Summary
Studies of mice deficient in classical major histocompatability complex class I (MHCI) revealed that MHCI plays an important role in neurodevelopment in the central nervous system. We previously studied the effects of recombinant MHCI molecules on wildtype retina explants and observed that MHCI can inhibit retina neurite outgrowth, with self-MHCI molecules having greater inhibitory effect than non-self MHCI molecules. Here, we examined classical MHCI’s effects on axon outgrowth from neurons of the peripheral nervous system (PNS). We used the embryonic dorsal root ganglia (DRG) explant model since their neurons express MHCI and because DRG explants have been widely used to assess the effects of molecules on axonal outgrowth from PNS neurons. We observed that picomolar levels of a recombinant self-MHCI molecule, but not non-self MHCI molecules, inhibited axon outgrowth from DRG explants. This differential sensitivity to self- versus non-self MHCI suggests that early in development, self-MHCI may “educate” PNS neurons to express appropriate MHCI receptors, as occurs during natural killer cell development. Furthermore, we observed that a MHCI tetramer stained embryonic DRG neurons, indicating the expression of classical MHCI receptors. These results suggest that MHCI and MHCI receptors play roles during early stages of PNS development and may provide new targets of therapeutic strategies to promote neuronal outgrowth after PNS injury.
Keywords: MHC, MHC receptor, neuron
1. Introduction
Studies of mice that were functionally classical MHCI (Ia)-deficient revealed that MHCI plays an important role in eliminating inappropriate synaptic connections [1,2]. This finding immediately raised questions as to whether neuronal recognition of MHCI is restricted to self-MHCI allele products and has specificity for the presented peptide. In the adaptive immune system, CD8+ T cells express TCRs that were generated by gene rearrangement and which are MHCI restricted and peptide specific [3-5]. In contrast, the MHCI receptors of NK cells of the innate immune system (e.g., the Ly49 in mice and KIR gene families in humans) are germline-encoded and can recognize one to several MHCI allele products often without specificity for the presented peptide [6,7]. Using a wildtype retina explant system, we showed that picomolar levels of recombinant soluble self-MHCI inhibited neurite outgrowth from retina ganglion cells (RGCs) [8]. Self-MHCI allele products had greater inhibitory neuroactivity than non-self MHCI molecules, regardless of the nature of the peptide presented--a pattern akin to MHCI recognition by some MHCI receptors of the innate immune system receptors [8]. Interestingly, retinas from MHCI-deficient mice were not affected by recombinant MHCI, suggesting that endogenous MHCI expression was necessary to “educate” neurons to express appropriate MHCI receptors, as occurs during NK cell development.
The neurobiological activity of recombinant MHCI on neurons of the PNS has not been explored. Since our previous study of mouse retina neurons suggested that MHCI expression was required for a neuronal “educational” process, we wanted to study an embryonic PNS tissue in which neurons expressed MHCI. There is evidence that embryonic DRG neurons of the PNS express MHCI [9,10] and that they upreglate their MHCI expression in response to inflammatory signals and injury [9,11-18]. Moreover, an embryonic mouse DRG explant model is widely used to study peripheral axonal outgrowth in vitro and to examine the effects of various factors on axon extension (reviewed in [19]). Here, we report for the first time, the effects of self- and nonself-MHCI molecules on axon outgrowth from mouse DRG cells and the staining of classical MHCI-receptors within this PNS tissue.
2. Materials and Methods
2.1 Mice
C57Bl/6 mice (H-2b, K b D b) mice were purchased from Jackson Laboratory (Bar Harbor, ME). After mating, the mice were checked daily for a vaginal plug. The day of plug formation was designated day 0 of pregnancy. All animal studies were approved by the UCLA Animal Research Committee.
2.2 MHCI monomers and tetramers
The recombinant Db MHCI monomer and tetramer presenting the ubiquitous mouse minor histocompatibility antigen H13a (SSVVGVWYL) [20], the non-self Dk monomer and tetramer presenting polyoma protein MT 389-397 (RRLGRTLLL) [21], as well as a human MHCI tetramer loaded with an EBV peptide were synthesized by the NIH Tetramer Core facility. These recombinant MHCI stocks were used in a separate concurrent study and shown to be biologically active and free of neuroinhibitory toxins [8].
