Abstract
Objective
To determine risk factors for the development of reactive arthritis (ReA) and examine factors associated with persistence of symptoms.
Methods
Patients with a new diagnosis of ReA and controls with a gastrointestinal (GI), urogenital, or sexually transmitted infection in the 3–6 months prior to study entry were prospectively enrolled in Guatemala City. ReA patients fulfilled ASAS criteria for peripheral spondyloarthropathy (SpA). Patients underwent history, examination, Achilles tendon ultrasound, and blood draw. Human leukocyte antigen (HLA) type and serum biomarkers were measured. T-tests and nonparametric equivalents were used to examine the association of clinical, laboratory, and imaging factors with ReA. Patients were contacted 2 years later to assess for persistence of symptoms.
Results
Study subjects included patients with ReA (N=32) and controls (N=32). ReA patients were most frequently infected in April whereas controls were most frequently infected in August. Two ReA patients and two controls were HLA-B27 positive. Serum cathepsin K and C-reactive protein were higher in ReA patients compared to controls (P = 0.03 for both), while total cholesterol and low density lipoprotein were lower (p=0.008 and 0.045 respectively). Among those with ReA, 15 (47%) patients had continued symptoms at 2 years. These patients had a lower matrix metalloproteinase-3 level at diagnosis than patients for whom ReA resolved (p=0.004).
Conclusion
HLA-B27 was not associated with development of ReA in Guatemala, however, month of infection was associated with ReA. The most striking finding was the persistence of arthritis at 2 years in nearly half of patients.
Keywords: reactive arthritis, spondyloarthropathy, epidemiology, risk factors, prognosis, biomarkers
INTRODUCTION
Reactive arthritis (ReA) is an inflammatory arthritis that generally develops within weeks after exposure to an infectious pathogen.(1) ReA is a sub-classification of the Spondyloarthropathies (SpA), a group of related multisystem inflammatory diseases that affect the spine, peripheral joints, and peri-articular structures. These conditions also share familial clustering, extra-articular manifestations (e.g. uveitis, inflammatory bowel disease, or subclinical gut inflammation), and an association with the HLA-B27 allele. ReA is characterized by an immune-mediated synovitis with some studies demonstrating intra-articular persistence of viable, non-culturable bacterial fragments.(2) The most common infections associated with ReA are caused by bacterial enteric pathogens such as Salmonella, Shigella, Campylobacter, and Yersinia. Sexually transmitted infections (STIs) such as Chlamydia, Mycoplasma, and HIV have also been correlated with the onset of ReA.(3–5) Symptoms of ReA tend to resolve within six months of the initial infection although, a proportion of patients with ReA may have persistent symptoms for months or years.(6) However, little is known about the course of ReA, including basic epidemiology, risk factors and prognostic factors, particularly within Central America. The epidemiology of SpA differs between the United States and Central America with a particularly wide variation in the prevalence of ReA. ReA is relatively rare in the US, but much more common in Central America, and specifically in Guatemala.(7) The relatively high prevalence of ReA in Guatemala offers the ideal opportunity to describe the genetic, phenotypic, and serologic aspects of ReA and to examine potential risk factors for disease development.
It remains unclear why some patients develop ReA following an infection and others do not. While relatively rare in the population, insights into why patients develop this form of SpA may inform risk factors for the development of other SpA types. HLA-B27 positivity has been considered a significant risk factor in the development of SpA in general. However, previous studies have shown that more than 50% of infection-related ReA cases in Europe are not associated with the HLA-B27 allele.(8) Enteric pathogens, including those listed above, are endemic causes of diarrheal illness in Guatemala.(9) Other potential risk factors for ReA include propensity for distinct inflammatory responses to an infectious exposure because of resident intestinal flora (the gut microbiome)(10, 11), other inflammatory disorders such as metabolic syndrome (e.g., obesity and hyperlipidemia are risk factors for psoriatic arthritis)(12, 13), nutritional status, occupational exposures, and genetic factors, specifically HLA genes.
The objective of this study was to identify potential risk factors for the development of ReA among Guatemalan patients with a known history of being infected with gastro-enteric, urologic, or sexually transmitted pathogens. A prospective study was performed to examine clinical, environmental, and genetic risk factors for the development of ReA. In addition, we examined the clinical features, serum biomarkers of inflammatory arthritis and systemic inflammation, and persistence of symptoms at two years among patients with ReA.
PATIENTS AND METHODS
Study Design
Prospective cross-sectional study with longitudinal follow up.
