Abstract
Background
Mechanisms underlying the heightened myocardial infarction risk among HIV-infected women (versus non-HIV-infected women) remain unclear. Our objectives were to assess epicardial adipose tissue (EAT) volume and its associations among asymptomatic women with and without HIV.
Methods
Fifty-five HIV-infected and 27 non-HIV-infected women without known cardiovascular disease who underwent cardiac CT and metabolic/immune phenotyping were included. EAT volume derived from CT was compared among women with and without HIV, and within-group EAT associations were assessed. Next, immune and atherosclerotic plaque parameters were compared among groups stratified by HIV serostatus and high/low EAT (defined in reference to median EAT for each serostatus group).
Results
Asymptomatic HIV-infected women and age-matched non-HIV-infected women with comparable mean BMI (28±1vs. 29±1kg/m2) had similar median volumes of EAT (54[41,79] vs. 65[41,78]cm3)(P>0.05); however, different within-group associations were noted. Markers of monocyte activation/arterial inflammation differed by [HIV serostatus/EAT volume] subgroup (CXCL10(P=0.02), sCD163(P=0.004), sCD14(P=0.03), Lp-PLA2(P=0.04); P for overall ANOVA) and were highest among HIV-infected women with excess EAT (vs. HIV-infected women without excess EAT, non-HIV-infected women with excess EAT, and non-HIV-infected women without excess EAT). The percentage of segments with non-calcified coronary plaque also differed by [HIV serostatus/EAT volume] subgroup and was highest among HIV-infected women with excess EAT.
Conclusions
Asymptomatic women with and without HIV have similar volumes of EAT, but drivers of EAT may differ between groups. HIV-infected women with excess EAT have highest-level immune activation and the highest percentage of non-calcified plaque. Future studies are needed to determine whether EAT contributes pathogenetically to HIV-associated cardiovascular disease in women.
Introduction
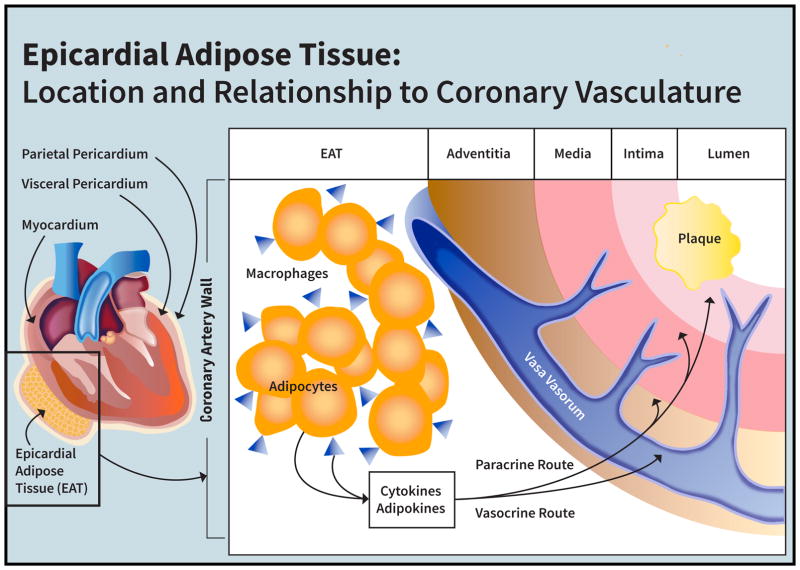
Mechanisms underlying the heightened myocardial infarction risk among HIV-infected women[1] remain unclear. Previous studies have shown that asymptomatic HIV-infected women (versus non-HIV-infected women) have a significantly higher percentage of non-calcified, rupture-prone coronary plaque in relation to increased systemic levels of pro-inflammatory cytokines and immune activation markers[2]. Indeed, persistent systemic immune activation among individuals with HIV is believed to contribute to heightened myocardial infarction risk[3] [4] [5]. Immunomodulatory cytokines are known to be produced by ectopic fat depots[6], and HIV infection and/or antiretroviral therapy predispose to ectopic fat deposition[7]. The epicardial adipose tissue (EAT) depot has distinct pathophysiologic relevance to atherosclerotic cardiovascular disease (CVD) risk[8]. EAT is an ectopic fat depot, which resides within the pericardial sac and shares a common blood supply with the coronary arteries it surrounds[8] (Figure 1). Cytokines secreted by EAT may be expected to exert deleterious vasocrine or paracrine effects on coronary arteries – increasing arterial inflammation and, in turn, engendering accelerated atherogenesis, plaque remodeling, and/or endothelial dysfunction. To this end, increased epicardial fat has been shown to relate to incident myocardial infarction in the general population[9].
