Abstract
AMD is a complex multifactorial disease characterized in its early stages by lipoprotein accumulations in BrM, seen on fundoscopic exam as drusen, and in its late forms by neovascularization (“wet”) or geographic atrophy of the RPE cell layer (“dry”). Genetic studies have strongly supported a relationship between the alternative complement cascade, in particular the common H402 variant in Complement Factor H (CFH) and development of AMD. However, the functional significance of the CFH Y402H polymorphism remains elusive. In this article, we critically review the literature surrounding the functional significance of this polymorphism. Furthermore, based on our group’s studies we propose a model in which CFH H402 affects CFH binding to heparan sulfate proteoglycans leading to accelerated lipoprotein accumulation in BrM and drusen progression. We also review the literature on the role of other complement components in AMD pathobiologies, including C3a, C5a and membrane attack complex (MAC) and on transgenic mouse models developed to interrogate in vivo the effects of the CFH H402 polymorphism.
1. Age-related macular degeneration (AMD)
Age-related macular degeneration (AMD) is a complex, progressive, chorioretinal degenerative disease that affects the central region of the retina known as the macula (Fig. 1), the area responsible for the majority of high acuity, photopic vision (vision under well-lit conditions) (Jager et al., 2008). Three major factors contribute to AMD: advanced age, environmental factors (particularly smoking) (Age-Related Eye Disease Study 2 Research, 2013; Age-Related Eye Disease Study Research, 2001; Christen et al., 1996; SanGiovanni et al., 2007; Seddon et al., 1994; Seddon et al., 2001; Seddon et al., 1996; Vingerling et al., 1996) and genetic risk factors, which will be described in detail below.
Figure 1.
Posterior eye ocular anatomy. (A) Cross-section schematic of a human eye showing major anatomical structures. (Image courtesy of Dr. Mikael Klingeborn) (B) Histological cross-section of retina and choroid in the perifoveal region of the macula. INL, inner nuclear layer; OPL outer plexiform layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments; RPE, retinal pigmented epithelium; BrM, Bruch’s membrane; CC, choriocapillaris (image courtesy of Christine A. Curcio, PhD; http://projectmacula). IS and OS are artifactually separated.
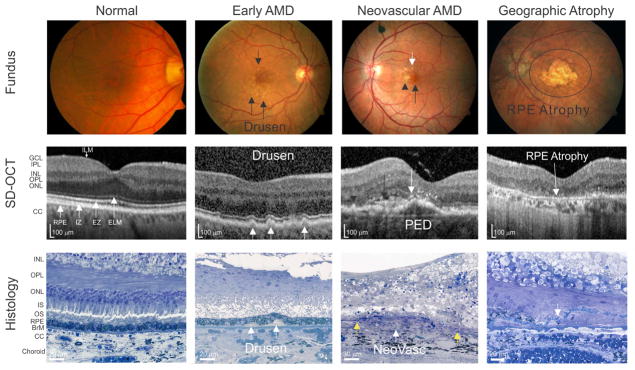
AMD is broadly classified into three categories based on fundoscopic appearance of the macula: early, intermediate and late stage. The pathognomonic lesions of early and intermediate AMD are lipid- and protein-rich deposits known as drusen (Fig. 2). The word drusen (singular, “druse”), is derived from the German word for geodes. Drusen are typically detected on fundoscopic clinical exam, but are now visible by a variety of imaging modalities including optical coherence tomography (OCT) (Fig. 2). The deposition of a few small (diameter less than 63 microns) drusen is normal in aged eyes and is generally not vision impairing. The presence of intermediate (diameter between 63 and 125 microns) or large (diameter greater than 125 microns) drusen in the macula typically leads to vision impairment and is indicative of early or intermediate AMD (Li et al., 2001; Stangos et al., 1995). The diagnosis of early and intermediate AMD places a patient at high risk of progressing to later stages of the disease (Ferris et al., 2013). Late-stage AMD is categorized as either ‘dry’ (Fig. 2) [geographic atrophy, with photoreceptor loss and extensive retinal pigmented epithelium (RPE) atrophy, also referred to as ‘atrophic’] or ‘wet’ (Fig. 2) [neovascular or exudative)], however, in many patients both ‘dry’ and ‘wet’ forms are evident (Ferris et al., 2013). The initial visual deficit in patients with aging and early AMD are in rod-mediated vision, especially rod-mediated dark adaptation and low luminance visual acuity (Owsley et al., 2016a; Owsley et al., 2001; Owsley et al., 2016b). Whereas, late-stage AMD is characterized by central scotoma and loss of central vision.
Figure 2.
Clinical and histological depictions of AMD. Fundus photographs (Fundus), Spectral Domain-Optical Coherence Tomography (SD-OCT) images and chorioretinal histological sections (Histology) showing the normal eye and the characteristic lesions of early AMD, neovascular AMD, and geographic atrophy. Color fundus photographs, SD-OCT and histological images are not obtained from the same eyes. Normal: The corresponding SD-OCT of a normal color fundus demonstrating normal retinal structures and layers and intact four hyperreflective lines in outer retina (white arrows: RPE, retinal pigmented epithelium; IZ, interdigitation zone; EZ, ellipsoid zone; ELM, external limiting membrane). No sub-RPE or subretinal drusenoid deposits are noted in either the SD-OCT or the histology image. The break between the IS and OS in histology is a fixation artifact. Early AMD: Images showing small drusen on the color fundus photo (black arrows), SD-OCT (white arrows) and soft drusen on histopathology (white arrows). Neovascular AMD: Color fundus photograph depicts drusen (white arrow), pigmentary changes (black arrow head) and a foveal subretinal hemorrhage (black arrow); SD-OCT demonstrates a thin layer of subretinal fluid (black arrow), subretinal hyperreflective material (black arrowhead), and a large pigment epithelial detachment (PED) with overlaying hyper-reflective foci (white arrow); histology illustrates a neovascular complex (NeoVasc) between the two free ends of breached Bruch’s membrane (yellow arrows). Geographic atrophy: Color fundus image and SD-OCT depicts a well-demarcated area of fovea-involving RPE atrophy, while histology shows absence of the RPE and a blue-stained line of persistent basal laminar deposits (white arrow). GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL outer plexiform layer; ONL, outer nuclear layer; ELM, external limiting membrane; EZ, ellipsoid zone; IZ, interdigitation zone; CC, choriocapillaris; IS, inner segments; OS, outer segments; RPE, retinal pigmented epithelium; BrM, Bruch’s membrane. (Fundus and OCT images courtesy of Drs. Eleonora Lad and Katayoon Baradaran Ebrahimi and histological images courtesy of Dr. Christine A. Curcio)
Despite the fact that AMD is the leading cause of irreversible vision loss in the elderly in industrialized nations (Klein et al., 2002) and is expected to affect 196 million people worldwide by 2020 (Wong et al., 2014), limited therapeutic options are currently available for those suffering from the disease. For example, the progression of early and intermediate stage to late-stage AMD pathology can be delayed through dietary supplementation with the AREDS/AREDS2 anti-oxidant and mineral-based formulations (Age-Related Eye Disease Study 2 Research, 2013; Age-Related Eye Disease Study Research, 2001). The AREDS2 formulation contains vitamin C, vitamin E, zinc oxide, cupric oxide, lutein and zeaxanthin (Age-Related Eye Disease Study 2 Research, 2013). The major pharmacologic advancement in the treatment of late exudative AMD has been the utilization of intravitreal anti-vascular endothelial growth factor therapies (Heier et al., 2012; Rosenfeld, 2011; Rosenfeld et al., 2006). There are currently no therapies that stop or reverse dry (early or late) AMD.
A limited understanding of the pathogenesis of the disease has hampered the development of therapies for AMD. Genetic studies have provided researchers with valuable clues into the mechanisms underpinning AMD (Black and Clark, 2016). In particular, in 2005 four separate studies identified single nucleotide polymorphism (SNP) variants within the complement factor H gene (CFH) on chromosome 1 that are associated with significantly increased risk for AMD (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005). CFH encodes complement factor H (CFH) a regulator of the complement pathway, which is an arm of the innate immune system. Identification of specific variants in CFH as major genetic risk factors for AMD was a watershed moment in the understanding of the disease and supported previous studies implicating the complement cascade and chronic inflammation in AMD pathogenesis (Anderson et al., 2002; Anderson et al., 2010; Hageman et al., 2001; Johnson et al., 2001). Subsequently, genetic risk associations were found in other complement genes (Fagerness et al., 2008; Gold et al., 2006b; Maller et al., 2007; Yates et al., 2007) and ushered in an era of intense study of the complement system and its components as therapeutic targets. In this review, we will discuss the literature surrounding the genetic influence of CFH on AMD and the current understanding of how CFH regulates the early events in AMD pathogenesis. We also report on the role of other complement components on AMD pathobiologies including C3a, C5a and MAC. Finally, we will discuss current transgenic mouse models developed to interrogate the in vivo role of CFH in AMD pathogenesis.
2. Drusen
In depth understanding of the early pathological hallmarks of AMD has been essential to our understanding of the pathobiology of the disease. It has long been recognized that drusen are a hallmark lesion of early and intermediate AMD; the size, number and extent of confluency of drusen influence the risk of progression to late AMD (Bird et al., 1995; Pauleikhoff et al., 1990; Sarks et al., 1988, 1994; Sarks et al., 2007). Drusen, which are visible as small yellowish deposits under the retina on fundus examination (Fig. 3), were first identified in 1855 by the Dutch ophthalmologist, Franciscus Donders (Donders, 1855). Depending on the type and extent of examination and classification, up to 80% of patients over 60 years of age have some evidence of macular drusen (Augood et al., 2006; Klein et al., 1993; Leske et al., 1994; Varma et al., 2004). Thus, drusen are a common but not disease-specific lesion for AMD. They also occur in a variety of other diseases affecting the posterior eye (Francis, 2006; LeBedis and Sakai, 2008; Shenoy et al., 2010). While, the presence of macular drusen is considered a strong risk factor for the development of both forms of late AMD, atrophic and exudative, the specific contributions of drusen to AMD progression are not known. Therefore, clarification of the origin of drusen, as well as their implications in retinal health, is essential to our understanding of disease pathogenesis and the development of therapeutics for AMD.
Figure 3.
Association between funduscopically detected drusen and drusen imaged by SD-OCT and post-mortem histological staining. (A) Fundoscopic image of a patient with early AMD with significant macular drusen (yellow lesions, a few indicated by white arrows). (B) Image showing drusen (white arrows) as depicted by SD-OCT. (C) Histologic image of RPE/choroid interface and a single small druse (black arrow) subjacent to the RPE. Labels as in Figure 1. (Fundus and OCT images courtesy of Drs. Eleonora Lad and Katayoon Baradaran Ebrahimi and histological image courtesy of Dr. Christine A. Curcio)
2.1 Drusen localization
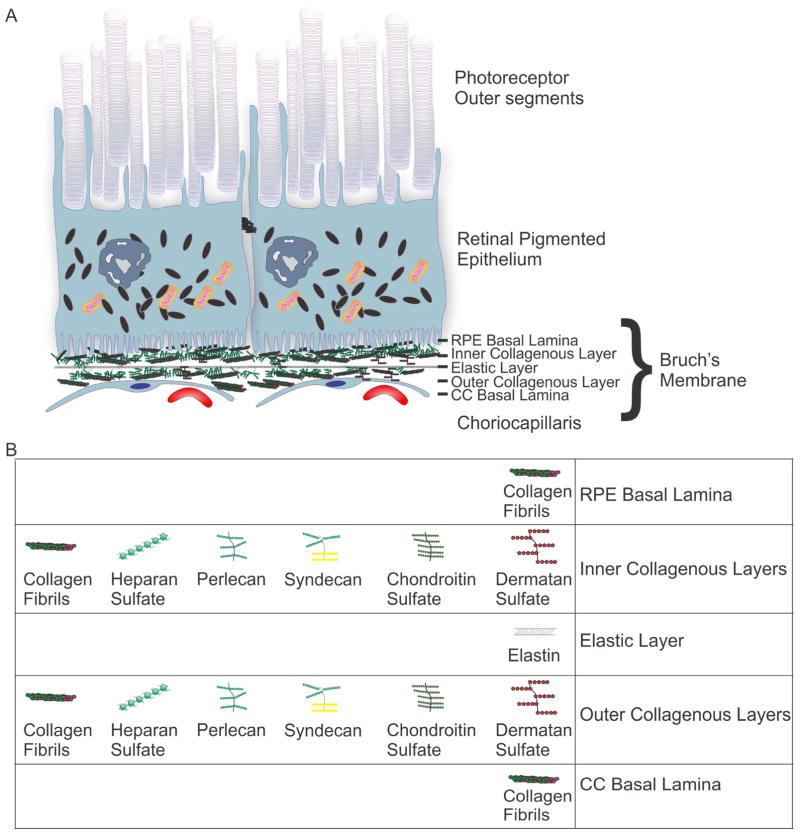
Early clinicopathological studies by S.H. Sarks described a linear deposit at the base of the RPE cells in association with inner surface of Bruch’s membrane (BrM) (Sarks, 1976). BrM is an organized pentalaminar extracellular matrix situated between the RPE and the capillary bed of the choroid known as the choriocapillaris (Hogan et al., 1971) (Fig. 4). BrM is not a true membrane; rather it is a thin (2–4μm thick), acellular matrix that includes the basal laminae of both the RPE and choroidal endothelial cells on its inner and outer aspects respectively (Fig. 4) (Curcio, 2013; Guymer et al., 1999). The five layers of BrM from the basal RPE side to the choroid are: RPE basal lamina, inner collagenous layer, elastic layer, outer collagenous layer and choriocapillaris basal lamina (Fig. 4). The structure of BrM is similar to vascular intima, with a subendothelial extracellular matrix and an elastic layer that is analogous to the internal elastic lamina of vessels (Curcio, 2013; Guymer et al., 1999). BrM differs from other vessel walls in that its abluminal surface abuts the basal lamina of an adjacent epithelium, which is also a characteristic of the renal glomerulus. This morphological similarity between BrM and the glomerulus, particularly the overlap between Type IV alpha3 and 4 collagens, (Butkowski et al., 1989; Chen et al., 2003; Harvey et al., 1998), as well as the fact that the luminal surfaces of each face a fenestrated vascular endothelium and basal lamina helps explain the overlap of some renal and retinal diseases, particularly AMD and Dense Deposit Disease (Curcio, 2013; Guymer et al., 1999; Mullins et al., 2001; Mullins et al., 2000). The inner collagenous layer is composed of a complex three-dimensional arrangement of collagen fibrils and proteoglycans with attached chains of all classes of glycosaminoglycans (GAGs) (e.g. heparan sulfate, chondroitin sulfate, dermatan sulfate and keratin sulfate) (Fig. 4B). Drusen form between the RPE basal lamina and the inner collagenous layer of BrM (Sarks, 1976). The composition, thickness and structural properties of BrM vary with age (Fisher, 1987; Lommatzsch et al., 2008; Okubo et al., 1999). In particular, collagen type IV content and collagen cross-linking increases with age, while collagen solubility decreases and advanced glycation end-product modifications accumulate (Guymer et al., 1999; Hamlin and Kohn, 1971; Handa et al., 1999; Ishibashi et al., 1998; Vater et al., 1979). In addition, lipid deposition increases with age (Curcio et al., 2011; Holz et al., 1994; Pauleikhoff et al., 1990) (discussed in more detail below). These age-related structural changes contribute to the increased hydraulic resistance seen in aged BrM (Ethier et al., 2004).
Figure 4.
Bruch’s membrane (BrM) layers and extracellular matrix composition. (A) Schematic of photoreceptor outer segments, RPE, BrM, and choriocapillaris (CC). BrM layers: basal lamina of the RPE, inner collagenous layer, elastic layer, outer collagenous layer and basal lamina of the choriocapillaris endothelial cells. (B) Extracellular matrix composition of BrM layers. The inner collagenous layer is composed of complex three-dimensional arrangement of GAGs including: collagen fibrils, heparan sulfate proteoglycans, perlecan, syndecan, chondroitin sulfate and dermatan sulfate.
