Abstract
Mice deficient in classical major histocompatibility complex class I (MHCI) have aberrations in neurodevelopment. The consequences of up-regulated neuronal MHCI expression have not been examined. We found that transgenic C57Bl/6 mice that are engineered to express higher levels of self-Db on their CNS neurons have alterations in their hippocampal morphology and retinogeniculate projections, as well as impaired neurorepair responses. Thus, enhanced neuronal classical MHCI expression can lead to aberrations in neural circuitry and neurorepair. These findings complement a growing body of knowledge concerning the neurobiological activities of MHCI and may have potential clinical relevance.
Keywords: major histocompatibility complex class I (MHCI), neurodevelopment, neurorepair, β2M, MHCI-deficient
1. Introduction
Classical MHCI (Ia) molecules play a central role in the dialog between cells and the immune system. MHCI is tripartite, consisting of a heavy chain with a peptide binding groove, β2 microglobulin (β2M) and a peptide fragment (8–11 amino acid residues) from a degraded protein (Natarajan et al., 1999). On the cell surface, MHCI molecules are screened by CD8+ T cells, which have MHCI receptors that were generated by gene rearrangement, in order to identify cells expressing foreign antigens (Heemels and Ploegh, 1995; Natarajan et al., 1999). MHCI also interacts with cells of the innate immune system (e.g., natural killer cells) that use germline-encoded MHCI receptors to identify cells with reduced MHCI levels that can be indicative of viral infection or transformation (Lanier, 2005; Raulet et al., 2001).
It was long thought that neurons express little or no MHCI, except in response to functional impairment or traumatic injury (Joly et al., 1991; Kimura and Griffin, 2000; Lampson, 1995; Neumann et al., 1995; Neumann et al., 1997; Wong et al., 1984). Studies by Shatz and colleagues demonstrated, however, that MHCI is expressed by CNS neurons, particularly those whose synapses undergo activity-dependent remodeling (Boulanger and Shatz, 2004; Corriveau et al., 1998; Huh et al., 2000). Mice that lack proteins involved in MHCI function (β2M, TAP1, or CD3ζ) have ectopic clusters of retinal inputs in their dorsal lateral geniculate nucleus (dLGN). Furthermore, they have increased synapsin levels in their hippocampus (Goddard et al., 2007), enhanced long-term potentiation (LTP), reduced long-term depression (LTD) in their hippocampus, and have altered motor learning (Goddard et al., 2007; Huh et al., 2000; McConnell et al., 2009). Additionally, a classical MHCI receptor of the innate immune system (PirB) is involved in synaptic plasticity in the visual cortex (Syken et al., 2006). Thus, besides MHCI’s central role in immune system function, MHCI is now thought to play an important role in neurodevelopment.
Studies of MHCI’s role in the nervous system have focused on mice that lack MHCI-associated proteins. The neurological aberrations observed in these MHCI-deficient mice beg the question what effect elevated neuronal MHCI expression might have. Several lines of evidence suggest that inappropriate neuronal MHCI expression could have neurological consequences. We recently reported that addition of picomolar amounts of recombinant MHCI to cultures of wild type neurons can inhibit neurite outgrowth (Escande-Beillard et al., 2010). This inhibition of neuronal outgrowth is a distinct biological activity from the previously described role of MHCI in pruning, or stabilizing synaptic connections (Huh et al., 2000; Oliveira et al., 2004; Syken et al., 2006). By extension, inappropriate expression of MHCI in the CNS during neurodevelopment may affect the establishment of neural circuitry.
To examine the consequences of enhanced neuronal MHCI expression on neurodevelopment, we studied transgenic C57BL/6 (H-2b) mice, termed “NSE-Db” mice, that have a transgene consisting of a neuron-specific enolase promoter (NSE) linked to a Db heavy chain cDNA (matching their endogenous H-2D MHCI allele) which express elevated levels of Db specifically on their CNS neurons (Rall et al., 1995). As in previous studies of MHCI-deficient mice, we analyzed the pattern of their retinogeniculate connections, synaptic marker levels in their hippocampus, and their hippocampal morphology and electrophysiology. Additionally, we studied their neuronal sprouting responses following a CNS injury. Together, the results provide lines of evidence that elevated neuronal MHCI expression can have physiological consequences, which may be of relevance for understanding neurodevelopmental disorders and the role of MHCI in neurorepair.
2. Materials and Methods
2.1 Animals
The generation of C57BL/6 NSE-Db mice which express elevated levels of Db on their neurons has been previously described (Rall et al., 1995). They have no obvious abnormalities in CNS anatomy or behavior (Rall et al., 1995). We crossed NSE-Db mice with C57BL/6 mice (The Jackson Laboratory, Bar Harbor, Maine) for 10 generations and then bred them to homozygosity for the transgene. C57BL/6 β2M−/− mice were purchased from the Jackson Laboratory. Age and sex matched C57BL/6 mice were used as wildtype controls. All studies were approved by the UCLA Animal Use and Care Committee.
2.2 Anterograde tracing
Eleven day old wildtype C57BL/6, MHCI-deficient C57BL/6 β2M−/− and NSE-Db mice were injected intraocularlly with 2–3 ul cholera toxin subunit B conjugated to Alexa Fluor 594 (Sigma) (1.0 mg/ml in saline), delivered at 2 uL/min. Forty eight hrs later, the animals were perfused intracardially with 4% paraformaldehyde (PFA) in saline. Brains were removed, postfixed overnight in 4% PFA at 4°C, transferred to 25% (wt/vol) sucrose in PBS (4°C), sectioned transversely at 50 µm with a cryostat and mounted onto a glass cover slip with DAPI-containing mounting medium (Vector). Blinded to the animal’s genotype, sections with equivalent extents of anterograde labeling in the dLGN were selected for analysis. A CCD camera linked to a computer with Metamorph software was used for taking the images of the sections using a Texas Red fluorescent filter on a Zeiss microscope. Image J was used to measure the labeled dLGN areas. The contour of contralateral projection was used to measure the total area of dLGN as described (11). For each brain, averages of the four largest total LGN and ipsilateral dLGN areas from the middle-third of the dLGN were used for measurements. All measurements excluded the ventral LGN, intrageniculate leaflet and the optical tract, as well as the background blood vessel staining, as per (Huh et al., 2000).