Figure 1. Epicardial Adipose Tissue: Location and Relationship to Coronary Vasculature.
EAT encases and shares a blood supply with the coronary vasculature such that cytokines and adipokines produced by EAT may, through vasocrine and/or paracrine pathways, reach and act upon incipient, subclinical coronary plaque.
No prior studies have explored the epicardial fat depot in relation to cardiovascular risk indices among HIV-infected and non-HIV-infected women. In the present study, we measured EAT volume among HIV-infected and non-HIV-infected women without known CVD who had previously undergone cardiac computed tomography angiography (CCTA) as well as detailed immune/metabolic phenotyping. We hypothesized that HIV-infected women would have higher volumes of EAT as compared with age-matched non-HIV-infected women. We further anticipated increased systemic immune activation and non-calcified coronary plaque among those HIV-infected women with the highest volumes of EAT.
Methods
Study Participants
Sixty HIV-infected and 30 non-HIV-infected women ages 18–60 years were recruited between 2011–2012 from the greater Boston area using similar strategies (newspaper advertisements, flyers in clinical settings and community centers)[2]. Subjects were excluded for a known history of CVD or for relative contraindications to iodinated contrast dye (including contrast allergy, serum creatinine >1.5 mg/dL or creatinine clearance <60mL/min) or to beta-blocker or nitroglycerin, which are administered as part of the CCTA protocol. For HIV-infected women, there was a requirement for stable ART for ≥3 months leading into the study. The study was approved by the Partners/MGH Institutional Review Board and participating subjects provided informed consent. Data on immune activation and plaque type in this cohort have been previously presented[2], but data on EAT (the primary focus of this analysis) have not. Fifty-five HIV-infected and 27 non-HIV-infected women from whom EAT measurements were available were included in these analyses.
Assessment of Medical History and Cardiovascular Risk Factors
Information related to subjects’ demographics, past medical and family history, tobacco or substance use, and current medication regimen was obtained. Women who self-reported amenorrhea for 12 or more consecutive months were considered post-menopausal. For HIV-infected women, information on prior and current ART use was elicited. Traditional cardiovascular risk was assessed using a validated aggregate point score via the Framingham equation[10].
Cardiac CT Angiography (CCTA)
CCTA for assessment of coronary plaque was conducted using a 64-slice CT scanner (Siemens Medical Solutions)[2, 11, 12]. Per-segment coronary plaque phenotype (calcified, non-calcified, or partially calcified) was assessed using the 18 segment Society of Cardiac Computed Tomography model[13].
Assessment of Ectopic Fat Depots using CT
EAT volume was measured from the non-contrast CT performed as part of the CCTA. Epicardial adipose tissue was defined as fat tissue residing within the pericardial sac having an attenuation between −195 to −45 Hounsfield Units (HU)[14]. EAT volume in cubic centimeters (cm3) was quantified using a dedicated semiautomated workstation (Multimodality Workplace (MMWP), Siemens Healthineers, Forchheim, Germany)[15]. Briefly, regions of interest were traced along the pericardium from the middle of the right pulmonary artery to the diaphragm at 10 mm intervals, with semiautomated interpolation. Abdominal visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) area were quantified on an axial noncontrast CT slice through the abdomen at the level of the L4 pedicle[11, 16].
Metabolic and Biochemical Parameters
Blood samples were drawn under 12-hour fasting conditions. Standard techniques were used to determine levels of total cholesterol, HDL, LDL, triglycerides, and glucose. For HIV-infected subjects, HIV viral load was assessed by ultrasensitive RT-PCR (Roche Cobas Amplicor, Pleasanton, California) (lower limit of detection, 50 copies/mL). For control subjects, a screening test for HIV was performed by ELISA, with subsequent confirmational testing by Western blot as indicated.
Markers of Inflammation and Immune Activation
Plasma sCD163 (Trillium, Bangor, ME), MCP-1, CXCL10, sCD14, and hsIL6 (R&D Systems, Minneapolis, MN) were quantified by ELISA. Lipoprotein-associated phospholipase A2 (Lp-PLA2) was assessed using a Vista Dimension 1500 system (Siemens Healthcare Diagnostics, Glasgow, DE).
