Abstract
Aim
Amplitude Spectrum Area (AMSA) and Median Slope (MS) are ventricular fibrillation (VF) waveform measures that predict defibrillation shock success. Cardiopulmonary resuscitation (CPR) obscures electrocardiograms and must be paused for analysis. Studies suggest waveform measures better predict subsequent shock success when combined with prior shock success. We determined whether this relationship applies during CPR.
Methods
AMSA and MS were calculated from 5-second pre-shock segments with and without CPR, and compared to logistic models combining each measure with prior return of organized rhythm (ROR).
Results
VF segments from 692 patients were analyzed during CPR before 1372 shocks and without CPR before 1283 shocks. Combining waveform measures with prior ROR increased areas under receiver operating characteristic curves for AMSA/MS with CPR (0.66/0.68 to 0.73/0.74, p<0.001) and without CPR (0.71/0.72 to 0.76/0.76, p<0.001).
Conclusions
Prior ROR improves prediction of shock success during CPR, and may enable waveform measure calculation without chest compression pauses.
Keywords: Ventricular fibrillation, amplitude spectrum area, resuscitation, defibrillation, cardiopulmonary resuscitation
Introduction
Sudden cardiac arrest caused by ventricular fibrillation (VF) is a major cause of death in the US.1 Defibrillation and cardiopulmonary resuscitation (CPR) are the definitive treatments for VF. However some shocks may fail to terminate VF, result in an organized rhythm, or produce return of spontaneous circulation.2,3 Moreover, shocks require cessation of CPR for rhythm analysis and shock delivery, a circumstance adversely associated with the likelihood of resuscitation.4–6 Ideally, shocks could be timed to achieve optimal likelihood of resuscitation while limiting interruption in CPR.
Quantitative measures of the VF electrocardiogram (ECG) are dynamic over the course of resuscitation7 and predict shock success,8 and thus have the potential to serve as real-time prognostic markers to guide therapy and shock timing. For instance, if a shock is likely to be unsuccessful due to prolonged VF, temporarily delaying shock and continuing CPR may improve outcome.9,10 However, chest compressions obscure the ECG, requiring a pause in CPR for accurate waveform measure calculation.11–15 A next-step strategy to predict shock outcome combines VF waveform measures with other features of the ECG to improve performance during CPR.
Research has suggested that prior rhythm and perfusion state are associated with subsequent likelihood of return of spontaneous circulation following shock,16 and that this information in combination with waveform measures may improve prediction of shock success.17 However, this relationship has not been tested during CPR, challenging real-world implementation of such a strategy. If a combination algorithm could more accurately predict shock success during CPR, then shock delivery might be timed based on a real-time prognostic likelihood calculated during continuous CPR.14,15
We sought to assess the predictive characteristics of two representative waveform measures, and to evaluate whether combining waveform measures with prior shock outcome improves prediction of shock success both with and without CPR.
Methods
Study design, population, and setting
The study was a retrospective cohort investigation of adult out-of-hospital cardiac arrest patients presenting with an initial rhythm of VF in King County, WA, from 2005–2014. Because the study in part evaluated the role of the ECG response to the prior shock, the primary study cohort was restricted to those who required at least two shocks. Cases were excluded if the defibrillator model did not record chest impedance, a public access defibrillator was used to deliver a shock prior to Emergency Medical Services (EMS) arrival, defibrillator data was missing or corrupted, or a 5-s VF ECG segment was not available before any of the first three subsequent shocks following initial shock. The study was approved by the Investigational Review Board at the University of Washington Human Subjects Division.