2.3 Electrophysiology
Transverse hippocampal slices (500 µm thick) from 4–6 week old C57BL/6 and NSE-Db male mice were prepared using a vibroslicer (Campden Instruments, UK) and the slices were incubated in artificial cerebrospinal fluid (ACSF; 120 mM NaCl, 25 NaHCO3, 3.3 mM KCl, 1.23 mM NaH2PO4, 2 mM CaCl2, 1.0 mM MgSO4, and 10 mM D-glucose, pH 7.4) at room temperature for at least 1 hr prior to recording. Brain slices were then placed in a submerged recording chamber and continuously perfused with warm (30±1°C), oxygenated ACSF at 2–3 mL/min. The Schaffer collateral-CA1 pathway was stimulated and the evoked field excitatory postsynaptic potentials (fEPSPs) were recorded from CA1 pyramidal neuron dendrites in the stratum radiatum. The basal synaptic transmission in each slice was assessed by the synaptic input-output curve constructed by plotting fEPSP slopes against presynaptic fiber volleys evoked at various stimulation intensities. The stimulus that yielded 50% of the maximum fEPSP slope was used for baseline collection. A 100 Hz high frequency stimulation (HFS) was then applied for 1 sec to induce LTP and post-HFS changes were monitored for 60 min. The potentiation of fEPSP was expressed as percent changes of fEPSP slopes from the baseline level. Experiments were performed blind to genotype.
2.4 Presynaptic marker density
For synaptophysin and growth-associated protein-43 (GAP-43) immunostaining, brains from P39 male C57BL/6 and NSE-Db mice were fixed in 4% paraformaldehyde at 4°C overnight, cryoprotected in 30% sucrose solution and frozen in 2-methylbutane. Transverse cryostat sections (25µm) were mounted on slides. After blocking endogenous peroxidase for 5 min at room temperature (RT), sections were permeabilized (0.3% Triton X-100 in PBS for 15 min at RT), blocked against non-specific binding (3% goat serum, 0.01% Triton X-100 in PBS for 30 min at RT), and incubated with polyclonal rabbit anti-synaptophysin (5.6 µg/ml, DakoCytomation) or mouse anti-GAP-43 (1:500, Sigma) monoclonal antibody (mAb) in blocking buffer overnight at 4°C. The next day, the sections were washed twice with PBS, incubated with HRP-conjugated secondary anti-rabbit or anti-mouse IgG, respectively (4.5 µg/ml, BioRad) for 2 hours at RT, washed twice in PBS and then developed with chromogen 3,3’ diaminobenzidine.
Representative color images of hippocampal regions were taken with a Nikon microscope (Microphot-FXA) with a 10x objective and a Sport digital camera (1400 Color). For quantification of synaptophysin and GAP-43 immunoreactivity, monochrome images were taken at the same light intensity using an inverted microscope (Axiovert 200, Zeiss) with a 20x air Plan-Neofluar objective equipped with a CCD camera (International Power Sources, Inc., Holliston, MA) and MetaMorph Software (version 6.2; Universal Imaging Corp). Synaptophysin staining densities were quantified in the polymorphic layer of dentate gyrus, stratum molecular of the dentate gyrus, stratum lucidum of area CA3, and stratum radiatum of area CA1–2. The optical density (OD) of synaptophysin immunostaining was measured as the average from equal areas within each layer of interest using Image J Analysis software. The OD of the corpus callosum on the same section served as the background value and was subtracted from the mean OD from each area of interest, as per (Masliah et al., 1991). The OD from 6 to 8 sections (150 µm intervals between each) were analysed in a blinded fashion and averaged. GAP-43 staining densities were quantified in the stratum lucidum, stratum moleculare and stratum lacunosum moleculare of CA1. The corpus callosum staining level on the same slice was used as the baseline for synaptophysin and GAP-43 staining (as per (Masliah et al., 1991)). The average staining density was determined from equal areas within each hippocampal layer in a blinded manner, as described above.
2.5 Hippocampal layer width measurements and cell counts
For neuronal nuclei (NeuN) staining, free-floating sections were stored in 30% sucrose, 1% Polyvinylpyrrolidone, 30% ethylene glycol and 0.1 M PBS, pH 7.2. Next, sections were permeabilized, blocked against non-specific binding (3% chick serum in PBS for 30 min at RT), and free-floating sections were incubated with mouse anti-NeuN mAb (5 µg/ml, Millipore) for 48 hours at 4°C. Subsequently, the sections were washed 3 times with PBS, incubated with Alexa 488 conjugated chicken anti-mouse IgG secondary (4 µg/ml, Molecular Probes) for 3 hours at RT, and washed three times in PBS. Finally, the sections were mounted with DAPI-containing Vectashield medium (Vector labs). We measured the width and number of neurons across the striatum pyramidal of CA1 and the striatum granulosum of the dentate gyrus, as well as the number of neurons per unit length (60 µm) within these two regions and in the striatum pyramidal of CA3. Since the width of CA3 pyramidal cell layer is variable within each section, we analyzed only the number of neurons per unit length within this area. Blinded to genotype, we analyzed equal regions within each layer, in at least 6 sections from at least 5 animals/group.
2.6 Perforant Path Lesioning
Perforant path lesions were performed as previously described (Drojdahl et al., 2002). Briefly, C57BL/6 or NSE-Db male mice (8–12 weeks old) were anesthetized and placed into a stereotaxic device. A trench was drilled into the skull 1 mm lateral lambda and extended ≈3 mm toward the temporal ridge. Using a micro-dissecting knife mounted on the stereotaxic device, the lesion was made 3 mm deep and 2.5 mm medial to lateral. The wound was closed and treated with antibiotic. After 14 days the mice were perfused with 4% PFA, their brains frozen, and slices mounted for AChE staining.
2.7 AChE Histochemistry
Histochemical analysis of AChE density was performed using a modification of a previously described protocol (Karnovsky and Roots, 1964). Briefly, 25 um coronal sections were preincubated in 10% buffered formalin for 20 minutes, washed in dH2O, and then incubated in cholinesterase stain (50 mM sodium acetate 2 mM copper sulfate, 10 mM glycine, 4 mM acetylthlocholine iodide, and 0.2 mM ethopropazine, pH 5.5) for 12–18 hours. The slices were then rinsed in dH2O and incubated in 2.5% sodium sulfide, pH 7 for 45 seconds. Sections were again rinsed in dH2O and then reacted with 1% silver nitrate for 5 seconds. The tissue sections were rinsed in dH2O, air dried, dehydrated in graded alcohols, cleared in xylene, and coverslipped. The density of AChE staining was determined using NIH imaging analysis software. A region of interest was drawn in the same location of the molecular layer of the dentate gyrus for each section and the optical density was calculated. Sprouting was determined as the ratio of density of AChE-positive fibers in the lesioned side versus the contralateral side averaged from 5–10 sections per mouse (n=6–8 mice/group). Statistical significance was determined using a one-tail Student’s t test.