Statistical Analysis
The Shapiro-Wilk test was performed to evaluate the normality of distribution of continuous variable data. Categorical variables are presented as proportions. Between-group comparisons of parameters were performed applying the Student’s t-test, the Wilcoxon Rank Sum Test, and the χ2 test, as appropriate, and these data are reported as mean ± standard error of the mean or median[interquartile range (IQR)], based on normality. Next, within each serostatus group, linear regression using Pearson’s correlation coefficient was applied to evaluate parameters associated with EAT. Among the HIV-infected women, determinants of epicardial fat volume were evaluated in multivariate regression modeling using dependent variables of interests selected from univariate analyses with P<0.10 as well as age, a recognized correlate of ectopic fat deposition. Finally, HIV-infected and non-HIV-infected women were substratified into groups with high/low EAT (defined in reference to median EAT for each serostatus group). Four-group comparisons were peformed to assess independent effects of HIV and EAT on indices of immune activation/inflammation and coronary plaque phenotype after determining there was no significant interaction between HIV serostatus and EAT volume on these variables. For immune parameters, multiple group comparisons were made using ANOVA for normally distributed variables after appropriate log transformation, and data are represented as mean ± standard error. For coronary plaque characteristics, multiple group comparisons were made using the Kruskal-Wallis Rank Sums test. Where significant four-group differences were observed, between-group comparisons were also made. Statistical significance was defined by P<0.05. Statistical analyses were performed with SAS JMP software (version 12.0).
Results
Baseline Characteristics among HIV-Infected and non-HIV-Infected Women
HIV and non-HIV groups were similar with respect to age, race, and prior history of tobacco use. The total Framingham Point Score was relatively low and did not differ based on HIV serostatus. With regards to menstrual status, a comparable percentage of women with and without HIV were post-menopausal by history (46 vs. 42%, HIV vs. non-HIV, P=0.75). Metabolic parameters related to hypertension, dyslipidemia, and dysglycemia were also similar between groups (Table 1).
Table 1.
Characteristics of Non-HIV-Infected and HIV-Infected Women
| Non-HIV-Infected Women (n=27) | HIV-Infected Women (n=55) | P value | |
|---|---|---|---|
| Demographics | |||
|
| |||
| Age, y | 47 ± 1 | 47 ± 1 | 0.84 |
| Race, % | |||
| Caucasian | 37 | 33 | 0.82 |
| African American | 59 | 62 | |
| Other | 4 | 5 | |
| Hypertension, % | 26 | 15 | 0.22 |
| Dyslipidemia, % | 7 | 15 | 0.33 |
| Current statin treatment, % | 4 | 9 | 0.35 |
| Diabetes mellitus, % | 15 | 16 | 0.86 |
| Current smoker, % | 56 | 49 | 0.58 |
| Current IVDU, % | 7 | 5 | 0.73 |
| Family history of premature CHD by NCEP, % | 42 | 20 | 0.04 |
| Total Framingham point score | 11 ± 1 | 11± 1 | 0.93 |
| Post-menopausal, % | 42 | 46 | 0.75 |
|
| |||
| Metabolic Parameters | |||
|
| |||
| Systolic blood pressure, mm Hg | 123 ± 3 | 117 ± 2 | 0.16 |
| Diastolic blood pressure, mm Hg | 79 ± 2 | 75 ± 1 | 0.12 |
| Fasting glucose, mg/dL | 83 ± 3 | 91 ± 6 | 0.24 |
| Hemoglobin A1c, % | 5.9 ± 0.1 | 5.8 ± 0.1 | 0.63 |
| Total cholesterol, mg/dL | 184 ± 5 | 189 ± 6 | 0.55 |
| HDL cholesterol, mg/dL | 60 ± 3 | 61 ± 3 | 0.79 |
| LDL cholesterol, mg/dL | 102 ± 5 | 106 ± 5 | 0.57 |