Study Population
Patients were prospectively enrolled from July 2014 to October 2014. Cases and controls were recruited concurrently and in the same time window. Cases were ascertained from two rheumatology clinics in Guatemala City (Asociacion Guatemalteca Anti Enfermedades Reumáticas (AGAR) outpatient clinic and Clinica Medica Especializada en Reumatologia (AGK) private clinic) and controls were obtained from primary care clinics within the Hospital Universitario Esperanza, Hospital Sanatorio Hermano Pedro, and Hospital Nacional de Traumatologia y Ortopedia. There has been much debate about how to define ReA.(14, 15) The definition of ReA in this study was presentation with inflammatory arthritis, a preceding infection within three to six months (restricted to gastroenteric, urological, or sexually transmitted infections), and a physician diagnosis of ReA. ReA was further defined by fulfillment of the ASAS criteria for peripheral SpA.(16) Additional inclusion criteria were age between 18 and 55 years of age and the ability to provide consent. Alternative diagnoses such as inflammatory bowel disease (Crohn’s disease or ulcerative colitis), active malignancy, psoriasis, or other inflammatory arthritis or joint diseases were exclusion criteria for this study.
Study Measurements
After obtaining informed consent, a rheumatologist performed a standardized clinical history and physical exam. Patients completed a pain numerical rating scale and the mini-nutrition assessment.(17, 18) All participants underwent ultrasound examination of the bilateral Achilles tendons, and blood draw. We selected the Achilles tendon as the site for ultrasound as it is among the most commonly involved entheses in SpA.(19) In October 2016, both patient and control groups completed a follow-up survey designed to detect any changes in arthritic symptomatology. This survey was most often conducted by phone.
Hypothesized clinical risk factors: for PsA
A priori, we developed a list of potential risk factors for development of ReA after an infection and these were included on the clinical research forms. Potential clinical risk factors included: sex, age, self-reported race, residence location, occupation, family history of SpA, psoriasis or IBD, smoking, obesity, metabolic comorbidities (e.g., diabetes, hypertension), timing of infection (as a proxy for type of infection as some infections may be more common during different times of the year), and use of antibiotics for the infection.
Laboratory assessments
Blood samples included serum and whole blood. Standard laboratory testing performed in the AGAR clinical laboratory included: sedimentation rate, fasting glucose, a fasting lipid panel (including total cholesterol, HDL cholesterol, LDL cholesterol, Triglycerides), and HbA1C. Blood and serum samples were stored in five 2cc Eppendorf tubes. All samples were stored at −80°C in Guatemala City until completion of the study. Samples were then shipped on dry ice to the University of Pennsylvania. Stool samples were collected for future microbiota analysis (cultures not isolated in this study).
HLA-typing and serum biomarker studies
HLA A, B, and C sequencing was performed by HistoGenetics laboratories, Ossinging, NY. Serum biomarkers were performed by the University of Pennsylvania Translational Core Laboratory. Dickkopf related protein 1 (DKK1) was measured using two-site sandwich ELISA kits from R and D systems. Cathepskin K was measured using two-site sandwich ELISA kits from LS Bio. Osteoprotegerin (OPG) was measured using two-site sandwich ELISA kits from BioVendor. Matrix metalloproteinase 3 (MMP-3) was measured using two-site sandwich ELISA kits from R and D systems. RANKL was measured using two-site sandwich ELISA kits from BioVendor. Sclerostin was measured using two-site sandwich ELISA kits from RnD systems. These biomarkers were selected because of their previous associations with ankylosing spondylitis or psoriatic arthritis and their hypothesized differential expression between ReA (or other forms of SpA) and controls.(20, 21)
Ultrasound
One rheumatologist trained in musculoskeletal ultrasound (MSKUS) blinded to clinical data, independently examined all patients’ and controls’ Achilles entheses MSKUS studies (Siemens G40 equipped with a 13–5 MHz linear transducer). Each tendon was scanned in both longitudinal and transverse planes. Power Doppler Ultrasound Enthesitis was defined based on the Outcome Measures in Rheumatology Ultrasound Task Force, which included hypo-echogenicity, increased thickness of the tendon insertion, calcifications, enthesophytes, erosions, and Doppler activity as elementary lesions of enthesitis.(22)
Statistical Analysis
Descriptive statistics were used to compare clinical and demographic characteristics among cases and controls. Differences in covariates between cases and controls were calculated using a student’s t-test for parametric variables, Wilcoxan-Rank Sum or Kruskal-Wallis for nonparametric continuous measures, and chi-square test for categorical or binary variables. Logistic regression was utilized to examine the association of studied potential risk factors with the development of ReA. The association of hypothesis predictors with case status were first tested. Those predictors with a p-value < 0.1 were included in a multivariable logistic regression model. We then corrected for age and sex in the model a priori. The results of the multivariable model were presented as odds ratios with 95% confidence intervals. Given the multiple tests conducted, we used a Bonferroni correction for all p-values and a corrected p-value of <0.05 was considered statistically significant. Differences in laboratory values and biomarkers were examined using univariable and age- and sex-adjusted linear regression models. From these models, beta coefficients and the resulting 95% confidence intervals are presented. Finally, HLA-serotypes are presented descriptively as we did not have enough power to test between differences in the large number of HLA genotypes identified. All analyses were performed using Stata 14.0 (College Station, TX).