2.8 Spontaneous alternation behavior
Spontaneous alternation behavior was tested as previously described (Scheff and Cotman, 1977). The procedure for determining percent alternation consisted of two test trials per day, one hour apart. Each trial consisted of a "forced run" followed by a "choice run." During the forced run, either the left, or right arm of the T-maze was blocked. Half of the mice were consistently allowed to enter only the right arm of the T-maze, and the other half could only enter the left arm. During the choice run, both arms of the T-maze were accessible. If during the choice run, the animal entered the previously blocked arm, an alternation was scored. Baseline levels of alternation were determined for 4 days (8 trials) prior to lesioning, and on days 6–8 and 12–14 post-lesion. The group mean alternation scores over time were compared using repeated measure analysis of variance methods (n=15 mice/group). The Tukey-Fisher least significant difference criterion was used to judge statistical significance for post hoc t-tests.
3. Results
3.1 Alterations in retinogeniculate projections and dLGN structure in NSE-Db mice
Deficiencies in synaptic refinement were first identified in MHCI-deficient mice by examining their retinogeniculate projections in the dLGN (Huh et al., 2000). To begin to study whether elevated neuronal classical MHCI levels had an effect on synaptic development, we examined retinogeniculate projections in NSE-Db mice. As an initial control study, we performed anterograde tracing of retina ganglion cell (RGC) afferents in P13 wildtype C57Bl6 and MHCI-deficient C57BL/6 β2M−/− mice and quantified the area of ipsilateral and contralateral retinogeniculate projections, as well as the overall dLGN size. We observed that β2M−/− mice had ectopic clusters and significantly larger ipsilateral dLGN area relative to wildtype mice, along with a larger ipsilateral/total dLGN ratio than wildtype mice (Supplemental Fig.), as previously reported by Huh et al. (11). We then studied the retinogeniculate projections in NSEDbmice. The ipsilateral dLGN area in NSE-Db mice was slightly smaller than that in wildtype mice, but the difference was not statistically significant (Fig. 1). We found that the area occupied by contralateral retinogeniculate projections in NSE-Db mice was significantly smaller compared to that in wildtype mice. Moreover, the total dLGN area was significantly smaller in NSE-Db mice (Fig. 1). Because the ipsilateral projection area and the total dLGN area were both reduced in NSE-Db mice, the ratio of ipsilateral projection area to the total dLGN in NSE-Db mice was similar to that in wildtype mice. The reduced contralateral projection area and the decreased dLGN size that we observed in NSE-Db mice complement the observations of increased projection area in MHCI-deficient-mice (Huh et al., 2000).
Fig. 1. Anterograde tracing of RGC afferents and quantitation of projection areas in the dLGN.
Representative images of ipsilateral (A, C) and contralateral (B, D) projections in wildtype and NSE-Db mice. Scale bar=0.067 mm. Quantification of ipsilateral (E), and contralateral projections (F), as well as total dLGN (G) areas in wildtype (open bars) and NSEDb (black bars) mice. H) Ipsilateral/dLGN area ratio. Data shown are mean+/−SEM. N = 13 wildtype, 15 NSE-Db mice. *=p<0.05, ***=p<0.005.
3.2 Normal basal synaptic transmission and LTP induction in the CA1 region of NSE-Db mice
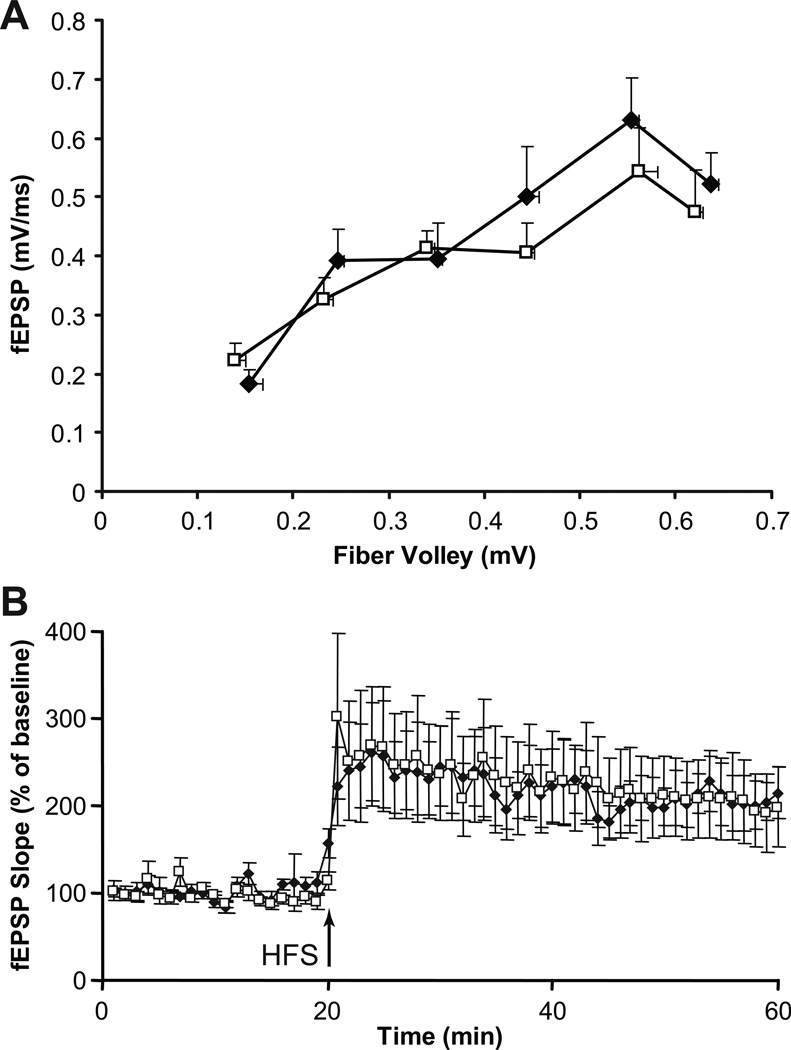
MHCI-deficient mice have enhanced LTP in the CA1 region of their hippocampus (Huh et al., 2000). Accordingly, we were interested in examining synaptic function in this region in the NSE-Db mice. We first characterized the synaptic input-output relationship in the Schaffer collateral-CA1 pathway but observed no significant differences between slices from wildtype C57Bl/6 and NSE-Db mice (Fig. 2A). To examine synaptic plasticity we recorded early-phase LTP triggered by a single HFS. Robust increases in fEPSP slopes were induced by the HFS and lasted for at least 60 min in both wildtype and NSE-Db mice with no statistical differences between the two groups (Fig. 2B). Thus, elevated neuronal MHCI expression did not significantly alter basal synaptic transmission nor the induction and expression of the early-phase LTP at CA1 synapses under our experimental conditions.
