Abstract
Background
We investigated whether epicardial adipose tissue (EAT) volume and density are related to early atherosclerosis, plaque inflammation and major adverse cardiac events (MACE, cardiac death and myocardial infarction) in asymptomatic subjects.
Methods
EAT volume and density were quantified from non-contrast cardiac CT in 456 asymptomatic individuals (age 60.3±8.3; 68% with CCS>0) from the prospective EISNER trial. EAT volume and density were examined in relation to coronary calcium score (CCS), inflammatory biomarkers and MACE.
Results
EAT volume was higher and EAT density lower in subjects with coronary calcium compared to subjects without [89 vs 74 cm³, p<0.001] [−76.9 vs −75.7 HU,p=0.024]. EAT volume was lowest in individuals with no coronary calcium and was significant higher in subjects with early atherosclerosis (CCS 1–99) [74 vs 87 cm³,p=0.016] and in subjects with more advanced atherosclerosis (CCS≥100) [89 cm³,p=0.002]). EAT volume was independently related to serum levels of PAI-1, and MCP-1 and inversely related to adiponectin and HDL-cholesterol (p<0.05). EAT density was inversely related to PAI-1 and LDL-cholesterol and positively associated to adiponectin, sICAM-1 and HDL-cholesterol (p<0.05). EAT density was more significantly associated with MACE [(HR 0.8, CI95%:0.7–0.98), p=0.029] than EAT volume or CCS.
Conclusion
EAT volume was higher and density lower in subjects with coronary calcium compared to subjects with CCS=0, with similar EAT volume in CCS<100 and CCS≥100. Lower EAT density and increased EAT volume were associated with coronary calcification, serum levels of plaque inflammatory markers and MACE, suggesting that dysfunctional EAT may be linked to early plaque formation and inflammation.
Keywords: Epicardial adipose tissue, EAT density and volume, inflammatory biomarkers, early subclinical atherosclerosis, metabolic abnormality, major adverse cardiovascular events
TOC Summary
Our quantification of EAT volume and density from non-contrast cardiac CT in 456 asymptomatic subjects showed similar EAT volume in subjects with early and more advanced atherosclerosis. Lower EAT density and increased EAT volume were associated with the presence of coronary calcification, MACE, serum levels of plaque inflammatory markers and lipids, suggesting that dysfunctional EAT may be linked to cardiovascular events, early plaque formation, metabolic abnormality and inflammation. Thus, independent of CCS categories, measurements of EAT volume and density from non-contrast cardiac CT provide additional information regarding the “activity” of atherosclerosis in asymptomatic individuals.
1. Introduction
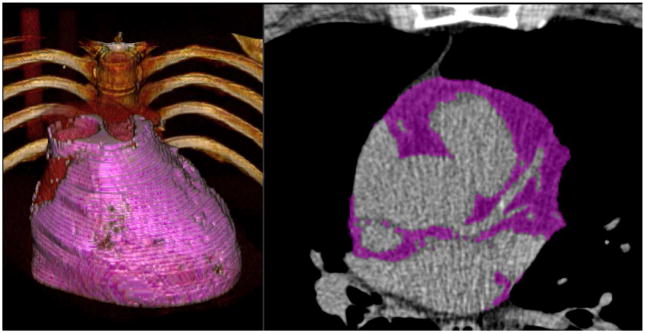
The pathophysiological process responsible for coronary artery disease (CAD) is atherosclerosis, an inflammatory disease resulting in coronary calcification (1). All stages of atherosclerosis involve inflammatory processes with the contribution of numerous inflammatory mediators, however, the exact mechanisms of atherosclerotic calcification remain poorly understood (2). Epicardial adipose tissue (EAT) is a local fat depot between the serous epicardium and the fibrous pericardium surrounding the coronary artery tree and can be quantified from the same non-contrast cardiac CT calcium scan, which is used to determine the Agatston-Score (Figure 1) (6). Prior research has demonstrated that increased EAT volume is independently related to adverse cardiovascular events (7,8,10). However, it remains unknown whether increased EAT volume is only restricted to subjects with more advanced atherosclerosis.
Figure 1.
Epicardial adipose tissue (EAT) quantification. Figure showing 3-D EAT quantification of a 55 years old asymptomatic (BMI 29.6 kg/m2) man with a history of hyperlipidemia, an EAT volume of 225 cm³ and a coronary calcium score (CCS) of 35.5. EAT is highlighted in purple color.
Different fat compositions such as inflammatory white adipose tissue, which has lower CT attenuation than the metabolically active and anti-inflammatory brown adipose tissue, might have a stronger influence on the atherosclerotic process (3,4,16). Previous studies described the quantification of visceral adipose tissue attenuation in CT as indirect measures of fat composition, unraveling a relationship between lower fat CT attenuation and incident metabolic risk factors as well as worsening of cardiovascular risk factors (14,15). In contrast to investigations of visceral adipose tissue CT attenuation, the relationship between EAT density measured by non-contrast cardiac CT and cardiovascular disease in asymptomatic individuals has been poorly investigated.