2.2 Drusen composition
Histochemical and biochemical analyses of drusen have provided great insight into their origin and to their contributions to AMD, however, it should be noted that the majority of drusen composition studies were not performed on the “soft” macular drusen typically associated with late-stage AMD. Drusen are lipid-rich, with lipid-containing particles occupying 37–44% of drusen volume and a typical druse contains approximately 112 ng lipid compared to 42 ng of protein (Wang et al., 2010).
2.2.1 Proteins in drusen
Much of our initial understanding of the composition of drusen and the proteins that have been implicated in the pathogenesis of AMD come from seminal studies using immunohistochemical and biochemical analyses of drusen in human donor eyes. Early immunohistochemical analyses showed that a large number of complement components, including CFH (Hageman et al., 2005; Johnson et al., 2006), Factor B (Gold et al., 2006a), C3 (Johnson et al., 2001; Mullins et al., 2000), C5 (Johnson et al., 2001)), complement activation products (including C5b-9 (Hageman et al., 2001; Johnson et al., 2001; Mullins et al., 2000) and activators of the complement cascade (including amyloid beta) (Dentchev et al., 2003; Johnson et al., 2002; Loffler et al., 1995), C-reactive protein (CRP) (Johnson et al., 2006) and immunoglobulin (Johnson et al., 2000; Mullins et al., 2000), are molecular constituents of drusen. The presence of complement components (including CFH, C3, C5, C6, C7, C8, C9) in drusen was also shown by proteomic analysis (Crabb et al., 2002). In addition, other complement-regulatory molecules, including vitronectin and clusterin, are also prevalent in drusen (Crabb et al., 2002; Hageman et al., 1999; Hawse et al., 2003; Jenne et al., 1991; Milis et al., 1993). These studies contributed to a comprehensive profiling of the molecular composition of drusen leading to a model of AMD pathogenesis based on the effects of chronic inflammation proposed by Hageman et al. (Hageman et al., 2001). This “inflammation” model of AMD implicates local complement activation as a major event in the pathogenesis of AMD (previously reviewed by (Anderson et al., 2002; Anderson et al., 2010; Hageman et al., 2001). Additional immunohistochemical and biochemical analyses showed significant amounts of apolipoproteins (including ApoE) (Crabb et al., 2002; Klaver et al., 1998; Mullins et al., 2000), as well as additional amyloids, plasma constituents, and cellular components (Mullins et al., 2000). Furthermore, in vitro analysis of sub-RPE deposits from cultured fetal RPE cells, showed RPE cell production of basally secreted ApoE-containing particles that trigger complement activation (Johnson et al., 2011).
2.2.2 Lipid in drusen
The lipid-containing particles in drusen resemble those that accumulate in BrM and basal linear deposits, which are hypothesized to be the precursors to drusen (Curcio et al., 2011). BrM lipid accumulation with age and basal linear deposits appear in the same histological plane as drusen and contain similar vesicular histological hallmarks (Curcio et al., 2011; Pauleikhoff et al., 1990).
Wolter and Falls used Oil Red O to stain for non-polar lipids and demonstrated the rich lipid content of drusen (Wolter and Falls, 1962). Further histochemical analysis of BrM and drusen identified the presence of phospholipids and unesterified and esterified cholesterol; these accumulate with age in both BrM and drusen (Curcio et al., 2001; Holz et al., 1994; Pauleikhoff et al., 1990). There is considerable variability among individuals in the lipid composition of their drusen (Pauleikhoff et al., 1990). While some drusen contain predominantly neutral lipids, others are dominated by phospholipids, however, all drusen contain esterified and unesterified cholesterol (Curcio et al., 2005; Holz et al., 1994; Li et al., 2007; Pauleikhoff et al., 1990; Pauleikhoff et al., 1994).
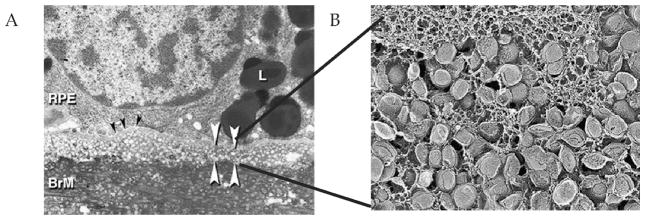
In the late 1990s and early 2000s genomic analysis of AMD patients revealed an association between lipid transport pathways, including apolipoproteins, and modification of risk for the development of AMD (Chen et al., 2010; Klaver et al., 1998; Neale et al., 2010; Souied et al., 1998). Correlating these findings with the lipid accumulation studies in BrM, Curcio et al. investigated the role of RPE-derived lipoproteins in age-related lipid accumulation, which led to their “Oil Spill” hypothesis of lipid accumulation in BrM (Curcio et al., 2011). Utilizing quick-freeze deep-etch, a freeze-fracture method to preserve lipids for electron microscopy, the authors observed round vesicles 60–80 nm in diameter with a core-shell structure that the authors later termed lipoprotein-like particles (Fig. 5). Subsequent analysis showed that these lipoproteins accumulate with age between the inner collagenous layer and elastic layer of BrM (Fig. 4), forming what they termed the “lipid wall” (Fig. 5) (Huang et al., 2007; Li et al., 2007). Isolation of lipoprotein-like particles from BrM extracts revealed two fractions one with 60–80 nm vesicles containing predominantly cholesterol esters, and a second containing a more heterogeneous population of vesicles predominantly comprised of unesterified cholesterol, lipids, and phospholipids (Li et al., 2005; Li et al., 2007; Wang et al., 2009b). Furthermore, cultured RPE-J cells, an immortalized rat RPE cell line, have been shown to secrete 60–80 nm ApoB-containing lipoprotein-like particles consistent with the lipoprotein-like particles seen in BrM, and in contrast to circulating high density lipoprotein (HDL) and low density (LDL) particles which range from 8–13 nm to 15–25 nm, respectively (Wang et al., 2009b). In addition, a recent study showed that long-term porcine RPE cultures form basal deposits with histochemically detectable lipid (Pilgrim et al., 2017). The experiments by Curcio et al. demonstrate that a portion of the lipid accumulation in BrM is made up of lipoprotein-like particles derived from the RPE. However, the origin of the RPE-derived lipids remains to be determined. There are two major cholesterol and fat sources for RPE cells: (1) daily phagocytosis of membrane-rich photoreceptor outer segments (OS); and (2) uptake of peripheral LDL particles from systemic circulation for retinal lipid supply (Peters et al., 2006; Rungger-Brandle et al., 1987; Tserentsoodol et al., 2006a; Tserentsoodol et al., 2006b). Based on the high content of linoleate (a lipid abundant in LDL), and low concentration of cholesteryl docosahexaenoate or cholesteryl stearate (abundant in OS), Curcio et al. concluded that the source of esterified cholesterol in BrM is from circulating LDL particles in blood processed by the RPE cells and deposited on the basal side of the RPE in BrM. (Bretillon et al., 2008; Li et al., 2005).
Figure 5.
Accumulation of BrM-associated lipoprotein-like particles leads to formation of lipid wall. (A) Transmission electron micrograph showing lipoprotein-like particles that accumulate between the RPE basal lamina (black arrowheads) and BrM inner collagenous layer as vesicles with lucent interiors forming the lipid wall (white arrowheads). (B) Quick freeze deep etch electron microscopic image of the lipoprotein-like particles in BrM. These pathological findings were posited to represent the RPE-derived “lipid-wall” lipoprotein layer that accumulates in BrM with age and may be a precursor to drusen formation. [Adapted from (Curcio et al., 2011)] (Images courtesy of Christine Curcio)
2.3 Basal laminar deposits and Sub-retinal drusenoid deposits (SDD)
In addition to drusen, other histologically evident deposits have been associated with AMD. Including the sub-RPE basal laminar deposits which, when found under the macula, are a defining feature of AMD and causally associated with macular risk of AMD (Rudolf et al., 2008a; Sarks, 1976). Notably, these deposits are the predominant lesion present in aged murine models of AMD (Toomey et al., 2015).
More recently, researchers have appreciated the presence of another extracellular deposits related to AMD located between the RPE and photoreceptors termed subretinal drusenoid deposits (SDD) (Spaide, 2013). SDD appear to have similar protein composition, including complement components (Rudolf et al., 2008b), to that of drusen. Thus, we suspect that the pathologic processes contributing to the biogenesis of SDD are similar to those contributing to the development of sub-RPE drusen. Due to their relatively recent identification and description, SDDs are not considered in detail here.
Together, histochemical and biochemical analyses of drusen and BrM proteins, lipids and lipoproteins have provided a framework that shaped our understanding of AMD pathobiology to include lipoprotein production by RPE cells and complement activation.
3. Complement system
In the early to late 2000s, two distinct lines of evidence showed a link between the complement cascade and AMD pathogenesis. First, immunohistological and proteomic analysis of post-mortem tissue from patients with AMD revealed the presence of complement proteins and activation products in drusen (Anderson et al., 2002; Anderson et al., 2010; Crabb et al., 2002; Hageman et al., 2005; Hageman et al., 2001; Mullins et al., 2000). Second, population-based genetic analyses showed several variants in complement proteins to be associated increased risk for AMD (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005). In this review, we will discuss these findings in greater detail. However, first we will explore the composition and function of the complement cascade.
3.1 Complement cascade
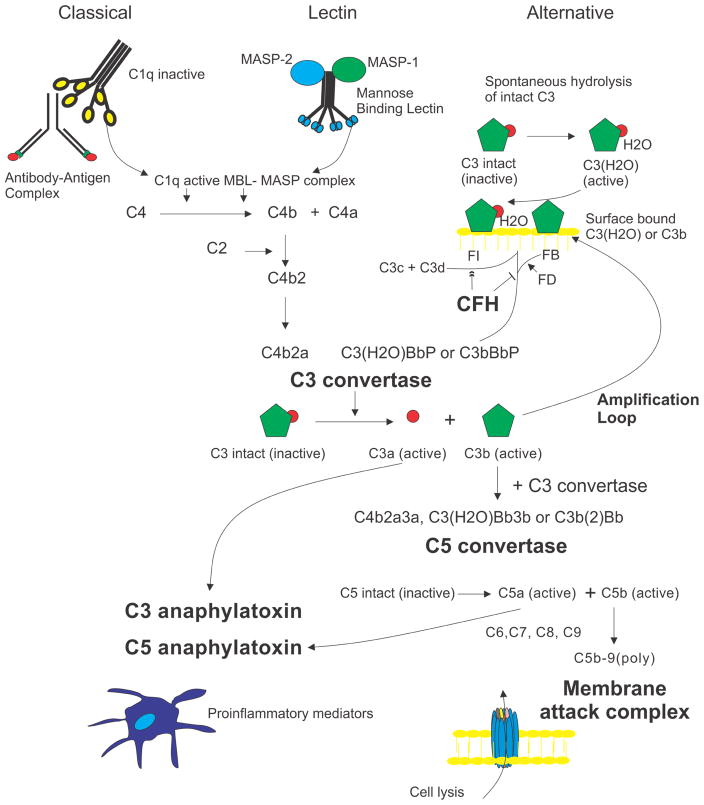
The complement system is amongst the oldest evolutionary components of the immune system. It was discovered in 1896 as a heat-labile fraction of serum that led to opsonization of bacteria. Biochemical characterization showed that the complement system is composed of over 30 proteins that function to mediate removal of apoptotic cells and eliminate pathogens (Sarma and Ward, 2011). Three separate pathways (i.e. classical, alternative and lectin pathways) converge to convert C3 to C3b forming the C3 convertase, an enzyme capable of initiating a cascade that results in the formation of the immunostimulatory breakdown products, C3a and C5a, and the membrane attack complex (MAC, C5b-9), a cell membrane pore that can cause cell lysis (Sarma and Ward, 2011) (Fig. 6).
Figure 6.
Overview of the complement system. Stimulated by antigen:antibody complexes, bacterial cell surfaces, and spontaneous hydrolysis, respectively, the classical, lectin, and alternative pathways converge to convert C3 to C3 convertase, an enzyme capable of initiating a cascade that results in 3 major effector functions: the formation of two anaphylatoxins, C3a and C5a, and generation of a cell membrane-spanning pore known as the membrane attack complex (MAC) that leads to cell lysis. There are two known inhibitory roles of the soluble regulator, CFH, within the complement cascade: 1) blocking the binding of Factor B to C3b and C3(H2O) and (2) acting as a co-factor to serum protease Factor I which functions to inactive C3b by cleaving it to generate the C3c and C3d inactivation products. MBL: Mannose binding lectin; MASPs: MBL-associated serine proteases; FB: Factor B; FD: Factor D; FI: Factor I.
3.2 Alternative pathway of complement
The classical and lectin complement pathways are initiated by the recognition of specific protein or carbohydrate targets. The immunoglobulins, IgM and IgG, and CRP activate the classical pathway. The lectin pathway is initiated by the interaction of mannose-binding lectin with repeating carbohydrate moieties found on bacterial surfaces. In contrast, the alternative pathway is initiated by the spontaneous hydrolysis of a thioester bond in C3 to form C3(H2O); hourly 0.2 to 0.4% of total C3 changes its conformation from C3 to C3(H20) (Pangburn and Muller-Eberhard, 1983) (Fig. 6). C3(H20) is capable of binding to Factor B (FB), resulting in a conformational change in FB. This conformational change allows Factor D, a constitutively active serum protease, to cleave FB to Factor Bb. Factor Bb is retained within the C3(H20)Bb complex where it acts as a serum protease for C3, cleaving it to C3b, which can in turn associate with FB to generate more C3 convertase (C3bBb). This auto-activation process is known as “tickover” (Sarma and Ward, 2011). Blurring the trichotomy between these pathways, however, is the role of the alternative pathway as an “amplification loop” of the complement cascade. In the “amplification loop”, C3b, generated from either the classical or lectin pathway, binds to FB to generate additional C3 convertase (Fig. 6). This “amplification loop” has been shown to be responsible for more than 80% of the C5a and membrane attack complex formation in both the classical and lectin pathways (Harboe et al., 2009; Harboe et al., 2004). Thus, the alternative pathway is responsible for the fluid–phase, spontaneous activation and amplification of C3 cleavage (Fig. 6).
3.3 Complement factor H (CFH)
Continuous control of the alternative pathway is necessary due to the spontaneous nature of the activation, its amplifying properties and its potential to provoke lysis in normal host tissues. The major negative fluid-phase regulator of the alternative complement cascade is Factor H (CFH). CFH is an abundant (218–654 μg/ml in serum) 155 kDa protein made up of twenty domains known as short consensus repeats (SCRs) or complement control protein (CCP) modules (Ansari et al., 2013; Schmidt et al., 2010). CFH SCRs 1–4 regulate the alternative pathway C3 convertase via two mechanisms: 1) CFH accelerates the decay of the C3 convertase and blocks its assembly by preventing the binding of FB to C3b and C3(H20) and 2) CFH promotes the irreversible inactivation of C3b to iC3b by the C3b serum protease, Factor I (FI); acting as a cofactor for FI-mediated cleavage of C3b. CFH functions not only in the fluid phase but also in the extracellular matrix and at host cell surfaces via its binding domains for polyanions, including heparan sulfate in SCRs 6–7 and 20 (Blaum et al., 2015; Clark et al., 2006). CFH thus regulates the spontaneous activation and amplification of C3 cleavage and assists in the degradation of active C3 cleavage products on host cells (Fig. 6).
4. Genetic discovery of the risk association of CFH with AMD
4.1 Genetics in AMD
Although Gass anticipated a genetic susceptibility in patients with AMD in 1972 (Gass, 1972), twin and population-based familial aggregation studies provided some of the earliest evidence of genetic susceptibility in AMD (Hammond et al., 2002; Klein et al., 2001; Klein et al., 1994; Meyers et al., 1995; Seddon et al., 1997). In fact, twin studies demonstrated that genetic factors account for 67% and 71% of the variation in disease risk for intermediate and advanced AMD, respectively (Seddon et al., 2005). Family based linkage analysis studies by R. J. Klein and colleagues were the first to report linkage to chromosome 1q25–31 in a large pedigree with an apparent autosomal dominant inheritance for “dry” AMD (Klein et al., 1998), which was then supported by a number of other linkage studies (Abecasis et al., 2004; Iyengar et al., 2004; Majewski et al., 2003; Seddon et al., 2003; Weeks et al., 2004). In contrast, candidate gene analysis, focusing on rare inherited macular dystrophies with phenotypic similarities to AMD, was limited in their ability to identify genes with significant risk association with AMD. Thus, although AMD pathobiology is dependent on aging, genetic heritability is a significant risk factor for the disease. In fact, it has long been appreciated that understanding the contributing genetic factors will provide insights into the relevant sequence of events in the aging chorioretina that lead to AMD.