2.3 DRG explant cultures
Lumbar level DRGs were dissected from E14 C57Bl/6 mice and placed into growth factor reduced (GFR) matrigel matrix (BD Biosciences) with 2.5S NGF (100 ng/ml, Invitrogen), in the presence or absence of the indicated MHCI monomer (500 pM, a concentration previously shown to inhibit neurite outgrowth from RGCs) [8] in an 8-chamber slide on ice (Lab-Tek Chamber slides). The slides were incubated at 37°C for 30-60 minutes to solidify the gel. Neurobasal medium with L-glutamine, antibiotics, B27 supplement (Gibco) 2.5S NGF with, or without, the indicated MHCI monomer (500 pM), was added to each chamber. The cultures were incubated for ≈48 hrs at 37°C in 5% CO2, after which the explants were fixed with 4% paraformaldehyde (PFA) and stored at 4°C. Images of the explants were captured using an inverted microscope (Zeiss Axiovert 200) with a 5x Plan-Neufluar objective connected to a CCD camera (International Power Sources, Inc., Holliston, MA). The images were analysed for the area covered by neurite projections using MetaMorph Software (version 6.2; Universal Imaging Corp) in a blinded fashion. The differences between groups was statistically analyzed using one-way ANOVA and Tukey-Fisher post-hoc testing.
2.4 MHCI immunocytochemistry
Embryonic day 14 DRGs were removed, dissociated and plated on polyornithine and laminin coated cover slips. They were grown 24 hrs in neurobasal medium with B27 and 2.5S NGF at 37°C in 5% CO2. Cultures were then fixed in 4% PFA, permeabilized with triton X-100 (0.2%) and co-stained with anti-MHCI monoclonal antibody OX-18 (Abcam Inc.) and the neuronal marker ßIII-tubulin and secondary antibodies alexa 594 conjugated anti-mouse IgG and alexa 488-conjugated anti-rabbit IgG, respectively. Control slides were incubated with anti-ßIII tubulin and secondary antibodies. Cells were mounted on DAPI-containing Vectashield medium (Vector labs).
2.5 MHCI tetramer staining of DRG cells
Fresh frozen DRG cryosections (15 μm) from E15 C57Bl/6 mice were incubated with a mouse allophycocyanin (APC)-labeled Db/H13a tetramer (8 μg/ml), nonself-Dk tetramer, human MHCI tetramer or medium only, overnight at 4°C. After washing, the sections were fixed in 4% PFA for 15 min, washed, incubated with goat anti-APC antibody (1:1000, Biomeda) and rabbit anti-βIII tubulin (1:500, Covance) at room temperature for 3 hrs. The section were then washed and incubated with Alexa 594 chicken anti-goat IgG and Alexa 488 chicken anti-rabbit IgG (both at 1:500, Molecular Probes) at room temperature for 3 hrs. After washing the sections were covered with mounting media containing DAPI (Vector, Burlingame, CA). Staining of experimental and control MHCI tetramers was imaged simultaneously using a Zeiss Axiovert 200 inverted microscope with a 20x/0.5 air or 40x/1.3 oil Plan-Neofluar objectives.
3. Results
3.1 MHCI inhibits the neurite outgrowth of embryonic DRG neurons
At embryonic day 14 (E14), mouse DRG neurons are in the process of projecting extensive axonal branches for target innervation. DRG explants from this stage provide an in vitro model to assess the effect of factors on PNS axonal outgrowth [19]. Previous studies have shown that human fetal DRGs express MHCI heavy chain and that embryonic rat DRGs express MHCI on their cell surface [9,10]. We show that cultured dissociated E14 mouse DRG neurons express MHCI on their cell body and neurites (Fig. 1).
Fig.1. MHCI expression on cultured E14 mouse DRG neurons.