The EMS system in King County is comprised of two tiers. The first tier consists of firefighter emergency medical technicians equipped with automated external defibrillators. The second tier consists of paramedics trained in advanced cardiac life support, ECG rhythm identification, and manual defibrillation. Resuscitation by EMS agencies in King County follows American Heart Association guidelines.18
Data collection and data definitions
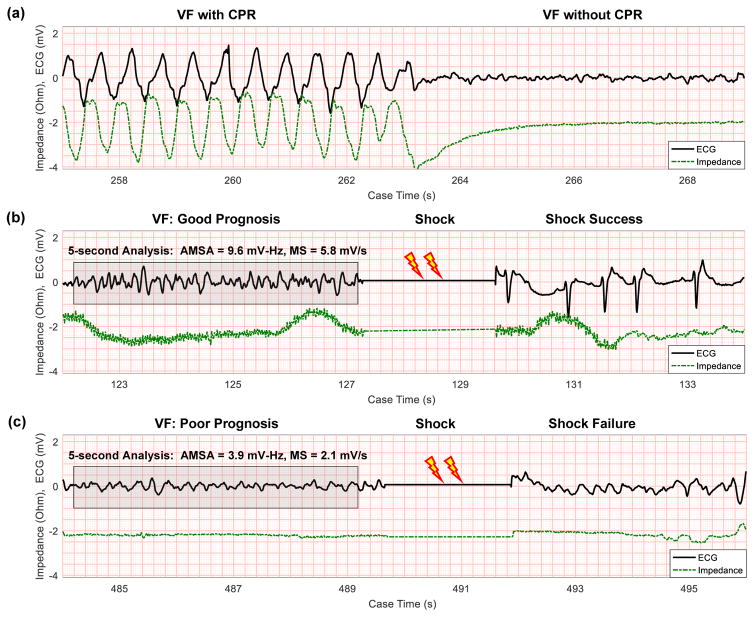
Cardiac arrest cases were collected using the Utstein template.19 ECGs from MRx defibrillators (Philips Healthcare, Bothell, WA) were recorded at a sample rate of 250 Hz, and ECGs from Lifepak 12 and Lifepak 15 defibrillators (Physio-Control, Redmond, WA) were recorded at 125 Hz and resampled to 250 Hz. For each patient, one 5-s VF ECG segment was collected with CPR and one segment was collected without CPR prior to the first three subsequent shocks following initial shock, when available. Presence of chest compressions was determined through manual review of the chest impedance signal (Figure 1). Presence of VF during CPR was verified during adjacent pauses in CPR and adjudicated by physician review if necessary.
Figure 1. ECG examples.
(a) VF ECG obscured during CPR is visible once compressions cease, with compressions confirmed by chest impedance. (b) AMSA and MS calculated from 5 seconds of CPR-free VF are high preceding a successful shock. Shock success is confirmed by return of organized rhythm in the ECG. (c) AMSA and MS calculated from 5 seconds of CPR-free VF are low preceding an unsuccessful shock. Impedance signals are shifted vertically for visibility. (AMSA = Amplitude Spectrum Area; CPR = Cardiopulmonary Resuscitation; ECG = Electrocardiogram; MS = Median Slope; VF = Ventricular Fibrillation.)
Quantitative waveform measures
We computed the Amplitude Spectrum Area (AMSA)20,21 and Median Slope (MS)16,22 as representative frequency-domain and time-domain quantitative waveform measures. Prior to waveform measure calculation, ECG segments were filtered with a 4th-order Butterworth filter from 4–30 Hz to reduce chest compression artifact and high-frequency noise. AMSA was calculated from 1–26 Hz23 as , where X0, …, Xm, …, XN/2 are the one-sided Discrete Fourier Transform magnitudes calculated from ECG voltages x0, …, xn, …, xN − 1 sampled at rate fs. The frequency values fm (in Hz) are defined in terms of the frequency index m and input length N, such that fm = mfs/N. Likewise, MS was calculated as MS = (median|xn+1 − xn| · fs) for n = 0, …, N − 2.
Shock success
Shock success was defined as return of organized rhythm (ROR) with at least two QRS complexes in a 5-s interval within two minutes following shock (Figure 1).24 Each shock was also annotated with a dichotomous variable, Prior ROR, indicating whether the prior shock was successful. To provide an estimate of inter-rater reliability, a second reviewer annotated ROR for a random subset of 20% of patients in the study group. Shocks with indeterminate ROR or Prior ROR were excluded.
Data analysis
We characterized cases according to inclusion in the primary study cohort using descriptive statistics. Patients in the study group were randomly divided into 30% training and 70% test groups. ECG segments were sorted based on training and test patient group and presence of CPR. Using the training segments, we developed logistic models to predict ROR: the first model type used a waveform measure alone (AMSA or MS) and the second used a waveform measure combined with Prior ROR. For instance, for AMSA combined with Prior ROR, the probability P of successful shock was calculated as P(ROR|AMSA, Prior ROR) = 1/(1+exp[−(β0 + β1 · AMSA + β2 · Prior ROR)]). The β parameters for both model types were trained with and without CPR separately. Waveform measure variables were log-transformed prior to use. Model performance was evaluated on test data.
Area under the receiver operating characteristic curve (AUC) was used to assess model performance for prediction of shock success. AUC values for receiver operating characteristic curves were compared using Delong’s method.25,26 Waveform measure medians were compared using a two-sided Wilcoxon rank-sum test. The Pearson phi coefficient was used to evaluate association of shock outcomes between adjacent shocks. Cohen’s kappa was used to estimate inter-rater reliability.
As a supplementary analysis to examine whether training dataset size or patient allocation to training or test groups affected results, AUCs were also estimated using 10-fold cross-validation across all data.27–29
MATLAB 2016b (The Mathworks, Natick, MA, USA) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical and signal analysis.