Dysfunctional EAT has been described as a source of inflammatory mediators and has been implicated in the precipitation of coronary atherosclerosis through its direct contact with the adventitia of the underlying coronary arteries, and paracrine inflammatory effects (7,8,11,12). In addition to that, results of the Framingham Heart study suggested a correlation between increased EAT volume and serum levels of inflammatory biomarkers (13). However, the relationship between EAT density and serum levels of inflammatory biomarkers has not been studied before.
Therefore, in this sub-analysis of the prospective EISNER (Early Identification of Subclinical Atherosclerosis using Non-invasivE Imaging Research) trial we aimed to investigate whether EAT volume and density are related to serum levels of plaque inflammatory markers in asymptomatic subjects as a potential trigger for the development of coronary atherosclerosis. Furthermore, we aimed to investigate whether increased EAT volume and lower EAT density could be observed in subjects with early coronary atherosclerosis or major adverse cardiac events (MACE, defined by cardiac death and myocardial infarction).
2. Methods
2.1. Study population
We evaluated 456 randomly selected subjects from the EISNER 1 and 4 sub-study (n=2614) of the prospective single-center EISNER trial (NCT00927693); in our subjects, both serum collection of inflammatory biomarkers and non-contrast cardiac-CT were performed. The EISNER trial represents a community-based cohort of asymptomatic subjects who underwent complete cardiovascular risk assessment and coronary calcium scanning. The EISNER participants were middle-aged subjects, with cardiovascular risk factors, but no prior known CAD. Subjects with any cardiac or cerebrovascular disease or chest pain, age ≥80 years, pregnancy, significant medical comorbidity or prior coronary calcium scanning were excluded (17). The trial was conducted under the guidelines of the Cedars-Sinai Medical Center Institutional Review Board, and all subjects gave written informed consent for the use of their data. In a sub-analysis of 292 individuals (64%) prospective long-term follow-up from 11/10/2000 to 10/08/2017 (mean 13.2 years ± 2.1 years, median 13.5 years) for subsequent MACE (defined by myocardial infarction and cardiac death) was performed. MACE was confirmed by direct contact between research staff and the subjects or their first-degree relatives, followed by a review of corresponding medical or death records by an independent cardiologist. In the long-term follow-up, 13 patients experienced myocardial infarctions and two patients deceased due to cardiac death.
2.2. Cardiac computed tomography
Non-contrast CT scans were acquired using either an Electron Beam CT (EBCT) scanner (e-Speed, manufactured by GE Healthcare, Milwaukee, WI, USA) or a 4-slice CT scanner (Somatom Volumezoom, Siemens Medical Solutions, Erlangen, Germany) with prospective ECG-triggering, and a tube voltage of 120 kVp; slice thickness was either 2.5 or 3.0 mm. Each scan was analyzed using semi-automatic commercially available calcium scoring software (ScImage, Inc., Los Altos, CA, USA) to measure the total Agatston coronary calcium score (CCS) as the sum of calcified plaque scores of all coronary arteries (6). CCS was reported in three categories as no coronary calcium (CCS of 0), early atherosclerosis (CCS 1–99) and more advanced atherosclerosis (CCS ≥100) (10,18).
2.3. Quantification of EAT density and volume
We defined EAT as all adipose tissue enclosed by the pericardium. EAT volume and density were quantified using semi-automated QFAT version 2.0 software from non-contrast CT developed at the Cedars-Sinai Medical Center (19). After estimating cardiac orientation and aligning a heart model the pericardium was automatically segmented in the non-contrast data sets. We used the pulmonary artery bifurcation as the superior limit and the level of the posterior descending artery as the inferior limit of the heart. Contour and slice limits were corrected by a CT reader with over 3 years of experience blinded to subject characteristics and clinical data. EAT volume (reported in cm³) and density [reported in Hounsfield units (HU)] were automatically calculated by including contiguous three-dimensional fat voxels between the HU limits of (−190, −30) enclosed by the visceral pericardium. EAT volume and density were assessed in relation to CCS of 0, 1–99 and ≥100.
2.4. Serum biomarkers
Serum samples were collected at the time of the CT scan and immediately centrifuged and stored at −80 degree°C until assayed. Adipocytokines and inflammatory serum biomarkers including interleukin 6 (IL-6), monocyte chemoattractant protein 1 (MCP-1), endothelial plasminogen activator inhibitor 1 (PAI-1), adiponectin, vascular cell adhesion molecule 1 (VCAM-1), soluble intercellular adhesion molecule 1 (sICAM-1), soluble endothelial cell-selective adhesion molecule (ESAM), lymphotoxin beta receptor (LTBR), myeloperoxidase (MPO), neutrophil gelatinase-associated lipocalin (NGAL), macrophage inflammatory protein 3 (MIP3A), chemokine (C-X-C motif) ligand 1 (CXCL1), peptidoglycan recognition proteins (PGRPs), caspase 3, angiotensinogen and C-reactive protein (CRP), were measured by an independent fully blinded laboratory (Biosite, San Diego, CA, USA). Furthermore, BNP, BNP 3–108, CK-MB, myoglobin, troponin I, D-dimer and a fasting lipid profile were measured in the serum samples of the individuals.