4.2 CFH risk alleles
Significant advances in sequencing technologies and genome-wide association studies (GWAS) have provided substantial additional insight into the genetic architecture of AMD phenotypes. The first successful GWAS, in 2005, lead to the identification of an intronic variant in the CFH gene that is strongly associated with AMD (Klein et al., 2005). Further focused resequencing within the original cohort, in search of a functional variant within the CFH gene, led to identification of a T to C change in exon 9 that was the most strongly associated non-synonymous SNP in the analysis (Klein et al., 2005). Three other groups concomitantly revealed that this common haplotype in the CFH gene predisposes individuals to AMD (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005). This risk haplotype was highly associated with a non-synonymous SNP resulting in a tyrosine (Y) to histidine (H) exchange at position 402 of the CFH protein (the “Y402H polymorphism”). This risk association has been supported in many subsequent studies and the presence of at least one CFH risk allele is estimated to account for a population attributable risk fraction for early and late AMD of 10% and 53%, respectively (Klein et al., 2013). Several studies specifically related the CFH risk allele to early AMD, drusen and disease progression (Delcourt et al., 2011; Dietzel et al., 2014; Gangnon et al., 2012; Holliday et al., 2013; Magnusson et al., 2006). The Y402H polymorphism is located in SCR 7, a region that is known to mediate CFH binding to polyanions such as heparin, glycosaminoglycans, and CRP (Fearon, 1978; Mold et al., 1984), but is not involved in regulation of complement activation (Rodriguez de Cordoba et al., 2004; Zipfel and Skerka, 2009). The functional implications of the Y402H polymorphism will be explored in detail below. Further analysis of the CFH gene has revealed five distinct haplotypes, two of which significantly alter AMD risk: the H1 common risk haplotype (containing the CFH Y402H polymorphism) and the H2 protective haplotype (containing the protective CFH I62V polymorphism) (Hageman et al., 2005).
4.3 Rare mutations in CFH
Due to the strong association between the H1 common risk haplotype of CFH and AMD, there was significant interest in the analysis of rare variants that alter the peptide sequences, truncate proteins or affect RNA splicing to corroborate a causal mechanism (Fritsche et al., 2016; Raychaudhuri, 2011; Raychaudhuri et al., 2011). In 2011, Raychaudhuri et al. identified a rare, but highly penetrant, non-synonymous variant in CFH at Arg1210 where Arginine is substituted by Cysteine (Raychaudhuri et al., 2011). Interestingly, this rare variant occurs in SCRs 19–20, a second region of CFH involved in binding to GAGs and which has been associated with extensive macular drusen accumulation and advanced disease (Ferrara and Seddon, 2015). The studies further suggest a role of CFH-HS interaction in drusen formation. Using whole exome sequencing in families with high AMD disease burden and no known common genetic risk alleles, Yu et al. identified two rare variants in CFH, R53C and D90G, which functionally appear to have decreased decay accelerating activity and cofactor mediated inactivation ability, respectively (Yu et al., 2014). More recently, studies looking for other rare mutations in complement component genes showed enrichment of rare variants in CFH in AMD patients in both SCRs 1–4 and SCRs 19–20 (Triebwasser et al., 2015).
4.4 Common polymorphisms in other alternative pathway components, C3, FB, and FI
Evidence for the role of the alternative pathway of complement activation in AMD was further supported by the subsequent identification of common polymorphisms in other components involved in the alternative pathway of the complement cascade. Variations in C3 (R102G), FB (R32Q), both common and rare variants in FI and new variants in CFH have also been linked to AMD risk (Fagerness et al., 2009; Gold et al., 2006a; Hageman et al., 2005; Kavanagh et al., 2015; Maller et al., 2006; Maller et al., 2007; Seddon et al., 2013; Spencer et al., 2008; Yates et al., 2007). In studies interrogating the functional impact of combinations of these variants, Heurich et al. demonstrated that the combination of the C3 (102G), FB (32R) and CFH (62V) AMD risk-associated variants correlates with six-fold higher serum hemolytic activity (indicative of increased activity of the complement cascade) compared with the combination of the protective variants C3 (102R), FB (32Q) and CFH (62I) (Heurich et al., 2011). This observation provides strong proof of principle that combinations of polymorphic variants of the complement activators and regulators or “complotypes” within individuals can influence complement dysregulation (Heurich et al., 2011).
4.5 Non-coding variants in CFH
Although the H402 substitution explains a large fraction of the genetic risk attributed to AMD, definitive proof for the involvement of CFH H402 is lacking due to the high linkage disequilibrium in CFH (Ennis et al., 2007; Fritsche et al., 2013). The linkage disequilibrium in CFH results in 5 common (occurring in at least 2% of genome subject chromosomes) haplotypes. The most common haplotype (H1) shows the highest association for AMD [2.46 odds ratio (OR), confidence interval (CI) 1.95–3.11] and is 95% associated with the CFH H402 amino acid substitution (Hageman et al., 2005). Ansari et al. were able to show that non-coding SNPs are associated with alterations in CFH plasma concentrations (Ansari et al., 2013). However, the cumulative effect of non-coding SNPs on CFH concentration is relatively small; AMD patients exhibit only 3.7% lower plasma CFH levels (428 μg/mL versus 412 μg/mL). Considering human plasma CFH concentrations are normally distributed within a 3-fold range (218–654 μg/mL) with a standard deviation of 62 μg/mL (15%), this statistically significant observation would appear to be biologically insignificant (Ansari et al., 2013).
4.6 CFH genetics and kidney disease
Membranoproliferative glomerulonephritis (MPGN) type II (MPGN II) is a rare kidney disease where mutations in CFH lead to chronic uncontrolled alternative pathway complement activation and glomerulonephritis in a subset of MPGNII patients (Neary et al., 2002). MPGN II is characterized pathologically by the accumulation of C3 and electron-dense deposits in the glomerular basement membrane, a structure similar to BrM where drusen form in AMD (Curcio, 2013). Interestingly, prior to discovery of a genetic link between CFH and AMD, it was well established that patients with MPGN II develop early ocular drusen (Colville et al., 2003; Mullins et al., 2001; Neary et al., 2002; O’Brien et al., 1993). This connection was supported by data showing that 70% of patients with MPGN II harbored the CFH H1 haplotype (Hageman et al., 2005). The association of CFH risk variants and ocular drusen in MPGN II provides further evidence for a role of CFH in the biogenesis of drusen.
Another kidney disease, atypical hemolytic uremic syndrome (aHUS), also has been associated with mutations and polymorphisms in the CFH gene (Buddles et al., 2000; Caprioli et al., 2003; Ying et al., 1999; Zipfel, 2001; Zipfel and Skerka, 2009; Zipfel et al., 2001). aHUS is characterized pathologically by renal endothelial injury, systemic small vessel thrombosis and hemolytic anemia (Zipfel, 2001). The renal endothelial and systemic thrombotic pathobiology seen in aHUS is pathologically distinct from MPGN II and there have been no studies linking aHUS and drusen formation. Genetically, aHUS is associated with mutations in C-terminal SCRs (16–20) of CFH (Rodriguez de Cordoba et al., 2004) and is not associated with the AMD and MPGN II common risk haplotype (H1) (Pickering et al., 2007). In addition, although the CFH R1210C mutation is associated with aHUS and AMD, AMD and aHUS appear to be mutually exclusive pathologies (Raychaudhuri et al., 2011; Recalde et al., 2016). Furthermore, Cfh gene deletion in mice results in renal pathology resembling MPGN II (Pickering et al., 2002), whereas, mice with deletion of SCRs 16–20 in CFH develop renal pathology resembling aHUS (Pickering et al., 2007). Thus, although MPGN II and AMD share pathologic and genetic similarities, mutations in the SCR 16–20 regions of CFH associate with the unique systemic pathology seen in aHUS (Pickering et al., 2007; Recalde et al., 2016). Recent studies have established that CFH interacts with the C-terminus of von Willebrand factor (VWF) thereby enhancing VWF’s cleavage by ADAMTS-13 (Feng et al., 2013; Nolasco et al., 2013). In fact, lower ADAMTS-13 activity has been found in patients with aHUS and may explain the clinical differences between MPGNII and aHUS (Remuzzi et al., 2002; Veyradier et al., 2003).
5. CFH variant functions
Since the identification of the risk association between the common CFH Y402H polymorphism and AMD, researchers have focused on identifying the mechanism by which the CFH H402 variant affects AMD pathobiology. Over the past decade several groups have published, sometimes conflicting, studies aimed at defining a CFH function that is altered in the CFH H402 variant and is relevant to AMD pathobiology. In the following sections, we will evaluate the available data.
5.1 Complement regulation
The canonical function of CFH protein is as a fluid phase inhibitor of the alternative pathway. It controls alternative pathway amplification by dissociating the enzymatic Bb domain from the C3 convertase and by catalyzing FI cleavage of C3b (Fearon, 1978). Structurally CFH is comprised of 20 tandem SCRs. The common polymorphisms of the H1 haplotype conferring risk for AMD occur in SCR7 while the complement-regulatory domains associated with the protein’s canonical functions are in SCRs1–4. Furthermore, functional assays comparing the co-factor activity of CFH H402 and Y402 in factor I mediated cleavage of C3b show no variant differences (Kelly et al., 2010). Although there are reports indicating that patients with the CFH H402 polymorphism have elevated C3d/C3 ratios (Smailhodzic et al., 2012) this has not been replicated by other studies (Ristau et al., 2014), and the majority of clinical studies have shown no relationship between the CFH H402 polymorphism and other proteins involved in complement activation (Hecker et al., 2010; Silva et al., 2012; Smailhodzic et al., 2012). Together these finding suggest that CFH H402 polymorphism does not directly alter the complement regulatory functions of the CFH protein.
5.2 Binding to C-reactive protein (CRP)
C-reactive protein (CRP) is an acute-phase reactant with multiple functions including the activation of the classical complement pathway and the upregulation of pro-inflammatory mediators by endothelial cells, neutrophils and macrophages (Black et al., 2004; Devaraj et al., 2004; Pasceri et al., 2000; Singh et al., 2006). CRP has been identified in drusen via immunohistochemistry (Johnson et al., 2006; Laine et al., 2007) and has been shown to be elevated in serum of AMD patients (Seddon et al., 2004); however, the precise function of CRP in the chorioretinal tissue and its effects on AMD progression are largely unknown. CRP levels were shown to be elevated in the choroid of CFH H402 patients compared to CFH Y402 (Johnson et al., 2006). Another study showed that the CFH H402 variant exhibited decreased binding to CRP and that binding occurred only to denatured CRP in the protomeric form, which was not thought to be of physiologic relevance (Hakobyan et al., 2008). This was revisited by Okemefuma et al., who showed that there was CRP binding at two different CFH sites (including SCR 7) under physiological conditions (Okemefuna et al., 2010). Other studies have shown that although CRP exists as a non-covalent pentamer composed of five identical subunits, it can dissociate into monomers under oxidative stress and upon interaction with bioactive lipids (Eisenhardt et al., 2009a; Eisenhardt et al., 2009b; Ji et al., 2007; Lauer et al., 2011; Thiele et al., 2014; Volanakis, 2001). Molins et al. showed that the monomeric form and not the pentameric form of CRP can bind to CFH at physiologically relevant concentrations and that the CFH H402 risk-variant binding was reduced compared to the CFH Y402 binding, which could increase inflammatory-mediated damage (Molins et al., 2016). In addition, Chirco et al. demonstrated that monomeric CRP is prevalent in the choriocapillaris and increased in patients with CFH H402 variant (Chirco et al., 2016), as originally shown by Johnson et al. (Johnson et al., 2006). Molins et al. propose that formation of monomeric CRP (either by spontaneous generation from the pentameric form or via activity of bioactive lipids on damaged cells under pathological conditions) leads to the activation of cytokines, a process that is attenuated by CFH, in a variant-dependent manner (Molins et al., 2016). However, future investigation into the pathophysiology surrounding monomeric CRP and its mechanistic relationship to the development of AMD in vivo is needed to clarify this relationship.
5.3 Interaction with heparan sulfate
The CFH molecule has two heparan sulfate (HS)-binding domains (SCRs 6–8 and SCRs 19–20) that facilitate binding to host cell surface GAGs, thereby protecting host tissue from complement damage (Schmidt et al., 2008). In a series of publications from 2006 to 2013, using both in vitro assays and ex vivo BrM-binding assays Clark et al. described CFH H402 as having decreased binding to BrM-associated HS compared to the CFH Y402 variant (Clark et al., 2006; Clark et al., 2010; Clark et al., 2013). These observations provided an explanation for how the H402 variant might increase AMD risk. Based on the fact that BrM is rich in HS, it was hypothesized that individuals with the CFH H402 variant have lowered levels of BrM-associated CFH due to its reduced affinity for HS. This likelihood was subsequently validated in post-mortem human BrM tissue by this same group of researchers (Keenan et al., 2014). Thus, lowered affinity for HS in the CFH H402 variant could lead to increased AMD risk by decreasing the total amount of complement-inhibiting CFH present in BrM. While very compelling, these studies relied on the use of recombinant fragments (SCRs 6–8) of the CFH protein instead of the full-length protein. We and others have had difficulty reproducing the observed decreased affinity of the CFH H402 variant for HS in experiments using the purified, full-length CFH protein (Kelly et al., 2010; Ormsby et al., 2008) (Fig. 7). We suspect that recombinant fragments may not fully represent the three-dimensional flexibility and/or post-translational modifications of the native protein; nor, do heparin plate-binding assays fully represent the complexity of HS proteoglycans present within human BrM. We have found that on BrM explants there is no difference between the binding of full-length CFH H402 and that of full-length CFH Y402 (Fig. 7). Thus, further studies of the effects of the CFH H402 variant on CFH binding to HS are warranted to resolve these discrepancies.
Figure 7.
CFH 402H variant does not show decreased binding to human BrM/choroid compared to the Y402 variant. Human BrM/choroid tissue punches from aged (73–79 years old), genotype-matched post-mortem donors were isolated, treated to remove adherent cells from BrM, and exposed to 1 μM full-length CFH Y402 (N=10) or CFH H402 (N=11) freshly isolated from homozygous blood bank donors as previously described (Kelly et al., 2010). Bound CFH was quantified by Western blot using CFH standard curves Equal binding was noted and type II error was calculated to establish statistical significance of the null hypothesis (β<0.05).
Crystal structure and mutation analyses of recombinant fragments of CFH suggest that the CFH H402 amino acid is involved in HS binding in a more complex manner than what was initially assumed (Prosser et al., 2007). Specifically, the authors suggest that the CFH H402 variant is involved in recognizing specific sulfation patterns, as supported experimentally by Clark et al. in 2013 (Clark et al., 2013; Prosser et al., 2007). A new paradigm was proposed in which specific sulfation patterns within the BrM extracellular matrix form “zip codes” whereby CFH is recruited (Keenan et al., 2014). Based on this paradigm the CFH H402 polymorphism alters the “zip code” recognition of CFH, which could result in lower levels of the H402 variant in BrM compared to the Y402 variant(Keenan et al., 2014). This paradigm, however, would be greatly strengthened by in vivo validation.
