendritic polarity establishment in 1 and 2 DIV primary hippocampal cultures from wildtype (open bars), KbDb-deficient (hatched bars) and NSE-Db (black bars) C57BL/6 mice +/− SEM. (A) Total neurite length per cell. (B) Percentage of polarized neurons. (C) Number of primary neurites per cell. (D) Average neurite length of stage II neurons. (E) Average dendrite length of stage III neurons. (F) Average axon length of stage III neurons. N=3–5 independent studies of each genotype, with all three genotypes studied side-by-side in two studies. *-p<0.05; **-p<0.01, ***-p<0.001
Further detailed analysis of neurite outgrowth from unpolarized (stage II) neurons in 1 DIV and 2 DIV hippocampal cultures revealed that the average length of individual minor neurites was significantly shorter on KbDb−/− neurons compared to those on wildtype neurons (Fig. 3D). Conversely, minor neurites were significantly longer on NSE-Db neurons relative to that of wildtype neurons. The main factor contributing to the decrease in the total neurite length on KbDb−/− stage II neurons was a reduction in the length of their minor neurites (Fig. S1B). Interestingly, the mean total neurite length per stage II NSE-Db neuron was 60% and 87% greater than that of 1 DIV and 2 DIV wildtype neurons (respectively, Fig. S1B), which is likely to be due to NSE-Db neurons having both longer neurites and more neurites per cell. These data indicate that enhanced Db expression affects not only the growth rate of existing minor neurites but also promotes new neurite formation. Taken together, these data suggest that MHCI molecules are not required for initial neurite formation, but their presence can accelerate neuritogenesis.
We found that MHCI deficiency not only slowed establishment of polarity, it also inhibited neurite outgrowth from polarized cells, with axons more affected than dendrites (Fig. 3E, 3F). The total neurite length of KbDb−/− polarized neurons was significantly reduced compared to that of wildtype and NSE-Db neurons at both 1 DIV and 2 DIV (Fig. S1C). Additionally, the average axon length from polarized KbDb−/− neurons was significantly less than that of wildtype and NSE-Db polarized neurons at both time points Conversely, the average axon length of NSE-Db neurons was significantly greater than that of wildtype and KbDb−/− neurons at 1 DIV. After 2 DIV, the average axon lengths of NSE-Db and wildtype neurons were similar, and both were significantly greater than that of KbDb−/− neurons (Fig. 3F). Thus, NSE-Db neurons initially have accelerated outgrowth, but after polarization their rate of outgrowth slows, pointing to other intrinsic factors that govern axon length and/or increasing transactions with other neurons in culture that limit axon growth.
3.2 β2M−/− neuron development is similar to that of wildtype neurons
Our observation that MHCI expression levels differentially modulate neuritogenesis at 1 DIV during which the neurons had relatively few, or no, interactions with nearby neurons, suggests that MHCI cis interactions mediate these effects. These MHCI cis interactions may involve closed MHCI molecules binding to classical MHCI receptors. Alternatively, MHCI on the cell surface can dissociate and the free heavy chain open conformers can re-associate with themselves, or with receptors that are involved in cell growth and modulate their activity (e.g., (Arosa et al., 2007; Due et al., 1986; Fehlmann et al., 1985; Phillips et al., 1986; Ramalingam et al., 1997; Rock et al., 1991; Santos et al., 2004; Schell et al., 2002; Schnabl et al., 1990)).
To differentiate between these scenarios, we next characterized the development of cultured C57BL/6 ϐ2M−/− hippocampal neurons. ϐ2M−/− neurons cannot produce closed MHCI, but they express MHCI heavy chain, some of which reaches the cell surface to form open conformers (e.g., (Allen et al., 1986; Bix and Raulet, 1992)). We hypothesized that if MHCI was promoting neurite outgrowth through closed MHCI interactions, then neurons from ϐ2M−/− mice should display slowed neurite outgrowth, similar to that displayed by KbDb−/− neurons. If however, open MHCI conformers promote neuritogenesis, neurons from ϐ2M−/− mice may not have the deficiencies in neuritogenesis that are displayed by KbDb−/− neurons.