Ethics Approval
This study protocol was approved by the Institutional Review Board of the University of Pennsylvania (#819438) and the ethics committee of Universidad Francisco Marroquin.
RESULTS
Thirty-two patients with ReA and thirty-two controls were prospectively enrolled. Demographics are shown in Table 1. The majority of patients were female (81% of patients with ReA and 63% of controls). Most patients self-identified as mestizo (mixed) race (50% of ReA patients and 50% of the controls), however more ReA patients identified as indigenous compared to controls although this was not statistically significant (P=0.05). The majority of patients with ReA (81%) and controls (88%) lived in Guatemala City. The prevalence of type II diabetes mellitus and hypertension were 6% and 16% respectively in patients with ReA compared to 0% and 6% of controls. Gastrointestinal (GI) infections were the most common infection reported by ReA patient (72%) and control (91%) groups. Genitourinary infection (GU) was reported in 31% of the cases and 13% of the controls. No sexually transmitted diseases were reported. Treatment of these infections with antibiotics was reported in 63% of cases and 47% of controls. The month of infection was different among patients with ReA and controls; patients with ReA were most frequently infected in April (28%) whereas controls were most frequently infected in August (28%) (P < 0.001) (Figure 1). This did not correlate with date of enrollment (data not shown).
Table 1.
Clinical and laboratory data of the studied groups
| Variables | Patients with ReA (n=32) | Controls (n=32) | P-value |
|---|---|---|---|
| Sex n (%) | 0.10 | ||
| Females | 26 (81%) | 20 (63%) | |
| Males | 6 (19%) | 12 (37%) | |
| Age (years) | 0.27 | ||
| Range | 18–55 | 18–54 | |
| Mean ± SD | 39 ± 11.46 | 36 ± 9.93 | |
| Race n (%) | 0.05 | ||
| Caucasian | 1 (3%) | 7 (22%) | |
| Mestiza | 16 (50%) | 16 (50%) | |
| Indigenous | 15 (47%) | 9 (28%) | |
| Residence n (%) | 0.50 | ||
| Ciudad Capital | 26 (81%) | 28 (88%) | |
| Interior de la Republica | 6 (19%) | 4 (12%) | |
| Relationship n (%) | 0.11 | ||
| Single | 6 (19 %) | 11 (34 %) | |
| Married | 20 (63%) | 18 (56 %) | |
| Partnered | 2 (6 %) | 2 (6 %) | |
| Divorced | 2 (6 %) | 0 (0 %) | |
| Widow | 2 (6 %) | 1 (3 %) | |
| Occupation n (%) | |||
| Healthcare | 3 (9 %) | 12 (38 %) | 0.007 |
| Manual Labor | 5 (16 %) | 3 (9 %) | 0.46 |
| Housekeeper | 11 (34 %) | 7 (22 %) | 0.27 |
| Business | 6 (19%) | 3 (9%) | 0.29 |
| Tobacco use n (%) | 1 (3 %) | 1 (3 %) | 1.00 |
| BMI mean ± SD | 25.13 ± 4.44 | 26.01 ± 4.26 | 0.42 |
| Selected Comorbidities n (%) | |||
| Diabetes Mellitus | 2 (6 %) | 0 (0 %) | 0.16 |
| Hypertension | 5 (16 %) | 2 (6 %) | 0.24 |
| Infection n (%) | |||
| Gastrointestinal | 23 (72 %) | 29 (91 %) | 0.06 |
| Genitourinary | 10 (31 %) | 4 (13 %) | 0.07 |
| STI | 0 (0%) | 0 (0%) | |
| Most frequent month of infection (%) | April (28 %) | August (28 %) | 0.00 |
| Antibiotics n (%) | |||
| Any | 20 (63 %) | 15 (47 %) | 0.22 |
Figure 1. Month of Infection.
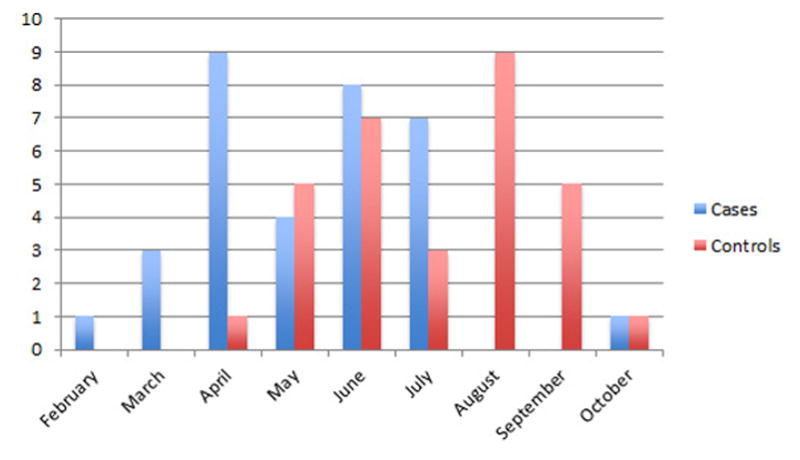
ReA patients (N=32) were most frequently infected with GI or GU pathogens in April (28%) whereas controls (N=32) were most frequently infected in August (28%).