Results
Study group
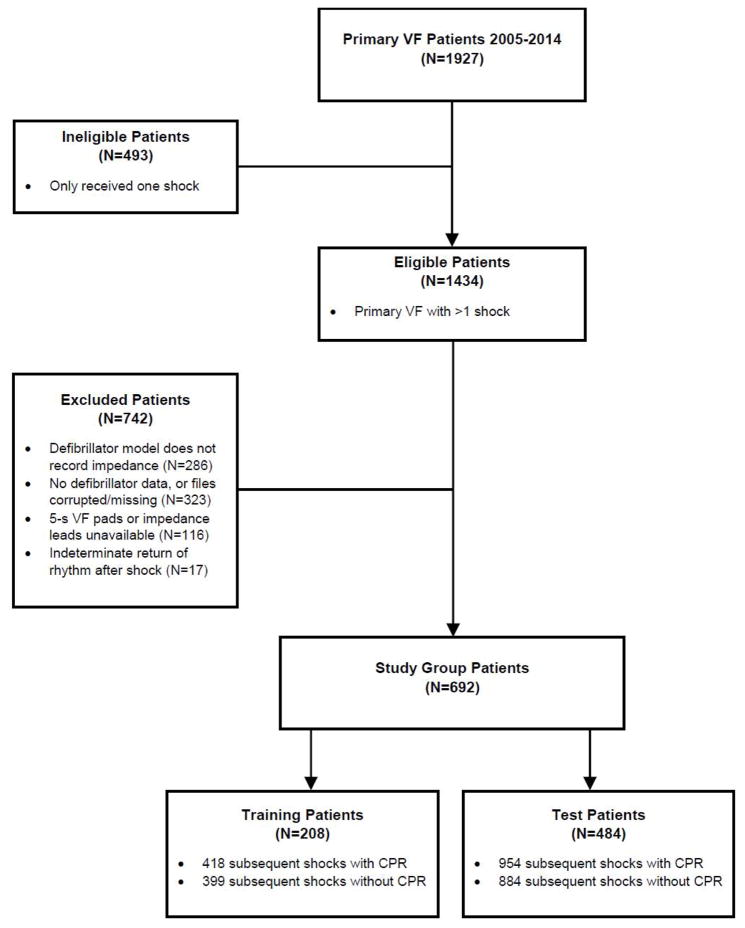
During the study period, there were 1434 primary VF patients who received more than one shock and were eligible for inclusion in the study (Figure 2). Of eligible patients, 742 (52%) were excluded, most commonly due to absence of defibrillator data or treatment with a device that did not record chest impedance. After exclusions, there were 692 (48%) patients included in the final study group. Demographic characteristics of included and excluded patients were similar (Table 1). Of the 692 patients included in the study group, 208 (30%) were randomly assigned to the training group and 484 (70%) to the test group.
Figure 2. Study cohort and exclusions.
(CPR = Cardiopulmonary Resuscitation; VF = Ventricular Fibrillation.)
Table 1. Patient Characteristics.
Demographics are shown for all primary VF cases in the 2005–2014 study period, for eligible cases with >1 shock, for excluded cases, and for study group cases. (CPR = Cardiopulmonary Resuscitation; EMS = Emergency Medical Services; IQR = Interquartile Range; ROSC = Return of Spontaneous Circulation; VF = Ventricular Fibrillation.)
| Primary VF cases 2005–2014 (N=1927) | Eligible cases with >1 shock (N=1434) | Excluded cases (N=742) | Study group cases (N=692) | |
|---|---|---|---|---|
| Female, n(%) | 454(23.6) | 333(23.2) | 168(22.6) | 165(23.8) |
| Age, median (IQR) | 62(52, 73) | 62(52, 73) | 63(52, 73) | 61(52, 73) |
| Cardiac etiology, n(%) | 1742(90.4) | 1314(91.6) | 672(90.6) | 642(92.8) |
| Location, n(%) | ||||
| Home | 1208(62.7) | 908(63.3) | 455(61.3) | 453(65.5) |
| Public | 627(32.5) | 467(32.6) | 248(33.4) | 219(31.6) |
| Nursing Home | 92(4.8) | 59(4.1) | 39(5.3) | 20(2.9) |
| Arrest before EMS arrival, n(%) | 1713(88.9) | 1300(90.7) | 645(86.9) | 655(94.7) |
| Witnessed, n(%) | 1482(76.9) | 1110(77.4) | 576(77.6) | 534(77.2) |
| Bystander CPR, n(%) | 1249(64.8) | 939(65.5) | 477(64.3) | 462(66.8) |
| EMS Response time (minutes), median (IQR) | 5(4, 6.6) | 5(4, 6.9) | 5(4, 7) | 5(4, 6.2) |
| Total shocks, median (IQR) | 3(1, 6) | 4(3, 7) | 4(3, 6) | 5(3, 7) |
| ROSC, n(%) | 1281(66.5) | 891(62.1) | 456(61.5) | 435(62.9) |
| Died in Field | 498(25.8) | 405(28.2) | 210(28.3) | 195(28.2) |
| Admit to hospital | 1254(65.1) | 876(61.1) | 439(59.2) | 437(63.2) |
| Survive to hospital discharge, n(%) | 818(42.4) | 550(38.4) | 276(37.2) | 274(39.6) |
VF segments were collected during CPR prior to 1372 subsequent shocks and without CPR prior to 1283 shocks. Of all VF segments collected, there were 418/399 segments with/without CPR from patients in the training group, and 954/884 segments with/without CPR from patients in the test group.