5.4 Binding of oxidative stress markers
Increased oxidative stress, particularly due to smoking, is associated with AMD pathogenesis (Beatty et al., 2000; Hollyfield et al., 2008; Seddon et al., 2006; Seddon et al., 1996). Using an unbiased proteomic approach to identify plasma proteins that interact with malondialdehyde (MDA), a marker of lipid peroxidation associated with oxidative stress, Weismann et al. discovered that CFH is a major MDA-binding protein and established it as an innate defense protein against MDA (Weismann et al., 2011). The binding of CFH to MDA results in masking of the pro-inflammatory activities of MDA (Weismann et al., 2011). It was further documented that the CFH H402 variant binds MDA less efficiently than the Y402 variant, predicting that patients with H402 variant have an impaired ability to regulate oxidative stressors and implicating it as a possible mechanism for the increased risk for AMD associated with the H402 variant (Weismann et al., 2011).
CFH interaction with oxidized phospholipids was further supported in a study by Shaw et al. (Shaw et al., 2012), demonstrating that the CFH H402 variant bound with less affinity to oxidized phospholipids than the normal Y402 form (Shaw et al., 2012). The implication that CFH regulates the inflammatory responses caused by MDA and oxidized phospholipids is particularly intriguing since these findings provide a link between oxidation motifs, lipids and CFH H402 risk association in AMD. Studies of a mouse model expressing a chimeric CFH transgene with the human SCRs 6–8 sequences of the H402 or Y402 CFH variants (chCFHTg mice, (Aredo et al., 2015)) indicate that such interactions are present in vivo. This model shows that the H402 variant in CFH SCR6–8 leads to higher levels of MDA-adducts compared to normal age-matched C57BL/6 controls, as well as increased microglial/macrophage uptake of MDA (Aredo et al., 2015).
6. Mouse Models of Complement Dysregulation and AMD
With the relationship between complement activation and AMD established in human patients, mouse models have provided a conduit to dissect the molecular mechanisms linking exaggerated complement responses and AMD pathobiology. While none of the available models fully recapitulate human AMD pathogenesis, studies of these models have provided important insights into the role of complement in numerous AMD-associated disease processes.
6.1 Complement effects in the laser-induced CNV mouse model
Early studies delving into the effects of complement in murine models of AMD focused on wet AMD, which is traditionally modeled by laser-induced choroidal neovascularization (CNV) in mice (described by (Ishibashi et al., 1987) and recently revisited by (Poor et al., 2014)). Laser-induced CNV is a frequently used experimental technique that induces breaks in BrM, stimulates neovascularization from the choriocapillaris, and resembles CNV seen in exudative AMD (Lambert et al., 2013). The laser-induced CNV mouse model of exudative AMD produces relatively quick results within a few weeks, but it may not reflect the progressive nature of AMD development and most likely reflects a wound healing response (Grossniklaus et al., 2010). Despite this caveat, the laser-induced CNV mouse model has been used to establish a role for complement in CNV development and progression, specifically the alternative pathway (Bora et al., 2006; Rohrer et al., 2011; Schnabolk et al., 2015), complement anaphylatoxins C3a and C5a (Coughlin et al., 2016; Nozaki et al., 2006) and MAC (Bora et al., 2005). In fact, over activation of the alternative pathway contributes to CNV lesion size as supported by increased laser-induced CNV lesion size in mice treated with siRNAs targeting mouse Cfh (Lyzogubov et al., 2010) and in CFH deficient mice (Lundh von Leithner et al., 2009). Complement activation in laser-induced CNV was detected at lesion sites in the posterior eye using systemic injection of monoclonal C3d antibodies (Thurman et al., 2013). Studies by Rohrer et al. showed that mice lacking the alternative pathway (FB deficient) exhibited reduced levels of CNV following laser treatment compared to mice lacking the classical pathway (C1q deficient) or MBL pathway (MBL deficient) (Rohrer et al., 2009). In addition, the same group showed that forced expression of FB in the RPE promotes laser-induced CNV lesions (Schnabolk et al., 2015). Using a novel recombinant protein that targets CFH-mediated alternative pathway inhibition to areas of complement activation (CR2-fH) (Huang et al., 2008), Rohrer and colleagues showed that pharmacological inhibition of the alternative pathway reduces laser-induced CNV (Rohrer et al., 2012; Rohrer et al., 2009). Recent studies by Poor et al. (2014) cite a need for repetition of some of the published laser CNV studies due to inconsistent strain- and vendor-dependent differences (Poor et al., 2014). However, multiple studies have confirmed the role of the alternative pathway and C5a in the laser-induced CNV model in mice (Coughlin et al., 2016; Nozaki et al., 2006). Specific mechanisms of action remain to be elucidated.
6.2 Complement and oxidative stress injury models
Smoking is one of the strongest environmental risk factors for development of AMD (Klein et al., 1993; Myers et al., 2014). Considering this association, researchers have utilized smoke-induced ocular injury to model ocular damage seen in AMD (Fujihara et al., 2008; Wang et al., 2009a). The alternative pathway also appears to play a role in smoke-induced ocular injury, as Factor B deficient mice have reduced visual function decline and less outer nuclear layer thinning compared to wild type mice treated with cigarette smoke (Woodell et al., 2013). In vivo studies in these alternative pathway deficient mice exposed to cigarette smoke, accompanied by studies of RPE cell cultures exposed to cigarette smoke extract, revealed a stress-mediated lipid accumulation in the ER and lipid secretion supporting a synergistic interaction of oxidative stress and the alternative pathway in AMD (Kunchithapautham et al., 2014). Cigarette smoke is thought to contribute to AMD pathobiology through the creation of free radicals (Church and Pryor, 1985) and direct activation of the complement cascade (Kew et al., 1985; Robbins et al., 1991) (recently reviewed in (Datta et al., 2017). The above results suggest that at least some of the retinal pathology resulting from smoking appears to be due to complement activation. In fact, treating mice chronically exposed to cigarette smoke with the complement inhibitor CR2-fH prevented smoke-induced retinal damage (Woodell et al., 2016).
6.3 Complement component knockout mice and aging
We and other groups have studied the combined effect of aging and complement deficiency on ocular phenotype in mouse models, attempting to link the age-associated risk of AMD with complement dysregulation (Coffey et al., 2007; Ding et al., 2015; Hoh Kam et al., 2013; Hoh Kam et al., 2016; Toomey et al., 2015). Initial investigation of aged (~2 year) Cfh−/− mice revealed that they had reduced scotopic electroretinograms, thinning of the outer nuclear layer and thinning of BrM (Coffey et al., 2007). Given the significant accumulation of lipid deposits in BrM seen in AMD (discussed above), thinning BrM in aged Cfh deficient mice was inconsistent with an AMD-like phenotype. However, subsequent analysis of Cfh deficient mice by our lab and Hoh Kam et al. (2013) showed that these mice indeed had thickened BrM and accumulation of basal laminar deposits compared to age-matched controls, showing that CFH influences the development of basal laminar deposits in vivo (Ding et al., 2015; Hoh Kam et al., 2013; Toomey et al., 2015). A study comparing the effect of rearing the mice in a pathogen free, barriered environment to a conventional open environment found that there was reduced pathology in the age-matched mice raised in a pathogen-free facility (Hoh Kam et al., 2016), which may have contributed to the lack of observed basal deposits in studies by Coffey et al. (2007). One study described C3 and C3b deposition on retinal vessels leading to decreased oxygen supply (Lundh von Leithner et al., 2009). Although our group has had trouble replicating increased C3 deposition in Cfh knockout mice, it is likely that CFH plays a complement dependent role in choroidal neovascularization (discussed further in section 6.5) (Ding et al., 2015; Toomey et al., 2015). In fact, using double knockout Cfh and C3 deficient mice Hoh Kam et al. showed that C3 deficiency did not facilitate the predicted rescue of the Cfh knockout ocular phenotype (Hoh Kam et al., 2013). The absence of CFH in Cfh knockout mice results in a functional deficiency of C3 in the circulation due to rapid consumption of C3b (Pickering et al., 2002; Toomey et al., 2015). Together our results support a role for CFH in regulating basal laminar deposit formation in vivo that is independent of its role in the complement cascade as detailed in the following section, although, some effects including visual function decline and RPE damage appear to occur in a complement-dependent manner (Toomey et al., 2015).
6.4 Multifactorial Mouse Model of Complement Dysregulation
Given the complexity of AMD pathogenesis, we developed a multifactorial, complement-dysregulated mouse model based on several risks associated with the human disease: advanced age, complement activation based on mouse Cfh gene knock out (Cfh−/−) or haploinsufficiency (Cfh+/−) (Pickering et al., 2002) and high fat, cholesterol-enriched (HFC) diet. Using this model we showed that Cfh+/− mice, when maintained on the HFC diet, exhibit a robust AMD-like ocular phenotype that includes formation of activated complement, basal laminar deposits, RPE cell pathology and visual function deficits (Fig. 8) (Toomey et al., 2015). Importantly, we showed that damage to the RPE layer and visual function decline was dependent on an intact complement cascade (Toomey et al., 2015). Remarkably, we additionally demonstrated that basal laminar deposit formation occurred in a CFH-dependent, but complement-independent manner (Toomey et al., 2015). Specifically, we found that there was greater basal laminar deposit accumulation in both the Cfh−/− and the Cfh+/− mice fed the HFC diet compared to age-matched normal C57BL/6 mice on HFC (Fig. 8A and B) (Toomey et al., 2015). This led us to hypothesize a non-canonical function of the CFH protein in regulating sub-RPE lipoprotein accumulation and basal laminar deposit formation (Toomey et al., 2015). Based on the assumption that sub-RPE deposit formation occurs following lipoprotein accumulation in BrM (Curcio et al., 2011), we showed that CFH and lipoproteins compete for novel binding sites in the HSPG-rich extracellular matrix of BrM (Fig. 9) (Toomey et al., 2015). We hypothesize that CFH and RPE-derived lipoproteins (potentially with oxidative modifications) interact at three-dimensional GAG motifs in BrM, which is altered by the CFH H402 polymorphism (Fig. 10). We propose that patients with the CFH H402 variant develop sub-RPE lipoprotein accumulation faster than patients with the CFH Y402 variant due to the reduced competition for GAG motifs in BrM by CFH H402. Consequently, increased BrM lipoprotein accumulation would lead to earlier development of drusen and resultant AMD pathology. Interestingly, individuals expressing the AMD-risk-associated CFH C1210 variant have increased macular drusen loads and develop later stages of AMD earlier than individuals with the R1210 variant (Ferrara and Seddon, 2015). This hypothesis provides a link between the CFH-binding properties altered by the Y402H polymorphism and the pathognomonic features of early AMD.
Figure 8.
Contrasting the ocular phenotypes of aged Cfh−/− and Cfh+/−~HFC mice shows a non-canonical function of CFH protein in regulating basal laminar deposit formation and complement-dependent impact on visual function decline. (A) Transmission electron micrographs showing basal laminar deposits along Bruch’s membrane (BrM, arrowhead). Large (>4mm high) deposits were often seen in the Cfh+/−~HFC and Cfh−/−~HFC mice. (B) Distribution of basal laminar deposit heights for the three mouse genotypes fed a HFC diet. These cumulative frequency curves illustrate the increased basal laminar deposit load present in both the Cfh−/− and Cfh+/− HFC-fed animals compared to wild type C57BL/6 HFC fed (B6~HFC) animals. Thus, mice with decreasing levels of CFH over the 8 week period of HFC diet challenge developed increased basal laminar deposit volumes, indicating that CFH plays a role in retarding basal laminar deposit formation (C) Scotopic electroretinogram (ERG) flash responses in wild type B6, Cfh+/− and Cfh−/− mice fed a normal diet (ND) or HFC diet. B6 and Cfh−/− mice showed no statistically significant depression of ERG amplitude with HFC diet. However, Cfh+/−~HFC mice showed a marked decrease in b-wave amplitude (middle graph). These data show that although Cfh+/− and Cfh−/− mice develop significant deposits only Cfh+/− mice, with an intact complement cascade, develop significant visual function loss. Data is presented as mean ± SE. * indicates post-hoc Tukey for a p<0.05 following a statistically significant genotype by diet interaction by ANOVA. N=6–8 per group. (Adapted from Toomey et al. PNAS 2015)
Figure 9.
CFH competes for lipoprotein-binding sites in BrM. (A–B) Human BrM samples from aged post-mortem donors were incubated with or without 1μM of human CFH protein. (A) Immunohistochemical staining for CFH (green) in the BrM samples. (B) FPLC fractionation of human BrM lysates shows endogenous lipoproteins present in aged BrM tissue are removed by the addition of CFH (1 μM CFH, green trace). The asterisk (*) indicates P < 0.05 for total cholesterol in each fraction comparing 0 μM CFH to 1 μM CFH (three independent experiments, each with n = 3) (From Toomey et al., PNAS 2015).
Figure 10.
Hypothesis linking CFH polymorphism and AMD pathobiology. Our group proposes that CFH and RPE-derived lipoproteins (with or without possible oxidative modifications) compete for binding sites in GAG motifs in BrM. Binding affinity for these sites differs in CFH Y402H variants such that decreased affinity in the H402 variant leads to increased lipoprotein accumulation in BrM and the formation of sub-RPE deposits (drusen).
Pierce and colleagues also tested the role of complement in basal laminar deposit formation in a mouse model of inherited macular dystrophy (Fernandez-Godino et al., 2015; Garland et al., 2014). Using a mouse model of inherited macular dystrophy (Doyne Honeycomb Retinal Dystrophy/Malattia Leventinese) caused by a mutation in EFEMP1 and in mouse RPE cell cultures these studies tested the effect of complement components on basal laminar deposit formation and showed that formation of deposits in vivo and in culture was mediated by C3a and not C5a (Fernandez-Godino et al., 2015; Garland et al., 2014). Our results in a separate model confirm that C5a does not appear to be involved in basal laminar deposit formation in mice (see below); furthermore, in vitro cigarette smoke extract exposure also appears to cause intracellular lipid accumulation that is dependent on C3aR (Kunchithapautham et al., 2014). The precise mechanism by which C3aR may affect sub-RPE deposit formation is unclear; however, Fernandez-Godino et al. proposed that the pro-inflammatory factors, IL-6 and IL-1-beta are involved in downstream events (Fernandez-Godino et al., 2015).
Calippe et al., have recently revealed an additional non-canonical role for CFH in inhibiting sub-retinal mononuclear phagocyte clearance in a Cx3cr1 model of chronic retinal inflammation and in Apolipoprotein E2 humanized transgenic mice (Calippe et al., 2017). The authors show that CFH via interaction with CD11b leads to reduced signaling through the TCD47-TSP1axis (Calippe et al., 2017). Most interestingly their paradigm predicts that the CFH protein promotes rather than protects against AMD pathology, a concept that has not been appreciated in many of the clinical studies and models involving CFH in AMD discussed above.
6.5 Effect of Targeting C5a in Mouse Models of AMD
Recently, our group has evaluated the role of C5a using systemic delivery of a monoclonal antibody targeting C5a. We show that anti-C5a appears to regulate monocyte and mononuclear phagocyte populations in the choroid in the setting of chronic complement dysregulation seen in the Cfh+/−~HFC model. Anti-C5a has anti-exudative properties relevant to the treatment of “wet” AMD, but does not appear to be capable of preventing basal laminar deposits, RPE damage or visual function decline seen in our model of early AMD (Toomey et al. 2017, in review). The anti-exudative properties of C5a were previously shown in several studies relying on the CNV laser model that implicated C5a in acute inflammation in the chorioretinal environment (Coughlin et al., 2016; Nozaki et al., 2006). Nozaki et al. proposed a role for C5a in recruiting monocytes and in production of VEGF contributing to CNV (Nozaki et al., 2006). We suspect that this monocyte recruitment is secondary to activation of adhesion molecules on choroidal endothelial cells by C5a (Skeie et al., 2010). Coughlin et al., propose that gamma-delta T-cells and IL-17 production in the choroid are dependent on C5a following laser-induced CNV (Coughlin et al., 2016). While these studies support a therapeutic advantage of anti-C5a for wet AMD based on its effects in the laser-induced CNV model, its effects on chronic inflammatory stress from complement activation in the chorioretinal environment underlying dry AMD pathobiology had not been addressed.
