Abstract
INTRODUCTION
This study tested the hypotheses that late-midlife obstructive sleep apnea (OSA) and short and long sleep duration are associated with dementia over 15 years of follow-up.
METHODS
1,667 ARIC study participants underwent in-home polysomnography (1996–1998) and were followed for dementia. Dementia was defined by a) hospitalization diagnosis codes (1996–2012) and b) a comprehensive neurocognitive exam (2011–2013) with adjudication.
RESULTS
OSA and sleep duration were not associated with risk of incident dementia. When using adjudicated outcomes, severe OSA (≥30 versus <5 apnea-hypopnea events/hour) was associated with higher risk of all-cause dementia [risk ratio (95%CI): 2.35 (1.06–5.18)] and Alzheimer’s disease dementia [1.66 (1.03–2.68)]; associations were attenuated with cardiovascular risk factor adjustment. Sleeping <7 versus 8–≤9 hours was associated with higher risk of all-cause dementia [2.00 (1.03–3.86)].
DISCUSSION
When adjudicated outcome definitions were employed, late-midlife OSA and short sleep duration were associated with all-cause and Alzheimer’s disease dementia in later life.
Keywords: Obstructive sleep apnea, sleep duration, dementia, mild cognitive impairment, Alzheimer’s disease, Atherosclerosis Risk in Communities (ARIC) study, Sleep Heart Health Study (SHHS)
1. Background1
Dementia and mild cognitive impairment (MCI) pose a major societal burden[1], which is projected to increase due to the aging of the U.S. and global population[2, 3]. Effective treatments for dementia and MCI are lacking, thus heightening the need to understand the etiology of these conditions and identify modifiable risk factors. These conditions have a long pre-clinical phase and for several dementia and MCI risk factors, levels assessed at midlife are more strongly associated with future dementia and MCI risk than are levels assessed later in life (e.g. hypertension,[4–6] diabetes,[6–10] smoking[6, 11]).
Evidence from both human and animal studies have suggested a link between obstructive sleep apnea (OSA) and habitual long and short sleep duration with risk of dementia and Alzheimer’s disease (AD)[12]. Mechanisms[13, 14] hypothesized to underlie these associations include chronic nocturnal hypoxemia[15] [16, 17], sleep fragmentation[18], mediation through cardiovascular disease (CVD) risk factors (e.g. hypertension, diabetes, inflammation), stroke (both clinical and subclinical) [17, 19, 20], increases in Aβ burden[21], and interaction with the APOE ε4 risk allele[22–26]. However, the relation between sleep and cognitive impairment is incompletely understood; many of the existing epidemiological studies were limited in that they did not measure sleep objectively, had a small sample size, and/or were cross-sectional or had short follow-up time, thus raising questions about the temporality of the relationship or reverse causation. In particular, information is lacking about the association between late-midlife sleep characteristics and development of dementia late in life.
Nearly 2,000 Atherosclerosis Risk in Communities (ARIC) Study participants had objective sleep measurements at ages 54–73 years as part of the Sleep Heart Health Study (SHHS) and were followed to ages 67–89 for neurocognitive outcomes. Using these data we tested the hypotheses that late-midlife OSA, and short and long habitual sleep duration, were independently associated with greater risk of developing incident dementia and MCI over approximately 15 years of follow-up. Additional analyses were conducted evaluating dementia and MCI of AD etiology.
2. Methods
2.1. Study Design
The ARIC cohort includes 15,792 mostly white and black individuals who in 1987–1989 were recruited from 4 U.S. communities[27]. In 1996–1998, which corresponded approximately with ARIC’s fourth clinical exam (visit 4), a total of 1,920 ARIC participants from the suburbs of Minneapolis, Minnesota and Washington County, Maryland centers underwent sleep measurements as part of the SHHS[28]. Since study enrollment, ARIC participants have been tracked continuously for hospitalizations and mortality through annual phone calls (twice-yearly since 2012), surveillance of local hospitals, and monitoring of state and national death indexes. Informed consent was obtained at each clinic visit, and study protocols were approved by relevant Institutional Review Boards.