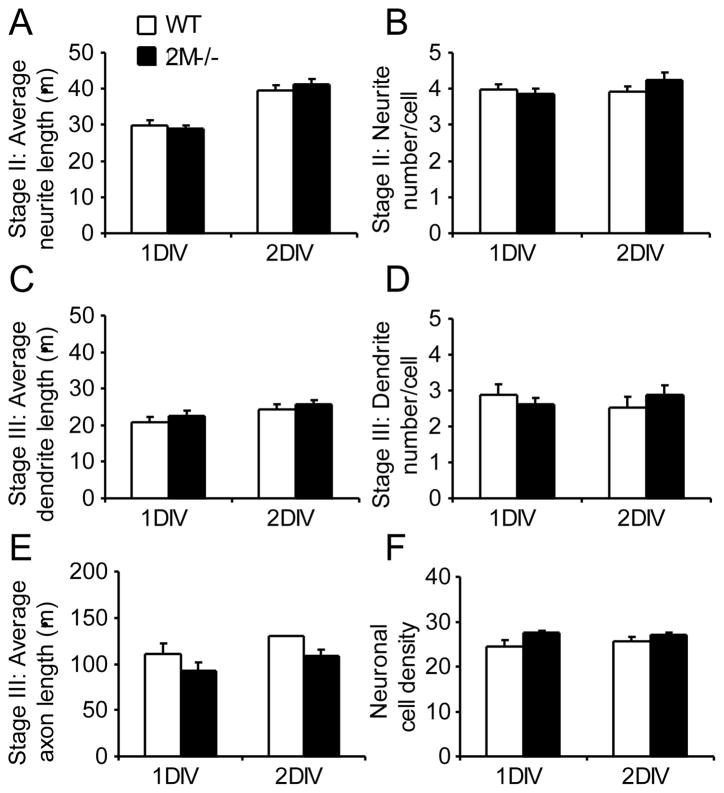
After culturing E15 wildtype and ϐ2M−/− hippocampal neurons side-by-side, we did not observe any significant differences between them in any of the measured parameters, including neurite length (Fig. 4A) and neurite number per cell (Fig. 4B) of stage II neurons, the dendrite length (Fig. 4C), dendrite number per cell (Fig. 4D), and axon length (Fig 4E) of stage III neurons, as well as neuronal density (Fig. 4F). Nor were there differences in the percentage of polarized neurons between wildtype and ϐ2M−/− hippocampal cultures (data not shown). Thus, unlike KbDb−/− mouse neurons, neurons from ϐ2M−/− mice displayed normal maturation. Evidently, the ability of ϐ2M−/− neurons to produce open MHCI conformers enabled normal neuritogenesis.
Figure 4. Development of β2M-deficient neurons is similar to that of wildtype neurons in vitro.
Quantitative analysis of mean neurite outgrowth in 1 and 2 DIV primary hippocampal cultures from wildtype (open bars) and β2M-deficient (black bars) C57BL/6 mice +/−SEM. (A) Average neurite length of stage II neurons. (B) Average neurite number of stage II neurons. (C) Average dendrite length of stage III neurons. (D) Average dendrite number of stage III neurons. (E) Average axon length of stage III neurons. (F) Neuronal cell density. Data shown are from two independent studies of side-by-side ϐ2M−/− and wildtype cultures.
3.3 Shifting open MHCI conformers towards closed conformers inhibits axon outgrowth
Multiple studies have demonstrated that the addition of exogenous ϐ2M to cultured immune cells and neurons promotes the dissociation of open MHCI complexes and the reformation of closed MHCI (e.g., (Arosa et al., 2007; Capps et al., 1993; Due et al., 1986; Glynn et al., 2011; Ramalingam et al., 1997)). To further test whether open MHCI conformers contribute to promoting neuritogenesis we added ϐ2M (3 μm) to some wildtype hippocampal cultures. Incubating wildtype E15 hippocampal neurons in the presence of ϐ2M had no ef fect on the average neuronal density, neurite length or neurite number of stage II neurons (Fig. S2 A, B, C) or neuronal cell polarization (data not shown). Moreover, it had no effect on dendrite length, or dendrite number on stage III neurons (Fig. S2 D, E). However, incubation with exogenous ϐ2M led to significantly shorter axons on stage III neurons (Fig. 5). This suggests that a reduction in open MHCI conformers and/or an increase in closed MHCI, has an inhibitory effect particularly on axon outgrowth from polarized neurons.
Figure 5. Exogenous β2M inhibits axon growth in hippocampal cultures from C57BL/6 mice.
Representative images of control (untreated) (A) and β2M-treated (B) 1 DIV neurons. Neurons were visualized by fluorescent labeling with anti-βIII-tubulin antibodies (green) and F-actin specific drug phalloidin (red). Scale bar = 20 μm. (C) Quantitative analysis of average axon length from control (open bar) and β2M-treated (black bar) hippocampal cultures +/− SEM. Data shown are from two independent experiments. **-p<0.01. μm.