Fig. 2. Normal basal synaptic transmission and LTP in the hippocampal CA1 region of NSE-Db mice.
(A) The synaptic input-output relationship at Schaffer collateral-CA1 synapses of wildtype (open squares) and NSE-Db mice (black diamonds). (B) Induction of early LTP by a single HFS in wildtype (open squares) and NSE-Db mouse hippocampus (black diamonds). For both A and B, N=1 slice per animal, 8–12 animals/group.
3.3 Reduced synaptophysin and GAP-43 immunoreactivity in the NSE-Db hippocampus
It has been reported that MHCI-deficient mice have higher levels of synapsin immunoreactivity in their hippocampus, suggesting an exuberance of synaptic connections (Goddard et al., 2007). To further evaluate whether enhanced neuronal MHCI expression can affect neural circuitry, we studied the expression of presynaptic markers synaptophysin and GAP-43 (Van Lookeren Campagne et al., 1990) in the hippocampus of NSE-Db and wildtype mice (Fig. 3). We observed that synaptophysin immunostaining was significantly lower in several of the NSE-Db mouse hippocampal regions that we examined, relative to that in wildtype mice (Fig. 3A, 3B). Specifically, in NSE-Db mice, synaptophysin staining was significantly reduced by 22%, 21% and 19% in the polymorphique layer, stratum moleculare of the dentate gyrus, and in the stratum lucidum of the CA3 region, respectively (Fig. 3B). There were no significant differences in synaptophysin immunostaining in the CA1 stratum radiatum of wildtype and NSE-Db mice, which may explain why we observed normal LTP in this region.
Fig. 3. Reduced synaptophysin and GAP-43 immunostaining in the hippocampal regions of NSE-Db mice.
(A) Representative images of anti-synaptophysin staining in the dentate gyrus (DG), CA3 and CA1 regions of wildtype (WT) and NSE-Db mice. Black boxes show sampling areas for each region. (B) Densitometric group data of synaptophysin immunoreactivity in hippocampal regions of wildtype and NSE-Db mice. Black bars=wildtype, open bars=NSE-Db mice. Data shown is mean ± SEM. (C) Representative images of anti-GAP-43 staining in different hippocampal regions of wildtype and NSE-Db mice. (D) Densitometric group data of GAP-43 immunoreactivity in hippocampal regions of wildtype and NSE-Db mice. N=8 slices/mouse, 8 mice/group. *=P<0.05, **=P<0.01, ***=P<0.001 by Student’s t-test. Scale bar=100 µm.
Consistent with the synaptophysin immunostaining results, we observed that in NSE-Db mice, GAP-43 immunoreactivity was reduced by 17% in the stratum moleculare of dentate gyrus and by 61% in the stratum lucidum of CA3 (Fig. 3C, 3D). No difference in GAP-43 immunostaining was observed in stratum lacunosum moleculare of CA1. Since the level of GAP-43 immunostaining in the polymorphique layer was at background levels, as reported by others (Masliah et al., 1991), we didn’t quantify GAP-43 staining in this region. The reduced synaptophysin and GAP-43 immunostaining in some hippocampal regions of NSE-Db mice may reflect fewer presynaptic terminals and/or reduced expression of these markers.
3.4 Reduced number of pyramidal neurons in the CA1 region of NSE-Db mouse hippocampus
To examine whether the reduced synaptophysin and GAP-43 immunostaining in hippocampal regions of NSE-Db mice was due to a change in the number of neurons, we determined the width and number of neurons across the pyramidal cell layer in CA1 and dentate granule cell layer of the dentate gyrus, as well as the number of neurons per unit length within different hippocampal regions.
We observed a small but significant decrease (13%, P<0.01) in the width of the pyramidal cell layer within CA1 (representative images shown in Fig. 4A, group data in Fig. 4B). This decrease in the width of CA1 was accompanied by a reduced number of cells across this layer (8%, P<0.05, Fig. 4C), as well as 8% fewer pyramidal cells per unit length (P<0.05, Fig. 4D). In contrast, there were no significant changes in these parameters within the granule cell layer of the dentate gyrus (Figs. 4A–D). Additionally, we did not observe changes in the cell number per unit length in the CA3 pyramidal cell layer. Thus, enhanced MHCI expression leads to reduced number of principal neurons within CA1, but not in other hippocampal regions that we examined.
Fig. 4. NSE-Db mice display reduced number of pyramidal neurons in the CA1 hippocampal region.
(A) Representative images of NeuN/DAPI stained cells in the CA1, CA3 and DG of the hippocampus. White boxes are representative counting frames for each region. Scale bar=100 µm. (B) Layer width, (C) cell number, and (D) cells per unit length in hippocampal regions of NSE-Db mice were calculated as described in Methods. N=6 sections from at least 5 animals/group. *=P<0.05, **=P<0.01 by Student’s t-test.
3.5 Deficient neurorepair responses in NSE-Db mice
Some neuropathological conditions and traumatic brain injury induce neuronal MHCI expression (Corriveau et al., 1998; Feuerstein et al., 1998; Thams et al., 2009; Zanon and Oliveira, 2006). Based on the ability of recombinant MHCI to inhibit neurite outgrowth in vitro (Escande-Beillard et al., 2010) and observations from the current studies, we hypothesized that higher levels of neuronal MHCI might alter neuronal repair mechanisms in vivo. To test this hypothesis, we utilized a well-characterized model of compensatory neuronal sprouting that occurs following the unilateral lesioning of the perforant path to the hippocampus (Cotman et al., 1973; Lynch et al., 1973; Steward, 1976). In response to this denervation, septal hippocampal cholinergic neurons sprout into the outer molecular layer (ML) of the hippocampus. This compensatory sprouting can be detected histologically by AChE staining and behaviorally by the restoration of the animal’s ability to perform hippocampal-dependent spatial tasks (Douglas and Raphelson, 1966; Lynch et al., 1973; Scheff and Cotman, 1977). Spontaneous alternation behavior has been established as a simple behavioral paradigm for assessing hippocampal-dependent spatial memory. After a unilateral perforant path lesion, wildtype animals display a deficit in spontaneous alternation behavior, followed by a gradual recovery to preoperative alternation levels (Douglas and Raphelson, 1966; Lynch et al., 1973; Scheff and Cotman, 1977).