The frequency of symptoms and signs of ReA are listed in Table 2. All ReA patients (100%) had peripheral arthritis, 91% had back pain, and 63% had uveitis (75% of these cases were diagnosed by ophthalmologist), 97% reported NSAID use, and 6 % of patients used glucocorticoids. The right SI joint was the most common tender joint. The insertion of the Achilles tendon was the most common tender enthesis in cases (69%) followed by the right medial femoral condyle (53%). Ultrasound examination identified Achilles enthesopathy in 44% of the cases and in 6% of the controls.
Table 2.
Spondyloarthropathy Characteristics
| Variables | Patients with ReA (n=32) |
|---|---|
| History of Peripheral Arthritis n (%) | 32 (100 %) |
| History of Sero-Negative SpAs n (%) | 3 (9 %) |
| First-Degree Relatives with SpAs n (%) | 2 (6 %) |
| Symptoms n (%) | |
| Back pain | 29 (91 %) |
| Uveitis | 20 (63 %) |
| SpA Medications n (%) | |
| NSAIDs | 31 (97 %) |
| Glucocorticoids | 2 (6 %) |
| Medicinal Herbs | 10 (31 %) |
| Abnormal Imaging (?*) | |
| Ultrasound Achilles Enthesopathy | 14 (44 %) |
| Tender Joints n (%) | |
| Right Sacroiliac | 27 (82 %) |
| Left Sacroiliac | 25 (76 %) |
| Right Ankle | 16 (49 %) |
| Left Ankle | 18 (55 %) |
| Right Elbow | 18 (55 %) |
| Left Elbow | 10 (30%) |
| Right Knee | 7 (21%) |
| Left Knee | 9 (27%) |
| Right Shoulder | 16 (50%) |
| Left Shoulder | 6 (18%) |
| Right Wrist | 4 (12%) |
| Left Wrist | 3 (9%) |
| Right Distal Interphalangeal | 0 (0%) |
| Left Distal Interphalangeal | 0 (0%) |
| Right Proximal Interphalangeal | 2 (6%) |
| Left Proximal Interphalangeal | 1 (3%) |
| Right Metacarpal | 5 (15%) |
| Left Metacarpal | 2 (6%) |
| Right Metatarsal | 1 (3%) |
|
| |
| Swollen Joints n (%) | |
| Right Ankle | 3 (9 %) |
| Left Ankle | 4 (13 %) |
| Right Elbow | 2 (6 %) |
| Left Elbow | 3 (9%) |
| Right Knee | 1 (3%) |
| Left Knee | 1 (3%) |
| Right Wrist | 2 (6%) |
| Left Wrist | 2 (6%) |
| Right Proximal Interphalangeal | 1 (3%) |
| Left Metacarpal | 1 (3%) |
| Tender Entheses | |
| Right Achilles Tendon | 22 (69 %) |
| Left Achilles Tendon | 22 (69 %) |
| Right Medial Femoral Condyle | 17 (53 %) |
| Left Medial Femoral Condyle | 16 (50%) |
| Right Lateral Femoral Condyle | 7 (22%) |
| Left Lateral Femoral Condyle | 7 (22%) |
| Right Plantar Fascia | 16 (50%) |
| Left Plantar Fascia | 15 (47%) |
Most controls did not report any SpA related symptoms, medication use, joint tenderness, or enthesopathy with the exception of 6% reporting uveitis, 16% reporting herbal medication use, and 6% found to have abnormal ultrasound Achilles enthesopathy imaging.
Only 2 subjects from each group were found to carry an HLA-B27 allele. The most frequent HLA-A allele was HLA A2 in both patients with ReA (44%) and controls (63%). The HLA B35 allele was the most prevalent HLA-B allele in both the patient (72%) and control (59%) groups, while the most prevalent HLA-C allele was HLA C7 in both cases (53%) and controls (53%).
Serum biomarkers with previously described associations with SpA were measured to determine whether there were differences in markers of systemic inflammation, new bone formation, and/or bone catabolism among the groups (Table 3). Serum cathepsin K (ng/mL) levels were elevated in patients with ReA compared to controls (2.70 vs 2.17, P = 0.028) and this remained significant after adjusting for age and sex. The other biomarkers tested were not significantly different between patients and controls.
Table 3.