3.4 Recombinant soluble closed MHCI inhibits axon outgrowth
We previously showed that recombinant self- (but not non-self) MHCI monomers, as well as soluble MHCI that was shed from neurons, could inhibit overall neurite outgrowth from cultured retina explants (Escande-Beillard et al., 2010; Washburn et al., 2011). To further analyze the effects of these closed MHCI conformers on neurite outgrowth we incubated wildtype E15 hippocampal cultures with a self-MHCI monomer, or a non-self MHCI monomer (200 pM) (Fig. 6). We found that neither monomer had a significant effect on neuronal cell density, minor neurite outgrowth, the number of neurites per cell, the dendrite length and number (Fig. S3 A, B, C, D, E) and neuronal cell polarization (data not shown). However, addition of a self-MHCI monomer, but not a non-self MHCI monomer, significantly inhibited axon outgrowth from stage III neurons (Fig. 6). Thus, unpolarized neurons are unaffected by soluble closed MHCI, but after they develop into polarized neurons, interaction with closed self-MHCI complexes inhibits axon outgrowth but has little effect on dendrite outgrowth.
Figure 6. Soluble self-MHCI monomer inhibits axon growth in hippocampal cultures from C57BL/6 mice.

Representative images of control (untreated) (A), nonself-MHCI (Dk/polyoma protein) monomer-treated (B) and self-MHCI (Db/H13a) monomer-treated 2 DIV neurons (C). Neurons were visualized by fluorescent labeling with anti-βIII-tubulin antibodies (green) and anti-MAP2 antibodies (red). Scale bar = 20 μm. (D) Quantitative analysis of average axon length from control (open bar), Dk (hatched bar)- and Db (black bar)-monomer treated hippocampal cultures +/− SEM. Data shown are from two independent experiments *-p<0.05; **-p<0.01.
4. Discussion
Factors that influence neuritogenesis and the establishment of neuronal polarity are of great interest for understanding neurodevelopment. Our previous studies showed that low levels of recombinant self-MHCI monomers, or soluble MHCI that was shed from neurons, inhibited neurite outgrowth from cultured embryonic retinas (Escande-Beillard et al., 2010; Washburn et al., 2011). Based on this, we anticipated that genetically reduced neuronal MHCI expression would promote neuritogenesis, while genetically increased neuronal MHCI expression would inhibit neuritogenesis. We observed, however, the converse. Cultured hippocampal neurons from KbDb−/− mice had diminished neurite extension and slower establishment of neuronal polarity compared to wildtype hippocampal neurons. In contrast, hippocampal neurons from NSE-Db mice had accelerated neurite outgrowth relative to hippocampal neurons from wildtype mice. Moreover, the number of primary neurites was increased on NSE-Db transgenic neurons compared to those of wildtype mice. These results are consistent with findings that IFNγ treatment, which induces neuronal heavy chain expression, promotes neurite outgrowth from cultured primary hippocampal neurons (Barish et al., 1991; Neumann et al., 1995). We did not observe the emergence of multiple axons from NSE-Db neurons indicating that elevated neuronal MHCI expression is insufficient to confer axon identity to immature neurites. We do not believe that differences in free ϐ2M levels among the tested mouse stains account for the differences in neuritogenesis that we observed because 1) neurite outgrowth from ϐ2M−/− neurons was similar to that of wildtype neurons and 2) exogenous ϐ2M had no effect on stage II wildtype neurons, while modulating the expression of the heavy chain had multiple effects on these neurons. Together, our observations provide the first direct evidence that neuronal MHCI can modulate the rate of neurite outgrowth and polarization. These are novel neurobiological activities of MHCI that are distinct from MHCI’s previously described role in synaptic remodeling (Glynn et al., 2011; Huh et al., 2000).