6.6 Modeling the CFH H402 variant in vivo
6.6.1 Chimeric transgenic CFH SCR6–8 expression driven by mouse ApoE promoter
The first study to model the CFH 402 variant in mouse was published by Ufret-Vincenty et al. in 2010. The authors used transgenic mice expressing the human CFH sequence for SCRs 6–8 (with either the Y402 or H402 variant), flanked by the mouse sequence for SCRs 1–5 and SCRs 9–20 under the control of the mouse apoE promoter crossed onto the Cfh knockout background (Ufret-Vincenty et al., 2010). They showed that this results in stable expression of chimeric CFH proteins in relevant tissues, liver and whole eye, and functional rescue of peripheral complement deficiencies seen in Cfh knockout mice. However, both the CFH H402 and CFH Y402 chimeric transgenic animals showed evidence of ocular pathology compared to wild type C57BL/6 controls, particularly accumulation of sub-retinal Iba1+ cells and basal laminar deposits (Ufret-Vincenty et al., 2010). This is unexpected since the Y402 variant of CFH is not associated with risk in human and the H402 variant, though associated with AMD risk in humans, is not a mutation, but is a common variant strongly associated with AMD risk and therefore does not singularly cause AMD pathology. The same group proposed this model could be used to investigate the role of altered protein-protein interactions in vivo. Chimeric SCRs 6–8 yielded interesting observations regarding the interaction of SCRs 6–8 and malondialdehyde (MDA) -modified proteins discussed earlier (Aredo et al., 2015).
6.6.2 Transgenic full-length CFH expression using bacterial artificial chromosomes
In 2015, our group published the ocular phenotype of transgenic mice created using gene constructs expressing full-length human CFH. Human bacterial artificial chromosomes (BAC) containing the CFH Y402 or CFH H402 gene variant were used to generate transgenic mice expressing the full-length Y402 and H402 variants of human CFH. These mice were then crossed with Cfh knockout mice to generate transgenic mice that were hemizygous (one human CFH gene and no copies of the mouse Cfh gene), and expressed the Y402 or H402 variant of human CFH but no murine CFH (Ding et al., 2015). We showed that the human CFH is expressed in relevant tissues, including the liver, kidney and eye, but at significantly lower levels than CFH in wild type mice. Expression of human CFH Y402, but not CFH H402, rescues the kidney phenotype seen in Cfh knockout mice (Ding et al., 2015). The CFH H402 mice also develop basal laminar deposits (Ding et al., 2015). However, these differences between the Y and H402 expressing mice were complicated by the fact that the CFH Y402 Cfh knockout mouse (CFH Y402:Cfh−/−) expresses 2-fold greater levels of CFH compared to the CFH H402 Cfh knockout mouse (Ding et al., 2015). In order to remove the confounding effect of different levels of expression of CFH in these mice, CFH H402:Cfh−/− mice were crossed to generate homozygous CFH HH402:Cfh−/− mice that express the same levels of CFH as the hemizygous CFH Y402:Cfh−/− animals (Ding et al., 2015). This increased expression of the H402 variant of CFH is sufficient to rescue these mice from the kidney damage seen in Cfh−/− and CFH H402:Cfh−/− mice (Ding et al., 2015). Work is now underway to definitively determine if expression of the AMD risk-associated H402 variant compared to the Y402 CFH leads to an AMD-phenotype in our multifactorial mouse model that invokes human CFH expression, advanced age and a high fat, cholesterol-enriched (HFC) diet.
7. Human Studies Implicating Complement Damage in AMD
Several groups have sought to relate circulating plasma levels of complement activation products and AMD. Two groups showed that increased levels of complement breakdown products, Factor Bb and C5a were independently associated with AMD (Reynolds et al., 2009; Silva et al., 2012). In addition, local complement activation was described in patients with AMD and accumulation of complement activation byproducts have been detected in drusen (Hageman et al., 2001; Loyet et al., 2012; Mullins et al., 2011; Mullins et al., 2014). MAC formation was increased in AMD patients compared to controls and appears to be primarily concentrated in the choriocapillaris and capillary pillars (Mullins et al., 2014). MAC also appears to be increased in the choriocapillaris of patients with the CFH Y402H polymorphism (Mullins et al., 2011). Furthermore, increased levels of the complement breakdown product C3a were detected in BrM/choroid extracts with increasing histological grade of AMD pathology in post-mortem tissue (Loyet et al., 2012).
There are two major effector functions of the complement cascade: (1) the formation of the MAC, resulting in cell lysis, and (2) the formation of the anaphylatoxins, C3a and C5a, resulting in immune cell recruitment (Sarma and Ward, 2011). Immunohistochemical analysis of MAC in RPE/choroid of AMD patients revealed that although significant MAC is found on and around drusen, it is generally not detected in association with RPE cell surfaces (Johnson et al., 2000; Mullins et al., 2011). It is thought that, due to the high levels of membrane-bound complement inhibitors, RPE are sufficiently protected from MAC formation (Yang et al., 2009). A series of experiments by Mullins and colleagues have investigated the role of MAC in AMD. Whitmore et al. proposed a model of AMD pathogenesis centering around MAC formation in the aging choriocapillaris (recently reviewed in (Whitmore et al., 2015)). Studies by this group have shown that MAC forms with age in the choriocapillaris and this is exacerbated in people with the CFH H402 variant (Mullins et al., 2011; Mullins et al., 2014). They propose that chronic MAC formation leads to vascular loss in the choroid, impacting the blood supply to BrM, the RPE and photoreceptors (Whitmore et al., 2015). In fact, hypoxia-induced metabolic stress has been shown to be sufficient to produce photoreceptor degeneration in mice (Kurihara et al., 2016). Due to the lack of an anti-mouse C5b-9 antibody, hypothesis driven experiments evaluating the role of MAC formation in either the RPE or choriocapillaris in mouse models has been difficult.
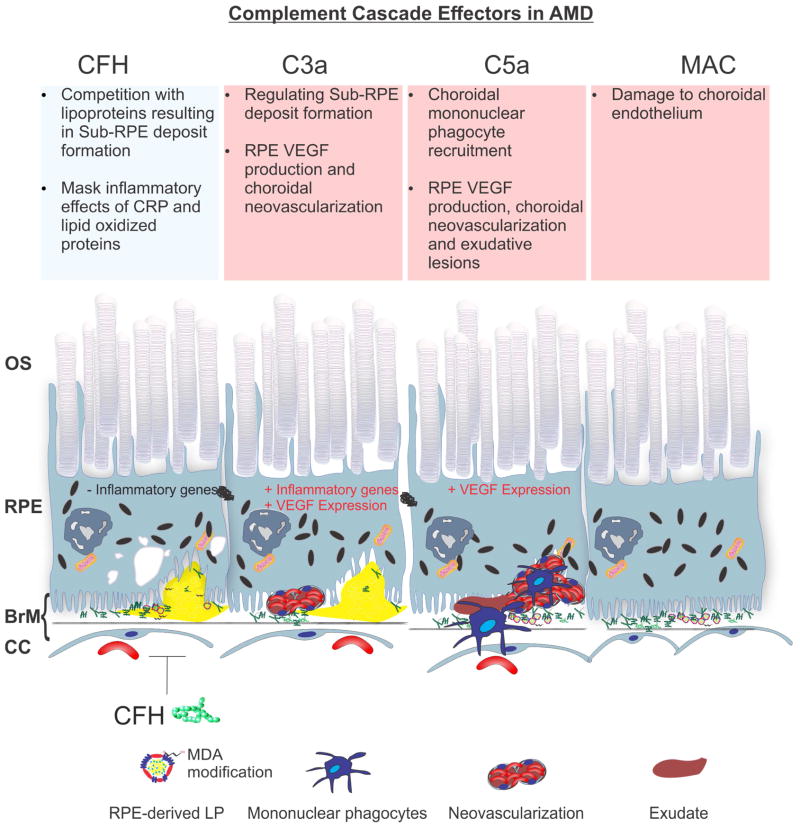
Collectively, a variety of studies have provided insight into the role specific effector functions of the complement cascade in AMD. Based on the findings in humans by Whitmore et al. (Whitmore et al., 2015), a hypothesis emerges in which MAC formation leads to significant damage to the choroidal endothelium and decreased blood supply to the RPE in mouse models as well. Decreased blood supply could lead to RPE hypoxia and subsequent damage to the RPE and photoreceptors (as suggested by the work in mouse by Kurihara et al. (Kurihara et al., 2016)). It appears that C5a plays a role in mononuclear phagocyte recruitment and choroidal neovascularization; whereas, C3a (Fernandez-Godino et al., 2015) and CFH (Toomey et al., 2015) play roles in sub-RPE deposit formation and choriocapillaris MAC formation (Whitmore et al., 2015) that may lead to choriocapillaris damage and visual function decline (Kurihara et al., 2016) (Fig. 11).
Figure 11.
Complement damage and AMD pathobiology. Analysis of human post-mortem tissue, mouse models and in vitro assays have elucidated that different components of the complement cascade appear to regulate or stimulate specific pathobiology in AMD. From left to right, CFH appears to influence sub-RPE deposit formation and RPE inflammatory responses, C3a appears to act as stimulus for sub-RPE deposit formation, RPE inflammatory responses and neovascularization, C5a appears to stimulate RPE inflammatory responses, mononuclear phagocyte recruitment, neovascularization and exudative lesions; MAC appears to mediate the destruction of choroidal endothelium and the formation of ghost capillaries in the choriocapillaris.
8. Summary and Future Directions
AMD is a complex multifactorial disease characterized in its early stages by lipoprotein accumulations in BrM and in its late forms by neovascularization (“wet” form) or geographic atrophy of the RPE cell layer (“dry” form). Genetic studies have strongly supported a relationship between the alternative complement cascade, including the common CFH H402 variant, and development of AMD. In the past decade, research has shown functional differences in the CFH H402 variant in terms of CRP binding, masking of lipoprotein peroxidation epitopes and binding to heparan sulfate proteoglycans. New data from ex vivo binding assays, in vivo mouse models and postmortem human tissue indicate that the CFH H402 variant may have reduced affinity for heparan sulfate proteoglycans leading to lipoprotein accumulation in BrM and ensuing drusen progression. The roles of additional complement components, including C3a, C5a and MAC, in AMD pathobiologies are also under investigation, as are ongoing studies in transgenic mice attempting to model effects of the H402 variant in CFH.
Acknowledgments
The authors acknowledge the funding support from the National Institutes of Health (EY026161 to CBR), P30 EY005722 (to V. Arshavsky), and T32 GM007171-Medical Scientist Training Program (to C.B.T.), an unrestricted grant from Research to Prevent Blindness (to the Duke Eye Center), and a grant from the Foundation Fighting Blindness (C.B.R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abecasis GR, Burt RA, Hall D, Bochum S, Doheny KF, Lundy SL, Torrington M, Roos JL, Gogos JA, Karayiorgou M. Genomewide scan in families with schizophrenia from the founder population of Afrikaners reveals evidence for linkage and uniparental disomy on chromosome 1. Am J Hum Genet. 2004;74:403–417. doi: 10.1086/381713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study 2 Research G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. American journal of ophthalmology. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Progress in retinal and eye research. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M, McKeigue PM, Skerka C, Hayward C, Rudan I, Vitart V, Polasek O, Armbrecht AM, Yates JR, Vatavuk Z, Bencic G, Kolcic I, Oostra BA, Van Duijn CM, Campbell S, Stanton CM, Huffman J, Shu X, Khan JC, Shahid H, Harding SP, Bishop PN, Deary IJ, Moore AT, Dhillon B, Rudan P, Zipfel PF, Sim RB, Hastie ND, Campbell H, Wright AF. Genetic influences on plasma CFH and CFHR1 concentrations and their role in susceptibility to age-related macular degeneration. Hum Mol Genet. 2013;22:4857–4869. doi: 10.1093/hmg/ddt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aredo B, Li T, Chen X, Zhang K, Wang CX, Gou D, Zhao B, He Y, Ufret-Vincenty RL. A chimeric Cfh transgene leads to increased retinal oxidative stress, inflammation, and accumulation of activated subretinal microglia in mice. Invest Ophthalmol Vis Sci. 2015;56:3427–3440. doi: 10.1167/iovs.14-16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augood CA, Vingerling JR, de Jong PT, Chakravarthy U, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Bentham G, Rahu M, Vioque J, Young IS, Fletcher AE. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE) Arch Ophthalmol. 2006;124:529–535. doi: 10.1001/archopht.124.4.529. [DOI] [PubMed] [Google Scholar]
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE, Klein R, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- Black JR, Clark SJ. Age-related macular degeneration: genome-wide association studies to translation. Genet Med. 2016;18:283–289. doi: 10.1038/gim.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrin D, Stehle T. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol. 2015;11:77–82. doi: 10.1038/nchembio.1696. [DOI] [PubMed] [Google Scholar]
- Bora NS, Kaliappan S, Jha P, Xu Q, Sohn JH, Dhaulakhandi DB, Kaplan HJ, Bora PS. Complement activation via alternative pathway is critical in the development of laser-induced choroidal neovascularization: role of factor B and factor H. J Immunol. 2006;177:1872–1878. doi: 10.4049/jimmunol.177.3.1872. [DOI] [PubMed] [Google Scholar]
- Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, Kaliappan S, Kaplan HJ, Bora NS. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- Bretillon L, Thuret G, Gregoire S, Acar N, Joffre C, Bron AM, Gain P, Creuzot-Garcher CP. Lipid and fatty acid profile of the retina, retinal pigment epithelium/choroid, and the lacrimal gland, and associations with adipose tissue fatty acids in human subjects. Exp Eye Res. 2008;87:521–528. doi: 10.1016/j.exer.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Buddles MR, Donne RL, Richards A, Goodship J, Goodship TH. Complement factor H gene mutation associated with autosomal recessive atypical hemolytic uremic syndrome. Am J Hum Genet. 2000;66:1721–1722. doi: 10.1086/302877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkowski RJ, Wieslander J, Kleppel M, Michael AF, Fish AJ. Basement membrane collagen in the kidney: regional localization of novel chains related to collagen IV. Kidney Int. 1989;35:1195–1202. doi: 10.1038/ki.1989.110. [DOI] [PubMed] [Google Scholar]
- Calippe B, Augustin S, Beguier F, Charles-Messance H, Poupel L, Conart JB, Hu SJ, Lavalette S, Fauvet A, Rayes J, Levy O, Raoul W, Fitting C, Denefle T, Pickering MC, Harris C, Jorieux S, Sullivan PM, Sahel JA, Karoyan P, Sapieha P, Guillonneau X, Gautier EL, Sennlaub F. Complement Factor H Inhibits CD47-Mediated Resolution of Inflammation. Immunity. 2017;46:261–272. doi: 10.1016/j.immuni.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Caprioli J, Castelletti F, Bucchioni S, Bettinaglio P, Bresin E, Pianetti G, Gamba S, Brioschi S, Daina E, Remuzzi G, Noris M, Familial HT International Registry of R. Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: the C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet. 2003;12:3385–3395. doi: 10.1093/hmg/ddg363. [DOI] [PubMed] [Google Scholar]
- Chen L, Miyamura N, Ninomiya Y, Handa JT. Distribution of the collagen IV isoforms in human Bruch’s membrane. Br J Ophthalmol. 2003;87:212–215. doi: 10.1136/bjo.87.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, Tan PL, Conley YP, Kanda A, Kopplin L, Li Y, Augustaitis KJ, Karoukis AJ, Scott WK, Agarwal A, Kovach JL, Schwartz SG, Postel EA, Brooks M, Baratz KH, Brown WL, Brucker AJ, Orlin A, Brown G, Ho A, Regillo C, Donoso L, Tian L, Kaderli B, Hadley D, Hagstrom SA, Peachey NS, Klein R, Klein BE, Gotoh N, Yamashiro K, Ferris F, III, Fagerness JA, Reynolds R, Farrer LA, Kim IK, Miller JW, Corton M, Carracedo A, Sanchez-Salorio M, Pugh EW, Doheny KF, Brion M, Deangelis MM, Weeks DE, Zack DJ, Chew EY, Heckenlively JR, Yoshimura N, Iyengar SK, Francis PJ, Katsanis N, Seddon JM, Haines JL, Gorin MB, Abecasis GR, Swaroop A. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirco KR, Scott Whitmore S, Wang K, Potempa LA, Halder JA, Stone EM, Tucker BA, Mullins RF. Monomeric C-reactive protein and inflammation in Age-related Macular Degeneration. J Pathol. 2016 doi: 10.1002/path.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen WG, Glynn RJ, Manson JE, Ajani UA, Buring JE. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. Jama. 1996;276:1147–1151. [PubMed] [Google Scholar]
- Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SJ, Higman VA, Mulloy B, Perkins SJ, Lea SM, Sim RB, Day AJ. His-384 allotypic variant of factor H associated with age-related macular degeneration has different heparin binding properties from the non-disease-associated form. The Journal of biological chemistry. 2006;281:24713–24720. doi: 10.1074/jbc.M605083200. [DOI] [PubMed] [Google Scholar]