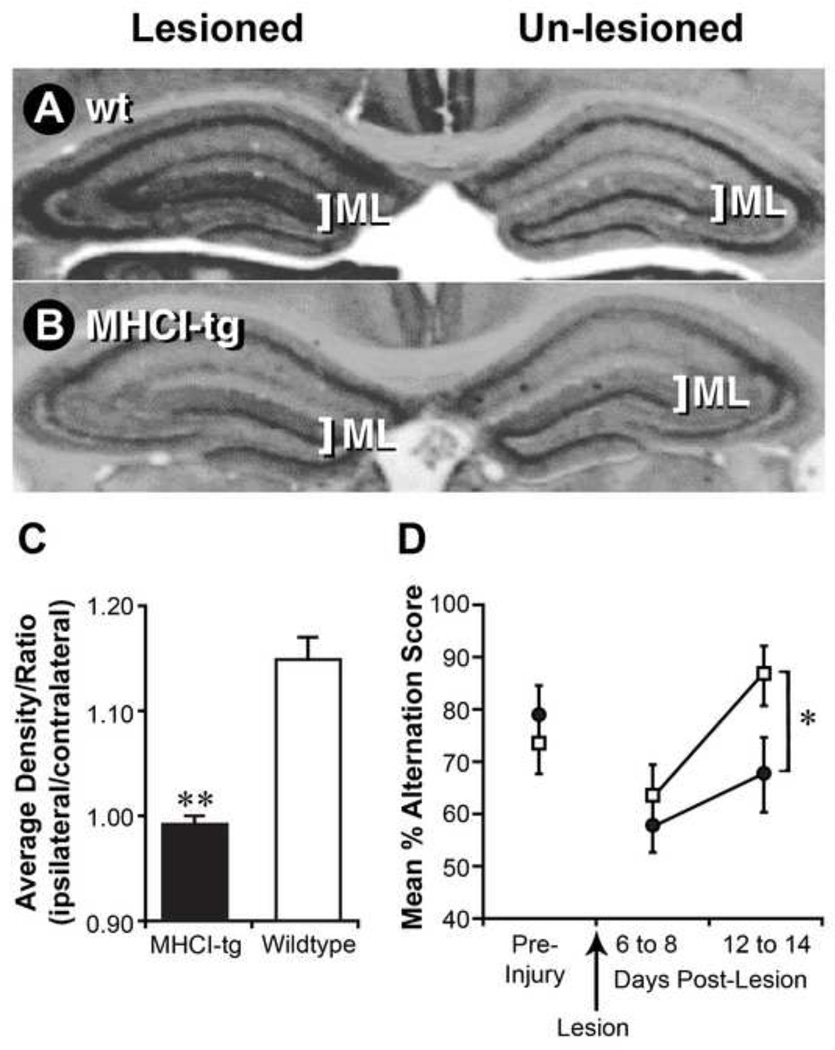
Before lesioning, wildtype and NSE-Db mouse brains had no apparent differences in their hippocampal cholinergic staining patterns and the mice displayed similar abilities in their performance of a hippocampal-dependent spatial task. Fourteen days post-unilateral perforant path lesioning, we compared the density of cholinergic fiber staining in the lesioned side hippocampus to that in the non-lesioned side on the same microsection. A robust compensatory sprouting response was seen in the hippocampal molecular layer on the lesioned side of wildtype mice (Figs. 5A, 5C). In NSE-Db mice, however, the density of AChE staining in the molecular layer on the lesioned side did not increase (Figs. 5B, 5C, p<0.001). Moreover, while lesioned wildtype mice completely recovered their ability to perform a hippocampal-dependent spatial task by 14 days post-lesioning, NSE-Db mice did not recover to their pre-lesion task performance ability within the observation period (Fig. 5D, p<0.05). Thus, both histological and behavioral testing indicate that ectopic expression of MHCI can inhibit compensatory sprouting responses in vivo.
Fig. 5. NSE-Db mice have deficiencies in compensatory sprouting responses after hippocampal lesioning.
The perforant path of wildtype (wt) C57BL/6 or NSE-Db mice was unilaterally lesioned. After 14 days, coronal brain sections were stained for AChE. Representative AChE stained sections from lesioned wt (A) and NSE-Db mice (B). The AChE staining patterns on unlesioned side of wt and NSE-Db mice were similar. On the lesioned side, compensatory cholinergic sprouting responses occurred in the molecular layer (ML) of wt, but not NSE-Db mice. (C) Group data of the mean ratio (lesioned/unlesioned) AChE staining density in the ML of wt and NSE-Db mice ± SEM. **=p<0.001. (N=6–8 mice/group). (D) Wt (□) and NSE-Db (●) mice showed similar ability to perform a hippocampal-dependent spatial task preoperatively and similar deficits immediately following lesioning. By 12–14 days post-lesion wt mice recovered their ability to perform the task, while NSE-Db mice displayed recovery deficits. Data shown is mean percent alternation ± SEM (*=p<0.05). (N=15 mice/group).
4. Discussion
Studies of MHCI-deficient mice revealed that MHCI expression is necessary for normal synaptic development. We were interested in the consequences of elevated neuronal MHCI expression. Here, we studied transgenic mice in which classical MHCI expression is specifically upregulated on CNS neurons.
Since MHCI-deficient mice are known to retain excess retinal innervation in their dLGN (11), we began by examining retinogeniculate connections in NSE-Db mice. We found that NSEDb mice had normal ipsilateral retinogeniculate projection areas. Their contralateral and total dLGN areas, however, were smaller compared to wildtype mice. Conceivably, the transgenic expression of Db in NSE-Db mice may have inhibited the outgrowth of some subset of RGC axons that were supposed to take the contralateral side of dLGN. Contralateral projections arrive in the dLGN before birth while the ipsilateral projections arrive after birth and endogenous MHCI expression in the dLGN is upregulated postnatally during activity-dependent remodeling (Huh et al., 2000). The precocious transgenic expression of Db in NSE-Db mice could have affected stability of the early contralateral connections, leading to the reduced contralateral area. The ipsilateral projections may be less affected by the additional transgenic Db expression because they arrive in the dLGN at a time at which endogenous MHCI levels are rising. Reduced RGC number is an unlikely explanation since there are no discernable differences in RGC cell number and morphology between NSE-Db and wildtype mice. It is of potential interest that mice lacking cAMP response element–binding protein (CREB) have deficiencies in binocular segregation similar to that seen in β2M/TAP−/− mice but also have smaller dLGNs (Huh et al., 2000; Pham et al., 2001) as we observed in NSE-Db mice. CREB is known to upregulate MHCI gene expression (Barco et al., 2002). The basis for the similar but divergent observations in the dLGN of MHCI-deficient, CREB−/− and NSE-Db mice remain to be elucidated.