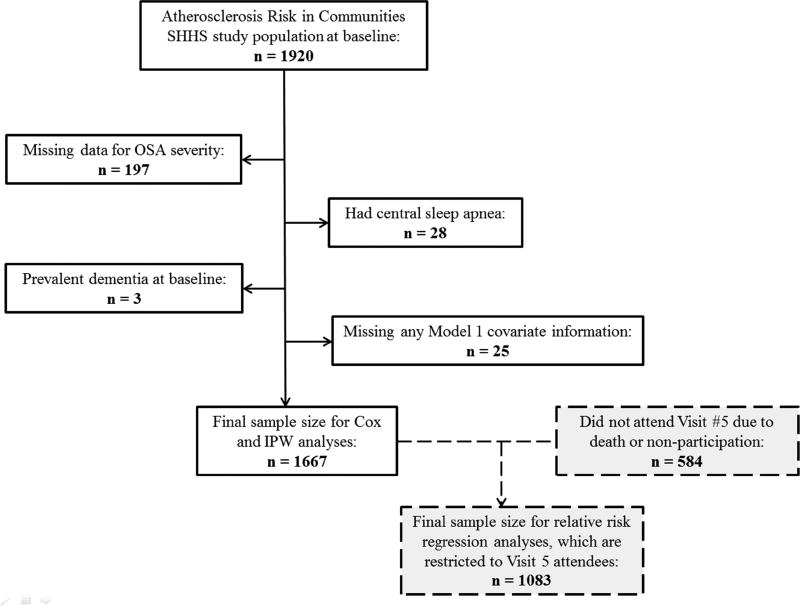
Of the 1,920 ARIC SHHS participants, for the present analysis we excluded individuals with missing data for OSA severity (n = 197), central sleep apnea (n = 28), prevalent dementia at the time of the SHHS exam per dementia hospitalization ICD codes (n = 3) and missing information on key covariates (n = 25). A flow chart is provided in Figure 1. Of the remaining 1,667 participants, all were followed for incident dementia, as defined below, and are included in OSA analyses. The maximal sample size for sleep duration analyses is 1,653 due to missing information on habitual sleep duration. Of the 1,667 participants, a total of 1,083 took part in the ARIC Neurocognitive exam (visit 5: 2011–2013). Only these 1,083 individuals were included in the analyses of adjudicated dementia, MCI and MCI or dementia due to AD. Of participants who did not take part, 359 had died and 225 did not participate for other reasons. Information on these individuals were included in the analyses, as described below.
Figure 1.
Participant flow chart for incidence and inverse probability-weighted analyses
IPW = inverse probability weighting
2.2. Sleep Measurements
Unattended polysomnography (PSG) was conducted in the participant’s homes (PS-2 System; Compumedics Limited, Abbotsford, Victoria, Australia), as has been detailed by SHHS Investigators[29]. An apnea was considered present if there was an absence or near absence of airflow (at least <25% of baseline) for ≥10 seconds[28, 29]. Hypopnea was defined as a decrease in the amplitude of the airflow below 70% of baseline for ≥10 seconds and an oxyhemoglobin desaturation of at least 4%. The apnea-hypopnea index (AHI) was calculated as the number of obstructive apneas (regardless of the oxygen desaturation level) plus hypopneas (with a ≥4% decrease in oxygen saturation) per hour of sleep. Participants were categorized into four OSA severity groups according to the AHI: <5.0 events/hr (normal), 5.0–14.9 events/hr (mild sleep apnea), 15–29.9 events/hr (moderate sleep apnea), ≥30.0 events/hr (severe sleep apnea). Percent time with oxygen saturation <90% was also calculated. Central sleep apnea events, which were defined by the absence of airflow with no associated respiratory effort detected, were excluded.