- Clark SJ, Perveen R, Hakobyan S, Morgan BP, Sim RB, Bishop PN, Day AJ. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch’s membrane in human retina. The Journal of biological chemistry. 2010;285:30192–30202. doi: 10.1074/jbc.M110.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SJ, Ridge LA, Herbert AP, Hakobyan S, Mulloy B, Lennon R, Wurzner R, Morgan BP, Uhrin D, Bishop PN, Day AJ. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. Journal of immunology. 2013;190:2049–2057. doi: 10.4049/jimmunol.1201751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey PJ, Gias C, McDermott CJ, Lundh P, Pickering MC, Sethi C, Bird A, Fitzke FW, Maass A, Chen LL, Holder GE, Luthert PJ, Salt TE, Moss SE, Greenwood J. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc Natl Acad Sci U S A. 2007;104:16651–16656. doi: 10.1073/pnas.0705079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville D, Guymer R, Sinclair RA, Savige J. Visual impairment caused by retinal abnormalities in mesangiocapillary (membranoproliferative) glomerulonephritis type II ("dense deposit disease") Am J Kidney Dis. 2003;42:E2–5. doi: 10.1016/s0272-6386(03)00665-6. [DOI] [PubMed] [Google Scholar]
- Coughlin B, Schnabolk G, Joseph K, Raikwar H, Kunchithapautham K, Johnson K, Moore K, Wang Y, Rohrer B. Connecting the innate and adaptive immune responses in mouse choroidal neovascularization via the anaphylatoxin C5a and gammadeltaT-cells. Sci Rep. 2016;6:23794. doi: 10.1038/srep23794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. The British journal of ophthalmology. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M. Structure, Function, and Pathology of Bruch’s Membrane. In: Ryan SJ, editor. Retina. 5. Elsevier Inc; 2013. pp. 465–481. [Google Scholar]
- Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Experimental eye research. 2005;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017 doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcourt C, Delyfer MN, Rougier MB, Amouyel P, Colin J, Le Goff M, Malet F, Dartigues JF, Lambert JC, Korobelnik JF. Associations of complement factor H and smoking with early age-related macular degeneration: the ALIENOR study. Invest Ophthalmol Vis Sci. 2011;52:5955–5962. doi: 10.1167/iovs.10-6235. [DOI] [PubMed] [Google Scholar]
- Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Molecular vision. 2003;9:184–190. [PubMed] [Google Scholar]
- Devaraj S, Kumaresan PR, Jialal I. Effect of C-reactive protein on chemokine expression in human aortic endothelial cells. J Mol Cell Cardiol. 2004;36:405–410. doi: 10.1016/j.yjmcc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Dietzel M, Pauleikhoff D, Arning A, Heimes B, Lommatzsch A, Stoll M, Hense HW. The contribution of genetic factors to phenotype and progression of drusen in early age-related macular degeneration. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. 2014;252:1273–1281. doi: 10.1007/s00417-014-2690-7. [DOI] [PubMed] [Google Scholar]
- Ding JD, Kelly U, Landowski M, Toomey CB, Groelle M, Miller C, Smith SG, Klingeborn M, Singhapricha T, Jiang H, Frank MM, Bowes Rickman C. Expression of human complement factor H prevents age-related macular degeneration-like retina damage and kidney abnormalities in aged Cfh knockout mice. Am J Pathol. 2015;185:29–42. doi: 10.1016/j.ajpath.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders FC. Beitraege zur pathologischen Anatomie des Auges. Arch Ophthalmol. 1855;1:139–165. [Google Scholar]
- Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Eisenhardt SU, Habersberger J, Murphy A, Chen YC, Woollard KJ, Bassler N, Qian H, von Zur Muhlen C, Hagemeyer CE, Ahrens I, Chin-Dusting J, Bobik A, Peter K. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ Res. 2009a;105:128–137. doi: 10.1161/CIRCRESAHA.108.190611. [DOI] [PubMed] [Google Scholar]
- Eisenhardt SU, Habersberger J, Peter K. Monomeric C-reactive protein generation on activated platelets: the missing link between inflammation and atherothrombotic risk. Trends Cardiovasc Med. 2009b;19:232–237. doi: 10.1016/j.tcm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Ennis S, Goverdhan S, Cree A, Hoh J, Collins A, Lotery A. Fine-scale linkage disequilibrium mapping of age-related macular degeneration in the complement factor H gene region. Br J Ophthalmol. 2007;91:966–970. doi: 10.1136/bjo.2007.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier CR, Johnson M, Ruberti J. Ocular biomechanics and biotransport. Annu Rev Biomed Eng. 2004;6:249–273. doi: 10.1146/annurev.bioeng.6.040803.140055. [DOI] [PubMed] [Google Scholar]
- Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2008 doi: 10.1038/ejhg.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2009;17:100–104. doi: 10.1038/ejhg.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon DT. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978;75:1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Liang X, Cruz MA, Vu H, Zhou Z, Pemmaraju N, Dong JF, Kroll MH, Afshar-Kharghan V. The interaction between factor H and Von Willebrand factor. PLoS ONE. 2013;8:e73715. doi: 10.1371/journal.pone.0073715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Godino R, Garland DL, Pierce EA. A local complement response by RPE causes early-stage macular degeneration. Hum Mol Genet. 2015;24:5555–5569. doi: 10.1093/hmg/ddv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara D, Seddon JM. Phenotypic Characterization of Complement Factor H R1210C Rare Genetic Variant in Age-Related Macular Degeneration. JAMA Ophthalmol. 2015;133:785–791. doi: 10.1001/jamaophthalmol.2015.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris FL, 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR Beckman Initiative for Macular Research Classification C. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RF. The influence of age on some ocular basement membranes. Eye (Lond) 1987;1(Pt 2):184–189. doi: 10.1038/eye.1987.35. [DOI] [PubMed] [Google Scholar]
- Francis PJ. Genetics of inherited retinal disease. J R Soc Med. 2006;99:189–191. doi: 10.1258/jrsm.99.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, Igo RP, Jr, Buitendijk GH, Sim X, Weeks DE, Guymer RH, Merriam JE, Francis PJ, Hannum G, Agarwal A, Armbrecht AM, Audo I, Aung T, Barile GR, Benchaboune M, Bird AC, Bishop PN, Branham KE, Brooks M, Brucker AJ, Cade WH, Cain MS, Campochiaro PA, Chan CC, Cheng CY, Chew EY, Chin KA, Chowers I, Clayton DG, Cojocaru R, Conley YP, Cornes BK, Daly MJ, Dhillon B, Edwards AO, Evangelou E, Fagerness J, Ferreyra HA, Friedman JS, Geirsdottir A, George RJ, Gieger C, Gupta N, Hagstrom SA, Harding SP, Haritoglou C, Heckenlively JR, Holz FG, Hughes G, Ioannidis JP, Ishibashi T, Joseph P, Jun G, Kamatani Y, Katsanis N, CNK, Khan JC, Kim IK, Kiyohara Y, Klein BE, Klein R, Kovach JL, Kozak I, Lee CJ, Lee KE, Lichtner P, Lotery AJ, Meitinger T, Mitchell P, Mohand-Said S, Moore AT, Morgan DJ, Morrison MA, Myers CE, Naj AC, Nakamura Y, Okada Y, Orlin A, Ortube MC, Othman MI, Pappas C, Park KH, Pauer GJ, Peachey NS, Poch O, Priya RR, Reynolds R, Richardson AJ, Ripp R, Rudolph G, Ryu E, Sahel JA, Schaumberg DA, Scholl HP, Schwartz SG, Scott WK, Shahid H, Sigurdsson H, Silvestri G, Sivakumaran TA, Smith RT, Sobrin L, Souied EH, Stambolian DE, Stefansson H, Sturgill-Short GM, Takahashi A, Tosakulwong N, Truitt BJ, Tsironi EE, Uitterlinden AG, van Duijn CM, Vijaya L, Vingerling JR, Vithana EN, Webster AR, Wichmann HE, Winkler TW, Wong TY, Wright AF, Zelenika D, Zhang M, Zhao L, Zhang K, Klein ML, Hageman GS, Lathrop GM, Stefansson K, Allikmets R, Baird PN, Gorin MB, Wang JJ, Klaver CC, Seddon JM, Pericak-Vance MA, Iyengar SK, Yates JR, Swaroop A, Weber BH, Kubo M, Deangelis MM, Leveillard T, Thorsteinsdottir U, Haines JL, Farrer LA, Heid IM, Abecasis GR, Consortium AMDG. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–439. 439e431–432. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, Kim IK, Cho D, Zack D, Souied E, Scholl HP, Bala E, Lee KE, Hunter DJ, Sardell RJ, Mitchell P, Merriam JE, Cipriani V, Hoffman JD, Schick T, Lechanteur YT, Guymer RH, Johnson MP, Jiang Y, Stanton CM, Buitendijk GH, Zhan X, Kwong AM, Boleda A, Brooks M, Gieser L, Ratnapriya R, Branham KE, Foerster JR, Heckenlively JR, Othman MI, Vote BJ, Liang HH, Souzeau E, McAllister IL, Isaacs T, Hall J, Lake S, Mackey DA, Constable IJ, Craig JE, Kitchner TE, Yang Z, Su Z, Luo H, Chen D, Ouyang H, Flagg K, Lin D, Mao G, Ferreyra H, Stark K, von Strachwitz CN, Wolf A, Brandl C, Rudolph G, Olden M, Morrison MA, Morgan DJ, Schu M, Ahn J, Silvestri G, Tsironi EE, Park KH, Farrer LA, Orlin A, Brucker A, Li M, Curcio CA, Mohand-Said S, Sahel JA, Audo I, Benchaboune M, Cree AJ, Rennie CA, Goverdhan SV, Grunin M, Hagbi-Levi S, Campochiaro P, Katsanis N, Holz FG, Blond F, Blanche H, Deleuze JF, Igo RP, Jr, Truitt B, Peachey NS, Meuer SM, Myers CE, Moore EL, Klein R, Hauser MA, Postel EA, Courtenay MD, Schwartz SG, Kovach JL, Scott WK, Liew G, Tan AG, Gopinath B, Merriam JC, Smith RT, Khan JC, Shahid H, Moore AT, McGrath JA, Laux R, Brantley MA, Jr, Agarwal A, Ersoy L, Caramoy A, Langmann T, Saksens NT, de Jong EK, Hoyng CB, Cain MS, Richardson AJ, Martin TM, Blangero J, Weeks DE, Dhillon B, van Duijn CM, Doheny KF, Romm J, Klaver CC, Hayward C, Gorin MB, Klein ML, Baird PN, den Hollander AI, Fauser S, Yates JR, Allikmets R, Wang JJ, Schaumberg DA, Klein BE, Hagstrom SA, Chowers I, Lotery AJ, Leveillard T, Zhang K, Brilliant MH, Hewitt AW, Swaroop A, Chew EY, Pericak-Vance MA, DeAngelis M, Stambolian D, Haines JL, Iyengar SK, Weber BH, Abecasis GR, Heid IM. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara M, Nagai N, Sussan TE, Biswal S, Handa JT. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS One. 2008;3:e3119. doi: 10.1371/journal.pone.0003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multistate models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130:1169–1176. doi: 10.1001/archophthalmol.2012.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland DL, Fernandez-Godino R, Kaur I, Speicher KD, Harnly JM, Lambris JD, Speicher DW, Pierce EA. Mouse genetics and proteomic analyses demonstrate a critical role for complement in a model of DHRD/ML, an inherited macular degeneration. Hum Mol Genet. 2014;23:52–68. doi: 10.1093/hmg/ddt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JD. Drusen and disciform macular detachment and degeneration. Trans Am Ophthalmol Soc. 1972;70:409–436. [PMC free article] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Group AMDGCS, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006a;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006b;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Progress in retinal and eye research. 2010;29:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymer R, Luthert P, Bird A. Changes in Bruch’s membrane and related structures with age. Prog Retin Eye Res. 1999;18:59–90. doi: 10.1016/s1350-9462(98)00012-3. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Progress in retinal and eye research. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. Faseb J. 1999;13:477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hakobyan S, Harris CL, van den Berg CW, Fernandez-Alonso MC, de Jorge EG, de Cordoba SR, Rivas G, Mangione P, Pepys MB, Morgan BP. Complement factor H binds to denatured rather than to native pentameric C-reactive protein. The Journal of biological chemistry. 2008;283:30451–30460. doi: 10.1074/jbc.M803648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin CR, Kohn RR. Evidence for progressive, age-related structural changes in post-mature human collagen. Biochimica et biophysica acta. 1971;236:458–467. doi: 10.1016/0005-2795(71)90226-1. [DOI] [PubMed] [Google Scholar]
- Hammond CJ, Webster AR, Snieder H, Bird AC, Gilbert CE, Spector TD. Genetic influence on early age-related maculopathy: a twin study. Ophthalmology. 2002;109:730–736. doi: 10.1016/s0161-6420(01)01049-1. [DOI] [PubMed] [Google Scholar]
- Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Investigative ophthalmology & visual science. 1999;40:775–779. [PubMed] [Google Scholar]
- Harboe M, Garred P, Karlstrom E, Lindstad JK, Stahl GL, Mollnes TE. The downstream effects of mannan-induced lectin complement pathway activation depend quantitatively on alternative pathway amplification. Molecular immunology. 2009;47:373–380. doi: 10.1016/j.molimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clinical and experimental immunology. 2004;138:439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SJ, Zheng K, Sado Y, Naito I, Ninomiya Y, Jacobs RM, Hudson BG, Thorner PS. Role of distinct type IV collagen networks in glomerular development and function. Kidney Int. 1998;54:1857–1866. doi: 10.1046/j.1523-1755.1998.00188.x. [DOI] [PubMed] [Google Scholar]
- Hawse JR, Hejtmancik JF, Huang Q, Sheets NL, Hosack DA, Lempicki RA, Horwitz J, Kantorow M. Identification and functional clustering of global gene expression differences between human age-related cataract and clear lenses. Molecular vision. 2003;9:515–537. [PMC free article] [PubMed] [Google Scholar]
- Hecker LA, Edwards AO, Ryu E, Tosakulwong N, Baratz KH, Brown WL, Charbel Issa P, Scholl HP, Pollok-Kopp B, Schmid-Kubista KE, Bailey KR, Oppermann M. Genetic control of the alternative pathway of complement in humans and age-related macular degeneration. Hum Mol Genet. 2010;19:209–215. doi: 10.1093/hmg/ddp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U View Groups V.S. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Heurich M, Martinez-Barricarte R, Francis NJ, Roberts DL, Rodriguez de Cordoba S, Morgan BP, Harris CL. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc Natl Acad Sci U S A. 2011;108:8761–8766. doi: 10.1073/pnas.1019338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye: An Atlas and Textbook. W. B. Sanders Company; Philadelphia: 1971. [Google Scholar]
- Hoh Kam J, Lenassi E, Malik TH, Pickering MC, Jeffery G. Complement component C3 plays a critical role in protecting the aging retina in a murine model of age-related macular degeneration. Am J Pathol. 2013;183:480–492. doi: 10.1016/j.ajpath.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Hoh Kam J, Morgan JE, Jeffery G. Aged complement factor H knockout mice kept in a clean barriered environment have reduced retinal pathology. Exp Eye Res. 2016;149:116–125. doi: 10.1016/j.exer.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Holliday EG, Smith AV, Cornes BK, Buitendijk GH, Jensen RA, Sim X, Aspelund T, Aung T, Baird PN, Boerwinkle E, Cheng CY, van Duijn CM, Eiriksdottir G, Gudnason V, Harris T, Hewitt AW, Inouye M, Jonasson F, Klein BE, Launer L, Li X, Liew G, Lumley T, McElduff P, McKnight B, Mitchell P, Psaty BM, Rochtchina E, Rotter JI, Scott RJ, Tay W, Taylor K, Teo YY, Uitterlinden AG, Viswanathan A, Xie S, Vingerling JR, Klaver CC, Tai ES, Siscovick D, Klein R, Cotch MF, Wong TY, Attia J, Wang JJ Wellcome Trust Case Control C. Insights into the genetic architecture of early stage age-related macular degeneration: a genome-wide association study meta-analysis. PLoS One. 2013;8:e53830. doi: 10.1371/journal.pone.0053830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang XP, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature Medicine. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz FG, Sheraidah G, Pauleikhoff D, Bird AC. Analysis of lipid deposits extracted from human macular and peripheral Bruch’s membrane. Arch Ophthalmol. 1994;112:402–406. doi: 10.1001/archopht.1994.01090150132035. [DOI] [PubMed] [Google Scholar]