2.5. Statistical analysis
Statistical analysis was performed using SPSS software (IBM® SPSS® statistics, version 24 for Windows, Armonk, NY, USA). All continuous variables were expressed as mean ± standard deviation (SD), and categorical variables as frequencies and percentage. Group-wise comparisons were performed with one-way analysis of variance (ANOVA) with Sidak correction to test between pairs as well as the Kruskal-Wallis test where the assumptions for ANOVA could not be met. Pearson or Spearman rank correlations were calculated to determine the relationships between EAT measures and serum biomarkers. Binary logistic regression was performed to examine EAT measures in relation to CCS and DM. We also evaluated the relationship between EAT measures and serum biomarkers using multivariable linear regression after adjusting for age, BMI, log-transformed CCS and number of traditional cardiovascular risk factors including DM, arterial hypertension, hyperlipidemia, smoking and family history of CAD. The association of EAT measures with MACE during follow-up was assessed using Cox regression models. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Study population
456 subjects (mean age 60.3±8.3 years; 64% male) were included in this analysis, with detailed characteristics of the study cohort summarized in Table 1. The mean number of cardiovascular risk factors per subject was 1.8 and in 312 patients (68%) there was evidence of coronary calcification.
Table 1.
Demographic and clinical characteristics of the study population. Mean ± SD are included for continuous variables.
| Demographic and clinical characteristics | Overall cohort (n=456) |
|---|---|
|
| |
| Demographics | |
| age | 60.3±8.3 years |
| Male | 291 (64%) |
| BMI (kg/m2) | 27.2±4.7 |
| CCS 0/CCS 1–99/CCS ≥100 | 31.5%/31.5%/37% |
|
| |
| Cardiovascular risk factors, lipid levels and blood pressure | |
| Diabetes mellitus | 51 (11%) |
| Hypertension | 252 (56%) |
| Hyperlipidemia | 314 (70%) |
| Smoking | 44 (10%) |
| Family history of CAD | 137 (30%) |
| ASCVD risk score | 11.3 ±9.5 |
| Cholesterol level | 207.3±42.0 |
| HDL-/LDL-cholesterol level | 53.7±16.3/127.0±37.5 |
| Systolic/diastolic blood pressure | 133.1±17.4/82.4±10.6 |
|
| |
| Medication (%) | |
| Lipid-lowering medication | 149 (34%) |
| Antihypertensive medication | 147 (36%) |
|
| |
| Cardiac CT measurements | |
| Coronary calcium score | 234±514 |
| EAT volume (cm3) | 83.9±38.0 |
| EAT density (HU) | −76.2±4.32 |
|
| |
| Prospective follow-up (n=292) | |
| Duration of follow-up (years) | 13.2±2.1 |
| Myocardial infarction and cardiac death | 15/292 |
BMI denotes body mass index; CAD, coronary artery disease; CCS, coronary calcium score; EAT, epicardial adipose tissue; HU, Hounsfield units; SD, standard deviation; ASCVD risk score, lifetime risk for atherosclerotic cardiovascular disease.
3.2. EAT volume and density in relation to presence of coronary artery calcium
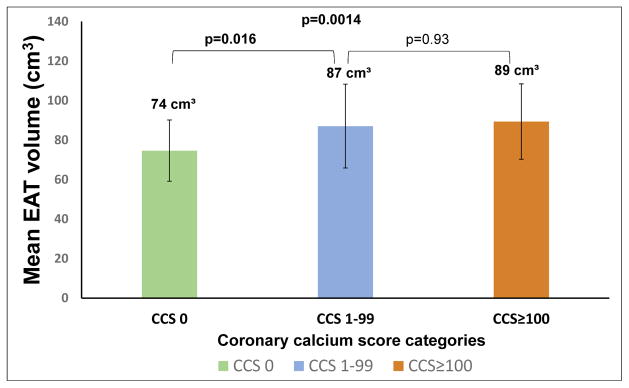
EAT volume was significantly greater in subjects with coronary calcium versus those without coronary calcium [89 cm³ (mean 95%CI:84.4–93.5 cm³), versus 74 cm³ (mean 95%CI:69.5–79.7 cm³), p<0.001] (Figure 2 and 4). However, comparing to early atherosclerosis (87 cm³±42), more advanced atherosclerosis did not exhibit significant difference in EAT volume (89 cm³±38, p=0.93) (Figure 4). EAT volume was also significantly higher in subjects with DM than in those without (111 cm³±47 vs. 80 cm³±35, p<0.001).
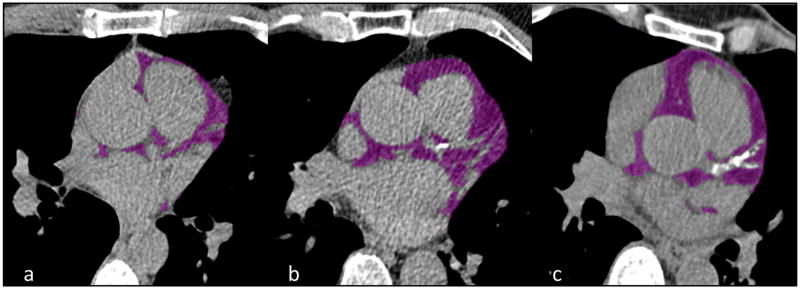
Figure 2.