| Triglycerides, mg/dL | 110 ± 13 | 108 ± 8 | 0.90 |
|
| |||
| Anthropometric Parameters | |||
|
| |||
| Body mass index, kg/m2 | 29 ± 1 | 28 ± 1 | 0.13 |
| VAT area, cm2 | 82 (50,103) | 74 (35,118) | 0.80 |
| SAT area, cm2 | 343 (246,508) | 277 (186,408) | 0.048 |
| EAT volume, cm3 | 65 (41,78) | 54 (41,79) | 0.31 |
|
| |||
| HIV-Related Parameters | |||
|
| |||
| Duration since HIV diagnosis, years | N/A | 15 ± 1 | N/A |
| Currently on antiretroviral therapy, % | N/A | 98 | N/A |
| Duration of antiretroviral therapy, years | N/A | 8 ± 1 | N/A |
| Current PI treatment, % | N/A | 58 | N/A |
| Duration of PI treatment, years | N/A | 4 ± 1 | N/A |
| Current NRTI treatment, % | N/A | 91 | N/A |
| Duration of NRTI treatment, years | N/A | 7 ± 1 | N/A |
| Current NNRTI treatment, % | N/A | 15 | N/A |
| Duration of NNRTI treatment, years | N/A | 2 ± 1 | N/A |
| Current integrase Inhibitor treatment, % | N/A | 35 | N/A |
| Duration integrase Inhibitor treatment, years | N/A | 1 ± 0 | N/A |
| CD4+ T-lymphocytes, cells/μL | N/A | 599 ± 42 | N/A |
| Nadir CD4+ T-lymphocytes, cells/μL | N/A | 193 ± 23 | N/A |
| CD8+ T-lymphocytes, cells/μL | N/A | 880 ± 58 | N/A |
| CD4/CD8 ratio | N/A | 0.84 ± 0.08 | N/A |
| Log HIV RNA viral load, copies/mL | N/A | 1.79 ± 0.06 | N/A |
| Undetectable HIV viral load <50copies/mL, % | N/A | 84 | N/A |
Data reported as mean ± standard error of mean for normally distributed variables, median (interquartile range) for non-normal distributions, and proportions for categorical variables.
Abbreviations: CHD, coronary heart disease; IVDU, intravenous drug use; NCEP, National Cholesterol Education Program; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; EAT, epicardial adipose tissue; PI, protease inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; N/A, non-applicable
For HIV-infected women, the average duration since HIV diagnosis was 15±1 years. Ninety-eight percent of women reported use of antiretroviral therapy. Mean duration of antiretroviral therapy was 8±1 years and mean CD4+ count was 599±42 cell/μL (Table 1).
Body Composition Parameters among HIV-Infected and Non-HIV-Infected Women
HIV-infected and non-HIV-infected women had similar BMI (28±1 vs. 29±1 kg/m2, P=0.13). Similar levels of ectopic fat deposition were demonstrated among the HIV versus non-HIV groups with regards to VAT (74 [35,118] vs. 82[50,103] cm2, P=0.80) and EAT (54 [41,79] vs. 65[41,78] cm3, P=0.31), whereas SAT was significant lower among the HIV group versus non-HIV group (277 [186,408] vs. 343 [246,508]cm2, P=0.048)) (Table 1).
Correlates of EAT among HIV-infected and Non-HIV-Infected Women
Among non-HIV-infected women, EAT was closely related to traditional CVD risk factors, as reflected in the total Framingham Point Score (r=0.44, P=0.02). In contrast, among HIV-infected women, EAT was not significantly related to traditional CVD risk factors reflected in this score and was instead related to unique HIV-specific parameters, including duration of NRTI use (r=0.27, P=0.045). Among HIV-infected women, there was also a trend towards a relationship between EAT and duration ART use (Table 2). In both groups, measures of ectopic fat deposition were related. Specifically, EAT strongly correlated with VAT among HIV-infected women (r=0.58, P≤0.0001)and non-HIV-infected women (r=0.55, P=0.003). The relationship of EAT to BMI was also significant in the HIV group (r=0.27, P=0.04), but EAT-BMI associations were weaker than EAT-VAT associations (Table 2).
Table 2.