Return of organized rhythm
ROR occurred after 801 (58%) of segments with CPR and 750 (58%) of segments without CPR. When stratified by shock number, the first, second, and third subsequent shocks all had similar rates of ROR. There was significant association between ROR from adjacent shocks (ϕ=0.36, p<0.001). Inter-rater reliability of ROR annotation between two reviewers, estimated from a random subset of 292 shocks from 138 patients, was high (K [95% CI] = 0.85 [0.78–0.91]).
Waveform measures
Median AMSA was 6.27 [IQR 4.03, 8.51] mV-Hz during CPR and 5.02 [IQR 3.06, 6.99] mV-Hz without CPR (p<0.001 for difference). Median MS was 3.51 [IQR 2.17, 4.86] mV/s during CPR and 2.80 [IQR 1.56, 4.05] mV/s without CPR (p<0.001 for difference). Median AMSA and MS were significantly higher before successful shocks compared to unsuccessful shocks both with and without CPR (p<0.001 for differences) (Supplementary Appendix A).
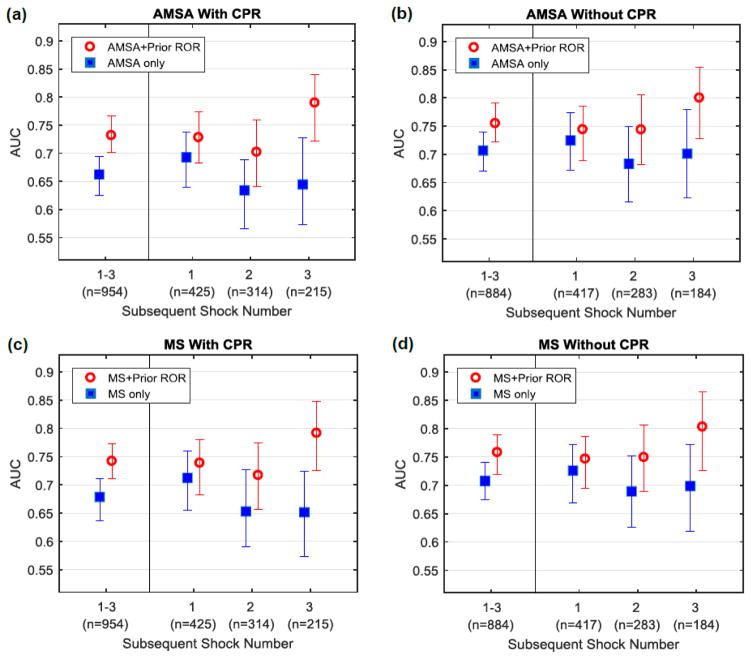
In all logistic models, waveform measures and Prior ROR were associated with a successful shock (Table 2). Predictive performance was significantly improved when Prior ROR was added to either waveform measure, regardless of whether CPR was ongoing (Table 3). For example, during CPR, AUC for AMSA alone was 0.66 [95% CI 0.63–0.70], while AUC for AMSA with Prior ROR was 0.73 [95% CI 0.70–0.76]. The increase in AUC due to Prior ROR varied across individual shocks and CPR state (Figure 3). For instance, during CPR, inclusion of Prior ROR increased the AMSA AUC by 0.04 for shock 2, 0.07 for shock 3, and 0.15 for shock 4 over that of AMSA alone (Supplementary Appendix B). Results using 10-fold cross-validation were similar (Supplementary Appendix C).
Table 2. Odds ratios.