Coronary calcium score (CCS) categories and epicardial adipose tissue (EAT) volume. Figure showing different degrees of CCS and EAT volume. Epicardial fat (purple) was identified on approximately the same slice in three different study individuals. The CCS and EAT volume increases from Figure 2a–2c. a CCS 0 and EAT volume 41 cm³, b CCS 73.8 and EAT volume 111 cm³, c CCS 538.6 and EAT volume 112 cm³.
Figure 4.
Epicardial adipose tissue (EAT) volume and coronary calcium score (CCS) categories. Figure showing the relation of mean EAT volume to CCS categories.
In a sub-analysis of the CCS groups CCS<100, CCS 100–399 and CCS ≥400, the mean EAT volume of group CCS<100 [87 cm³, mean 95%CI:80.7–94.9 cm³] was higher compared to CCS group 100–399 [85.6 cm³ (mean 95%CI:78.7–92.4 cm³) p=0.998] and not significantly lower compared to CCS group ≥400 [96.5 cm³ (mean 95%CI: 86–106.9 cm³), p=0.537]. In binary logistic regression analysis, EAT volume ≥125 cm³ was the patient characteristic with the strongest association to DM [Odds Ratio (OR) 2.2 (95%CI: 1.1–4.6), p=0.03], over log-transformed CCS (p=0.014), BMI (p<0.001), family history of CAD (p=0.036) and other risk factors (p>0.05).
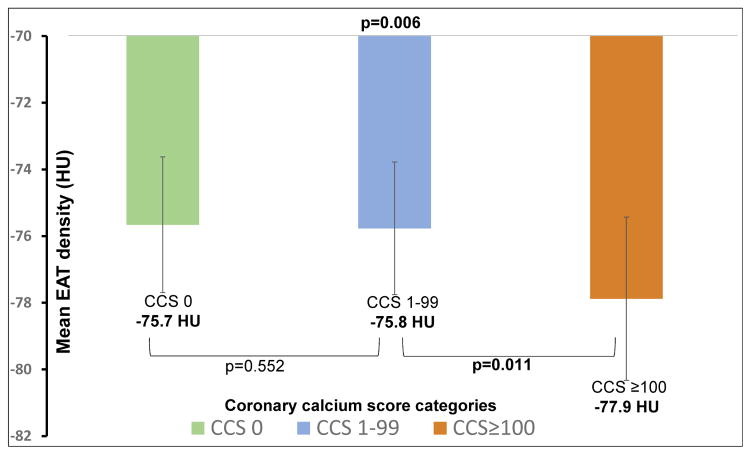
EAT density was significantly lower in subjects with coronary calcium compared to subjects with no coronary calcium [−76.9 HU (mean 95%CI: −77.6 to −76.2 HU) vs −75.7 HU (mean 95%CI: −76.4 to −74.9 HU), p=0.024]. In comparison of three CCS categories no significant difference was observed in the mean EAT density between subjects with no coronary calcium (−75.7 HU±4.07) and subjects with early atherosclerosis (−75.8 HU±3.98, p=0.552). However, more advanced atherosclerosis was associated with lower EAT density (−77.9 HU±4.90) when compared to subjects with no coronary calcium (p=0.002) (Figure 3). Furthermore, subjects with more advanced atherosclerosis showed a significant lower EAT density compared to subjects with early atherosclerosis (p=0.011) (Figure 3). As CCS increased from 0 to 100–399 and then to ≥400, the mean EAT density decreased from −75.7 HU to −78.2 HU (mean 95%CI: −79.7 to −76.6 HU, p=0.01) and then to −78.5 HU (mean 95%CI: −80.6 to −76.4 HU, p=0.023) respectively.
Figure 3.
Epicardial adipose tissue (EAT) density and coronary calcium score (CCS) categories. Figure showing the relation of mean EAT density to CCS categories.
In bivariate correlation analysis EAT density was inversely correlated to EAT volume (r=−0.69, p<0.001), BMI (r=−0.21, p=0.001), log-transformed CCS (r=−0.17, p=0.006) and CCS categories (r=−0.18, p=0.004). The presence of DM or other patient characteristics such as age, arterial hypertension, hyperlipidemia, smoking and family history of CAD were not correlated with EAT density.
3.3. EAT density and volume in relation to serum levels of inflammatory biomarkers and MACE
In bivariate correlation analysis EAT density was significantly correlated with serum levels of adiponectin, sICAM-1 and HDL-cholesterol and inversely correlated with PAI-1 and triglyceride (Table 2). In multivariate regression analysis adjusted for age, BMI, EAT volume, CCS and number of cardiovascular risk factors, EAT density was independently positively correlated with serum levels of adiponectin, sICAM-1 and HDL-cholesterol and inversely correlated with PAI-1, LDL-cholesterol and triglyceride (all p<0.05, Table 2).
Table 2.
Relationship of EAT density with levels of serum biomarkers
| Serum biomarkers | Correlation (EAT density) | p-value | Adjusted p-value* | Coefficient Beta* | Standard Error* |
|---|---|---|---|---|---|
| PAI-1 | −0.29 | <0.001 | 0.013 | −0.191 | 0.078 |
| Adiponectin | 0.20 | 0.01 | 0.011 | 0.192 | 0.082 |
| sICAM-1 | 0.14 | 0.03 | 0.039 | 0.176 | 0.091 |
| HDL-cholesterol | 0.30 | <0.001 | 0.014 | 0.194 | 0.075 |
| Triglyceride | −0.28 | <0.001 | 0.049 | −0.163 | 0.057 |
| LDL-cholesterol | −0.11 | 0.091 | 0.004 | −0.247 | 0.077 |
multivariate regression analysis adjusted for age, BMI, EAT volume, CCS and number of cardiovascular risk factors; only serum biomarkers with a p-value <0.05 are listed.