Univariate Associations of Epicardial Adipose Tissue Volume Among Non-HIV-Infected and HIV-Infected Women
| Log EAT Volume (cm3) | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | Non-HIV-Infected Women (n=27) | HIV-Infected Women (n=55) | ||
| r | P value | r | P value | |
| Total Framingham point score | 0.44 | 0.02 | 0.001 | 0.99 |
| BMI, kg/m2 | 0.15 | 0.45 | 0.27 | 0.04 |
| Log VAT area, cm2 | 0.55 | 0.003 | 0.58 | ≤0.0001 |
| Duration since HIV diagnosis, years | N/A | N/A | 0.08 | 0.58 |
| Duration of ART, years | N/A | N/A | 0.24 | 0.08 |
| Duration of PI use, years | N/A | N/A | 0.03 | 0.85 |
| Duration of NRTI use, years | N/A | N/A | 0.27 | 0.045 |
| Duration of NNRTI use, years | N/A | N/A | −0.02 | 0.90 |
| Log HIV RNA viral load, copies/mL | N/A | N/A | 0.11 | 0.45 |
| CD4+ T-lymphocytes, cells/μL | N/A | N/A | 0.06 | 0.67 |
| Nadir CD4+ T-lymphocytes, cells/μL | N/A | N/A | −0.22 | 0.11 |
| Fasting glucose, mg/dL | 0.56 | 0.002 | 0.02 | 0.91 |
| Triglycerides, mg/dL | 0.39 | 0.04 | 0.06 | 0.66 |
r represents Pearson’s correlation coefficient. EAT, epicardial adipose tissue; BMI, body mass index; VAT, visceral adipose tissue; ART, antiretroviral therapy; PI, protease inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; N/A, non-applicable
Multivariate Modeling to Assess Variables Contributing to Epicardial Fat Deposition in HIV-infected Women
Among HIV-infected women, VAT area (β estimate 0.2527, P=0.0002) was a significant and independent predictor of EAT (overall model R2=0.365, P≤0.0001) after controlling for age and duration of NRTI use (Supplemental Table 1). Given the potential co-linearity of EAT and VAT, identical modeling replacing VAT with BMI was performed. In this modeling, BMI (β estimate 1.9887, P=0.02) remained a significant predictor of EAT (overall model R2=0.233, P=0.004). Duration of NRTI use also remained a significant predictor of EAT in this modeling (β estimate 1.7400, P=0.05) (Supplemental Table 1).
Immune Activation/Arterial Inflammation and Coronary Plaque Characteristics Among HIV-Infected and Non-HIV-Infected Women Stratified by EAT
Among HIV-infected women, EAT did not directly correlate with systemic immune or plaque parameters on univariate analysis. To further assess for any potential independent effects of HIV and EAT on indices of immune activation/inflammation and coronary plaque phenotype, comparisons of these parameters were made across four groups stratified by HIV serostatus and by high/low EAT (defined in reference to median EAT within each serostatus group). These comparisons were perfomed after determination that there was no significant interaction between HIV serostatus and EAT volume on these variables. Groups stratified by HIV serostatus and high/low EAT differed significantly with respect to markers of systemic immune activation, including CXCL10 (P=0.02), sCD163 (P=0.004), and sCD14 (P=0.03), and highest levels of these markers were observed among HIV-infected women with excess EAT (Table 3). In addition, these groups differed significantly overall with respect to the arterial inflammation marker Lp-PLA2 (P=0.04) (Table 3). In contrast, no significant trends were seen across groups with respect to generalized markers of inflammation or relevant metabolic parameters, including hsIL6, fasting glucose, and triglycerides.
Table 3.
Immune, Inflammatory, Metabolic, and Coronary Plaque Parameters among Non-HIV-Infected and HIV-Infected Women Stratified by Within-Group Median Epicardial Adipose Tissue Volume
| Non-HIV-Infected Below Median EAT Volume (n=13) |
HIV-Infected Below Median EAT Volume (n=27) |
Non-HIV-Infected Above Median EAT Volume (n=14) |
HIV-Infected Above Median EAT Volume (n=28) |
Overall P Value | |
|---|---|---|---|---|---|
| Markers of Monocyte/Macrophage Activation* | |||||
|
| |||||
| MCP-1, pg/mL | 166 ± 31 | 235 ± 22 | 249 ± 31 | 262 ± 22 | 0.10 |
| CXCL10, pg/mL | 131 ± 60 | 285 ± 43 | 179 ± 60 | 305 ± 42 | 0.02a,c,f |
| sCD163, ng/mL | 984 ± 237 | 1642 ± 171 | 1506 ± 237 | 1896 ± 167 | 0.004a,c |
| sCD14, ng/mL | 1594 ± 406 | 2021 ± 293 | 1310 ± 406 | 2488 ± 287 | 0.03f |
|
| |||||
| Inflammatory Markers* | |||||
|
| |||||
| hsIL6, pg/mL | 1.68 ± 0.32 | 1.56 ± 0.25 | 1.94 ± 0.32 | 2.20 ± 0.24 | 0.32 |
| Lp-PLA2, nmol/min/mL | 135 ± 14 | 174 ± 10 | 168 ± 14 | 176 ± 10 | 0.04a,b,c |