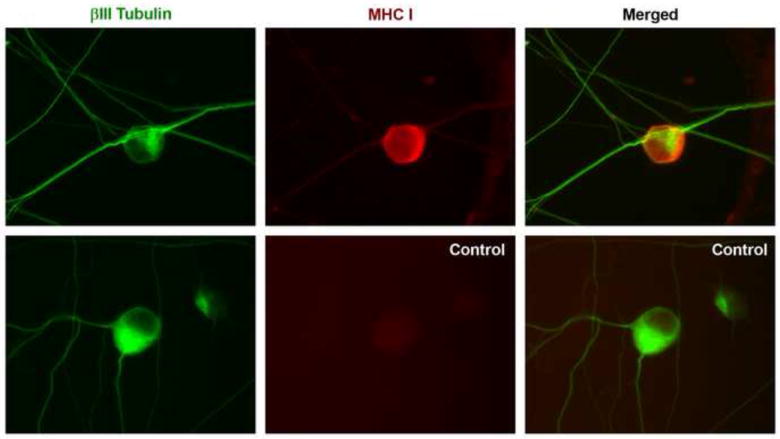
(A) Representative images of E14 DRG cultures stained with anti-ßIII tubulin (green), anti-MHCI (OX-18, red) and appropriate secondary antibodies (top row), or control cultures stained with anti-ßIII tubulin, and all secondary antibodies (bottom row).
We cultured E14 C57Bl/6 mouse lumbar DRG explants with media alone, or with a recombinant self-MHCI monomer (Db/H13a), a non-self mouse MHCI monomer (Dk/polyoma), or a human MHCI monomer at 500 pM, a concentration previously shown to inhibit neurite outgrowth from RGCs [8]. After 48 hours of incubation, the cultures were fixed and DRG neurite outgrowth was measured in a blinded fashion.
We observed that the areas of neurite outgrowth from DRG explants incubated with the non-self-Dk or a human MHCI monomer were similar to that in cultures incubated with media alone. Representative images of neurite outgrowth from DRG explants are shown in Fig. 2, and the average area of neurite outgrowth for each group are shown in Fig. 3. In contrast, the area of neurite outgrowth in DRG explants cultured with the Db monomer was on average 20% smaller than that in control cultures (p=0.004). Explants cultured with Db had a similar number of neurites as those cultured in media alone, or other MHCI monomers, consistent with our previous observations of the effects of MHCI monomers on retina explants [8]. Apparently, self-MHCI molecules can have an inhibitory effect on DRG neurite outgrowth, and E14 DRG cells express MHCI receptors that can distinguish self- from non-self MHCI molecules. These findings extend our previous observations using E14 retina explant cultures [8].
Fig. 2. Representatives composite micrographs of neurite outgrowth from DRG explants cultured in the presence or absence of different MHCI monomers.
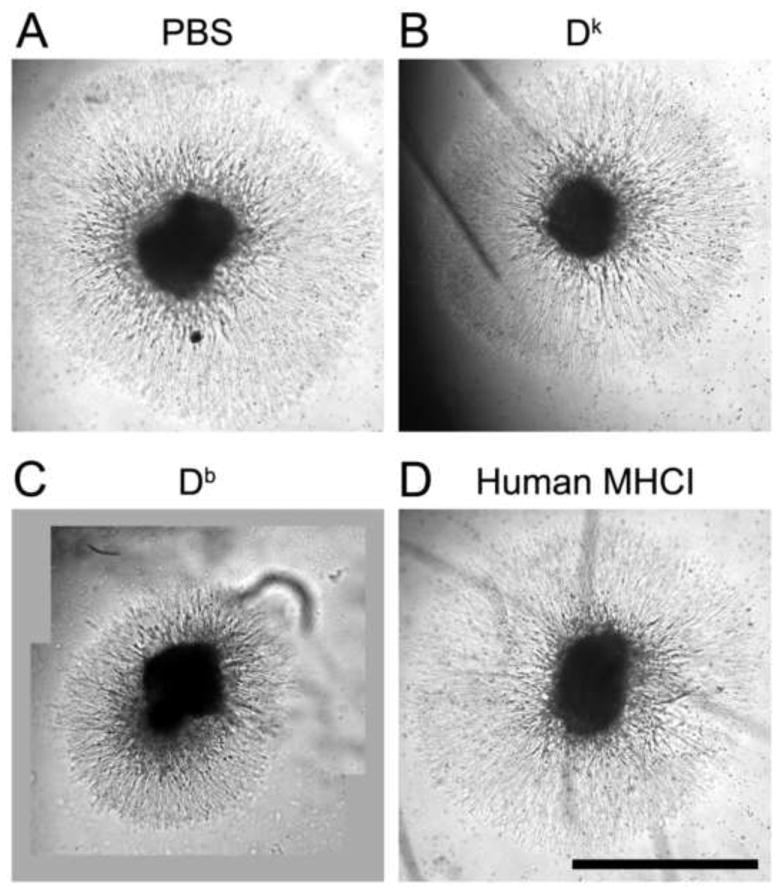
DRG C57Bl/6 explants were cultured in medium alone (A), or with Dk (B), Db (C), or human MHCI monomer, as described in Materials and Methods. Scale Bar = 1.0 mm.