The 1 DIV neurons had little, or no, contact with neighboring neurons, suggesting that the differences in neuronal development that we observed in the three different strains of mice arose largely from differences in MHCI cis interactions with other cell surface molecules. Hippocampal neurons express classical MHCI receptors such as Ly49 and PirB (Syken et al., 2006; Zohar et al., 2008) and closed MHCI can bind to these receptors either in cis or in trans (e.g., (Back et al., 2009; Chalifour et al., 2009; Held and Mariuzza, 2008; Masuda et al., 2007)). Alternatively, MHCI open conformers can bind in cis with insulin receptors, EGF receptors, IL-2 receptors, and perhaps IGF receptors (Arosa et al., 2007; Due et al., 1986; Jelonek et al., 1998; Ramalingam et al., 1997; Santos et al., 2004). Heavy chain-insulin receptor heterodimers have been confirmed by immunoprecipitation, cross-linking, and fluorescence resonance energy transfer-based proximity measurements (e.g., (Arosa et al., 2007; Chvatchko et al., 1983; Due et al., 1986; Edidin and Reiland, 1990; Fehlmann et al., 1985; Phillips et al., 1986; Ramalingam et al., 1997; Verland et al., 1989)). Open MHCI conformer interactions with some of these receptors has been shown to trigger phosphorylation cascades (Arosa et al., 2007; Ramalingam et al., 1997; Santos et al., 2004) and open MHCI conformer binding to insulin receptors increases the insulin receptor’s affinity for insulin (Arosa et al., 2007; Due et al., 1986; Ramalingam et al., 1997). The activation of insulin receptors and IGF receptors are known to stimulate neurite outgrowth (e.g., (Bhat, 1983; Chiu et al., 2008; Patel et al., 1993; Recio-Pinto and Ishii, 1988)) and the establishment of neuronal polarity (Sosa et al., 2006).
Previous studies which assessed the levels of open and closed MHCI on the cell surface utilized monoclonal antibodies that are specific for open or closed forms of particular HLAs on human cells (reviewed in (Arosa et al., 2007)). Such antibodies, however, are not available for analyzing the levels of open and closed murine MHCI. To try to differentiate whether the differences in neuritogenesis between the three mouse strains were due to differences in closed or open MHCI cis interactions with cell surface proteins, we analyzed the development of cultured ϐ2M−/− neurons that can produce open, but not closed, MHCI. We found that unlike neurons from KbDb−/− mice that displayed delayed maturation, neurons from ϐ2M−/− mice displayed normal outgrowth at both 1 DIV and 2 DIV. Because there were few, if any, contacts between neurons after 1 DIV, we favor the notion that open MHCI conformer cis interactions with other cell surface proteins promote neuronal development. This could be mediated through interactions with growth promoting receptors (as previously described (Arosa et al., 2007; Due et al., 1986; Ramalingam et al., 1997; Santos et al., 2004)), or by inhibiting the activity of a negative regulator of neuritogenesis.
Additionally, we observed that culturing wildtype neurons in the presence of exogenous ϐ2M, which promotes the dissociation of open MHCI complexes and the reformation of closed MHCI (Arosa et al., 2007; Capps et al., 1993; Due et al., 1986; Glynn et al., 2011; Ramalingam et al., 1997) had no effect on the development of unpolarized neurons, but it reduced axon outgrowth from polarized neurons. These data further suggest that open MHCI conformer interactions with other cell surface proteins promote axon outgrowth. The reformation of closed MHCI and its interaction in cis and trans with MHCI receptors may have also contributed to the reduced axon outgrowth.
We previously showed that exogenous recombinant self-MHCI monomers, as well as soluble MHCI that was shed from neurons, inhibited neurite outgrowth from cultured embryonic retina explants (Escande-Beillard et al., 2010; Washburn et al., 2011). Our more detailed analysis in the present study revealed that self- and non-self recombinant MHCI monomers had no effect on unpolarized neurons, and that self-MHCI (but not non-self MHCI) specifically affected axon outgrowth of polarized neurons. Thus, while open MHCI conformer cis interactions can modulate unpolarized neuronal growth, closed soluble self-MHCI trans interactions only affected polarized neurons. This suggests that the mechanisms to recognize self-MHCI in trans are not present and/or functional until after neuronal polarization. It is notable that, while increased endogenous heavy chain expression promoted NSE-Db neuron outgrowth, exogenous MHCI monomer inhibited this process. These divergent outcomes support the scenario that open conformer cis interactions promote neuritogenesis, while closed MHCI interactions (in cis or trans) inhibit axon outgrowth on differentiated neurons. In vivo, closed MHCI could exert neuroinhibitory activity through interacting in cis or trans with classical MHCI receptors or by masking neuritogenesis-promoting receptors, similar to what has been described for MHCI-Ly49 cis interactions ((Back et al., 2009; Chalifour et al., 2009). Evaluation of these possibilities will be aided by the identification of the hippocampal neuronal MHCI receptor(s).
