Abstract
Objective
Correlational studies have linked shortened sleep to adolescents’ report of post-concussion symptoms and cognitive performance during concussion assessments. This study tested whether those are cause-effect relationships.
Design
3-week randomly-counterbalanced, within-subjects, crossover experiment
Setting
Adolescents slept at home with weekly visits to an outpatient clinic for sleep monitor uploads and outcome assessments.
Participants
24 healthy 14–17.9-year-olds
Conditions
After an initial sleep-stabilization period, adolescents experienced 5-night spans of Short Sleep (SS; 6.5 hours/night in bed) versus Healthy Sleep Opportunity (HS; 9.5 hours/night in bed).
Main Outcome Measures
Cognitive indexes and Post-Concussion Symptom Severity (PCSS) from the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT).
Results
Adolescents reported significantly worse symptoms on the PCSS after SS than HS, even after excluding items manifestly related to sleep. Verbal Memory was also worse after SS than HS, though the effect was small. The manipulation did not significantly affect other cognitive indexes.
Conclusions
A realistic “dose” of short sleep, similar to what many adolescents experience regularly on school nights, can cause or contribute to symptom reports during concussion assessments. Consistent with prior sleep research, one-on-one cognitive tests appear to be less sensitive than measures of emotional and behavioral functioning to the effects of short sleep.
Keywords: Sleep Restriction, Adolescents, Concussion
Introduction
On pre-injury evaluations, adolescent athletes who sleep less tend to self-report more concussion symptoms1–3 and may have lower cognitive test scores.2,3 If indeed short sleep affects symptoms and cognition in young athletes, it could complicate interpretation of concussion evaluation scores, since adolescents normatively get 1–3 hours less sleep than is recommended on school nights.4 Here we move beyond the correlations reported in prior work to test whether experimentally shortened sleep causes healthy adolescents to experience concussion-related symptoms and worsens cognition on a commercially-marketed concussion assessment tool. We assessed adolescents in the context of an experimental protocol that induces five nights of shortened sleep (SS) versus healthy sleep (HS) opportunity. We hypothesized that, compared to HS, after SS (a) indexes of both cognitive functioning and subjective symptoms would worsen, and (b) the effect on subjective symptoms would remain true even after removing symptoms that are manifestly related to sleep.
Methods
The study was approved and overseen by the local ethics board. Adolescent participants provided informed assent and their parents provided informed consent.
Participants
Healthy 14–17.9 year-olds were recruited through flyers posted throughout a regional pediatric healthcare system. Eligibility was screened by telephone and verified during the first study visit. Exclusions included psychiatric disorder, history of neurological illness or injury (including head injury with loss of consciousness), BMI>30, IQ<70, daily consumption of >1 coffee or energy drink or >2 caffeinated soft drinks, illegal substance use, or use of medication that can affect sleep or daytime alertness.
Procedures
The sleep manipulation mirrors our prior work.5 Briefly, each participant underwent a 3-week protocol in the summer of 2016. Wake time throughout was set at the time the participant would need to awaken to arrive at our office at 8 am. During the initial sleep stabilization week, participants were asked to awaken at that time, but were permitted to self-select their bedtimes. That week allowed participants to adjust to a steady morning-rising schedule; those who did not rise on time were ineligible for randomization.
Eligible participants then entered a within-subjects, randomly counterbalanced cross-over experiment comparing 5 night spans (Monday – Friday nights) of SS versus HS. Adolescents adjusted their bedtime to allow for 6.5 hours/night in bed during SS and 9.5 hours/night during HS. During the two-night Saturday/Sunday washout, bedtimes were adjusted to allow ≥8 hours/night in bed. Participants were instructed not to nap and to limit caffeine intake. Participants came in for office visits on the Saturday morning after the stabilization, SS, and HS conditions, at which time we measured our primary outcomes.
Measures
Participant sleep was monitored via a Micro-Motionlogger SleepWatch® (Ambulatory Monitoring, Inc.). Trained coders uploaded data and screened for artifacts with the support of nightly sleep diaries and follow-up queries to participants. A validated algorithm6 then derived sleep onset, offset, and sleep period (offset minus onset).