Information about habitual sleep duration during the workdays and weekends was queried through the following questions on the SHHS Sleep Habits Questionnaire: How much sleep do you usually get at night (or in your main sleep period): on weekdays or workdays? and on weekends or nonwork days? Habitual sleep duration per night (hr) was calculated as follows: [(habitual total sleep time during the workdays)*5 + (habitual total sleep time during the weekends)*2]/7. Habitual sleep duration was then categorized as <7, 7 to ≤8, 8 to ≤9 and ≥9 hours per night.
2.3. Outcome ascertainment
Ascertainment of dementia and MCI during follow-up was performed through different mechanisms. First, of the 6,538 ARIC participants attending visit 5 (2011–2013) (many of whom did not participate in SHHS), 6,471 underwent a detailed neurocognitive assessment, and a selected subset received a neurological exam and brain magnetic resonance imaging (MRI)[30]. Second, the modified telephone interview for cognitive status (TICSm), a validated phone-based cognitive assessment, was performed in 1,461 participants who were alive at the time of visit 5 but unable or unwilling to participate, while an additional 505 informants of participants deceased or unable to complete the TICSm assessment were interviewed as previously described[30]. Finally, possible cases of dementia in participants who did not provide information through any of the previous methods were ascertained from diagnosis codes from hospitalizations.
Based on this information we defined the outcomes of interest for the present analysis according to the methodology previously utilized in ARIC[30]. Incident dementia from 1996 to 2012 was defined combining all the potential diagnostic sources described above (i.e. visit 5 assessment, TICSm, hospitalization codes). Second, syndromic dementia and MCI were adjudicated by an expert panel, only in participants attending the visit 5 exam (n = 6,538), based on full neuropsychological assessment plus a functional activities questionnaire (FAQ), a clinical dementia rating (CDR) interview (administered separately to the participant and the informant) and a neuropsychiatric inventory (NPI) interview (administered to the informant only). Interviews were conducted using standardized protocols and by certified staff. Quality control of examiner performance was monitored by review of audiotaped recordings. Etiologic diagnoses were assigned by a panel of physicians and neuropsychologists, for individuals who were seen in-person and given diagnoses of dementia or MCI[30]. Reviewers were allowed to diagnose more than one etiology, but they were required to designate one etiology as primary. The diagnosis of dementia or MCI due to AD etiology was defined based on the presence of the cognitive syndrome that is not of abrupt onset and includes memory impairment and the absence of features of other specific diagnoses sufficient to cause the cognitive impairment. The criteria from the National Institute on Aging-Alzheimer's Association (NIA-AA) workgroups[31, 32] were followed. Due to a low prevalence in this sample of dementia and MCI attributed to other etiologies (e.g. CVD-related, Lewy body disease), we evaluate only the risk of all-cause dementia and dementia due to AD etiology.
2.4. Other covariates
Information on potential confounders and effect modifiers was collected at ARIC clinic visit 4, which occurred shortly before the sleep study visit, unless otherwise noted (see Supplemental Methods for details).
2.5. Statistical Analysis
For the 1,667 participants in our study, descriptive characteristics are provided stratified according to visit 5 participation status, OSA severity categories, and sleep duration categories, respectively. Cox proportional hazards regression was used to study the association of OSA with hazard of dementia. Follow-up time began on the date of the sleep study and accrued until a dementia hospitalization ICD code, loss-to-follow-up, death, December 31, 2012, or the visit 5 exam date. The proportional hazards assumption was checked by plotting of log(−log) survival curves and testing the interaction between the exposures and time.
We also evaluated the association of mid-life OSA to risk of several neurocognitive study adjudicated outcomes using relative risk regression using generalized linear models with a Poisson distribution and a log link[33]: i) dementia or MCI, ii) dementia, iii) MCI, iv) dementia or MCI due to AD. For these analyses selection bias may have occurred as a result of differential participation and survival to visit 5. As such, we used inverse probability weighting (IPW)[34, 35] to adjust for attrition due to either death or failure to attend the follow-up neurocognitive exam (censoring). Further information on our methodology is provided in the Supplemental Methods.