Fig. 3. Inhibition of DRG neurite outgrowth by self-, but not non-self, MHCI.

(A) Group data of total area under neurites in cultures incubated with media alone, or with a Dk, Db, or human MHCI monomer. Axonal outgrowth is expressed as the mean percentage of outgrowth relative that of control cultures grown in media (alone) ± SEM. For each group 5-8 explants were examined in a blinded fashion in at least 3 independent experiments. ** p=0.004
To verify that the neuroinhibitory factor in the Db/H13a stock was in fact the Db/H13a monomer, we subtracted the Db/H13a stock with a conformation-dependent anti-Db monoclonal antibody (mAb), or with an isotype matched anti-Dk mAb, using protein A sepharose beads. Db/H13a stock that was preabsorbed with anti-Dk mAb fully retained its neuroinhibitory activity--the extent of neurite outgrowth from DRG cultured in its presence was similar to that of DRGs cultured with unsubtracted Db/H13a (Fig. 4). In contrast, neurite outgrowth from explants grown in the presence of Db/H13a stock that was preabsorbed with anti-Db mAb was similar to that of explants grown in media alone (Fig. 4). Thus, the neuroactive factor in the Db/H13a preparation is conformationally correct Db/H13a.
Fig. 4. The neuroinhibitory activity of Db monomer is removed by anti-Db, but not anti-Dk mAb.
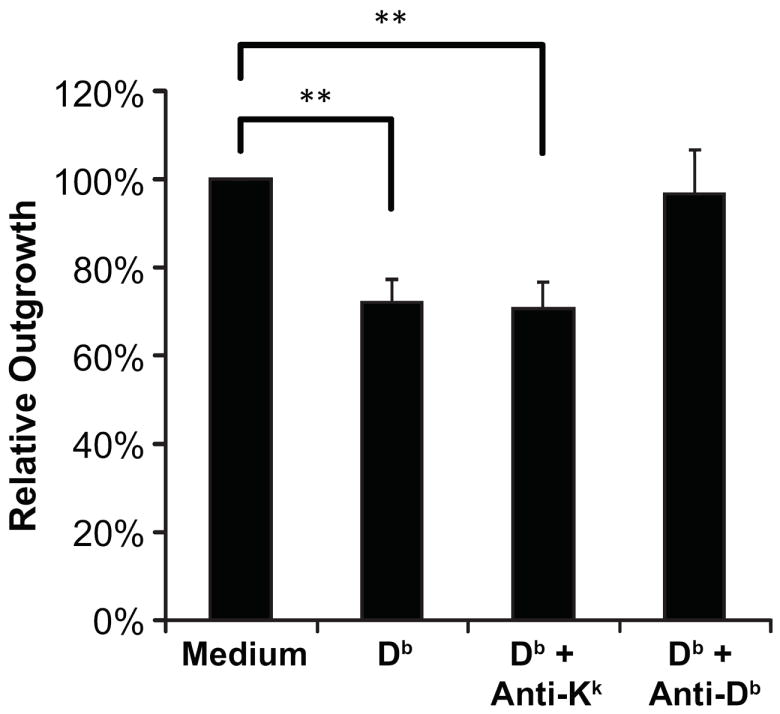
Quantification of DRG neurite outgrowth in cultures incubated with medium alone, with Db monomer, or Db monomer that had been subtracted with either an isotype matched anti-Dk or an anti-Db mAb. Axonal outgrowth is expressed as the mean percentage of outgrowth relative that of control cultures grown in media (alone) ± SEM. N=5-8 DRG cultures for each group in at least three independent experiments. ** p < 0.01 by Student’s t test.