- Huang JD, Presley JB, Chimento MF, Curcio CA, Johnson M. Age-related changes in human macular Bruch’s membrane as seen by quick-freeze/deep-etch. Experimental eye research. 2007;85:202–218. doi: 10.1016/j.exer.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181:8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Miller H, Orr G, Sorgente N, Ryan SJ. Morphologic observations on experimental subretinal neovascularization in the monkey. Invest Ophthalmol Vis Sci. 1987;28:1116–1130. [PubMed] [Google Scholar]
- Ishibashi T, Murata T, Hangai M, Nagai R, Horiuchi S, Lopez PF, Hinton DR, Ryan SJ. Advanced glycation end products in age-related macular degeneration. Archives of ophthalmology. 1998;116:1629–1632. doi: 10.1001/archopht.116.12.1629. [DOI] [PubMed] [Google Scholar]
- Iyengar SK, Song D, Klein BE, Klein R, Schick JH, Humphrey J, Millard C, Liptak R, Russo K, Jun G, Lee KE, Fijal B, Elston RC. Dissection of genomewide-scan data in extended families reveals a major locus and oligogenic susceptibility for age-related macular degeneration. Am J Hum Genet. 2004;74:20–39. doi: 10.1086/380912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. The New England journal of medicine. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- Jenne DE, Lowin B, Peitsch MC, Bottcher A, Schmitz G, Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem. 1991;266:11030–11036. [PubMed] [Google Scholar]
- Ji SR, Wu Y, Zhu L, Potempa LA, Sheng FL, Lu W, Zhao J. Cell membranes and liposomes dissociate C-reactive protein (CRP) to form a new, biologically active structural intermediate: mCRP(m) FASEB J. 2007;21:284–294. doi: 10.1096/fj.06-6722com. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Forest DL, Banna CD, Radeke CM, Maloney MA, Hu J, Spencer CN, Walker AM, Tsie MS, Bok D, Radeke MJ, Anderson DH. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:18277–18282. doi: 10.1073/pnas.1109703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Experimental eye research. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Experimental eye research. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH, Johnson LV. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci U S A. 2006;103:17456–17461. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh D, Yu Y, Schramm EC, Triebwasser M, Wagner EK, Raychaudhuri S, Daly MJ, Atkinson JP, Seddon JM. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet. 2015;24:3861–3870. doi: 10.1093/hmg/ddv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan TD, Pickford CE, Holley RJ, Clark SJ, Lin W, Dowsey AW, Merry CL, Day AJ, Bishop PN. Age-Dependent Changes in Heparan Sulfate in Human Bruch’s Membrane: Implications for Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2014;55:5370–5379. doi: 10.1167/iovs.14-14126. [DOI] [PubMed] [Google Scholar]
- Kelly U, Yu L, Kumar P, Ding JD, Jiang H, Hageman GS, Arshavsky VY, Frank MM, Hauser MA, Rickman CB. Heparan sulfate, including that in Bruch’s membrane, inhibits the complement alternative pathway: implications for age-related macular degeneration. J Immunol. 2010;185:5486–5494. doi: 10.4049/jimmunol.0903596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew RR, Ghebrehiwet B, Janoff A. Cigarette smoke can activate the alternative pathway of complement in vitro by modifying the third component of complement. J Clin Invest. 1985;75:1000–1007. doi: 10.1172/JCI111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, van Broeckhoven C, de Jong PT. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63:200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein BE, Klein R, Lee KE. Incidence of age-related cataract over a 10-year interval: the Beaver Dam Eye Study. Ophthalmology. 2002;109:2052–2057. doi: 10.1016/s0161-6420(02)01249-6. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Lee KE, Moore EL, Danforth L. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am J Epidemiol. 2001;154:207–211. doi: 10.1093/aje/154.3.207. [DOI] [PubMed] [Google Scholar]
- Klein ML, Mauldin WM, Stoumbos VD. Heredity and age-related macular degeneration. Observations in monozygotic twins. Arch Ophthalmol. 1994;112:932–937. doi: 10.1001/archopht.1994.01090190080025. [DOI] [PubMed] [Google Scholar]
- Klein ML, Schultz DW, Edwards A, Matise TC, Rust K, Berselli CB, Trzupek K, Weleber RG, Ott J, Wirtz MK, Acott TS. Age-related macular degeneration. Clinical features in a large family and linkage to chromosome 1q. Arch Ophthalmol. 1998;116:1082–1088. doi: 10.1001/archopht.116.8.1082. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137:190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- Klein R, Myers CE, Meuer SM, Gangnon RE, Sivakumaran TA, Iyengar SK, Lee KE, Klein BE. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131:383–392. doi: 10.1001/jamaophthalmol.2013.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, Sangiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunchithapautham K, Atkinson C, Rohrer B. Smoke exposure causes endoplasmic reticulum stress and lipid accumulation in retinal pigment epithelium through oxidative stress and complement activation. J Biol Chem. 2014;289:14534–14546. doi: 10.1074/jbc.M114.564674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Gantner ML, Usui Y, Schultz A, Bravo S, Aguilar E, Wittgrove C, Friedlander M, Paris LP, Chew E, Siuzdak G, Friedlander M. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife. 2016:5. doi: 10.7554/eLife.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine M, Jarva H, Seitsonen S, Haapasalo K, Lehtinen MJ, Lindeman N, Anderson DH, Johnson PT, Jarvela I, Jokiranta TS, Hageman GS, Immonen I, Meri S. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J Immunol. 2007;178:3831–3836. doi: 10.4049/jimmunol.178.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM, Rakic JM, Noel A. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nature protocols. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- Lauer N, Mihlan M, Hartmann A, Schlotzer-Schrehardt U, Keilhauer C, Scholl HP, Charbel Issa P, Holz F, Weber BH, Skerka C, Zipfel PF. Complement regulation at necrotic cell lesions is impaired by the age-related macular degeneration-associated factor-H His402 risk variant. J Immunol. 2011;187:4374–4383. doi: 10.4049/jimmunol.1002488. [DOI] [PubMed] [Google Scholar]
- LeBedis CA, Sakai O. Nontraumatic orbital conditions: diagnosis with CT and MR imaging in the emergent setting. Radiographics. 2008;28:1741–1753. doi: 10.1148/rg.286085515. [DOI] [PubMed] [Google Scholar]
- Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- Li CM, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, Curcio CA. Lipoprotein-like particles and cholesteryl esters in human Bruch’s membrane: initial characterization. Invest Ophthalmol Vis Sci. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- Li CM, Clark ME, Rudolf M, Curcio CA. Distribution and composition of esterified and unesterified cholesterol in extra-macular drusen. Exp Eye Res. 2007;85:192–201. doi: 10.1016/j.exer.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Li J, Tso MO, Lam TT. Reduced amplitude and delayed latency in foveal response of multifocal electroretinogram in early age related macular degeneration. Br J Ophthalmol. 2001;85:287–290. doi: 10.1136/bjo.85.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler KU, Edward DP, Tso MO. Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest Ophthalmol Vis Sci. 1995;36:24–31. [PubMed] [Google Scholar]
- Lommatzsch A, Hermans P, Muller KD, Bornfeld N, Bird AC, Pauleikhoff D. Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. 2008;246:803–810. doi: 10.1007/s00417-007-0749-4. [DOI] [PubMed] [Google Scholar]
- Loyet KM, Deforge LE, Katschke KJ, Jr, Diehl L, Graham RR, Pao L, Sturgeon L, Lewin-Koh SC, Hollyfield JG, van Lookeren Campagne M. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:6628–6637. doi: 10.1167/iovs.12-9587. [DOI] [PubMed] [Google Scholar]
- Lundh von Leithner P, Kam JH, Bainbridge J, Catchpole I, Gough G, Coffey P, Jeffery G. Complement factor h is critical in the maintenance of retinal perfusion. Am J Pathol. 2009;175:412–421. doi: 10.2353/ajpath.2009.080927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzogubov VV, Tytarenko RG, Jha P, Liu J, Bora NS, Bora PS. Role of ocular complement factor H in a murine model of choroidal neovascularization. Am J Pathol. 2010;177:1870–1880. doi: 10.2353/ajpath.2010.091168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KP, Duan S, Sigurdsson H, Petursson H, Yang Z, Zhao Y, Bernstein PS, Ge J, Jonasson F, Stefansson E, Helgadottir G, Zabriskie NA, Jonsson T, Bjornsson A, Thorlacius T, Jonsson PV, Thorleifsson G, Kong A, Stefansson H, Zhang K, Stefansson K, Gulcher JR. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med. 2006;3:e5. doi: 10.1371/journal.pmed.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski J, Schultz DW, Weleber RG, Schain MB, Edwards AO, Matise TC, Acott TS, Ott J, Klein ML. Age-related macular degeneration--a genome scan in extended families. Am J Hum Genet. 2003;73:540–550. doi: 10.1086/377701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J, George S, Purcell S, Fagerness J, Altshuler D, Daly MJ, Seddon JM. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38:1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39:1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- Meyers SM, Greene T, Gutman FA. A twin study of age-related macular degeneration. American journal of ophthalmology. 1995;120:757–766. doi: 10.1016/s0002-9394(14)72729-1. [DOI] [PubMed] [Google Scholar]
- Milis L, Morris CA, Sheehan MC, Charlesworth JA, Pussell BA. Vitronectin-mediated inhibition of complement: evidence for different binding sites for C5b-7 and C9. Clin Exp Immunol. 1993;92:114–119. doi: 10.1111/j.1365-2249.1993.tb05956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold C, Kingzette M, Gewurz H. C-reactive protein inhibits pneumococcal activation of the alternative pathway by increasing the interaction between factor H and C3b. J Immunol. 1984;133:882–885. [PubMed] [Google Scholar]
- Molins B, Fuentes-Prior P, Adan A, Anton R, Arostegui JI, Yague J, Dick AD. Complement factor H binding of monomeric C-reactive protein downregulates proinflammatory activity and is impaired with at risk polymorphic CFH variants. Sci Rep. 2016;6:22889. doi: 10.1038/srep22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Aptsiauri N, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye (Lond) 2001;15:390–395. doi: 10.1038/eye.2001.142. [DOI] [PubMed] [Google Scholar]
- Mullins RF, Dewald AD, Streb LM, Wang K, Kuehn MH, Stone EM. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Experimental eye research. 2011;93:565–567. doi: 10.1016/j.exer.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. Faseb J. 2000;14:835–846. [PubMed] [Google Scholar]
- Mullins RF, Schoo DP, Sohn EH, Flamme-Wiese MJ, Workamelahu G, Johnston RM, Wang K, Tucker BA, Stone EM. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184:3142–3153. doi: 10.1016/j.ajpath.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Klein BE, Gangnon R, Sivakumaran TA, Iyengar SK, Klein R. Cigarette smoking and the natural history of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2014;121:1949–1955. doi: 10.1016/j.ophtha.2014.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, Bernstein PS, Li B, Frederick JM, Zhang K, Brantley MA, Jr, Lee AY, Zack DJ, Campochiaro B, Campochiaro P, Ripke S, Smith RT, Barile GR, Katsanis N, Allikmets R, Daly MJ, Seddon JM. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc Natl Acad Sci U S A. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JJ, Conlon PJ, Croke D, Dorman A, Keogan M, Zhang FY, Vance JM, Pericak-Vance MA, Scott WK, Winn MP. Linkage of a gene causing familial membranoproliferative glomerulonephritis type III to chromosome 1. J Am Soc Nephrol. 2002;13:2052–2057. doi: 10.1097/01.asn.0000022006.49966.f8. [DOI] [PubMed] [Google Scholar]
- Nolasco L, Nolasco J, Feng S, Afshar-Kharghan V, Moake J. Human complement factor H is a reductase for large soluble von Willebrand factor multimers--brief report. Arterioscler Thromb Vasc Biol. 2013;33:2524–2528. doi: 10.1161/ATVBAHA.113.302280. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien C, Duvall-Young J, Brown M, Short C, Bone M. Electrophysiology of type II mesangiocapillary glomerulonephritis with associated fundus abnormalities. Br J Ophthalmol. 1993;77:778–780. doi: 10.1136/bjo.77.12.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okemefuna AI, Nan R, Miller A, Gor J, Perkins SJ. Complement factor H binds at two independent sites to C-reactive protein in acute phase concentrations. The Journal of biological chemistry. 2010;285:1053–1065. doi: 10.1074/jbc.M109.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo A, Rosa RH, Jr, Bunce CV, Alexander RA, Fan JT, Bird AC, Luthert PJ. The relationships of age changes in retinal pigment epithelium and Bruch’s membrane. Investigative ophthalmology & visual science. 1999;40:443–449. [PubMed] [Google Scholar]
- Ormsby RJ, Ranganathan S, Tong JC, Griggs KM, Dimasi DP, Hewitt AW, Burdon KP, Craig JE, Hoh J, Gordon DL. Functional and structural implications of the complement factor H Y402H polymorphism associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:1763–1770. doi: 10.1167/iovs.07-1297. [DOI] [PubMed] [Google Scholar]
- Owsley C, Huisingh C, Clark ME, Jackson GR, McGwin G., Jr Comparison of Visual Function in Older Eyes in the Earliest Stages of Age-related Macular Degeneration to Those in Normal Macular Health. Curr Eye Res. 2016a;41:266–272. doi: 10.3109/02713683.2015.1011282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C, Jackson GR, White M, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–1202. doi: 10.1016/s0161-6420(01)00580-2. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G, Jr, Clark ME, Jackson GR, Callahan MA, Kline LB, Witherspoon CD, Curcio CA. Delayed Rod-Mediated Dark Adaptation Is a Functional Biomarker for Incident Early Age-Related Macular Degeneration. Ophthalmology. 2016b;123:344–351. doi: 10.1016/j.ophtha.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn MK, Muller-Eberhard HJ. Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Annals of the New York Academy of Sciences. 1983;421:291–298. doi: 10.1111/j.1749-6632.1983.tb18116.x. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology. 1990;97:171–178. [PubMed] [Google Scholar]
- Pauleikhoff D, Sheraidah G, Marshall J, Bird AC, Wessing A. Biochemical and histochemical analysis of age related lipid deposits in Bruch’s membrane. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 1994;91:730–734. [PubMed] [Google Scholar]
- Peters S, Reinthal E, Blitgen-Heinecke P, Bartz-Schmidt KU, Schraermeyer U. Inhibition of lysosomal degradation in retinal pigment epithelium cells induces exocytosis of phagocytic residual material at the basolateral plasma membrane. Ophthalmic Res. 2006;38:83–88. doi: 10.1159/000090268. [DOI] [PubMed] [Google Scholar]
- Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- Pickering MC, de Jorge EG, Martinez-Barricarte R, Recalde S, Garcia-Layana A, Rose KL, Moss J, Walport MJ, Cook HT, de Cordoba SR, Botto M. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. The Journal of experimental medicine. 2007;204:1249–1256. doi: 10.1084/jem.20070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim MG, Lengyel I, Lanzirotti A, Newville M, Fearn S, Emri E, Knowles JC, Messinger JD, Read RW, Guidry C, Curcio CA. Subretinal Pigment Epithelial Deposition of Drusen Components Including Hydroxyapatite in a Primary Cell Culture Model. Invest Ophthalmol Vis Sci. 2017;58:708–719. doi: 10.1167/iovs.16-21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poor SH, Qiu Y, Fassbender ES, Shen S, Woolfenden A, Delpero A, Kim Y, Buchanan N, Gebuhr TC, Hanks SM, Meredith EL, Jaffee BD, Dryja TP. Reliability of the mouse model of choroidal neovascularization induced by laser photocoagulation. Invest Ophthalmol Vis Sci. 2014;55:6525–6534. doi: 10.1167/iovs.14-15067. [DOI] [PubMed] [Google Scholar]