We did not observe significant alternations in basal synaptic transmission or LTP in the CA1 region of NSE-Db mice. Consistent with this observation, our subsequent analysis of synaptophysin immunoreactivity indicated that there were no discernable changes in presynaptic terminals in the CA1 striatum radiatum. In contrast, LTP magnitude is increased in CA1 region of β2M/TAP1−/− mice (Huh et al., 2000). Evidently, a profound deficiency in MHCI produced a discernable change in synaptic plasticity in CA1 while an increase in the expression of one MHCI allele product did not. Notably, β2M/TAP1−/− mice not only have deficiencies in classical MHCI, but also in nonclassical MHCI molecules such as CD1, H2-M2 and Qa molecules of the innate immune system which require β2M and TAP (Bai et al., 1998; Bauer et al., 1997; Brutkiewicz et al., 1995; Chun et al., 2001; Lindahl et al., 1997; Soloski et al., 1995). Additionally, in NSE-Db mice, activity-dependent regulation of endogenous MHCI gene expression may help preserve normal levels of synaptic plasticity via homeostatic mechanisms. In contrast, in β2M/TAP1−/− mice, changes in long-term synaptic plasticity may be amplified due to impaired homeostatic regulation, as previous studies indicated a lack of synaptic scaling in β2M/TAP1 deficient neurons following prolonged tetrodotoxin treatment (Goddard et al., 2007). Finally, our results do not exclude the possibility that other forms of long-term synaptic plasticity, such as the later-phase LTP induced by multiple HFS, could be altered in NSE-Db mice.
We observed significant reductions in presynaptic markers synaptophysin and GAP43 in several regions of the NSE-Db hippocampus relative to wild type mice. This may be due to 1) excessive synaptic pruning, consistent with the observations of synaptic refinement deficiencies in mice lacking functional MHCI (Huh et al., 2000) and/or 2) deficits in axon outgrowth/branching and presynaptic button size/number, consistent with recombinant MHCI’s inhibitory effects on neurite outgrowth in vitro (Escande-Beillard et al., 2010) and the deficiencies we observed in NSE-Db dLGN morphology and compensatory neuronal sprouting. We observed significant changes in the number of neurons within CA1 pyramidal layer and the width of this layer, suggesting that in some regions, enhanced neuronal MHCI expression can lead to cell elimination, perhaps as part of the circuit refinement process. The lower presynaptic marker levels may also be partly due to reduced neuronal cell numbers, but we do not favor this scenario because markers levels were reduced in the stratum lucidum without changes in the number or density of CA3 pyramidal cells or in the granule cells of the dentate gyrus which project to that region.
We do not believe that these alterations in NSE-Db mice are merely the consequence of the transgene integration site because: 1) upregulating MHCI expression in wildtype mice by treating with IFNγ leads to greater pruning of presynaptic neurons and reduced synaptophysin immunoreactivity after peripheral nerve transection (Zanon and Oliveira, 2006); 2) neurite outgrowth from wildtype neurons is inhibited by recombinant MHCI in vitro (Escande-Beillard et al., 2010); 3) our observations are consistent with findings of increased synapsin levels in hippocampal neurons from MHCI-deficient mice (Goddard et al., 2007); and 4) our findings complement evidence from MHCI-deficient mice that MHCI is involved in synaptic pruning (Huh et al., 2000; Zohar et al., 2008).
We do not know the extent to which Db expression is elevated in specific hippocampal regions of NSE-Db mice. The staining of classical MHCI has been on neurons is technically challenging which contributed to the long-held notion that neurons do not express MHCI. Most anti-mouse MHCI antibodies were developed to stain native MHCI for FACS analysis and do not recognize denatured MHCI in fixed brain sections, or the antibodies do not distinguish between classical and nonclassical MHCI (reviewed in (Boulanger and Shatz, 2004)). Retinas from E14 NSE-Db mice express about 2-fold higher levels of Db heavy chain and normal levels of β2M (our unpublished observation), but this analysis, as well as in situ hybridization, does not provide information about MHCI levels on the neuronal cell surface since the formation of MHCI is likely to be limited by the availability of β2M in NSE-Db mice. Accordingly, the initial study of NSE-Db mice functionally demonstrated enhanced Db expression on the cell surface of purified hippocampal primary neurons by their greater adherence to anti-Db coated slides and greater susceptibility to CD8+ mediated killing after loading with a Db-restricted LCMV peptide or LCMV infection in vivo (Rall et al., 1995). One antibody (ER-H52) was recently reported to stain MHCI on peripheral nerves (Thams et al., 2009), but it does not sufficiently stain MHCI in the mouse hippocampus (our observations, and S. Thams, personal communication). Consequently, we do not know the exact extent to which Db is expressed in each hippocampal region of NSE-Db mice. However, the reduced level of synaptophysin is consistent with the increased levels of synapsin immunoreactivity in hippocampal neurons of MHCI-deficient mice (Goddard et al., 2007). It is also consistent with the greater pruning of presynaptic neurons and reduced synaptophysin immunoreactivity in wildtype mice treated with after IFNγ after peripheral nerve transection (Zanon and Oliveira, 2006), the deficits in synaptic pruning in MHCI-deficient mice (Huh et al., 2000; Zohar et al., 2008) and the reduced neurite outgrowth in of cultured neurons in the presence of recombinant MHCI (Escande-Beillard et al., 2010).
Experimentally induced seizures and traumatic injury cause neurons to express MHCI (Corriveau et al., 1998; Thams et al., 2009; Zanon and Oliveira, 2006). The MHCI-mediated inhibition of neurite outgrowth that we observed in vitro lead us to hypothesize that ectopic expression of neuronal MHCI could counteract neuronal repair mechanisms in vivo. Before lesioning, wildtype and NSE-Db mice had similar hippocampal cholinergic staining patterns and similar ability to perform a hippocampal-dependent spatial task. Following perforant path lesioning, however, NSE-Db mice displayed limited compensatory neuronal sprouting responses, which were manifested by their failure to recover the ability to perform the spatial task. It is possible that the lesioning process itself locally induced MHCI expression that affected the compensatory sprouting response. However, the neurons that sprout to compensate for the denervation are some distance from the lesion site and no T cells are recruited to this area (Fagan and Gage, 1994). If the lesion did induce endogenous MHCI expression in the area of sprouting, this is likely to have occurred to the same extent in both experimental and control mice. However, these contentions require further testing once more sensitive probes for Db become available. Based on the MHCI-mediated inhibition of neurite outgrowth that we observed in vitro, we believe that MHCI expressed in the septum of transgenic mice inhibited compensatory sprouting of the septal neurons. It is also possible that sprouted septal neurons that reached the hippocampus were pruned by hippocampal MHCI. We favor the inhibition mechanism since compensatory sprouting responses occur in wildtype mice even though their hippocampal neurons express MHCI (Huh et al., 2000). The neurobiological activity of MHCI, on both synapses and neurite outgrowth, may explain in part why MHCI expression is tightly regulated in the CNS.