3.2 MHCI tetramer staining of classical MHCI receptors on DRG cells
Given that recombinant MHCI inhibits E14 DRG neurite outgrowth, it is expected that DRG cells express classical MHCI receptors. The molecular identity of DRG cell MHCI receptor(s) is unknown. MHCI tetramers have been widely used to stain immune cells expressing cognate MHCI receptors [22,23]. Our previous study demonstrated that MHCI tetramers can be used to detect the expression of classical MHCI (Ia) receptors in mouse retinas [8]. Classical MHCI tetramers do not bind to nonclassical MHCI receptors.
To detect MHCI receptors on DRG cells, we incubated fresh frozen embryonic C57Bl/6 mouse lumbar DRG tissue sections in PBS alone, or PBS containing a non-self mouse MHCI Dk tetramer, a human MHCI tetramer, or the self-Db/H13a tetramer followed by detection antibodies (as described in Materials and Methods). The DRG sections incubated in PBS alone, or probed with the Dk or human MHCI tetramer, did not display any cell-specific labeling (Fig. 5 A-F). In contrast, the vast majority of DRG cells in the central portion and dorsomedial parts of the DRG were clearly labeled by the Db tetramer (Fig. 5 G, H). There were also many weakly Db-tetramer stained cells in the peripheral DRG regions. Staining was observed on both small-diameter and large-diameter neurons (Fig. 5 I, J). The Db tetramer staining was co-localized with that of anti-βIII tubulin, a neuronal marker. (Fig. 5 G-J). Additionally, tetramer staining was observed along axons that also stained with anti-βIII tubulin (not shown). Thus, classical MHCI receptors are expressed on DRG neurons at early stages of development.
Fig. 5. MHCI tetramer staining of classical MHCI receptors in DRG cells.
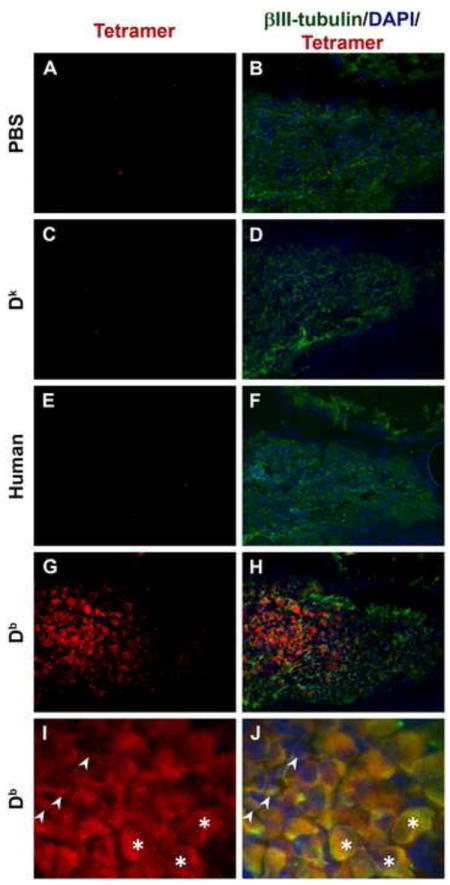
Data show representative micrographs of fresh frozen DRG sections stained with different tetramers (red), ßIII-tubulin (green) and DAPI (blue). Panels show tissue sections incubated without tetramer (A, B), with Dk tetramer (C, D), human MHCI tetramer (E, F), and Db tetramer (G, H). Panels A-H were taken at 20x, and panels I and J show Db monomer staining at 40x magnification. In panels I and J, arrows point to small neurons and asterisks are placed on large neuronal cell bodies.
4. Discussion
Shatz and colleagues demonstrated that classical MHCI is expressed in neurons throughout the CNS and that mice that are MHCI-deficient have abnormalities in synaptic connections and electrophysiology [1,16]. Moreover, mice lacking PirB, a classical MHCI (Ia) receptor of the innate immune system, have abnormalities in their visual cortex [24]. Thus, besides its central role in immune system function, classical MHCI also plays an important role in neurodevelopment.