EAT denotes epicardial adipose tissue; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PAI-1, endothelial plasminogen activator inhibitor 1; sICAM-1, soluble intercellular adhesion molecule 1
As summarized in Table 3, in bivariate correlation analysis increased EAT volume was significantly correlated with serum levels of PAI-1, MCP-1, VCAM-1, triglyceride, myoglobin and inversely correlated with adiponectin, angiotensinogen and HDL-cholesterol (all p<0.05), while CCS was not significantly correlated with levels of serum biomarkers (p>0.05). In multivariate regression analysis adjusted for age, BMI, CCS and number of cardiovascular risk factors, EAT volume remained independently associated with PAI-1, MCP-1 and triglyceride and inversely associated with adiponectin and HDL-cholesterol (all p<0.05, Table 3).
Table 3.
Relationship of EAT volume with levels of serum biomarkers
| Serum biomarkers | Correlation (EAT volume) | p-value | Adjusted p-value* | Coefficient Beta* | Standard Error* |
|---|---|---|---|---|---|
| PAI-1 | 0.29 | <0.001 | 0.001 | 0.174 | 0.055 |
| MCP-1 | 0.20 | <0.001 | <0.001 | 0.237 | 0.059 |
| Adiponectin | −0.18 | <0.001 | 0.028 | −0.123 | 0.058 |
| VCAM-1 | 0.12 | 0.026 | 0.130 | 0.088 | 0.060 |
| HDL-cholesterol | −0.27 | <0.001 | <0.001 | −0.252 | 0.050 |
| Triglyceride | 0.26 | <0.001 | <0.001 | 0.198 | 0.050 |
| Myoglobin | 0.15 | 0.006 | 0.091 | 0.098 | 0.060 |
| Angiotensinogen | −0.13 | 0.015 | 0.103 | −0.092 | 0.061 |
multivariate regression analysis adjusted for age, BMI, CCS and number of cardiovascular risk factors; only serum biomarkers with a p-value <0.05 are listed.
EAT denotes epicardial adipose tissue; HDL, high-density lipoprotein; MCP-1, monocyte chemoattractant protein 1; PAI-1, endothelial plasminogen activator inhibitor 1; sICAM-1, soluble intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1
292 (64%) subjects completed long-term follow-up (mean long-term follow-up 13.2 years ± 2.1 years, median 13.5 years). In univariate analysis, the subjects who experienced cardiac death or myocardial infarction had significantly higher EAT volumes and significantly lower EAT densities compared to subjects without MACE [135 cm³ (mean CI95%: 111.4–158.6 cm³) versus 79.3 cm³ (mean CI95%: 75.4–83.2 cm³), p<0.001] and [−81.3 HU (mean CI95%: −83.6.4 to −79.0 HU) versus −75.8 HU (mean CI95%: −76.3 to −75.3 HU, p<0.001]. In multivariable Cox analysis adjusted for log-transformed CCS and ASCVD risk score (Pooled Cohort Equations), EAT volume ≥125 cm³ was the only variable independently associated with MACE [(Hazard Ratio (HR) 4.6, CI95%: 1.6–13.1), p=0.004] (Table 4). When EAT density was included, EAT density was more significantly associated with MACE [(HR 0.8, CI95%: 0.7–0.98), p=0.029] than EAT volume or log-transformed CCS [(HR 1.5, CI95%: 1.1–2.1), p=0.017] (Table 4).
Table 4.
Multivariable Cox analysis of patient characteristics and major adverse cardiac events
| Patient characteristics | Hazard Ratio (95% CI) | p-value |
|---|---|---|
| Model 1 | ||
| ASCVD risk score | - | 0.83 |
| Log-transformed CCS | 1.2 (0.99–1.5) | 0.053 |
| EAT volume ≥125 cm3 | 4.6 (1.6–13.1) | 0.004 |
|
| ||
| Model 2 (=Model 1 + EAT density) | ||
| ASCVD risk score | - | 0.72 |
| Log-transformed CCS | 1.5 (1.1–2.1) | 0.017 |
| EAT volume ≥125 cm3 | 2.3 (0.6–8.8) | 0.2 |
| EAT density | 0.8 (0.7–0.9) | 0.029 |
4. Discussion
There are three main findings in our study investigating the relation of EAT density and volume quantified using non-contrast cardiac CT with early coronary atherosclerosis, plaque inflammatory markers and MACE in asymptomatic subjects. First, EAT volume was significantly increased in subjects with early atherosclerosis compared to subjects with no coronary calcium, and similar to subjects with advanced atherosclerosis; suggesting increased EAT volume might not be restricted only to more advanced atherosclerosis. Second, mean EAT density and volume were significantly related to serum levels of lipids and plaque inflammatory markers. Third, increased EAT volume and lower EAT density were related to MACE.