Together, these findings suggest a new model of MHCI’s actions during early neurodevelopment. In this model, MHCI exerts neurobiological activity not only through its interaction with classical MHCI receptors (such as PirB and Ly49), but also through its ability to bind to other types of surface proteins in ways that alter those protein’s activity. We propose that neuritogenesis is modulated by the balance between open MHCI conformer cis interactions with other cell membrane molecules that promote neuritogenesis, and closed MHCI interactions with classical MHCI receptors (in cis or trans) which inhibit axon outgrowth on polarized neurons, as shown diagrammatically in Fig. 7. Since increased open MHCI conformers on cortical neurons limit synapse formation (Glynn et al., 2011), it appears that open MHCI conformer promotion of neurite outgrowth is associated with reduced synaptogenesis. Together, these results suggest that MHCI-associated signals for neuronal growth can counter-regulate those for synaptogenesis. The balance of closed and open MHCI on the neuronal surface may be modulated by the cell’s metabolic state, electrical activity, heavy chain and ϐ2M expression levels, the expression levels of other proteins that can bind in cis or trans to closed and open MHCI, membrane reorganization and turn-over, MHCI interactions with neurotransmitter receptors, complement cleavage of the heavy chain, and other factors (Arosa et al., 2007; Boulanger, 2009; Boulanger et al., 2001; Fourgeaud et al., 2010; Glynn et al., 2011; Washburn et al., 2011).
Fig. 7. Diagrammatic representation of the proposed open and closed MHCI interactions in cis and trans that modulate neuritogenesis.
A) On the cell surface, closed MHCI (left) consisting of a heavy chain (α1, α2 and α3 domains shown in black), ϐ2M (grey rectangle) and a cytosolic peptide (stippled) can dissociate forming an open conformer (right). The free heavy chain’s cytoplasmic tail becomes phosphorylated (P) (reviewed in (Arosa et al., 2007)). B) The open conformers (center) can bind in cis to other free heavy chains, forming homodimers (left side), or associate with other cell surface molecules (such as insulin receptor, EGF receptor, IGF receptor, transferrin receptor, CD8αϐ and others), represented by a grey diamond) forming heterodimers (right side). We depict the open MHCI binding to a receptor through the heavy chain’s peptide-empty α1 and α2 domains based on the observations of Jelonek et al. Heterodimer formation modulates the receptor’s intracellular signaling in response to ligand binding and/or its internalization in ways that promote neurite outgrowth and neuronal polarization. C) Closed MHCI can bind classical MHCI receptors on same cell surface in cis preventing the receptor from interacting with MHCI on another cell (Back et al., 2009; Chalifour et al., 2009; Held and Mariuzza, 2008; Masuda et al., 2007) (left). Classical MHCI receptors that interact in trans with closed MHCIs on another cell surface send an inhibitory intracellular signal for axon outgrowth (center). Similarly, classical MHCI receptors can also bind soluble closed MHCI molecules that are shed from MHCI-expressing cells, which leads to inhibitory signals for axon outgrowth (Escande-Beillard et al., 2010; Washburn et al., 2011) (right side).
Consistent with our model, conformation-dependent anti-MHCI mAbs (i.e., they recognize closed MHCI) enhance neurite outgrowth from cultured neurons (Zohar et al., 2008), which may be due to their preventing closed MHCI interactions with receptors that convey inhibitory signals. Anti-MHCI also reduced synapsin-I levels, consistent with the notion that increased neurite outgrowth is paired with reduced synaptogenesis. Conversely, anti-Ly49 mAbs reduced neurite outgrowth and increased synapsin levels (Zohar et al., 2008). This antibody is thought to mimic MHCI-Ly49 interactions, which according to our model, would explain the reduced neurite outgrowth and increased synapsin. These explanations are, however, conjectural and much work remains to be done towards elucidating how MHCI interactions modulate neuritogenesis and synaptogenesis.
MHCI has now been shown to play multiple roles in the nervous system, including axon and dendrite morphogenesis, neuronal polarity and synaptic development. Reducing, or elevating, neuronal MHCI expression leads to abnormalities in mouse CNS development (Glynn et al., 2011; Huh et al., 2000; Wu et al., 2011a). By extension, it is reasonable to hypothesize that abnormalities in neuronal MHCI expression during critical periods of human neurodevelopment could have deleterious consequences. Along this line, treating pregnant rodents with immunostimulants that may induce fetal neuronal MHCI expression cause the offspring to have autism-like behavioral deficits (e.g., (Goines and Van de Water, 2010; Malkova et al., 2012; Patterson, 2011)). Moreover, mutations in molecules involved in synaptogenesis and neuritogenesis have been associated with a number of human neuropathological conditions and the MHCI region has been associated with schizophrenia and autism (Boulanger and Shatz, 2004; Stefansson et al., 2009; Torres et al., 2006). Conceivably, modulating the balance of open and closed MHCI on neurons may provide new approaches to mitigate some neuropathological conditions.