Laboratory Data and Biomarkers
| Lab Value | Patients with ReA (n=32) Mean ± SD | Controls (n=32) Mean ± SD | p-value | Univariate β coeff. (95% CI) | Age-and-sex adjusted β coeff. (95% CI) |
|---|---|---|---|---|---|
| Sedimentation rate | 21.44 ± 25.25 | 20.19±23.11 | NS | −1.25 (−13.35, 10.85) | 2.66 (−9.14, 14.46) |
| Total cholesterol | 153.94±39.62 | 188.78±48.76 | 0.0008 | 34.84 (12.64, 57.04) | 32.47 (9.92, 55.01) |
| High density lipoprotein | 55.76±15.30 | 60.07±17.43 | NS | 4.31 (−3.90, 12.50) | 5.60 (−2.81, 14.01) |
| Low density lipoprotein | 71.8±34.0 | 89.33±31.71 | 0.045 | 17.53 (1.12 33.96) | 15.46 (−1.50, 32.41) |
| Triglycerides | 131.53±49.91 | 180.06±115.80 | NS | 48.53 (3.97, 93.09) | 40.10 (−3.60, 83.8) |
| Hemoglobin | 5.74±1.68 | 5.25±0.73 | NS | −0.48 (−1.13, 0.16) | −0.28 (−0.89, 0.33) |
| Glucose | 95.94±41.65 | 91.88±37.37 | NS | −4.06 (−23.84, 15. 71) | −0.90 (−20.84 19.04) |
| Cathepsin K (ng/mL) | 2.70±0.95 | 2.17±0.61 | 0.028 | −0.53 (−0.93, −0.13) | −0.51 (−0.92, −0.09) |
| Creatinine (pg/mL) | 0.73 ± 0.14 | 0.78 ± 0.17 | NS | 0.05 (−0.03, 0.13) | 0.004 (−0.05, 0.06) |
| hsCRP (mg/dL) | 4.17±5.34 | 2.77 ± 3.87 | 0.03 | −1.40 (−3.76, 0.96) | −1.11 (−3.54, 1.33) |
| DKK-1(pg/mL) | 3416.16 ± 1388.72 | 3069.59 ± 1204.33 | NS | −346.56 (−996.13, 303.00) | −106.40 (−725.51, 512.71) |
| MMP-3 (ng/mL) | 14.14 ±9.16 | 13.07 ± 6.58 | NS | −1.08 (−5.06 2.91) | −3.0 (−6.42, 0.43) |
| OPG (pmol/L) | 6.12 ±1.84 | 5.24 ± 1.27 | NS | −0.88(−1.67, −0.09) | −0.65 (−1.42, 0.13) |
| Sclerostin (pg/mL) | 179.73 ±75.57 | 171.00 ± 46.55 | NS | −8.72 (−40.09, 22.64) | −2.98 (−32.52, 26.56) |
| sRANKL (pmol/L) | 530.88 ± 511.01 | 778.63 ± 713.17 | NS | 247.74 (−62.39, 557.77) | 298.18 (−9.72, 606.09) |
|
| |||||
| HLA typing | |||||
|
| |||||
| HLA-A2 (%) | 14 (44 %) | 20 (63 %) | NS | ||
| HLA-B35 (%) | 23 (72 %) | 19 (59 %) | NS | ||
| HLA-C7 (%) | 17 (53 %) | 17 (53 %) | NS | ||
| HLA-B27 (%) | 2 (6 %) | 2 (6 %) | NS | ||
| HLA-B15 (%) | 2 (6 %) | 2 (6 %) | NS | ||
p-values were generated using the Wilcoxan Rank Sum test for non-parametric data.
All patients completed a follow up survey at 2 years (in person or by phone). Among patients with ReA, 15 (47%) patients had continued symptoms at 2 years. One control received a new diagnosis of psoriasis. Uveitis resolved in all but one of the ReA patients. There were no significant differences in clinical features at baseline among patients with ReA who had persistent joint symptoms and patients in whom the process resolved with one exception. Levels of MMP3 were significantly lower in patients who had persistent joint symptoms: mean MMP3 18.3 (SD 9.5) vs 9.4 (6.1) among those with resolve symptoms versus those with persistent symptoms respectively (p=0.004).
DISCUSSION
We report the results of a study to examine the epidemiology of ReA in Guatemala including examining potential risk factors for the development of ReA and the clinical characteristics. As expected, SpA characteristics were common, including a high prevalence of Achilles enthesitis (67% on exam, 44% on ultrasound).(6) However, we found a few differences from classic depictions of ReA. First, approximately half of patients were female. While this seems surprising given previous studies have reported that ReA is a male predominant disease, we have also learned from recent epidemiology studies in AS that women comprise a larger proportion of patients with AS than previous recognized when newer definitions are used.(15) Additionally, one other study of ReA in the US also found a higher risk for ReA among women.(23) There was a higher than expected prevalence of sacroiliac (SI) joint tenderness (82% and 76% of patients reported tenderness in right and left SI joints, respectively) although low back pain has previously been reported as a common accompanying symptom in ReA.(6) Uveitis was also highly prevalent in this population and resolved in most patients. Most interesting was that nearly half of patients had persistent symptoms at 2 years.