Odds ratios are shown for Prior ROR, AMSA, and MS. All variables were significantly associated with successful shock outcome. Logistic models were trained using N=208 patients in the training set (418 shocks with CPR and 399 shocks without CPR). Standardized odds ratios are also presented for continuous variables. (AMSA = Amplitude Spectrum Area; CI = Confidence Interval; CPR = Cardiopulmonary Resuscitation; MS = Median Slope; ROR = Return of Organized Rhythm.)
| Logistic Model | Variable | With CPR | Without CPR | ||
|---|---|---|---|---|---|
|
| |||||
| Odds Ratio [95% CI] | Standardized Odds Ratio [95% CI] | Odds Ratio [95% CI] | Standardized Odds Ratio [95% CI] | ||
|
| |||||
| AMSA only | AMSA (mV-Hz) | 1.22 [1.14–1.31] | 1.98 [1.58–2.48] | 1.31 [1.21–1.42] | 2.21 [1.75–2.78] |
|
| |||||
| AMSA + Prior ROR | AMSA (mV-Hz) | 1.16 [1.08–1.24] | 1.68 [1.33–2.12] | 1.21 [1.11–1.32] | 1.75 [1.37–2.24] |
| Prior ROR | 3.58 [2.31–5.55] | - | 3.48 [2.17–5.56] | - | |
|
| |||||
| MS only | MS (mV/s) | 1.43 [1.28–1.61] | 2.19 [1.70–2.81] | 1.57 [1.37–1.79] | 2.47 [1.89–3.22] |
|
| |||||
| MS + Prior ROR | MS (mV/s) | 1.32 [1.17–1.48] | 1.82 [1.41–2.35] | 1.38 [1.20–1.59] | 1.91 [1.45–2.53] |
| Prior ROR | 3.44 [2.21–5.35] | - | 3.33 [2.07–5.34] | - | |
Table 3. AUCs for logistic model performance on test data.
AUC values for prediction of shock success on N = 484 test patients (954 shocks with CPR, 884 shocks without CPR) are significantly higher for Prior ROR combined with waveform measures versus waveform measures alone. Differences may not sum due to rounding. (AMSA = Amplitude Spectrum Area; AUC = Area under the Receiver Operating Characteristic Curve; CI = Confidence Interval; CPR = Cardiopulmonary Resuscitation; MS = Median Slope.)
| Logistic Model | AUC [95% CI] with CPR | AUC [95% CI] without CPR |
|---|---|---|
| AMSA only | 0.66 [0.63–0.70] | 0.71 [0.67–0.74] |
| AMSA + Prior ROR | 0.73 [0.70–0.76] | 0.76 [0.72–0.79] |
| (Change) | 0.07 [0.04–0.10]* | 0.04 [0.02–0.07]* |
| MS only | 0.68 [0.64–0.71] | 0.71 [0.68–0.75] |
| MS + Prior ROR | 0.74 [0.71–0.77] | 0.76 [0.72–0.79] |
| (Change) | 0.06 [0.04–0.09]* | 0.05 [0.03–0.07]* |
p<0.001 for change
Figure 3. AUC values for prediction of ROR on test data.
AUC values with 95% confidence intervals for predicting shock success are shown for all subsequent shocks using (a) AMSA with CPR, (b) AMSA without CPR, (c) MS with CPR, and (d) MS without CPR. AUC values are presented for all subsequent shocks combined and also by individual shock number. AUC values are significantly higher for waveform measures in combination with Prior ROR versus waveform measures alone. Improvement in AUC due to inclusion of Prior ROR is greater as shock sequence progresses. (AMSA = Amplitude Spectrum Area; AUC = Area under the Receiver Operating Characteristic Curve; CPR = Cardiopulmonary Resuscitation; MS = Median Slope; ROR = Return of Organized Rhythm.)
Discussion
In this investigation of out-of-hospital arrest, we confirmed that two representative VF waveform measures, AMSA and MS, predict shock outcome as determined by return of rhythm on the ECG. We observed that the measures retain some prognostic capability during CPR but AUC is reduced compared to CPR-free analysis. Moreover, in novel investigation, we demonstrated that inclusion of previous shock outcome improves waveform measures during CPR and offsets the reduction in waveform measure performance caused by CPR artifact. Combining information from waveform measures and the resuscitation history thus offers a step forward towards achieving near-continuous CPR while guiding treatment and its timing.
Ideally cardiac arrest treatment would be tailored to match individual pathophysiology in order to achieve the greatest likelihood of resuscitation.30 Shocks that are less likely to result in ROR could be temporarily postponed to allow additional perfusion with CPR (or other interim treatments) that might enhance subsequent shock success.8–10 Conversely, shocks more certain to result in ROR could be administered immediately. Such an approach would not necessarily delay receipt of an immediate shock, since rhythm analysis (to determine whether the rhythm is shockable or non-shockable) and prognostic assessment could both be performed concurrently. However, current protocol uses a uniform approach that delivers shocks using fixed timing based on information available at predetermined 2-minute intervals.15 Outcomes might be improved if care could be guided by real-time physiologic measures throughout the course of resuscitation, especially if these measures incorporated accumulating information about patient-specific response to therapy. The approach is largely untested in human resuscitation, but such “patient-specific” resuscitation is supported by the results of experimental research.31,32
Multiple prior studies have highlighted the ability of waveform measures to predict shock outcome, i.e. return of rhythm or survival.8 However these studies require CPR-free ECG epochs in order to calculate quantitative waveform measures. Such interruptions undermine the goals of continuous circulatory support during resuscitation.18 Given this understanding, we previously evaluated the prognostic ability of waveform measures using ever-shorter CPR-free segments, and found that measures retain comparable prediction even as the CPR-free segment length approaches <1 second.24 Nonetheless, even these brief interruptions are logistically challenging to coordinate, and would not support continuous VF waveform assessment during CPR.