|
| |||||
| Plaque Characteristics** | |||||
|
| |||||
| Presence of coronary plaque, % | 23 | 40 | 54 | 33 | 0.41 |
| Segments with noncalcified plaque, % | 33 ± 19 [0 (0,100)] | 71 ± 10 [71 (63,100)] | 19 ± 13 [0 (0,44)] | 78 ± 12 [88 (54,100)] | 0.009 (0.047)d,f |
| Segments with mixed plaque, % | 13 ± 16 [0 (0,40)] | 27 ± 9 [25 (0,33)] | 51 ± 11 [42 (31,75)] | 17 ± 10 [0 (0,33)] | 0.13 (0.13) |
| Segments with calcified plaque, % | 53 ± 14 [60 (0,100)] | 3 ± 8 [0 (0,0)] | 40 ± 9 [33 (0,67)] | 5 ± 9 [0 (0,13)] | 0.003 (0.01)a,c,d,f |
|
| |||||
| Metabolic Parameters | |||||
|
| |||||
| Fasting glucose, mg/dL | 78 ± 10 | 91 ± 7 | 88 ± 10 | 90 ± 7 | 0.75 |
| Triglycerides, mg/dL | 85 ± 17 | 113 ± 12 | 134 ± 17 | 104 ± 12 | 0.24 |
Abbreviations: EAT, epicardial adipose tissue; MCP-1, monocyte chemoattractant protein-1; CXCL10, C-X-C motif chemokine 10; sCD163, soluble CD163; sCD14, soluble CD14; hsIL6, high sensitivity interleukin 6; Lp-PLA2, lipoprotein-associated phospholipase A2
Data reported as mean ± standard error; overall P value by ANOVA. Data for markers of monocyte/macrophage activation and generalized inflammation are presented prior to log transformation only for purposes of clinical interpretation; the appropriate statistical test was applied after log-transformation.
For plaque segment data, non-normally distributed data also reported as [median (IQR)]; overall P value by Kruskal-Wallis test.
P < 0.05 Non-HIV-Infected Below Median EAT vs. HIV-Infected Below Median EAT
P < 0.05 Non-HIV-Infected Below Median EAT vs. Non-HIV-Infected Above Median EAT
P < 0.05 Non-HIV-Infected Below Median EAT vs. HIV-Infected Above Median EAT
P < 0.05 HIV-Infected Below Median EAT vs. Non-HIV-Infected Above Median EAT
P < 0.05 HIV-Infected Below Median EAT vs. HIV-Infected Above Median EAT
P < 0.05 Non-HIV-Infected Above Median EAT vs. HIV-Infected Above Median EAT
With respect to coronary plaque, although the prevalence of any subclinical coronary atherosclerotic plaque did not differ among groups, groups showed predispositions to distinct atherosclerotic phenotypes. Groups stratified by HIV serostatus and high/low EAT differed significantly overall with respect to the percentage of segments with non-calcified plaque (P=0.047), with the highest percentage of non-calcified plaque noted among HIV-infected women with excess EAT (Table 3). By contrast, several indices related to calcified plaque were greatest among non-HIV-infected women, including the percentage of segments with calcified plaque (Table 3).
Discussion
In our community sample of women without known CVD, EAT volume did not differ by HIV serostatus. However, unique associations of EAT were noted among HIV-infected women versus non-HIV-infected women. Moreover, relationships between EAT, systemic immune activation, and subclinical non-calcified coronary artery plaque were discerned in analyses stratifiying subjects by HIV serostatus and high/low EAT.
Contrary to our primary hypothesis, HIV-infected women in our cohort did not have higher volumes of EAT than non-HIV-infected women with similar age and BMI. In contrast, parallel CT-based studies conducted in all-male cohorts have revealed higher EAT volumes among HIV-infected men versus non-HIV-infected men[17] [18]. Lo et al. demonstrated EAT volumes were higher among a community sample of asymptomatic HIV-infected men (n=78) versus non-HIV-infected men with similar BMI (n=32 )[17]. Brener et al. confirmed in the larger MACS cohort that EAT volumes were higher among HIV-infected men (n=579) versus non-HIV-infected men (n=353), despite that the HIV-infected cohort was younger, with lower BMI[18]. To assess the generalizability of our null findings on EAT volume differences in women with and without HIV, additional comparisons of EAT volumes in diverse all-women cohorts, both in the US and abroad, are needed. Notably, in a large cohort of HIV-infected women and men, EAT volume has been shown to be associated with male sex [19] and to progress over time more markedly among men (vs. women)[20]. These findings support the notion of sex-specific differences in the diathesis towards ectopic fat deposition in HIV, suggesting that insights on EAT derived from all-male/ predominantly-male HIV-infected cohorts may not be applicable to HIV-infected women.