Cognition and post-concussion symptoms were assessed using Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT Applications, Inc). This computerized battery yields four primary cognitive composites (Verbal Memory, Visual Memory, Reaction Time, and Processing Speed) and a 21-item self-report Post-Concussion Symptom Scale (PCSS). We computed PCSS Total Score and a Modified PCSS Score that removed items manifestly related to sleep (fatigue, trouble falling asleep, sleeping more/less than usual, and drowsiness).1
Analytic Approach
Effects across SS vs. HS were tested using paired-sample t-tests for the sleep and cognitive indexes, and Wilcoxon tests for PCSS scores. We also explored potential effect modifiers by computing difference scores for each outcome and testing whether these differences varied by the participant’s sex, race, or age using Wilcoxon tests or Spearman correlations. Alpha was set at .05 for the hypothesis-driven primary analyses, and .01 for exploratory analyses.
Results
Of 36 adolescents who arrived for the initial visit, 5 were not randomized due to ineligibility or poor adherence and 7 dropped out. Table 1 provides information on the remaining 24 participants at the initial visit.
Table 1.
Sample features at the time of randomization. Categorical variables are listed in percentages and normally-distributed variables are summarized as mean ± standard deviation.
| Percent or Mean ± SD | |
|---|---|
| Female (%) | 46% |
| Ethnicity (%): | |
| White | 63% |
| Black | 33% |
| Multiracial | 4% |
| Age (years) | 15.77 ± 1.03 |
| Stabilization Week Sleep Onset (time) | 00:06 ± 1:07 |
| Stabilization Week Sleep Offset (time) | 06:52 ± 0:40 |
| Stabilization Week Sleep Period (hours onset to offset) | 6.77 ± 0.99 |
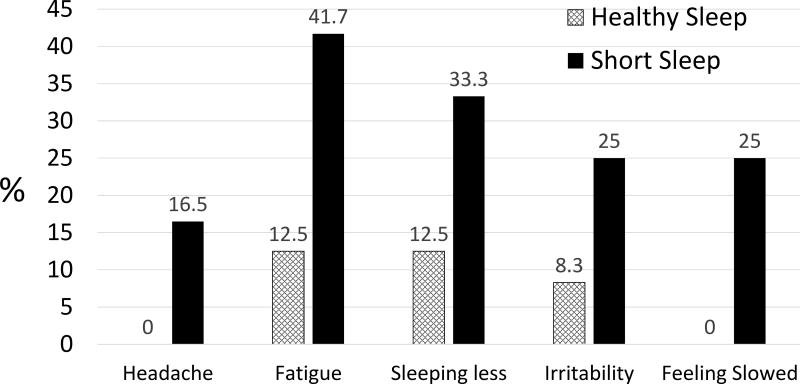
As shown in Table 2, adolescents averaged 2.29 hours more sleep per night during HS than SS. Adolescents showed slightly but significantly weaker verbal memory during SS than HS, p=.039. Effects on the other cognitive indexes were non-significant, and effect sizes were small to negligible. Teens reported significantly greater post-concussion symptoms during SS than HS on both the PCSS and modified PCSS, with large effects, p<.001. Teens reported worse symptoms during SS on 18 of the 21 items. Figure 1 shows the frequency of complaints across conditions on the items that showed at least medium effects (Cohen’s d >.50). Findings were not modified by sex, age, or ethnicity.
Table 2.
Changes in Sleep and ImPACT Scores Across Experimental Conditions. Normally-distributed variables are presented as mean ± standard deviation, while the skewed variables are presented as median (25th, 75th percentiles).
| Short Sleep (SS) | Healthy Sleep (HS) | Effect Size (d) |
p | |
|---|---|---|---|---|
| Sleep Onset (time) | 00:39 ± 0:33 | 22:15 ± 0:36 | 4.16 | <.001 |
| Sleep Offset (time) | 06:56 ± 0:21 | 06:49 ± 0:27 | 0.27 | .160 |
| Sleep Period (hours) | 6.28 ± 0.58 | 8.57 ± 0.65 | 3.71 | <.001 |
| Verbal Memory | 84.96 ± 9.26 | 87.79 ± 8.70 | 0.32 | .039 |
| Visual Memory | 75.00 ± 13.24 | 74.21 ± 14.96 | 0.06 | .783 |
| Visual-Motor Speed | 39.52 ± 6.59 | 40.10 ± 6.21 | 0.09 | .397 |
| Reaction Time | 0.62 ± 0.11 | 0.60 ± 0.07 | 0.19 | .156 |
| PCSS Total Score | 3.00 (0.00, 7.00) | 0.00 (0.00, 2.75) | 0.88 | .005 |
| Modified PCSS Score | 1.00 (0.00, 3.00) | 0.00 (0.00, 1.00) | 0.92 | .004 |
PCSS = Postconcussive Symptom Scale. Modified PCSS equals the PCSS minus 5 items that are directly linked to sleep (see text). Effect sizes and significance (p) values were calculated from within-subjects t-tests for all but the PCSS and Modified PCSS, for which we used the Wilcoxon Test. By convention, d = .20 reflects a small effect, d = .50 reflects a medium effect, and d ≥ .80 reflects a large effect.