- Prosser BE, Johnson S, Roversi P, Herbert AP, Blaum BS, Tyrrell J, Jowitt TA, Clark SJ, Tarelli E, Uhrin D, Barlow PN, Sim RB, Day AJ, Lea SM. Structural basis for complement factor H linked age-related macular degeneration. The Journal of experimental medicine. 2007;204:2277–2283. doi: 10.1084/jem.20071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S. Mapping rare and common causal alleles for complex human diseases. Cell. 2011;147:57–69. doi: 10.1016/j.cell.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S, Iartchouk O, Chin K, Tan PL, Tai AK, Ripke S, Gowrisankar S, Vemuri S, Montgomery K, Yu Y, Reynolds R, Zack DJ, Campochiaro B, Campochiaro P, Katsanis N, Daly MJ, Seddon JM. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nature genetics. 2011;43:1232–1236. doi: 10.1038/ng.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalde S, Tortajada A, Subias M, Anter J, Blasco M, Maranta R, Coco R, Pinto S, Noris M, Garcia-Layana A, Rodriguez de Cordoba S. Molecular Basis of Factor H R1210C Association with Ocular and Renal Diseases. J Am Soc Nephrol. 2016;27:1305–1311. doi: 10.1681/ASN.2015050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi G, Galbusera M, Noris M, Canciani MT, Daina E, Bresin E, Contaretti S, Caprioli J, Gamba S, Ruggenenti P, Perico N, Mannucci PM Italian Registry of R Familial H.U.S.T.T.P.T.t.p.h.u.s. von Willebrand factor cleaving protease (ADAMTS13) is deficient in recurrent and familial thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Blood. 2002;100:778–785. doi: 10.1182/blood-2001-12-0166. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–5827. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristau T, Paun C, Ersoy L, Hahn M, Lechanteur Y, Hoyng C, de Jong EK, Daha MR, Kirchhof B, den Hollander AI, Fauser S. Impact of the common genetic associations of age-related macular degeneration upon systemic complement component C3d levels. PLoS One. 2014;9:e93459. doi: 10.1371/journal.pone.0093459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RA, Nelson KJ, Gossman GL, Koyama S, Rennard SI. Complement activation by cigarette smoke. Am J Physiol. 1991;260:L254–259. doi: 10.1152/ajplung.1991.260.4.L254. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41:355–367. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Coughlin B, Bandyopadhyay M, Holers VM. Systemic human CR2-targeted complement alternative pathway inhibitor ameliorates mouse laser-induced choroidal neovascularization. J Ocul Pharmacol Ther. 2012;28:402–409. doi: 10.1089/jop.2011.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Coughlin B, Kunchithapautham K, Long Q, Tomlinson S, Takahashi K, Holers VM. The alternative pathway is required, but not alone sufficient, for retinal pathology in mouse laser-induced choroidal neovascularization. Mol Immunol. 2011;48:e1–8. doi: 10.1016/j.molimm.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Long Q, Coughlin B, Wilson RB, Huang Y, Qiao F, Tang PH, Kunchithapautham K, Gilkeson GS, Tomlinson S. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:3056–3064. doi: 10.1167/iovs.08-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld PJ. Bevacizumab versus ranibizumab for AMD. N Engl J Med. 2011;364:1966–1967. doi: 10.1056/NEJMe1103334. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008a;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008b;87:402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungger-Brandle E, Englert U, Leuenberger PM. Exocytic clearing of degraded membrane material from pigment epithelial cells in frog retina. Invest Ophthalmol Vis Sci. 1987;28:2026–2037. [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Clemons TE, Davis MD, Ferris FL, 3rd, Gensler GR, Kurinij N, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007;125:671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2(Pt 5):552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- Sarks JP, Sarks SH, Killingsworth MC. Evolution of soft drusen in age-related macular degeneration. Eye (Lond) 1994;8(Pt 3):269–283. doi: 10.1038/eye.1994.57. [DOI] [PubMed] [Google Scholar]
- Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of Basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:968–977. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. The British journal of ophthalmology. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CQ, Herbert AP, Kavanagh D, Gandy C, Fenton CJ, Blaum BS, Lyon M, Uhrin D, Barlow PN. A new map of glycosaminoglycan and C3b binding sites on factor H. J Immunol. 2008;181:2610–2619. doi: 10.4049/jimmunol.181.4.2610. [DOI] [PubMed] [Google Scholar]
- Schmidt CQ, Herbert AP, Mertens HD, Guariento M, Soares DC, Uhrin D, Rowe AJ, Svergun DI, Barlow PN. The central portion of factor H (modules 10–15) is compact and contains a structurally deviant CCP module. J Mol Biol. 2010;395:105–122. doi: 10.1016/j.jmb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabolk G, Coughlin B, Joseph K, Kunchithapautham K, Bandyopadhyay M, O’Quinn EC, Nowling T, Rohrer B. Local production of the alternative pathway component factor B is sufficient to promote laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015;56:1850–1863. doi: 10.1167/iovs.14-15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. American journal of ophthalmology. 1997;123:199–206. doi: 10.1016/s0002-9394(14)71036-0. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. Jama. 1994;272:1413–1420. [PubMed] [Google Scholar]
- Seddon JM, Cote J, Page WF, Aggen SH, Neale MC. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123:321–327. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. Jama. 2004;291:704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Santangelo SL, Book K, Chong S, Cote J. A genomewide scan for age-related macular degeneration provides evidence for linkage to several chromosomal regions. Am J Hum Genet. 2003;73:780–790. doi: 10.1086/378505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. Jama. 1996;276:1141–1146. [PubMed] [Google Scholar]
- Seddon JM, Yu Y, Miller EC, Reynolds R, Tan PL, Gowrisankar S, Goldstein JI, Triebwasser M, Anderson HE, Zerbib J, Kavanagh D, Souied E, Katsanis N, Daly MJ, Atkinson JP, Raychaudhuri S. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45:1366–1370. doi: 10.1038/ng.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PX, Zhang L, Zhang M, Du H, Zhao L, Lee C, Grob S, Lim SL, Hughes G, Lee J, Bedell M, Nelson MH, Lu F, Krupa M, Luo J, Ouyang H, Tu Z, Su Z, Zhu J, Wei X, Feng Z, Duan Y, Yang Z, Ferreyra H, Bartsch DU, Kozak I, Zhang L, Lin F, Sun H, Feng H, Zhang K. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc Natl Acad Sci U S A. 2012;109:13757–13762. doi: 10.1073/pnas.1121309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Babu K, Prabhakaran VC. Large drusen following long-standing retinal detachment. Indian J Pathol Microbiol. 2010;53:329–330. doi: 10.4103/0377-4929.64316. [DOI] [PubMed] [Google Scholar]
- Silva AS, Teixeira AG, Bavia L, Lin F, Velletri R, Belfort R, Jr, Isaac L. Plasma levels of complement proteins from the alternative pathway in patients with age-related macular degeneration are independent of Complement Factor H Tyr(4)(0)(2)His polymorphism. Mol Vis. 2012;18:2288–2299. [PMC free article] [PubMed] [Google Scholar]
- Singh U, Devaraj S, Dasu MR, Ciobanu D, Reusch J, Jialal I. C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Arterioscler Thromb Vasc Biol. 2006;26:2469–2475. doi: 10.1161/01.ATV.0000241572.05292.fb. [DOI] [PubMed] [Google Scholar]
- Skeie JM, Fingert JH, Russell SR, Stone EM, Mullins RF. Complement component C5a activates ICAM-1 expression on human choroidal endothelial cells. Invest Ophthalmol Vis Sci. 2010;51:5336–5342. doi: 10.1167/iovs.10-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smailhodzic D, Klaver CC, Klevering BJ, Boon CJ, Groenewoud JM, Kirchhof B, Daha MR, den Hollander AI, Hoyng CB. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology. 2012;119:339–346. doi: 10.1016/j.ophtha.2011.07.056. [DOI] [PubMed] [Google Scholar]
- Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde JP, Munnich A, Kaplan J, Coscas G, Soubrane G. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. American journal of ophthalmology. 1998;125:353–359. doi: 10.1016/s0002-9394(99)80146-9. [DOI] [PubMed] [Google Scholar]
- Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33:1800–1808. doi: 10.1097/IAE.0b013e31829c3765. [DOI] [PubMed] [Google Scholar]
- Spencer KL, Olson LM, Anderson BM, Schnetz-Boutaud N, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL. C3 R102G polymorphism increases risk of age-related macular degeneration. Human molecular genetics. 2008;17:1821–1824. doi: 10.1093/hmg/ddn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangos N, Voutas S, Topouzis F, Karampatakis V. Contrast sensitivity evaluation in eyes predisposed to age-related macular degeneration and presenting normal visual acuity. Ophthalmologica. 1995;209:194–198. doi: 10.1159/000310612. [DOI] [PubMed] [Google Scholar]
- Thiele JR, Habersberger J, Braig D, Schmidt Y, Goerendt K, Maurer V, Bannasch H, Scheichl A, Woollard KJ, von Dobschutz E, Kolodgie F, Virmani R, Stark GB, Peter K, Eisenhardt SU. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. 2014;130:35–50. doi: 10.1161/CIRCULATIONAHA.113.007124. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Kulik L, Orth H, Wong M, Renner B, Sargsyan SA, Mitchell LM, Hourcade DE, Hannan JP, Kovacs JM, Coughlin B, Woodell AS, Pickering MC, Rohrer B, Holers VM. Detection of complement activation using monoclonal antibodies against C3d. J Clin Invest. 2013;123:2218–2230. doi: 10.1172/JCI65861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey CB, Kelly U, Saban DR, Bowes Rickman C. Regulation of age-related macular degeneration-like pathology by complement factor H. Proc Natl Acad Sci U S A. 2015;112:E3040–3049. doi: 10.1073/pnas.1424391112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebwasser MP, Roberson ED, Yu Y, Schramm EC, Wagner EK, Raychaudhuri S, Seddon JM, Atkinson JP. Rare Variants in the Functional Domains of Complement Factor H Are Associated With Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2015;56:6873–6878. doi: 10.1167/iovs.15-17432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006a;12:1319–1333. [PubMed] [Google Scholar]
- Tserentsoodol N, Sztein J, Campos M, Gordiyenko NV, Fariss RN, Lee JW, Fliesler SJ, Rodriguez IR. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Molecular vision. 2006b;12:1306–1318. [PubMed] [Google Scholar]
- Ufret-Vincenty RL, Aredo B, Liu X, McMahon A, Chen PW, Sun H, Niederkorn JY, Kedzierski W. Transgenic mice expressing variants of complement factor H develop AMD-like retinal findings. Invest Ophthalmol Vis Sci. 2010;51:5878–5887. doi: 10.1167/iovs.09-4457. [DOI] [PubMed] [Google Scholar]
- Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP Los Angeles Latino Eye Study G. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111:1288–1297. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Vater CA, Harris ED, Jr, Siegel RC. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. The Biochemical journal. 1979;181:639–645. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyradier A, Obert B, Haddad E, Cloarec S, Nivet H, Foulard M, Lesure F, Delattre P, Lakhdari M, Meyer D, Girma JP, Loirat C. Severe deficiency of the specific von Willebrand factor-cleaving protease (ADAMTS 13) activity in a subgroup of children with atypical hemolytic uremic syndrome. J Pediatr. 2003;142:310–317. doi: 10.1067/mpd.2003.79. [DOI] [PubMed] [Google Scholar]
- Vingerling JR, Hofman A, Grobbee DE, de Jong PT. Age-related macular degeneration and smoking. The Rotterdam Study. Arch Ophthalmol. 1996;114:1193–1196. doi: 10.1001/archopht.1996.01100140393005. [DOI] [PubMed] [Google Scholar]
- Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–197. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Handa JT, Neufeld AH. Changes in retinal pigment epithelium related to cigarette smoke: possible relevance to smoking as a risk factor for age-related macular degeneration. PLoS One. 2009a;4:e5304. doi: 10.1371/journal.pone.0005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Clark ME, Crossman DK, Kojima K, Messinger JD, Mobley JA, Curcio CA. Abundant lipid and protein components of drusen. PLoS One. 2010;5:e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li CM, Rudolf M, Belyaeva OV, Chung BH, Messinger JD, Kedishvili NY, Curcio CA. Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile. Investigative ophthalmology & visual science. 2009b;50:870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks DE, Conley YP, Tsai HJ, Mah TS, Schmidt S, Postel EA, Agarwal A, Haines JL, Pericak-Vance MA, Rosenfeld PJ, Paul TO, Eller AW, Morse LS, Dailey JP, Ferrell RE, Gorin MB. Age-related maculopathy: a genomewide scan with continued evidence of susceptibility loci within the 1q31, 10q26, and 17q25 regions. Am J Hum Genet. 2004;75:174–189. doi: 10.1086/422476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore SS, Sohn EH, Chirco KR, Drack AV, Stone EM, Tucker BA, Mullins RF. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1–29. doi: 10.1016/j.preteyeres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter JR, Falls HF. Bilateral confluent drusen. Arch Ophthalmol. 1962;68:219–226. doi: 10.1001/archopht.1962.00960030223013. [DOI] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- Woodell A, Coughlin B, Kunchithapautham K, Casey S, Williamson T, Ferrell WD, Atkinson C, Jones BW, Rohrer B. Alternative complement pathway deficiency ameliorates chronic smoke-induced functional and morphological ocular injury. PLoS One. 2013;8:e67894. doi: 10.1371/journal.pone.0067894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodell A, Jones BW, Williamson T, Schnabolk G, Tomlinson S, Atkinson C, Rohrer B. A Targeted Inhibitor of the Alternative Complement Pathway Accelerates Recovery From Smoke-Induced Ocular Injury. Invest Ophthalmol Vis Sci. 2016;57:1728–1737. doi: 10.1167/iovs.15-18471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Tyrrell J, Han I, Jaffe GJ. Expression and modulation of RPE cell membrane complement regulatory proteins. Investigative ophthalmology & visual science. 2009;50:3473–3481. doi: 10.1167/iovs.08-3202. [DOI] [PubMed] [Google Scholar]
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT Genetic Factors in AMDSG. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Ying L, Katz Y, Schlesinger M, Carmi R, Shalev H, Haider N, Beck G, Sheffield VC, Landau D. Complement factor H gene mutation associated with autosomal recessive atypical hemolytic uremic syndrome. Am J Hum Genet. 1999;65:1538–1546. doi: 10.1086/302673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Triebwasser MP, Wong EK, Schramm EC, Thomas B, Reynolds R, Mardis ER, Atkinson JP, Daly M, Raychaudhuri S, Kavanagh D, Seddon JM. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum Mol Genet. 2014;23:5283–5293. doi: 10.1093/hmg/ddu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF. Hemolytic uremic syndrome: how do factor H mutants mediate endothelial damage? Trends Immunol. 2001;22:345–348. doi: 10.1016/s1471-4906(01)01972-x. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C, Caprioli J, Manuelian T, Neumann HH, Noris M, Remuzzi G. Complement factor H and hemolytic uremic syndrome. Int Immunopharmacol. 2001;1:461–468. doi: 10.1016/s1567-5769(00)00047-3. [DOI] [PubMed] [Google Scholar]