Hence we evaluated the prognostic characteristics of two well-characterized waveform measures – A MSA and MS – during uninterrupted CPR. As part of the investigation, we applied a bandpass filter to improve the usability of the ECG waveform (Supplementary Appendix D). The fundamental frequency of chest compressions is approximately 2 Hz, while VF frequencies are primarily between 3–8 Hz.33 However, harmonics and higher-frequency CPR artifact lie within the spectral range of the VF signal, such that removal via filtering cannot be easily accomplished without degrading the VF signal itself.15 Waveform measures therefore cannot be calculated optimally during CPR even after filtering the ECG.11–13,34 Since AMSA is computed from the product of each frequency and its magnitude, and MS computes the pointwise derivative, both measures increasingly emphasize higher-frequency content. Consequently, AMSA and MS may still be relatively better-suited to evaluate VF during chest compressions as they inherently ignore some CPR artifact. In our investigation, AMSA and MS did indeed predict ROR after shock during CPR, though the AUC was significantly less compared to CPR-free analysis (Supplementary Appendix D).
We sought to evaluate whether additional information might be combined with quantitative waveform measures to increase prognostic accuracy during CPR. Research has suggested that prior rhythm is associated with subsequent likelihood of return of spontaneous circulation following shock, and that this information may improve prediction when combined with waveform measures calculated during CPR-free epochs.16,17 However little is known about whether ROR from prior shock would improve prediction when combined with waveform measures calculated during CPR. We observed that Prior ROR was associated with the response to a subsequent shock. Moreover, Prior ROR in combination with waveform measures significantly increased AUC for prognosis regardless of whether CPR artifact was present. Additionally the increase in AUC by incorporating Prior ROR was greater as shock sequence progressed, suggesting that patient response to shock may be increasingly related to previous shock response over the course of resuscitation.
In this study we demonstrated that when prior shock outcome is known, it can be leveraged to improve waveform measure prognostic ability during CPR. Clinical benefit of a prognostic algorithm able to assess VF viability during CPR would be contingent on ability to also classify ECG rhythms (i.e. detect VF) during CPR. Unfortunately, current protocol for rhythm analysis still requires a pause in chest compressions for rhythm classification.15 In recent experimental work, however, we demonstrated the possibility of real-time rhythm classification during CPR by incorporating ECG history over time.35 This finding further highlights the value of integrating information accumulated over the course of resuscitation to improve accuracy during CPR. By including historical information from the ECG, patient-specific treatment guided by a real-time prognostic algorithm concurrent with real-time rhythm classification may be possible without interruption of chest compressions. Further efforts to develop such a prognostic algorithm would involve improving individual waveform measures as well as refining methods to integrate continuous readings (rather than using a limited number of isolated segments) to increase prognostic accuracy.
In summary, the AUC that resulted from the combination of waveform measures with prior shock success during CPR was improved over the AUC of waveform measures alone. Waveform measures during CPR combined with Prior ROR also exceeded the predictive capability of CPR-free waveform measures alone. These results support – at least in concept – that historical ECG information in addition to analysis of isolated segments can overcome some of the prognostic challenges introduced by CPR artifact, and in turn may enable guided resuscitation with continuous CPR.
Limitations
We used waveform measures as well as ROR from prior shock to develop a prognostic model that could predict subsequent ROR. This method incorporates previous shock success and thus would not apply to prediction of initial shock outcome. Overall predictive performance was modest, thus limiting potential clinical application of the specific measures used. The study group restriction to subsequent shocks may also have affected results due to exclusion of patients resuscitated with a single shock; for instance, waveform measure AUC values in this study were less compared to a previous investigation of initial shocks.24
We used ROR as a shock-specific electrical outcome. ROR had excellent inter-reviewer reliability in the current study, but since ROR includes non-perfusing organized rhythms, its utility as a surrogate clinical outcome may be limited. Other definitions of shock success such as return of spontaneous circulation could also serve as a shock-specific outcome and may produce different results. Each shock was treated as an independent sample for training and test, though multiple clips from the same patient were included in the study. This approach may produce correlation among ECG segments, although only a maximum of three subsequent shocks were collected from a single patient.