Among both HIV-infected women and non-HIV-infected women in our cohort, EAT associated most strongly with VAT. Previously, associations between EAT and VAT have been explored in all-male cohorts[17] and in mixed cohorts of individuals with HIV[19] [21]. Beyond the association with VAT, however, EAT had differential associations with non-traditional versus traditional CV risk factors in our cohorts of women with/without HIV. Specifically, among the group of HIV-infected women, EAT was associated with duration of NRTI therapy and tended to be associated with duration of ART, but was not associated with total Framingham Point Score. In contrast, among the group of non-HIV-infected women, EAT was strongly associated with traditional CVD risk factors encompassed in the total Framingham Point Score. These findings suggest that though overall levels of epicardial fat may not be different among women with and without HIV, unique mechanisms potentially contribute to ectopic fat deposition within each group.
Among HIV-infected women, EAT did not directly correlate with systemic immune or plaque parameters. However, groups stratified by HIV serostatus and high/low EAT differed significantly with respect to select immune activation/arterial inflammation markers including CXCL10, sCD163, sCD14, and Lp-PLA2, and highest levels of these markers were observed among HIV-infected women with excess EAT. Though levels of these markers were highest among HIV-infected women with excess EAT, statistically significant differences in levels of these markers could not be discerned between the groups of HIV-infected women with high vs. low EAT, and this may have been due to the relatively small sample size of the HIV cohort. However, our testing showing the absence of an interaction between HIV and EAT suggests that both HIV status and EAT may be independently contributing to the highest-level immune activation observed among HIV-infected women with excess EAT. Previous work contextualizes our findings on the relationship between EAT volume and systemic markers of immune activation and arterial inflammation among women with HIV. Longenecker et al. demonstrated in a subset of the SATURN-HIV cohort (n=100, 77% men) that EAT volume correlated with systemic levels of the general inflammatory biomarker C-reactive protein (CRP) and the monocyte activation marker soluble CD163. In our community sample of women, biomarkers which were highest among those HIV-infected women with excess EAT included 3 markers of monocyte activation – sCD163, sCD14, and CXCL10 – as well as Lp-PLA2, a marker of arterial inflammation.
We further demonstrate, in our cohort of women, that the percentage of segments with non-calcified plaque differed in groups stratified by HIV serostatus and high/low EAT and was highest among HIV-infected women with excess EAT. Non-calcified plaque, as compared with calcified plaque, is believed to be more prone to rupture, resulting in myocardial infarction[22]. The relationships between EAT, systemic immune activation markers, and atherosclerotic plaque type have never before been assessed among HIV-infected women. Notably, Brener et al. showed in a subset of the all-male MACS cohort that EAT volume was associated with subclinical coronary plaque (total plaque and non-calcified plaque) even after adjustment for traditional CVD risk factors and HIV serostatus[18]. There is biologic plausibility to the notion that EAT might influence atherosclerotic plaque morphology in HIV. As noted, EAT encases and shares a blood supply with the coronary vasculature such that cytokines and adipokines produced by EAT may, through vasocrine and/or paracrine pathways, reach and act upon incipient, subclinical coronary plaque[8](Figure 1). An ongoing study assessing EAT samples from individuals with and without HIV undergoing coronary artery bypass grafting will shed light on how inflammatory cytokine expression by EAT may influence systemic immune activation and coronary atherosclerotic plaque morphology in HIV (NCT01899196).
Study limitations include a relatively small sample size and recruitment of women from one geographic region in Massachusetts (northeastern United States). Moreover, though the groups of HIV-infected women and non-HIV-infected women were relatively well-matched on many parameters, there was a higher prevalence of family history of CVD among the group of non-HIV-infected women, with potential influence on measured parameters. Finally, the cross-sectional study design precludes conclusive inferences on causality. However, our singular focus of women with and without HIV represents a strength of this study, which adds to a body of literature on epicardial fat in all-male cohorts. Another study strength is the very close matching of our HIV-infected and non-HIV-infected cohorts, which demonstrated similar age and BMI. A third strength resides in our ability to relate epicardial adipose fat to subclinical coronary atherosclerosic plaque morphology on CCTA and to traditional, metabolic, and immune risk factors in this well-phenotyped group of women with and without HIV.