We enrolled a control population to determine whether there were differences in demographic, clinical or genetic differences that would suggest a reason why some patients developed ReA compared to patients from a similar source population who were also exposed to similar infections, who did not. We found qualitative differences in the month(s) of infection, which may suggest that different pathogens may play a role in risk of developing ReA. A study of intestinal microbiota in these cohorts is currently underway to better investigate this possibility.
We found that there were differences in the race/ethnicity of cases and controls (although not statistically significant), suggesting potential genetic variances. We examined limited genetic characteristics, in particular the HLA- A, B, and C allele phenotypes. The HLA-B27 allele has been strongly linked to SpA, in particular ankylosing spondylitis but studies have also suggested a link with ReA.(24, 25) A retrospective study by Sheehan et al., suggested that 60–90% of patients diagnosed with ReA in the United Kingdom in the 1960s and 1970s carried the HLA-B27 allele.(26) Our study suggests that HLA-B27 is not an important risk factor in Central America. The HLA-B27 allele was not associated with ReA in this population and no single HLA-A, B, or C allele was associated with ReA although it is important to note that the study was not powered to detect a difference, particularly in this Central American population where a lower prevalence of HLA-B27 would be expected. Work previously published by Garcia Kutzbach et al. reporting that the association with HLA-B27 was weak in SpA cohorts from El Salvador, Costa Rica, and Guatemala (prevalence of HLA-B27: 4–57%).(27) Studies in the U.S. have also found that the prevalence of HLA-B27 is lower among Hispanic populations compared to Caucasians (28) and have found no association between HLA-B27 and reactive arthritis .(23) The lack of association with HLA-B27 in this study is also supported by recent work suggesting that C. trachomatis-induced ReA in an animal model was independent of HLA-B27 and genetic studies identifying other genotypes that are associated with spondyloarthropathies.(25, 29)
Previous studies have found that SpA, in particular AS and PsA, are associated with metabolic alterations that increase the incidence of type II diabetes and hypertension in affected patients.(30) While there was a higher prevalence of hypertension and diabetes in patients with ReA compared to controls, this was not statistically significant. It may be that the duration of systemic inflammation that leads to these metabolic disorders is shorter in patients with ReA, and therefore the metabolic syndrome association cannot be identified. Small sample size also limited the ability to determine differences between groups.
In this study, we assessed serum biomarkers to determine whether there were pathophysiologic differences between patients with ReA and controls. Serum Cathepsin K levels were significantly higher in ReA patients when compared to controls (P = 0.028). Cathepsin K is a protease, secreted by osteoclasts, that is responsible for the degradation of type I collagen (90% of organic bone matrix) in bone resorption processes; this protease is activated by proinflammatory cytokines (e.g., IL6, TNF) and IL17 may also influence this pathway. In one small study, serum cathepsin K was higher in patients with AS than healthy controls, although the difference was not statistically significant (possibly related to the small sample size). Similarly, in another study, serum cathepsin K levels rose following TNFi therapy, although this was a similarly small study so the values were not statistically significant.(31, 32) These results raise the question as to whether Cathepsin K plays a role in the pathogenesis or activation of symptoms in ReA. MMP3 has been associated with disease activity and prognosis in AS.(20) While MMP did not differentiate between cases and controls in our study, it was associated with persistence of disease at two years. Finally, total cholesterol and LDL were lower in our patients with ReA; this is expected, as these tend to decline in the setting of active inflammation.(33)
Our study has several strengths. This is the first study to examine potential risk factors for ReA, including serum biomarkers, by systemically comparing patients with ReA to controls from the same source population with a prior GI or GU infection. The prospective design allowed for standardized clinical evaluation and uniform protocols for blood sampling, stool sampling, and blinded imaging procedures. Additionally, we used the ASAS peripheral SpA criteria to define ReA. Finally, this is one of few studies, to follow patients for persistence or resolution of symptoms at two years in a cohort of patients with ReA. (15, 34–36)
This study also has limitations. This study was initially constructed as a pilot study for the genetic and microbial analysis of a sample of ReA patients compared to controls; we intended to look for large differences. The sample size studied would not allow for detection of subtle differences between groups including an association between HLA-B27 in ReA in a population in which HLA-B27 is low. Next, socio-demographically, the majority of subjects were residents of Guatemala City (84.4%). Therefore, our results may not be generalizable to the Guatemalan population or to Central America in general. Also GI and GU infections were self-reported by patients. It is possible that these infections were viral rather than bacterial, although approximately half received antibiotics. Ideally, a cohort study would include patients that have documented bacterial infections followed prospectively for the development of ReA. However, given the relatively low incidence of ReA, this was not possible. Additionally, viral infections have similarly been implicated in stimulating inflammatory arthritis. Sexually transmitted infections were also not observed in our study population. It is possible that patients were reluctant to share their infectious sexual history with study investigators, or patients who had an STI may have specifically avoided enrollment in our study. Finally, we were able to follow up with patients via telephone to ascertain whether or not they had joint pain or any other new symptoms or diagnoses related to SpA but we were not able to bring patients back for a follow up visit in most cases and thus have limited information on the clinical examination.