The study included ECG segments from multiple defibrillator models with different filtering bandwidths. This heterogeneity underscores the generalizability of the findings across different hardware platforms, but due to waveform measure relationship to high frequency content, may cause variation in waveform measure magnitude between devices and may reduce combined AUC. The study was retrospective and so did not actually incorporate ROR status or waveform measures in real-time, and results therefore may not be indicative of prospective investigation.
Conclusion
AMSA and MS retained shock-specific prognostic value when generated during CPR, and their predictive ability was improved by incorporating outcome from the prior shock. Future efforts may continue to refine and evaluate how historical ECG information predicts outcome and may potentially guide patient-specific care.
Supplementary Material
Highlights.
Ventricular fibrillation waveform measures predict defibrillation shock outcome.
CPR artifact obscures the ECG and reduces waveform measure accuracy.
Outcomes (i.e., return of organized rhythm) of repeated shocks are correlated.
Waveform measures during CPR improve if combined with prior shock outcome.
Measures during CPR combined with prior outcome match measures alone without CPR.
Acknowledgments
We acknowledge the EMS personnel of King County for consistently striving towards optimal resuscitation and for enabling this study. Philips Healthcare and Physio-Control provided technical guidance on defibrillator data extraction. This study was supported in part by grants provided to the University of Washington by the Laerdal Foundation, Philips Healthcare, the Washington State Life Sciences Discovery Fund, and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (T32EB001650). The content of this study is solely the responsibility of the authors and does not necessarily represent the views of the funding organizations, King County EMS, Philips Healthcare, or Physio-Control.
Abbreviations
- AMSA
Amplitude Spectrum Area
- AUC
Area under Receiver Operating Characteristic Curve
- CI
Confidence Interval
- CPR
Cardiopulmonary Resuscitation
- ECG
Electrocardiogram
- EMS
Emergency Medical Services
- IQR
Interquartile Range
- ROR
Return of Organized Rhythm
- MS
Median Slope
- VF
Ventricular Fibrillation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rea TD, Eisenberg MS, Sinibaldi G, White RD. Incidence of EMS-treated out-of-hospital cardiac arrest in the United States. Resuscitation. 2004;63:17–24. doi: 10.1016/j.resuscitation.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Kudenchuk PJ, et al. Transthoracic Incremental Monophasic Versus Biphasic Defibrillation by Emergency Responders (TIMBER) Circulation. 2006;114:2010–2018. doi: 10.1161/CIRCULATIONAHA.106.636506. [DOI] [PubMed] [Google Scholar]
- 3.Ristagno G, Li Y, Fumagalli F, Finzi A, Quan W. Amplitude spectrum area to guide resuscitation—A retrospective analysis during out-of-hospital cardiopulmonary resuscitation in 609 patients with ventricular fibrillation cardiac arrest. Resuscitation. 2013;84:1697–1703. doi: 10.1016/j.resuscitation.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer TF, Walker RG, Chapman FW, Koster RW. Association between chest compression interruptions and clinical outcomes of ventricular fibrillation out-of-hospital cardiac arrest. Circulation. 2015;132:1030–1037. doi: 10.1161/CIRCULATIONAHA.115.014016. [DOI] [PubMed] [Google Scholar]
- 5.Cheskes S, et al. Perishock Pause: An Independent Predictor of Survival from Out-of-Hospital Shockable Cardiac Arrest. Circulation. 2011;124:58–66. doi: 10.1161/CIRCULATIONAHA.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu T, et al. Adverse Outcomes of Interrupted Precordial Compression During Automated Defibrillation. Circulation. 2002;106:368–372. doi: 10.1161/01.cir.0000021429.22005.2e. [DOI] [PubMed] [Google Scholar]
- 7.Schoene P, et al. Course of quantitative ventricular fibrillation waveform measure and outcome following out-of-hospital cardiac arrest. Hear Rhythm. 2014;11:230–236. doi: 10.1016/j.hrthm.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Callaway CW, Menegazzi JJ. Waveform analysis of ventricular fibrillation to predict defibrillation. Curr Opin Crit Care. 2005;11:192–9. doi: 10.1097/01.ccx.0000161725.71211.42. [DOI] [PubMed] [Google Scholar]
- 9.Cobb LA, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–8. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 10.Wik L, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation. JAMA. 2003;289:1389–95. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 11.Lo MT, et al. A new method to estimate the amplitude spectrum analysis of ventricular fibrillation during cardiopulmonary resuscitation. Resuscitation. 2013;84:1505–1511. doi: 10.1016/j.resuscitation.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Tang W. Techniques for artefact filtering from chest compression corrupted ECG signals: Good, but not enough. Resuscitation. 2009;80:1219–20. doi: 10.1016/j.resuscitation.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Neurauter A, et al. Improving countershock success prediction during cardiopulmonary resuscitation using ventricular fibrillation features from higher ECG frequency bands. Resuscitation. 2008;79:453–9. doi: 10.1016/j.resuscitation.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Strohmenger HU. Predicting defibrillation success. Curr Opin Crit Care. 2008;14:311–6. doi: 10.1097/MCC.0b013e3282fc9a9c. [DOI] [PubMed] [Google Scholar]