Figure 1.
Prevalence (in percent) of post-concussive symptoms that were most sensitive to shortened sleep. Eighteen of the 21 symptoms on the PCSS worsened overall during short sleep; the five shown are those that had at least a medium effect size (d ≥.50).
Discussion
Findings confirm that shortened sleep, similar to what many adolescents in western countries experience during a school week,4 increases symptoms commonly seen after concussions. This effect occurred in healthy adolescents in the absence of head injury and across a range of symptoms, not just symptoms that are manifestly sleep-related. For example, short sleep tripled the prevalence of irritability and fatigue. It also caused headache and subjective mental slowing in 1/6 to 1/4 of adolescents who were asymptomatic when well-rested.
These experimental findings complement and extend prior correlational work that found consistent associations of short sleep with post-concussive symptoms and weaker associations with cognitive test scores.1–3 Short sleep worsened performance on tests involving short-term verbal learning/memory in our sample, but the effect was small, and effects on other cognitive composites were even smaller. Prior research has suggested that shortened sleep has a much greater effect on mood and behavior than on one-on-one test scores, possibly because of the brief, highly-structured nature of one-on-one tests.7
This study was limited by a modestly-sized sample, short-term 2-condition sleep manipulation, and focus on healthy adolescents. Findings suggest the need for a clinical trial examining the impact of sleep optimization on post-concussion recovery. In the meantime, clinicians should consider the sleep of their adolescent athletes during concussion evaluations and when making treatment decisions. Current recommendations state that even non-concussed adolescents get 8–10 hours of sleep/night.8,9 Given that this seldom occurs on school nights, problem-solving with families to optimize nocturnal sleep might result in notable symptom improvement.
Clinical Relevance.
Clinicians should consider the sleep of adolescents during concussion evaluations and when making treatment decisions. Given the ubiquity of short sleep during adolescence, problem-solving with families to optimize nocturnal sleep may improve symptoms.
Acknowledgments
Aspects of this project were supported by the United States National Institutes of Health (NIH; R01HL120879) and the Division of Sports Medicine at Cincinnati Children’s Hospital Medical Center.
Footnotes
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest to report.
References
- 1.Silverberg ND, Berkner PD, Atkins JE, Zafonte R, Iverson GL. Relationship Between Short Sleep Duration and Preseason Concussion Testing. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2016;26:226–31. doi: 10.1097/JSM.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 2.Sufrinko A, Johnson EW, Henry LC. The influence of sleep duration and sleep-related symptoms on baseline neurocognitive performance among male and female high school athletes. Neuropsychology. 2016;30:484–91. doi: 10.1037/neu0000250. [DOI] [PubMed] [Google Scholar]
- 3.McClure DJ, Zuckerman SL, Kutscher SJ, Gregory AJ, Solomon GS. Baseline neurocognitive testing in sports-related concussions: the importance of a prior night's sleep. The American journal of sports medicine. 2014;42:472–8. doi: 10.1177/0363546513510389. [DOI] [PubMed] [Google Scholar]
- 4.National Sleep Foundation. Summary of Findings:2006 Sleep In America Poll. Washington, DC: National Sleep Foundation; 2006. [Google Scholar]
- 5.Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. Journal of Child Psychology and Psychiatry. 2014;55:180–90. doi: 10.1111/jcpp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-week identification: An empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 7.Beebe DW. A brief primer on sleep for pediatric and child clinical neuropsychologists. Child Neuropsychology. 2012;18:313–38. doi: 10.1080/09297049.2011.602014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paruthi S, Brooks LJ, D'Ambrosio C, et al. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785–6. doi: 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremblay MS, Carson V, Chaput JP, et al. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl Physiol Nutr Metab. 2016;41:S311–27. doi: 10.1139/apnm-2016-0151. [DOI] [PubMed] [Google Scholar]