4.1. EAT volume and density in relation to presence of coronary artery calcium
In our study EAT volume was significantly higher in subjects with coronary calcium compared to subjects with no coronary calcium, however, without significant difference in subjects with a CCS of 1–99 compared to CCS 100–399 or CCS ≥400. Mahabadi et al. described an association of EAT volume with coronary events even among subjects with no or early atherosclerosis (CCS<100) potentially supporting the hypothesis that EAT might be linked with future coronary events through different mechanistic pathways than coronary calcification, such as a link to early and noncalcified plaque burden (10). Our study further showed that subjects in early atherosclerosis group had higher amounts of epicardial fat, similar to advanced atherosclerosis.
EAT density was significantly lower in subjects with coronary calcium or a CCS ≥100 compared to subjects with a CCS of 0. The study of Franssens et al. also described an association between lower EAT density and greater extent of coronary artery calcification (4). Recently Abazid et al. reported that lower EAT density (HR 0.879; 95%CI:0.817–0.946; p=0.001) is independently associated with a higher prevalence of subclinical CAD as defined by a CCS>0.
Brown adipose tissue, a metabolically active adipose tissue with anti-inflammatory and anti-apoptotic effect, is in contrast to white adipose tissue related to higher CT attenuation and more common in the healthy state (4,16,20). Our finding that lower EAT density is related to higher BMI and higher CCS, as recently described for lower CT attenuation of visceral adipose tissue (14,15), suggests the presence of inflammatory white EAT is linked to higher cardiovascular risk.
4.2. EAT volume and density in relation to serum levels of lipids and inflammatory biomarkers and MACE
In multivariate regression analysis EAT volume and density were associated with serum levels of lipids. Hell et al. also reported that EAT density was inversely correlated to plasma levels of LDL-cholesterol and triglyceride and positively correlated with plasma levels of HDL-cholesterol (24). In the study of Harada et al. increased EAT volume was also related to serum levels of triglyceride and HDL-cholesterol (32). This association of lipid levels and EAT measures may be explained by the established association between increased EAT volume and metabolic syndrome as well as by the storage of triglycerides in EAT to supply free fatty acids for myocardial energy production (5).
In our study, increased EAT volume was independently associated with serum levels of PAI-1 and MCP-1 and inversely associated with serum levels of adiponectin, which was described previously (27,28). To our knowledge this is the first description of a significant positively association of EAT density with serum levels of adiponectin and inversely correlation with serum levels of PAI-1, independently of potential confounders such as EAT volume, age, cardiovascular risk factors, BMI and CCS.
Monocyte chemoattractant protein 1 (MCP-1) is an adipocytokine that recruits monocytes into the developing atheroma and might play a central role in vascular inflammation and progression of atherosclerosis (29).
The activity of plasminogen activator inhibitor-1 (PAI-1) has been shown to play a key role in thrombus formation upon unstable atherosclerotic plaque rupture through the inhibition of fibrin clot breakdown (30). A close correlation between plasma level of PAI-1 with increased rate of myocardial infarction was described previously (31).
Adiponectin is secreted by adipose tissue and a low adiponectin expression has been reported to be associated with acute coronary syndrome and progression of atherosclerosis because of its anti-inflammatory and anti-atherogenic effects (23). In the study of Otake et al. a reduced serum level of adiponectin was associated with an increase in necrotic core ratio in both culprit and non-culprit lesions in patients with acute coronary syndrome (9).
Our study is also the first to show that subjects who experienced cardiac death or myocardial infarction had significantly higher EAT volume and significantly lower EAT density compared to subjects without MACE. In multivariable Cox analysis adjusted for CCS and ASCVD risk score, EAT volume ≥125 cm³ was the only variable independently associated with MACE, consistent with previous studies (8,10). Interestingly, EAT density, when included in the Cox analysis model, was more significantly associated with MACE than EAT volume suggesting that the measurement of EAT density in addition to EAT volume in non-contrast cardiac CT datasets might add valuable information in improving cardiovascular risk assessment in asymptomatic individuals. Lu et al recently reported that subjects with high-risk plaque had significantly lower mean EAT density compared to subjects without high-risk plaque (21). Though this relationship did not remain significant in multivariable analysis (21), the composition of EAT might play an important role in the development of high-risk plaque features increasing the risk for MACE.
Adipose tissue with lower CT attenuation is related to adipocyte hypertrophy and hyperplasia following excess lipid accumulation in diet-induced obesity and insulin resistance (4,14,25). This unfavorable metabolic and dysfunctional state is associated with systemic low-grade inflammation (4,14,25). Adipocyte hypertrophy leading to a reduced capillary density, can induce hypoxia resulting in necrosis among the adipose tissue and might trigger an inflammation process to induce coronary atherosclerosis through its direct contact with the coronary arteries (12,15,26). In summary, inflammatory white adipose tissue, which has lower CT attenuation than the metabolically active and anti-inflammatory brown adipose tissue, might trigger the atherosclerotic process (4,16).