Supplementary Material
Quantitative analysis of average (A) neuronal cell density (B) total neurite length per unpolarized (stage II) neuron and (C) total neurite length per polarized (stage III) neuron in 1 and 2 DIV primary hippocampal cultures from wildtype (open bar), KbDb-deficient (hatched bar) and NSE-Db (black bar) C57BL/6 mice +/− SEM. N=3–5 independent studies of each genotype, with all three genotypes studied side-by-side in two studies. ***-p<0.001
Primary neurons were untreated (control, open bars) or incubated with exogenous ϐ2M (black bars). Quantitative analysis of (A) neuronal cell density, (B) average neurite length and number (C) on stage II neurons, and average dendrite length (D) and number (E) on stage III neurons after 1 DIV +/− SEM.
E15 C57BL/6 hippocampal neurons we incubated with media alone (open bars), a nonself Dk monomer (hatched bars), or a self-MHCI Db monomer (black bars). Quantitative analysis of (A) neuronal cell density, (B) average neurite length and (C) number on stage II neurons and (D) average dendrite length and (E) number on stage III neurons in 2 DIV primary hippocampal cultures +/− SEM.
Acknowledgments
We thank Drs. Iryna Ethell, Lorraine Washburn and Olena Bukalo for their comments on the manuscript. We thank Matthew Dizon, Julia Chui, Terry Hsieh and other members of the Kaufman lab for their assistance. This work was supported by NIH grants R21NS053847 and R21NS047383 to D.L.K.
Footnotes
All authors declare that there are no conflicts of interest
Author contributions
Conceived and designed the experiments: TB, JT, DLK. Performed the experiments: TB, HD, WX, SG, YJ, LW, TW, GB, BM. Analyzed the data: TB, DLK. Wrote the paper: TB, DLK
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen H, Fraser J, Flyer D, Calvin S, Flavell R. Beta 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc Natl Acad Sci U S A. 1986;83:7447–7451. doi: 10.1073/pnas.83.19.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosa FA, Santos SG, Powis SJ. Open conformers: the hidden face of MHC-I molecules. Trends Immunol. 2007;28:115–123. doi: 10.1016/j.it.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Back J, Malchiodi EL, Cho S, Scarpellino L, Schneider P, Kerzic MC, Mariuzza RA, Held W. Distinct conformations of Ly49 natural killer cell receptors mediate MHC class I recognition in trans and cis. Immunity. 2009;31:598–608. doi: 10.1016/j.immuni.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish ME, Mansdorf NB, Raissdana SS. Gamma-interferon promotes differentiation of cultured cortical and hippocampal neurons. Dev Biol. 1991;144:412–423. doi: 10.1016/0012-1606(91)90433-4. [DOI] [PubMed] [Google Scholar]
- Bhat NR. Insulin dependent neurite outgrowth in cultured embryonic mouse brain cells. Brain Res. 1983;313:315–318. doi: 10.1016/0165-3806(83)90231-6. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix M, Raulet D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J Exp Med. 1992;176:829–834. doi: 10.1084/jem.176.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Huh GS, Shatz CJ. Neuronal plasticity and cellular immunity: shared molecular mechanisms. Curr Opin Neurobiol. 2001;11:568–578. doi: 10.1016/s0959-4388(00)00251-8. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Capps GG, Robinson BE, Lewis KD, Zuniga MC. In vivo dimeric association of class I MHC heavy chains. Possible relationship to class I MHC heavy chain-beta 2-microglobulin dissociation. J Immunol. 1993;151:159–169. [PubMed] [Google Scholar]
- Chalifour A, Scarpellino L, Back J, Brodin P, Devevre E, Gros F, Levy F, Leclercq G, Hoglund P, Beermann F, Held W. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatchko Y, Van Obberghen E, Kiger N, Fehlmann M. Immunoprecipitation of insulin receptors by antibodies against Class 1 antigens of the murine H-2 major histocompatibility complex. FEBS Lett. 1983;163:207–211. doi: 10.1016/0014-5793(83)80820-5. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due C, Simonsen M, Olsson L. The major histocompatibility complex class I heavy chain as a structural subunit of the human cell membrane insulin receptor: implications for the range of biological functions of histocompatibility antigens. Proc Natl Acad Sci U S A. 1986;83:6007–6011. doi: 10.1073/pnas.83.16.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M, Reiland J. Dynamic measurements of the associations between class I MHC antigens and insulin receptors. Mol Immunol. 1990;27:1313–1317. doi: 10.1016/0161-5890(90)90036-y. [DOI] [PubMed] [Google Scholar]