Overall, we show that EAT volume did not differ significantly among women with and without HIV in our cohorts with similar age and BMI. In both groups of women, EAT was associated with VAT, but there were also unique within-group associations – to HIV-specific parameters in the cohort of women with HIV and to traditional cardiovascular risk parameters in the cohort of women without HIV. Notably, markers of immune activation and arterial inflammation, as well as the percentage of segments with non-calcified plaque, differed in groups stratified by HIV serostatus and high/low EAT and were highest among HIV-infected women with excess EAT. In order to test whether EAT contributes pathogenetically to CVD among women with HIV, future studies may assess whether strategies which reduce EAT also result in parallel reductions in immune activation and pathologic plaque remodeling in this population.
Supplementary Material
Acknowledgments
The investigators would like to thank the nursing staff on the MGH CRC for their dedicated efforts, the volunteers who participated in this study, and Arch Macinnes for his work on the medical illustration in Figure 1.
Principal contributions of the authors are project conception/design (SS, MTL, TGN, MVZ), subject recruitment and implementation of the original study protocol (KVF, SEL, JL), data acquisition and analysis and database management (SS, MTL, TRH, TKO, AM, THB, MVZ), statistical analysis and interpretation (SS, MTL, TKO, LAS, AM, AJC, MVZ), drafting of the manuscript (SS, MTL, MVZ), and critical revision of the manuscript (SS, MTL, KVF, AJC, THB, VAT, JL, SEL, TGN, MVZ).
Funding
This work was supported by an investigator-initiated grant from Bristol Myers Squibb, Inc and by NIH M01RR01066, UL1RR025758 and UL1TR001102 to the Harvard Clinical and Translational Science Center from the National Center for Research Resources. Support was also received from NIH/NIDDK P30045061, Pilot and Feasibility Grant, Nutrition and Obesity Research Center at Harvard. SS was supported in part by NIH/NHLBI K23 HL136262. MTL was supported in part by the American Roentgen Ray Society Scholarship and the MGH Executive Committee on Research. JL was supported in part by NIH/NHLBI F32 HL088991 and NIH/NHLBI K23 HL092792. SEL and MVZ were supported in part by NIH/NIAID R01AI123001. Funding sources had no role in the design of the study, data analysis, or writing of the manuscript.
Footnotes
Clinical Trial Registration
Disclosure Statement
SS, MTL, KVF, TRH, TKO, LAS, AM, AJC, THB, SEL, TGN have nothing to declare. JL participated in a Scientific Advisory Board meeting for Gilead. TGN participated in a Scientific Advisory Board meeting for GlaxoSmithKline. MVZ participated in a Scientific Advisory Board meeting for Roche Diagnostics and received investigator-initiated grant support to her institution from Gilead Sciences. All authors’ disclosures are unrelated to this manuscript.
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–1746. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boccara F, Lang S, Meuleman C, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61:511–523. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 5.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728–741. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 6.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol. 2014;34:1820–1826. doi: 10.1161/ATVBAHA.114.303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown TT, Glesby MJ. Management of the metabolic effects of HIV and HIV drugs. Nat Rev Endocrinol. 2012;8:11–21. doi: 10.1038/nrendo.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–371. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 9.Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 10.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 11.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raff GL, Abidov A, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample: The Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 15.Lu MT, Park J, Ghemigian K, et al. Epicardial and paracardial adipose tissue volume and attenuation - Association with high-risk coronary plaque on computed tomographic angiography in the ROMICAT II trial. Atherosclerosis. 2016;251:47–54. doi: 10.1016/j.atherosclerosis.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 17.Lo J, Abbara S, Rocha-Filho JA, Shturman L, Wei J, Grinspoon SK. Increased epicardial adipose tissue volume in HIV-infected men and relationships to body composition and metabolic parameters. AIDS. 2010;24:2127–2130. doi: 10.1097/QAD.0b013e32833c055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brener M, Ketlogetswe K, Budoff M, et al. Epicardial fat is associated with duration of antiretroviral therapy and coronary atherosclerosis. AIDS. 2014;28:1635–1644. doi: 10.1097/QAD.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guaraldi G, Scaglioni R, Zona S, et al. Epicardial adipose tissue is an independent marker of cardiovascular risk in HIV-infected patients. AIDS. 2011;25:1199–1205. doi: 10.1097/QAD.0b013e3283474b9f. [DOI] [PubMed] [Google Scholar]
- 20.Zona S, Raggi P, Bagni P, et al. Parallel increase of subclinical atherosclerosis and epicardial adipose tissue in patients with HIV. Am Heart J. 2012;163:1024–1030. doi: 10.1016/j.ahj.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Longenecker CT, Jiang Y, Yun CH, et al. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int J Cardiol. 2013;168:4039–4045. doi: 10.1016/j.ijcard.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990–999. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.