From our results, the serum level of plaque inflammatory markers, the presence of coronary calcium and MACE were associated to an increased EAT volume and to lower EAT density. Such excess and potentially inflammatory EAT has been implicated in the precipitation of coronary atherosclerosis through its direct contact with the adventitia of the underlying coronary arteries, and paracrine inflammatory effects (7,8,11,12). Indeed, convincing data indicate that dysfunctional EAT emits adipocytokines through paracrine or “vasocrine” mechanisms, interacts directly with the underlying coronary vasculature (7,11,12,27). Our findings support the hypothesis that a dysfunction of EAT may be linked to subclinical atherosclerosis in asymptomatic subjects not only by shared cardiovascular risk factors but also through its underlying source of inflammatory mediators potentially triggering atherosclerosis (11). Consequently, the CT measurements of quantity and quality of EAT might add valuable information in the cardio-vascular risk assessment of asymptomatic individuals.
Our study represents a large, community-based cohort of asymptomatic subjects without known CAD, utilizing precise semi-automated methods to measure EAT volume and density, and availability of a large panel of serum biomarkers. To our knowledge, our study is the first to examine EAT density as well as volume and in relation to inflammatory serum biomarkers and MACE in asymptomatic subjects. Thus, the interference between serum levels of plaque inflammatory biomarkers and EAT measures requires further investigation.
5. Limitations
Several other limitations of our study deserve additional consideration besides the single-center design. Injection of contrast media was not done because of the population-based study design including asymptomatic subjects. Consequently, measurements of noncalcified plaque features, coronary artery stenosis and pericoronary fat were not possible. The EISNER trial included only asymptomatic subjects with no prior history of CAD. Thus, our results may not be applicable to symptomatic subjects or subjects with known CAD, but are rather more representative of the general asymptomatic population.
6. Conclusion
EAT volume was significantly increased in subjects with coronary calcification without a significant difference in EAT volume in subjects with early or advanced atherosclerosis, suggesting increased EAT volume might not be restricted to subjects with more advanced atherosclerosis. Lower EAT density and increased EAT volume were associated with presence of coronary calcification, serum levels of plaque inflammatory markers and lipids as well as with MACE, suggesting that dysfunctional EAT may be linked to adverse cardiac events, early plaque formation, metabolic abnormality, and inflammation. Thus, measurements of EAT volume and density from non-contrast cardiac CT add information regarding the “activity” of the atherosclerotic process, potentially adding meaningful to cardio-vascular risk assessment in asymptomatic individuals, independent of coronary calcium score categories.
Acknowledgments
Funding: This work was funded by National Institute of Health/National Heart, Lung, and Blood Institute grant 1R01HL133616 (to Dr. Dey) and in part by the Bundesministerium für Bildung und Forschung (01EX1012B, Spitzencluster Medical Valley).
This research was supported by grant 1R01HL133616 from the National Heart, Lung, and Blood Institute/National Institute of Health (NHLBI/NIH) (PI: Damini Dey) and in part by the Bundesministerium für Bildung und Forschung (01EX1012B, Spitzencluster Medical Valley). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the Bundesministerium für Bildung und Forschung.
Abbrevations
- BMI
body mass index
- CAD
coronary artery disease
- CCS
coronary calcium score
- CRP
C-reactive protein
- EAT
epicardial adipose tissue
- DM
diabetes mellitus
- HDL
high-density lipoprotein
- IL-6
interleukin 6
- LDL
low-density lipoprotein
- MACE
major adverse cardiac events
- MCP-1
monocyte chemoattractant protein 1
- PAI-1
endothelial plasminogen activator inhibitor 1
- sICAM-1
soluble intercellular adhesion molecule
- 1 VCAM-1
vascular cell adhesion molecule 1
Footnotes
Conflict of interest statement: None.
Disclosure: The authors have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis-an inflammatory disease. The New England journal of medicine. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Abazid RM, Smettei OA, Kattea MO, Sayed S, Saqqah H, Widyan AM, Opolski M. Relation Between Epicardial Fat and Subclinical Atherosclerosis in Asymptomatic Individuals. Journal of thoracic imaging. 2017;32(6):378–382. doi: 10.1097/RTI.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 4.Franssens BT, Nathoe HM, Visseren FL, van der Graaf Y, Leitner T. Relation of Epicardial Adipose Tissue Radiodensity to Coronary Artery Calcium on Cardiac Computed Tomography in Patients at High Risk for Cardiovascular Disease. The American journal of cardiology. 2017;119(9):1359–65. doi: 10.1016/j.amjcard.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 5.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. American heart journal. 2007;153(6):907–17. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 7.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Wolf PA, O’Donnel CJ, Hofmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. European heart journal. 2009;30(7):850–6. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, Berman DS. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovascular imaging. 2010;3(4):352–60. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otake H, Shite J, Shinke T, Watanabe S, tanino Y, Ogasawara D, Sawada T, Hirata K, Yokoyama M. Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. The American journal of cardiology. 2008;101(1):1–7. doi: 10.1016/j.amjcard.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K, Dragano N, Moebus S, Jöckel KH, Erbel R, Möhlenkamp S. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. Journal of the American College of Cardiology. 2013;61(13):1388–95. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 11.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 12.Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H, Fukuda D, Soeki T, Kitagawa T, Takanashi S, Sata M. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(5):1077–84. doi: 10.1161/ATVBAHA.112.300829. [DOI] [PubMed] [Google Scholar]