- Escande-Beillard N, Washburn L, Zekzer D, Wu ZP, Eitan S, Ivkovic S, Lu Y, Dang H, Middleton B, Bilousova TV, Yoshimura Y, Evans CJ, Joyce S, Tian J, Kaufman DL. Neurons preferentially respond to self-MHC class I allele products regardless of peptide presented. J Immunol. 2010;184:816–823. doi: 10.4049/jimmunol.0902159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlmann M, Peyron JF, Samson M, Van Obberghen E, Brandenburg D, Brossette N. Molecular association between major histocompatibility complex class I antigens and insulin receptors in mouse liver membranes. Proc Natl Acad Sci U S A. 1985;82:8634–8637. doi: 10.1073/pnas.82.24.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Davenport CM, Tyler CM, Cheng TT, Spencer MB, Boulanger LM. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc Natl Acad Sci U S A. 2010;107:22278–22283. doi: 10.1073/pnas.0914064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn MW, Elmer BM, Garay PA, Liu XB, Needleman LA, El-Sabeawy F, McAllister AK. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci. 2011;14:442–451. doi: 10.1038/nn.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Van de Water J. The immune system’s role in the biology of autism. Curr Opin Neurol. 2010;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol. 2008;8:269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelonek MT, Classon BJ, Hudson PJ, Margulies DH. Direct binding of the MHC class I molecule H-2Ld to CD8: interaction with the amino terminus of a mature cell surface protein. J Immunol. 1998;160:2809–2814. [PubMed] [Google Scholar]
- Joseph MS, Bilousova T, Zdunowski S, Wu ZP, Middleton B, Boudzinskaia M, Wong B, Ali N, Zhong H, Yong J, Washburn L, Escande-Beillard N, Dang H, Edgerton VR, Tillakaratne NJ, Kaufman DL. Transgenic mice with enhanced neuronal major histocompatibility complex class I expression recover locomotor function better after spinal cord injury. J Neurosci Res. 2011;89:365–372. doi: 10.1002/jnr.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda A, Nakamura A, Maeda T, Sakamoto Y, Takai T. Cis binding between inhibitory receptors and MHC class I can regulate mast cell activation. J Exp Med. 2007;204:907–920. doi: 10.1084/jem.20060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci U S A. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Li H, Mariuzza RA, Margulies DH. MHC class I molecules, structure and function. Rev Immunogenet. 1999;1:32–46. [PubMed] [Google Scholar]
- Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- Olsson L, Goldstein A, Stagsted J. Regulation of receptor internalization by the major histocompatibility complex class I molecule. Proc Natl Acad Sci U S A. 1994;91:9086–9090. doi: 10.1073/pnas.91.19.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RA, Kurian P, Raizada MK, Crews FT. Insulin stimulates phosphatidylinositol 3-kinase activity in rat neuronal primary cultures. J Neurochem. 1993;61:360–363. doi: 10.1111/j.1471-4159.1993.tb03578.x. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Moule ML, Delovitch TL, Yip CC. Class I histocompatibility antigens and insulin receptors: evidence for interactions. Proc Natl Acad Sci U S A. 1986;83:3474–3478. doi: 10.1073/pnas.83.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MB. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam TS, Chakrabarti A, Edidin M. Interaction of class I human leukocyte antigen (HLA-I) molecules with insulin receptors and its effect on the insulin-signaling cascade. Mol Biol Cell. 1997;8:2463–2474. doi: 10.1091/mbc.8.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio-Pinto E, Ishii DN. Insulin and insulinlike growth factor receptors regulating neurite formation in cultured human neuroblastoma cells. J Neurosci Res. 1988;19:312–320. doi: 10.1002/jnr.490190306. [DOI] [PubMed] [Google Scholar]
- Rock KL, Gamble S, Rothstein L, Gramm C, Benacerraf B. Dissociation of beta 2-microglobulin leads to the accumulation of a substantial pool of inactive class I MHC heavy chains on the cell surface. Cell. 1991;65:611–620. doi: 10.1016/0092-8674(91)90093-e. [DOI] [PubMed] [Google Scholar]