Our studies have focused on examining the neurobiological activity of MHCI on wildtype neurons. In previous studies we showed that neurite outgrowth from cultured RGCs was especially sensitive to self-MHCI monomers compared to non-self MHCI molecules, regardless of the nature of the peptide presented [8]. This pattern is akin to MHCI recognition by many MHCI receptors of the innate immune system [6,7]. In the current studies, we examined whether PNS neurons were also sensitive to MHCI-mediated inhibition of neurite outgrowth. We observed that picomolar levels of a self-MHCI molecule inhibited DRG neurite outgrowth. However, the nonself-MHCI molecules had little no effect on DRG neurite outgrowth. There is evidence suggesting that embryonic DRGs express MHCI [9,10]. The differential sensitivity of DRG neuronal outgrowth to self- vs. nonself-MHCI molecules suggests that MHCI acted early in development to direct DRG neurons to express the appropriate self-MHCI receptors. The coordination of neuronal MHCI receptor expression with the MHCI haplotype of the host may be a necessity because most classical MHCI receptors have specificity for particular MHCI allele products and MHCI haplotypes are highly variable among outbred populations. Notably, in the innate immune system, NK cells undergo an “educational” process so that they express appropriate Ly49 MHCI receptors (out of a family of possible receptors) for the particular MHCI haplotype of the host [6,7,25,26], as well as a “licensing” process to ensure that mature NK cells are self-tolerant [26,27]. It is possible that a similar educational process occurs on neurons, so that they express appropriate MHCI receptors for the animal’s MHCI haplotype.
Although the effect of exogenous MHCI on neurite outgrowth was rather modest, it should be born in mind that retention of just a small percent of spinal axon connections after spinal cord injury can have a large effect on an animal’s ability to recover locomotor function [28]. Additionally, in the CNS, subtle abnormalities in neuronal connections are thought to underlie neurodevelopmental disorders as autism and schizophrenia [29,30]. Thus, modest changes in neuronal outgrowth may have important neurobiological consequences in vivo.
For embryonic DRG neurons to be “educated” by self-MHCI and for them to be sensitive to self-MHCI, they must express classical self-MHCI receptors. MHCI tetramers have been widely used to stain classical MHCI receptors on cells of the innate and adaptive immune system [22,23,31]. Nonclassical MHCI (Ib) receptors do not bind classical MHCI and are not known to be involved in neurodevelopment. We previously used MHCI tetramer staining to identify the cell types in the developing retina that express classical MHCI receptors [8]. Here, we observed that a self-, but not non-self, MHCI tetramers stained cells in E15 DRG sections and these cells also stained with the neuronal marker ßIII tubulin. Neurons in the central DRG region were strongly stained by the Db tetramer while neurons on the DRG periphery were weakly stained--which were not observed in sections stained with control MHCI tetramers. The physiological basis for the differences in DRG region tetramer staining intensity is unclear. We observed tetramer staining of both large and small diameter neurons which represent different sensory neuron subtypes [32]. The expression of MHCI receptors early in DRG development, along with the observation that DRG neuronal outgrowth is modulated by self-MHCI, is consistent with the notion that some DRG neurons have been “educated” and capable of interacting with MHCI on target neurons.
The identity of the DRG neuronal MHCI receptor is unknown. PirB is a classical MHCI receptor in the innate immune system which is involved in synapse formation in the visual cortex [33]. The expression PirB by DRG cells and the effect of PirB deficiency on PNS development have not been reported. While early evidence implicated CD3ζ involvement in the neuronal MHCI receptor [1], more recent studies argue against TCR involvement [33], and our own studies have found that an activating anti-CD3ε mAb has no effect on retina neuronal outgrowth in vitro (unpublished observation). Ly49 is expressed on cortical mouse neurons and anti-Ly49 antibodies modulate neurite outgrowth [34], however, humans do not have Ly49 genes. Additionally, KIR-like genes are expressed in some mouse brain regions [35]. Accordingly, it remains an open question whether the DRG neuronal MHCI receptor is one of these known MHCI receptors, one or more of the many MHCI receptor-related genes whose function are not yet understood [36], or a completely novel gene(s).
The expression of both MHCI receptors and MHCI in embryonic DRGs suggests that MHCI interaction with it’s receptor may play a role during the early stages of DRG development. These early interactions may educate developing DRG neurons to express appropriate MHCI receptors that can subsequently interact with the MHCI on their target cells in the spinal cord and muscle. Spinal cord motor neurons express MHCI [37], and MHCI is expressed by embryonic tissues throughout the periphery [38]. The MHCI and MHCI receptors expressed by developing DRG cells may also be involved in intra-DRG remodelling, and their interactions may occur in both in cis (on the same cell) and in trans (between different DRG cells) as has been reported for MHCI interactions with Ly49 [34,39].