Our observations suggest that inappropriate neuronal MHCI expression during critical periods of human neurodevelopment could be deleterious. A growing number of studies have shown that treatment of pregnant rodents with immunostimulants such as bacterial endotoxin (lipopolysaccharide), the double stranded RNA viral mimic polyinosinic: polycytidylic acid (poly I:C), turpentine (an inducer of local inflammation) or viral infection, cause the offspring to have behavioral abnormalities (Ballabh et al., 2004; Banks et al., 1995; Broadwell and Sofroniew, 1993; Goines and Van de Water, 2010b; Johnson et al., 2007; Meyer et al., 2009; Patterson, 2007). The behavioral aberrations are thought to be due to maternal immune responses that passed through the fetal blood brain barrier and affected neurodevelopment. Many inflammatory cytokines can pass through the BBB (Ballabh et al., 2004; Banks et al., 1995; Broadwell and Sofroniew, 1993; Goines and Van de Water, 2010b) and some can induce neuronal MHCI expression (Drew et al., 1993; Neumann et al., 1995; Neumann et al., 1997; Wong et al., 1984). Interestingly, prenatal enforced expression of the anti-inflammatory cytokine IL-10 attenuates the behavioral abnormalities in the offspring of mice treated with polyI:C (Meyer et al., 2008). In humans, viral infections during pregnancy are associated with schizophrenia and perhaps autism in offspring (Ciaranello and Ciaranello, 1995; Meyer et al., 2009; Patterson, 2007). Furthermore, the presence of maternal antibodies against brain proteins during gestation may be a risk factor for autism in offspring (reviewed in (Goines and Van de Water, 2010a)). If environmental factors such as an infection or maternal anti-brain autoantibodies induced a transient increase in fetal neuronal MHCI expression leading to altered neurodevelopment, there would not be a genetic link with MHCI, nor tell-tale evidence such as elevated neuronal MHCI levels or immune cell infiltrates in brain tissue from older schizophrenia or autism patients. Interestingly, the MHC region was recently genetically linked with schizophrenia (Stefansson et al., 2009) although autoantibodies and immune cell infiltrates in the CNS are not hallmarks of this disorder. While a transient inflammatory signal could also induce expression of some nonclassical MHCI genes, these molecules are largely involved in presenting particular microbial antigens and there is no evidence that they play a role in neurodevelopment. In the future, will be of interest to generate transgenic mice in which the Cre-Lox system can be used to up-regulate MHCI expression during specific periods of neurodevelopment in order to further test the hypothesis that inflammatory responses during pregnancy can induce fetal neuronal MHCI expression and alter embryonic neurodevelopment.
In conclusion, our studies demonstrate that mice with enhanced neuronal classical MHCI expression have aberrations in neural circuit formation and neurorepair. A further understanding of the role MHCI can play in the nervous system may lead to new inroads to understanding factors that can contribute to neuropsychiatric disorders, as well as new approaches to promote recovery in some neuropathological conditions.
Supplementary Material
Representative images of ipsilateral (A, C) and contralateral (B, D) projections in wildtype and β2M−/− mice. Scale bar = 0.067 mm. Quantification of ipsilateral (E), and contralateral projections (F), as well as total dLGN (G) areas in wildtype (open bars) and β2M−/− (black bars) mice. (H) Ipsilateral/dLGN area ratio. Data shown are mean+/−SEM. N=13 wildtype, 3 β2M−/− mice. * p<0.05
ACKNOWLEDGEMENTS
We thank Sebastian Thams for technical advise on ERH52 immunostaining, Oswald Steward, Nick Brecha, Larry Zipursky, Michael Sofroniew, David Hovda, J. Patrick Kesslak, Marie-Francoise Chesselet, Maio Tan, Carolyn Houser, Olena Bukalo, current and former members of the Kaufman lab for their help and advice, and Michael Oldstone for the NSE-Db mice. This work was supported by NIH grants R21NS053847 and R21NS047383 to D.L.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare that there are no conflicts of interest
Author contributions: Conceived and designed the experiments: Z-PW LW NE-B C-WX DLK. Performed the experiments: Z-PW LW MB JQ HD. Analyzed the data: Z-PW LW MB JQ TVB C-WX JT. Wrote the paper: Z-PW LW TVB C-WX JT DLK.