- 15.Affatato R, Li Y, Ristagno G. See through ECG technology during cardiopulmonary resuscitation to analyze rhythm and predict defibrillation outcome. Curr Opin Crit Care. 2016;22:199–205. doi: 10.1097/MCC.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 16.Eilevstjønn J, Kramer-Johansen J, Sunde K. Shock outcome is related to prior rhythm and duration of ventricular fibrillation. Resuscitation. 2007;75:60–7. doi: 10.1016/j.resuscitation.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 17.He M, et al. Combining Amplitude Spectrum Area with Previous Shock Information Using Neural Networks Improves Prediction Performance of Defibrillation Outcome for Subsequent Shocks in Out-Of-Hospital Cardiac Arrest Patients. PLoS One. 2016;11:e0149115. doi: 10.1371/journal.pone.0149115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link MS, et al. Part 7: Adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S444–S464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs I, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation. Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 20.Povoas HP, Bisera J. Electrocardiographic waveform analysis for predicting the success of defibrillation. Crit Care Med. 2000;28:N210–1. doi: 10.1097/00003246-200011001-00010. [DOI] [PubMed] [Google Scholar]
- 21.Marn-Pernat A, Weil MH, Tang W, Pernat A, Bisera J. Optimizing timing of ventricular defibrillation. Crit Care Med. 2001;29:2360–5. doi: 10.1097/00003246-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Neurauter A, et al. Prediction of countershock success using single features from multiple ventricular fibrillation frequency bands and feature combinations using neural networks. Resuscitation. 2007;73:253–63. doi: 10.1016/j.resuscitation.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Firoozabadi R, Nakagawa M, Helfenbein ED, Babaeizadeh S. Predicting defibrillation success in sudden cardiac arrest patients. J Electrocardiol. 2013;46:473–479. doi: 10.1016/j.jelectrocard.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Coult J, et al. Short ECG segments predict defibrillation outcome using quantitative waveform measures. Resuscitation. 2016;109:16–20. doi: 10.1016/j.resuscitation.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 26.Robin X, et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eftestol T, Sunde K, Ole Aase S, Husoy JH, Steen PA. Predicting Outcome of Defibrillation by Spectral Characterization and Nonparametric Classification of Ventricular Fibrillation in Patients With Out-of-Hospital Cardiac Arrest. Circulation. 2000;102:1523–1529. doi: 10.1161/01.cir.102.13.1523. [DOI] [PubMed] [Google Scholar]
- 28.Watson JN, Addison PS, Clegg GR, Steen PA, Robertson CE. Practical issues in the evaluation of methods for the prediction of shock outcome success in out-of-hospital cardiac arrest patients. Resuscitation. 2006;68:51–9. doi: 10.1016/j.resuscitation.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Figuera C, et al. Machine learning techniques for the detection of shockable rhythms in automated external defibrillators. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0159654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rea T, et al. Association between survival and early versus later rhythm analysis in out-of-hospital cardiac arrest: Do agency-level factors influence outcomes? Ann Emerg Med. 2014;64:1–8. doi: 10.1016/j.annemergmed.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg RA, Hilwig RW, Kern KB, Ewy GA. Precountershock cardiopulmonary resuscitation improves ventricular fibrillation median frequency and myocardial readiness for successful defibrillation from prolonged ventricular fibrillation: A randomized, controlled swine study. Ann Emerg Med. 2002;40:563–570. doi: 10.1067/mem.2002.129866. [DOI] [PubMed] [Google Scholar]
- 32.Hayes MM, Berg RA, Otto CW. Monitoring during cardiac arrest: are we there yet? Curr Opin Crit Care. 2003;9:211–7. doi: 10.1097/00075198-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Strohmenger HU, Lindner KH, Brown CG. Analysis of the ventricular fibrillation ECG signal amplitude and frequency parameters as predictors of countershock success in humans. Chest. 1997;111:584–9. doi: 10.1378/chest.111.3.584. [DOI] [PubMed] [Google Scholar]
- 34.Gong Y, Chen B, Li Y. A review of the performance of artifact filtering algorithms for cardiopulmonary resuscitation. J Healthc Eng. 2013;4:185–202. doi: 10.1260/2040-2295.4.2.185. [DOI] [PubMed] [Google Scholar]
- 35.Kwok H, Coult J, Drton M, Rea TD, Sherman L. Adaptive rhythm sequencing: A method for dynamic rhythm classification during CPR. Resuscitation. 2015;91:26–31. doi: 10.1016/j.resuscitation.2015.02.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.