Embryonic DRG neurons express MHCI and upreglate MHCI expression in response to inflammatory signals and injury [9,11-18]. Our in vitro studies of recombiant MHCI’s effects on RGCs and DRGs, as well a previous observations of exuberant synaptic connections in MGHCI-deficent mice [1] suggest that elevated neuronal MHCI levels may be deleterious for neurorepair. However, after sciatic nerve transection, lesioned motor neurons of MHCI-deficient mice had more extensive synaptic detachments than wildtype mice and also had hampered axon regeneration [17,40]. Additionally, we recently observed that transgenic mice with enhanced expression of neuronal MHCI had better recovery of locomotor function after a spinal cord injury [41]. The mechanisms underlying these contrasting findings are currently unclear.
In conclusion, our data indicate similarities between the neurobiological activity of MHCI in the CNS and PNS. Our findings also suggest the presence of a self-MHCI educational system for neuronal MHCI receptors in the CNS and PNS. MHCI and MHCI receptors may provide new targets that can be modulated to help promote healthy neuronal function in the CNS and PNS in neurological disorders or to promote neurorepair after injury.
Acknowledgments
This work was supported by NIH grants R21NS053847 and R21NS047383 to D.L.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci U S A. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viret C, Janeway CA., Jr MHC and T cell development. Rev Immunogenet. 1999;1:91–104. [PubMed] [Google Scholar]
- 4.Berg LJ, Kang J. Molecular determinants of TCR expression and selection. Curr Opin Immunol. 2001;13:232–241. doi: 10.1016/s0952-7915(00)00209-0. [DOI] [PubMed] [Google Scholar]
- 5.Nemazee D. Receptor selection in B and T lymphocytes. Annu Rev Immunol. 2000;18:19–51. doi: 10.1146/annurev.immunol.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 8.Escande-Beillard N, Washburn L, Zekzer D, Wu ZP, Eitan S, Ivkovic S, Lu Y, Dang H, Middleton B, Bilousova TV, Yoshimura Y, Evans CJ, Joyce S, Tian J, Kaufman DL. Neurons preferentially respond to self-MHC class I allele products regardless of peptide presented. J Immunol. 2010;184:816–823. doi: 10.4049/jimmunol.0902159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollas BB, Hartle HT, Wigdahl B. Effect of gamma-interferon on the expression of major histocompatibility complex class I and II gene products in neural cells isolated from the developing human fetal peripheral nervous system. Fetal diagnosis and therapy. 1992;7:62–74. doi: 10.1159/000263652. [DOI] [PubMed] [Google Scholar]
- 10.Neumann H, Schmidt H, Wilharm E, Behrens L, Wekerle H. Interferon gamma gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med. 1997;186:2023–2031. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong GH, Bartlett PF, Clark-Lewis I, Battye F, Schrader JW. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- 12.Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 13.Fujimaki H, Hikawa N, Nagoya M, Nagata T, Minami M. IFN-gamma induces expression of MHC class I molecules in adult mouse dorsal root ganglion neurones. Neuroreport. 1996;7:2951–2955. doi: 10.1097/00001756-199611250-00030. [DOI] [PubMed] [Google Scholar]
- 14.Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampson LA. Interpreting MHC class I expression and class I/class II reciprocity in the CNS: reconciling divergent findings. Microsc Res Tech. 1995;32:267–285. doi: 10.1002/jemt.1070320402. [DOI] [PubMed] [Google Scholar]
- 16.Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 17.Zanon RG, Oliveira AL. MHC I upregulation influences astroglial reaction and synaptic plasticity in the spinal cord after sciatic nerve transection. Exp Neurol. 2006;200:521–531. doi: 10.1016/j.expneurol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Payes AC, Zanon RG, Pierucci A, Oliveira AL. MHC class I upregulation is not sufficient to rescue neonatal alpha motoneurons after peripheral axotomy. Brain Res. 2008;1238:23–30. doi: 10.1016/j.brainres.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Tonge D, Edstrom A, Ekstrom P. Use of explant cultures of peripheral nerves of adult vertebrates to study axonal regeneration in vitro. Prog Neurobiol. 1998;54:459–480. doi: 10.1016/s0301-0082(97)00072-5. [DOI] [PubMed] [Google Scholar]