References
- Bai A, Broen J, Forman J. The pathway for processing leader-derived peptides that regulate the maturation and expression of Qa-1b. Immunity. 1998;9:413–421. doi: 10.1016/s1074-7613(00)80624-x. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Bauer A, Huttinger R, Staffler G, Hansmann C, Schmidt W, Majdic O, Knapp W, Stockinger H. Analysis of the requirement for beta 2-microglobulin for expression and formation of human CD1 antigens. Eur J Immunol. 1997;27:1366–1373. doi: 10.1002/eji.1830270611. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 1993;120:245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, beta 2-microglobulin-dependent surface expression of functional mouse CD1.1. J Exp Med. 1995;182:1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun T, Grandea AG, 3rd, Lybarger L, Forman J, Van Kaer L, Wang CR. Functional roles of TAP and tapasin in the assembly of M3-N-formylated peptide complexes. J Immunol. 2001;167:1507–1514. doi: 10.4049/jimmunol.167.3.1507. [DOI] [PubMed] [Google Scholar]
- Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. doi: 10.1146/annurev.ne.18.030195.000533. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Matthews DA, Taylor D, Lynch G. Synaptic rearrangement in the dentate gyrus: histochemical evidence of adjustments after lesions in immature and adult rats. Proc Natl Acad Sci U S A. 1973;70:3473–3477. doi: 10.1073/pnas.70.12.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Raphelson AC. Spontaneous alternation and septal lesions. J Comp Physiol Psychol. 1966;62:320–322. doi: 10.1037/h0023657. [DOI] [PubMed] [Google Scholar]
- Drew PD, Lonergan M, Goldstein ME, Lampson LA, Ozato K, McFarlin DE. Regulation of MHC class I and beta 2-microglobulin gene expression in human neuronal cells. Factor binding to conserved cis-acting regulatory sequences correlates with expression of the genes. J Immunol. 1993;150:3300–3310. [PubMed] [Google Scholar]
- Drojdahl N, Hegelund IV, Poulsen FR, Wree A, Finsen B. Perforant path lesioning induces sprouting of CA3-associated fibre systems in mouse hippocampal formation. Exp Brain Res. 2002;144:79–87. doi: 10.1007/s00221-002-1025-9. [DOI] [PubMed] [Google Scholar]
- Escande-Beillard N, Washburn L, Zekzer D, Wu ZP, Eitan S, Ivkovic S, Lu Y, Dang H, Middleton B, Bilousova TV, Yoshimura Y, Evans CJ, Joyce S, Tian J, Kaufman DL. Neurons preferentially respond to self-MHC class I allele products regardless of peptide presented. J Immunol. 2010;184:816–823. doi: 10.4049/jimmunol.0902159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Gage FH. Mechanisms of sprouting in the adult central nervous system: cellular responses in areas of terminal degeneration and reinnervation in the rat hippocampus. Neuroscience. 1994;58:705–725. doi: 10.1016/0306-4522(94)90449-9. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation. 1998;5:143–159. doi: 10.1159/000026331. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Van de Water J. The immune system's role in the biology of autism. Curr Opin Neurol. 2010a doi: 10.1097/WCO.0b013e3283373514. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Van de Water J. The immune system's role in the biology of autism. Curr Opin Neurol. 2010b;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemels MT, Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Suidan GL, McDole J, Pirko I. The CD8 T cell in multiple sclerosis: suppressor cell or mediator of neuropathology? Int Rev Neurobiol. 2007;79:73–97. doi: 10.1016/S0074-7742(07)79004-9. [DOI] [PubMed] [Google Scholar]
- Joly E, Mucke L, Oldstone MB. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A "Direct-Coloring" Thiocholine Method for Cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kimura T, Griffin DE. The role of CD8(+) T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J Virol. 2000;74:6117–6125. doi: 10.1128/jvi.74.13.6117-6125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson LA. Interpreting MHC class I expression and class I/class II reciprocity in the CNS: reconciling divergent findings. Microsc Res Tech. 1995;32:267–285. doi: 10.1002/jemt.1070320402. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lindahl KF, Byers DE, Dabhi VM, Hovik R, Jones EP, Smith GP, Wang CR, Xiao H, Yoshino M. H2-M3, a full-service class Ib histocompatibility antigen. Annu Rev Immunol. 1997;15:851–879. doi: 10.1146/annurev.immunol.15.1.851. [DOI] [PubMed] [Google Scholar]
- Lynch G, Deadwyler S, Cotman G. Postlesion axonal growth produces permanent functional connections. Science. 1973;180:1364–1366. doi: 10.1126/science.180.4093.1364. [DOI] [PubMed] [Google Scholar]
- Masliah E, Fagan AM, Terry RD, DeTeresa R, Mallory M, Gage FH. Reactive synaptogenesis assessed by synaptophysin immunoreactivity is associated with GAP-43 in the dentate gyrus of the adult rat. Exp Neurol. 1991;113:131–142. doi: 10.1016/0014-4886(91)90169-d. [DOI] [PubMed] [Google Scholar]
- McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci U S A. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13:208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Li H, Mariuzza RA, Margulies DH. MHC class I molecules, structure and function. Rev Immunogenet. 1999;1:32–46. [PubMed] [Google Scholar]
- Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Thams S, Lidman O, Piehl F, Hokfelt T, Karre K, Linda H, Cullheim S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc Natl Acad Sci U S A. 2004;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- Pham TA, Rubenstein JL, Silva AJ, Storm DR, Stryker MP. The CRE/CREB pathway is transiently expressed in thalamic circuit development and contributes to refinement of retinogeniculate axons. Neuron. 2001;31:409–420. doi: 10.1016/s0896-6273(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MB. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Cotman CW. Recovery of spontaneous alternation following lesions of the entorhinal cortex in adult rats: possible correlation to axon sprouting. Behav Biol. 1977;21:286–293. doi: 10.1016/s0091-6773(77)90374-1. [DOI] [PubMed] [Google Scholar]
- Soloski MJ, DeCloux A, Aldrich CJ, Forman J. Structural and functional characteristics of the class IB molecule, Qa-1. Immunol Rev. 1995;147:67–89. doi: 10.1111/j.1600-065x.1995.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Reinnervation of dentate gyrus by homologous afferents following entorhinal cortical lesions in adult rats. Science. 1976;194:426–428. doi: 10.1126/science.982024. [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Thams S, Brodin P, Plantman S, Saxelin R, Karre K, Cullheim S. Classical major histocompatibility complex class I molecules in motoneurons: new actors at the neuromuscular junction. J Neurosci. 2009;29:13503–13515. doi: 10.1523/JNEUROSCI.0981-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lookeren Campagne M, Oestreicher AB, Van Bergen en Henegouwen PM, Gispen WH. Ultrastructural double localization of B-50/GAP43 and synaptophysin (p38) in the neonatal and adult rat hippocampus. J Neurocytol. 1990;19:948–961. doi: 10.1007/BF01186822. [DOI] [PubMed] [Google Scholar]
- Wong GH, Bartlett PF, Clark-Lewis I, Battye F, Schrader JW. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Zanon RG, Oliveira AL. MHC I upregulation influences astroglial reaction and synaptic plasticity in the spinal cord after sciatic nerve transection. Exp Neurol. 2006;200:521–531. doi: 10.1016/j.expneurol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Zohar O, Reiter Y, Bennink JR, Lev A, Cavallaro S, Paratore S, Pick CG, Brooker G, Yewdell JW. Cutting edge: MHC class I-Ly49 interaction regulates neuronal function. J Immunol. 2008;180:6447–6451. doi: 10.4049/jimmunol.180.10.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative images of ipsilateral (A, C) and contralateral (B, D) projections in wildtype and β2M−/− mice. Scale bar = 0.067 mm. Quantification of ipsilateral (E), and contralateral projections (F), as well as total dLGN (G) areas in wildtype (open bars) and β2M−/− (black bars) mice. (H) Ipsilateral/dLGN area ratio. Data shown are mean+/−SEM. N=13 wildtype, 3 β2M−/− mice. * p<0.05