- Santos SG, Powis SJ, Arosa FA. Misfolding of major histocompatibility complex class I molecules in activated T cells allows cis-interactions with receptors and signaling molecules and is associated with tyrosine phosphorylation. J Biol Chem. 2004;279:53062–53070. doi: 10.1074/jbc.M408794200. [DOI] [PubMed] [Google Scholar]
- Schell TD, Mylin LM, Tevethia SS, Joyce S. The assembly of functional beta(2)-microglobulin-free MHC class I molecules that interact with peptides and CD8(+) T lymphocytes. Int Immunol. 2002;14:775–782. doi: 10.1093/intimm/dxf041. [DOI] [PubMed] [Google Scholar]
- Schnabl E, Stockinger H, Majdic O, Gaugitsch H, Lindley IJ, Maurer D, Hajek-Rosenmayr A, Knapp W. Activated human T lymphocytes express MHC class I heavy chains not associated with beta 2-microglobulin. J Exp Med. 1990;171:1431–1442. doi: 10.1084/jem.171.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa L, Dupraz S, Laurino L, Bollati F, Bisbal M, Caceres A, Pfenninger KH, Quiroga S. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat Neurosci. 2006;9:993–995. doi: 10.1038/nn1742. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Torres AR, Sweeten TL, Cutler A, Bedke BJ, Fillmore M, Stubbs EG, Odell D. The association and linkage of the HLA-A2 class I allele with autism. Hum Immunol. 2006;67:346–351. doi: 10.1016/j.humimm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Verland S, Simonsen M, Gammeltoft S, Allen H, Flavell RA, Olsson L. Specific molecular interaction between the insulin receptor and a D product of MHC class I. J Immunol. 1989;143:945–951. [PubMed] [Google Scholar]
- Vugmeyster Y, Glas R, Perarnau B, Lemonnier FA, Eisen H, Ploegh H. Major histocompatibility complex (MHC) class I KbDb −/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc Natl Acad Sci U S A. 1998;95:12492–12497. doi: 10.1073/pnas.95.21.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn LR, Zekzer D, Eitan S, Lu Y, Dang H, Middleton B, Evans CJ, Tian J, Kaufman DL. A potential role for shed soluble major histocompatibility class I molecules as modulators of neurite outgrowth. PLoS One. 2011 doi: 10.1371/journal.pone.0018439. Published online 2011 March 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZP, Washburn L, Bilousova TV, Boudzinskaia M, Escande-Beillard N, Querubin J, Dang H, Xie CW, Tian J, Kaufman DL. Enhanced neuronal expression of major histocompatibility complex class I leads to aberrations in neurodevelopment and neurorepair. J Neuroimmunol. 2011a;232:8–16. doi: 10.1016/j.jneuroim.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZP, Bilousova T, Escande-Beillard N, Dang H, Hsieh T, Tian J, Kaufman DL. Major histocompatibility complex class I-mediated inhibition of neurite outgrowth from peripheral nerves. Immunol Lett. 2011b;135:118–123. doi: 10.1016/j.imlet.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar O, Reiter Y, Bennink JR, Lev A, Cavallaro S, Paratore S, Pick CG, Brooker G, Yewdell JW. Cutting edge: MHC class I-Ly49 interaction regulates neuronal function. J Immunol. 2008;180:6447–6451. doi: 10.4049/jimmunol.180.10.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantitative analysis of average (A) neuronal cell density (B) total neurite length per unpolarized (stage II) neuron and (C) total neurite length per polarized (stage III) neuron in 1 and 2 DIV primary hippocampal cultures from wildtype (open bar), KbDb-deficient (hatched bar) and NSE-Db (black bar) C57BL/6 mice +/− SEM. N=3–5 independent studies of each genotype, with all three genotypes studied side-by-side in two studies. ***-p<0.001
Primary neurons were untreated (control, open bars) or incubated with exogenous ϐ2M (black bars). Quantitative analysis of (A) neuronal cell density, (B) average neurite length and number (C) on stage II neurons, and average dendrite length (D) and number (E) on stage III neurons after 1 DIV +/− SEM.
E15 C57BL/6 hippocampal neurons we incubated with media alone (open bars), a nonself Dk monomer (hatched bars), or a self-MHCI Db monomer (black bars). Quantitative analysis of (A) neuronal cell density, (B) average neurite length and (C) number on stage II neurons and (D) average dendrite length and (E) number on stage III neurons in 2 DIV primary hippocampal cultures +/− SEM.