- 20.Mendoza LM, Paz P, Zuberi A, Christianson G, Roopenian D, Shastri N. Minors held by majors: the H13 minor histocompatibility locus defined as a peptide/MHC class I complex. Immunity. 1997;7:461–472. doi: 10.1016/s1074-7613(00)80368-4. [DOI] [PubMed] [Google Scholar]
- 21.Lukacher AE, Wilson CS. Resistance to polyoma virus-induced tumors correlates with CTL recognition of an immunodominant H-2Dk-restricted epitope in the middle T protein. J Immunol. 1998;160:1724–1734. [PubMed] [Google Scholar]
- 22.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 23.Hanke T, Takizawa H, McMahon CW, Busch DH, Pamer EG, Miller JD, Altman JD, Liu Y, Cado D, Lemonnier FA, Bjorkman PJ, Raulet DH. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11:67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- 24.Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 25.Veinotte LL, Wilhelm BT, Mager DL, Takei F. Acquisition of MHC-specific receptors on murine natural killer cells. Crit Rev Immunol. 2003;23:251–266. doi: 10.1615/critrevimmunol.v23.i4.10. [DOI] [PubMed] [Google Scholar]
- 26.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 28.Young W. Spinal cord regeneration. Science. 1996;273:451. doi: 10.1126/science.273.5274.451. [DOI] [PubMed] [Google Scholar]
- 29.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura A, Kobayashi E, Takai T. Exacerbated graft-versus-host disease in Pirb-/-mice. Nat Immunol. 2004;5:623–629. doi: 10.1038/ni1074. [DOI] [PubMed] [Google Scholar]
- 32.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 33.Syken J, Shatz CJ. Expression of T cell receptor beta locus in central nervous system neurons. Proc Natl Acad Sci U S A. 2003;100:13048–13053. doi: 10.1073/pnas.1735415100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zohar O, Reiter Y, Bennink JR, Lev A, Cavallaro S, Paratore S, Pick CG, Brooker G, Yewdell JW. Cutting edge: MHC class I-Ly49 interaction regulates neuronal function. J Immunol. 2008;180:6447–6451. doi: 10.4049/jimmunol.180.10.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryceson YT, Foster JA, Kuppusamy SP, Herkenham M, Long EO. Expression of a killer cell receptor-like gene in plastic regions of the central nervous system. J Neuroimmunol. 2005;161:177–182. doi: 10.1016/j.jneuroim.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Trowsdale J, Barten R, Haude A, Stewart CA, Beck S, Wilson MJ. The genomic context of natural killer receptor extended gene families. Immunol Rev. 2001;181:20–38. doi: 10.1034/j.1600-065x.2001.1810102.x. [DOI] [PubMed] [Google Scholar]
- 37.Linda H, Hammarberg H, Cullheim S, Levinovitz A, Khademi M, Olsson T. Expression of MHC class I and beta2-microglobulin in rat spinal motoneurons: regulatory influences by IFN-gamma and axotomy. Exp Neurol. 1998;150:282–295. doi: 10.1006/exnr.1997.6768. [DOI] [PubMed] [Google Scholar]
- 38.Arcellana-Panlilio MY, Schultz GA. Temporal and spatial expression of major histocompatibility complex class I H-2K in the early mouse embryo. Biol Reprod. 1994;51:169–183. doi: 10.1095/biolreprod51.2.169. [DOI] [PubMed] [Google Scholar]
- 39.Back J, Malchiodi EL, Cho S, Scarpellino L, Schneider P, Kerzic MC, Mariuzza RA, Held W. Distinct conformations of Ly49 natural killer cell receptors mediate MHC class I recognition in trans and cis. Immunity. 2009;31:598–608. doi: 10.1016/j.immuni.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thams S, Oliveira A, Cullheim S. MHC class I expression and synaptic plasticity after nerve lesion. Brain Res Rev. 2008;57:265–269. doi: 10.1016/j.brainresrev.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Wu Z-P, Washburn L, Bilousova TV, Boudzinskaia M, Escande-Beillard N, Querubin J, Dang H, Xie C-W, Tian J, Kaufman DL. Enhanced neuronal expression of major histocompatibility complex class I leads to aberrations in neurodevelopment and neurorepair. J Neuroimmunol. doi: 10.1016/j.jneuroim.2010.09.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