In summary, we found that month of infection were associated with development of reactive arthritis but there were few other factors that differentiated cases from controls, including HLA A, B, and C genotypes. Furthermore, we found that ReA was associated with a high prevalence of uveitis and nearly half of patients with ReA continued to have persistent symptoms at two years. Overall relatively little is understood about why patients develop ReA, or the SpA in general. It is also unclear whether ReA should be differentiated from early peripheral SpA as the outcomes may be similar. Further studies are needed to determine why some patients develop ReA and to better understand prognostic factors for persistence of inflammatory arthritis. (37)
Acknowledgments
Financial Support: This work was funded by the Guatemala-Penn Partners Program and NIH Fogarty International Center grant D43TW008317. AO was supported by NIH K23 AR063764 and the Rheumatology Research Foundation. JUS was supported by NIH K23 AR064318.
Footnotes
Conflicts of Interest: AO has consulted for Novartis and Pfizer and has received grant support from Pfizer and Novartis. JUS has consulted for Novartis, Janssen and UCB. AGK served as a principal investigar for studies funded by MS&D, Novartis, Pfzier, and Lilly. The remaining authors do not report a conflict of interest.
References
- 1.Carter JD, Hudson AP. Reactive arthritis: clinical aspects and medical management. Rheum Dis Clin North Am. 2009;35:21–44. doi: 10.1016/j.rdc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Berthelot JM, de la Cochetiere MF, Potel G, Le Goff B, Maugars Y. Evidence supporting a role for dormant bacteria in the pathogenesis of spondylarthritis. Joint, bone, spine : revue du rhumatisme. 2013;80:135–40. doi: 10.1016/j.jbspin.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Stolwijk C, Boonen A, van Tubergen A, Reveille JD. Epidemiology of spondyloarthritis. Rheum Dis Clin North Am. 2012;38:441–76. doi: 10.1016/j.rdc.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter JD, Hudson AP. Recent advances and future directions in understanding and treating Chlamydia-induced reactive arthritis. Expert review of clinical immunology. 2016:1–10. doi: 10.1080/1744666X.2017.1233816. [DOI] [PubMed] [Google Scholar]
- 5.Hannu T. Reactive arthritis. Best Pract Res Clin Rheumatol. 2011;25:347. doi: 10.1016/j.berh.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt SK. Reactive Arthritis. Infectious disease clinics of North America. 2017;31:265–77. doi: 10.1016/j.idc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Kutzbach A, Montenegro A, Iraheta I, Bara C, Saenz R. Epidemiology of spondyloarthropathies in Central America. Am J Med Sci. 2011;341:295–7. doi: 10.1097/MAJ.0b013e31820f8cf1. [DOI] [PubMed] [Google Scholar]
- 8.Sieper J, Braun J, Kingsley GH. Report on the Fourth International Workshop on Reactive Arthritis. Arthritis Rheum. 2000;43:720–34. doi: 10.1002/1529-0131(200004)43:4<720::AID-ANR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Diaz SL, Jarquin C, Morales AJ, Morales M, Valenzuela C. Burden of salmonellosis and shigellosis in four departments of Guatemala, 2010. Revista panamericana de salud publica = Pan American journal of public health. 2015;38:326–32. [PubMed] [Google Scholar]
- 10.Paget SA. The microbiome, autoimmunity, and arthritis: Cause and Effect. Trans Am Clin Climatol Assoc. 2012;123:257–67. [PMC free article] [PubMed] [Google Scholar]
- 11.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569–78. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Love TJ, Zhu Y, Zhang Y, Wall-Burns L, Ogdie A, Gelfand JM, et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71:1273–7. doi: 10.1136/annrheumdis-2012-201299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Li WQ, Han J, Sun Q, Qureshi AA. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis & rheumatology (Hoboken, NJ) 2014;66:304–10. doi: 10.1002/art.38227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun J, Kingsley G, van der Heijde D, Sieper J. On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. J Rheumatol; Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis; Berlin, Germany. July 3–6, 1999; 2000. pp. 2185–92. [PubMed] [Google Scholar]
- 15.Misra R, Gupta L. Epidemiology: Time to revisit the concept of reactive arthritis. Nat Rev Rheumatol. 2017;13:327–8. doi: 10.1038/nrrheum.2017.69. [DOI] [PubMed] [Google Scholar]