- 13.Tadros TM, Massaro JM, Rosito GA, Hoffmann U, Vasan RS, Larson MG, Keaney JF, Jr, Lipinska I, Meigs JB, Kathiresan S, O'Donnell CJ, Fox CS, Benjamin EJ. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring, Md) 2010;18(5):1039–45. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132(17):1639–47. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS. Association of Changes in Abdominal Fat Quantity and Quality With Incident Cardiovascular Disease Risk Factors. Journal of the American College of Cardiology. 2016;68(14):1509–21. doi: 10.1016/j.jacc.2016.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldiss P, Davies G, Woods R, Budge H, Sacks HS, Symonds ME. 'Browning' the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. International journal of cardiology. 2017;228:265–74. doi: 10.1016/j.ijcard.2016.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong ND, Rana JS, Orakzai R, Hayes SW, Friedman JD, Thomson LE, Polk D, Min J, Budoff MJ, Berman DS. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. Journal of the American College of Cardiology. 2011;57(15):1622–32. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 19.Dey D, Suzuki Y, Suzuki S, Ohba M, Slomka PJ, Polk D, Shaw LJ, Berman DS. Automated quantitation of pericardiac fat from noncontrast CT. Investigative radiology. 2008;43(2):145–53. doi: 10.1097/RLI.0b013e31815a054a. [DOI] [PubMed] [Google Scholar]
- 20.Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME. Adult epicardial fat exhibits beige features. The Journal of clinical endocrinology and metabolism. 2013;98(9):E1448–55. doi: 10.1210/jc.2013-1265. [DOI] [PubMed] [Google Scholar]
- 21.Lu MT, Park J, Ghemigian K, Mayrhofer T, Puchner SB, Liu T, Fleg JL, Udelson JE, Truong QA, Ferencik M, Hoffmann U. Epicardial and paracardial adipose tissue volume and attenuation - Association with high-risk coronary plaque on computed tomographic angiography in the ROMICAT II trial. Atherosclerosis. 2016;251:47–54. doi: 10.1016/j.atherosclerosis.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy RA, Register TC, Shively CA, Carr JJ, Ge Y, Heilbrun ME, Cummings SR, Koster A, Nevitt MC, Satterfield S, Tylvasky FA, Strotmeyer ES, Newman AB, Simonsick EM, Scherzinger A, Goodpaster BH, Launer LJ, Eiriksdottir G, Sigurdsson S, Sigurdsson G, Gudnason V, Lang TF, Kritchevsky SB, Harris TB. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(1):109–17. doi: 10.1093/gerona/glt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barseghian A, Gawande D, Bajaj M. Adiponectin and vulnerable atherosclerotic plaques. Journal of the American College of Cardiology. 2011;57(7):761–70. doi: 10.1016/j.jacc.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Hell MM, Ding X, Rubeaux M, Slomka P, Gransar H, Terzopoulos D, Hayes S, Marwan M, Achenbach S, Berman DS, Dey D. Epicardial adipose tissue volume but not density is an independent predictor for myocardial ischemia. Journal of cardiovascular computed tomography. 2016;10(2):141–9. doi: 10.1016/j.jcct.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Baba S, Jacene HA, Engles JM, Honda H, Wahl RL. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2010;51(2):246–50. doi: 10.2967/jnumed.109.068775. [DOI] [PubMed] [Google Scholar]
- 26.Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, Schwartz AR, Halberg N, Scherer PE, Semenza GL, Powell DR, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. American journal of respiratory and critical care medicine. 2013;188(2):240–8. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurovich-Horvat P, Kallianos K, Engel LC, Szymonifka J, Schlett CL, Koenig W, Hoffmann U, Truong QA. Relationship of thoracic fat depots with coronary atherosclerosis and circulating inflammatory biomarkers. Obesity (Silver Spring, Md) 2015;23(6):1178–84. doi: 10.1002/oby.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gauss S, Klinghammer L, Steinhoff A, Raaz-Schrauder D, Marwan M, Achenbach S, Garlichs CD. Association of systemic inflammation with epicardial fat and coronary artery calcification. Inflammation research: official journal of the European Histamine Research Society [et al] 2015;64(5):313–9. doi: 10.1007/s00011-015-0809-x. [DOI] [PubMed] [Google Scholar]
- 29.Deo R, Khera A, McGuire DK, Murphy SA, de Meo Neto JP, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. Journal of the American College of Cardiology. 2004;44(9):1812–8. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 30.Sobel BE. Increased plasminogen activator inhibitor-1 and vasculopathy. A reconcilable paradox Circulation. 1999;99(19):2496–8. doi: 10.1161/01.cir.99.19.2496. [DOI] [PubMed] [Google Scholar]
- 31.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nature medicine. 1996;2(7):800–3. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 32.Harada K, Amano T, Kataoka T, Takeshita M, Harada K, Kunimura A, Takayama Y, Shinoda N, Kato B, Uetani T, Kato M, Marui N, Ishii H, Matsubara T, Murohara T. Impact of abdominal and epicardial fat on the association between plasma adipocytokine levels and coronary atherosclerosis in non-obese patients. Atherosclerosis. 2014;237(2):671–6. doi: 10.1016/j.atherosclerosis.2014.10.014. [DOI] [PubMed] [Google Scholar]