- 16.Rudwaleit M, van deer Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 17.Cereda E, Pedrolli C, Klersy C, Bonardi C, Quarleri L, Cappello S, et al. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA(R) Clinical nutrition (Edinburgh, Scotland) 2016;35:1282–90. doi: 10.1016/j.clnu.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13:782–8. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino MA, Olivieri I. Enthesitis. Best Pract Res Clin Rheumatol. 2006;20:473–86. doi: 10.1016/j.berh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Reveille JD. Biomarkers for diagnosis, monitoring of progression, and treatment responses in ankylosing spondylitis and axial spondyloarthritis. Clinical rheumatology. 2015;34:1009–18. doi: 10.1007/s10067-015-2949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandran V. Soluble biomarkers may differentiate psoriasis from psoriatic arthritis. The Journal of rheumatology Supplement. 2012;89:65–6. doi: 10.3899/jrheum.120247. [DOI] [PubMed] [Google Scholar]
- 22.Terslev L, Gutierrez M, Schmidt WA, Keen HI, Filippucci E, Kane D, et al. Ultrasound as an Outcome Measure in Gout. A Validation Process by the OMERACT Ultrasound Working Group. The Journal of rheumatology. 2015;42:2177–81. doi: 10.3899/jrheum.141294. [DOI] [PubMed] [Google Scholar]
- 23.Townes JM, Deodhar AA, Laine ES, Smith K, Krug HE, Barkhuizen A, et al. Reactive arthritis following culture-confirmed infections with bacterial enteric pathogens in Minnesota and Oregon: a population-based study. Annals of the rheumatic diseases. 2008;67:1689–96. doi: 10.1136/ard.2007.083451. [DOI] [PubMed] [Google Scholar]
- 24.Mathieu A, Paladini F, Vacca A, Cauli A, Fiorillo MT, Sorrentino R. The interplay between the geographic distribution of HLA-B27 alleles and their role in infectious and autoimmune diseases: a unifying hypothesis. Autoimmunity reviews. 2009;8:420–5. doi: 10.1016/j.autrev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Reveille JD. An update on the contribution of the MHC to AS susceptibility. Clinical rheumatology. 2014;33:749–57. doi: 10.1007/s10067-014-2662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan NJ. The ramifications of HLA-B27. Journal of the Royal Society of Medicine. 2004;97:10–4. doi: 10.1258/jrsm.97.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Kutzbach A, Montenegro A, Iraheta I, et al. Epidemiology of Spondyloarthropathies in Central America. Am J Med Sci. 2011;341:295–7. doi: 10.1097/MAJ.0b013e31820f8cf1. [DOI] [PubMed] [Google Scholar]
- 28.Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci. 2013;245:431–6. doi: 10.1097/maj.0b013e318294457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baillet AC, Rehaume LM, Benham H, O’Meara CP, Armitage CW, Ruscher R, et al. High Chlamydia Burden Promotes Tumor Necrosis Factor-Dependent Reactive Arthritis in SKG Mice. Arthritis & rheumatology (Hoboken, NJ) 2015;67:1535–47. doi: 10.1002/art.39041. [DOI] [PubMed] [Google Scholar]
- 30.Ogdie A, Schwartzman S, Husni ME. Recognizing and managing comorbidities in psoriatic arthritis. Current opinion in rheumatology. 2015;27:118–26. doi: 10.1097/BOR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 31.Neidhart M, Baraliakos X, Seemayer C, Zelder C, Gay RE, Michel BA, et al. Expression of cathepsin K and matrix metalloproteinase 1 indicate persistent osteodestructive activity in long-standing ankylosing spondylitis. Annals of the rheumatic diseases. 2009;68:1334–9. doi: 10.1136/ard.2008.092494. [DOI] [PubMed] [Google Scholar]
- 32.Wendling D, Cedoz JP, Racadot E. Serum levels of MMP-3 and cathepsin K in patients with ankylosing spondylitis: effect of TNFalpha antagonist therapy. Joint, bone, spine : revue du rhumatisme. 2008;75:559–62. doi: 10.1016/j.jbspin.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Annals of the rheumatic diseases. 2009;68:460–9. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 34.Leirisalo-Repo M, Helenius P, Hannu T, Lehtinen A, Kreula J, Taavitsainen M, et al. Long-term prognosis of reactive salmonella arthritis. Annals of the rheumatic diseases. 1997;56:516–20. doi: 10.1136/ard.56.9.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leirisalo-Repo M, Skylv G, Kousa M. Follow-up study of Reiter’s disease and reactive arthritis. Factors influencing the natural course and the prognosis. Clinical rheumatology. 1987;6(Suppl 2):73–82. doi: 10.1007/BF02203388. [DOI] [PubMed] [Google Scholar]
- 36.Kaarela K, Jantti JK, Kotaniemi KM. Similarity between chronic reactive arthritis and ankylosing spondylitis. A 32–35-year follow-up study. Clinical and experimental rheumatology. 2009;27:325–8. [PubMed] [Google Scholar]
- 37.Turina MC, Yeremenko N, van Gaalen F, van Oosterhout M, Berg IJ, Ramonda R, et al. Serum inflammatory biomarkers fail to identify early axial spondyloarthritis: results from the SpondyloArthritis Caught Early (SPACE) cohort. RMD open. 2017;3:e000319. doi: 10.1136/rmdopen-2016-000319. [DOI] [PMC free article] [PubMed] [Google Scholar]