For both the Cox and relative risk regression analyses, a series of models were estimated using covariate information collected at approximately the time of the sleep exam. Model 1 adjusted for age, sex, center, education attainment and the APOE ε4 risk allele. Model 2 further adjusted for BMI, smoking status and leisure time physical activity. Model 3 also adjusted for characteristics hypothesized to be on the causal pathway between OSA and incident dementia/MCI (i.e. diabetes, antihypertensive medications, C-reactive protein, and systolic blood pressure). Cox and relative risk regression analyses were repeated with sleep duration categories as the exposure. Analyses were conducted in SAS 9.3 (SAS, Inc., Cary, NC).
3. Results
At the time of the sleep study, the 1,667 participants included in our analytic sample were on average (±SD) 62.7±5.5 years old and 52.6% were female. Participant characteristics stratified by neurocognitive study participation status are presented in Table 1. Those who did not participate had lower educational attainment, and were more likely to be current smokers, have diabetes, and use antihypertensive medications.
Table 1.
Baseline characteristics at the sleep examination (1996–1998) stratified by neurocognitive study (2011–2013) participation status: The Atherosclerosis Risk in Communities (ARIC) study.
| Neurocognitive Study Participation Status* | ||
|---|---|---|
|
| ||
| Participant (n=1083) |
Nonparticipant (n=584) |
|
|
|
||
| Age, years | 61.4 (5.1) | 65.2 (5.4) |
| Female, % | 54.6 | 48.8 |
| Education level, % | ||
| <High school | 8.3 | 15.9 |
| High school graduate | 46.2 | 48.0 |
| College/Graduate school | 45.5 | 36.1 |
| APOE genotype, % | ||
| e4/e4 | 2.0 | 4.3 |
| e2/e4 or e3/e4 | 24.0 | 25.2 |
| Other | 69.5 | 64.5 |
| Missing | 4.4 | 6.0 |
| Smoking status, % | ||
| Current | 8.4 | 13.9 |
| Former | 48.7 | 48.5 |
| Never | 42.9 | 37.6 |
| Body mass index, kg/m2 | 28.5 (4.9) | 29.1 (5.4) |
| Prevalent diabetes, % | 9.0 | 17.8 |
| C-reactive protein, mg/L* | 2.1 (3.4) | 2.5 (4.6) |
| Leisure index | 2.5 (0.5) | 2.4 (0.5) |
| Antihypertensive medications, % | 30.8 | 46.2 |
| Systolic blood pressure, mmHg | 123.2 (16.6) | 129.9 (19.2) |
Data shown as mean (SD) or percentage except for *geometric mean (interquartile range)
Neurocognitive study attendance status defined as attending ARIC visit 5 and not missing dementia status
3.1. Obstructive sleep apnea and incident dementia
Of our sample, a total of 102 (6.1%) had severe OSA, 213 (12.8%) had moderate OSA, 503 (30.2%) had mild OSA, and 849 (50.9%) had a normal sleep breathing pattern. Participant characteristics at the time of the sleep study, stratified by OSA categorization, are presented in Table 2. After a median follow-up of 14.9 years (25th percentile 14.0; 75th percentile 15.8; minimum 0.19; maximum 17.5), a total of 145 individuals had incident dementia as defined by objective measurements at the neurocognitive examination and by hospitalization ICD codes identified throughout the 15 years of follow-up. As shown in Supplemental Table 1, there was no association between OSA severity and incident dementia [Model 1 HRsevere OSA vs. normal = 1.09 (0.56–2.12)].
Table 2.
Baseline characteristics by obstructive sleep apnea (OSA) categories, Atherosclerosis Risk in Communities (ARIC) study, 1996–1998
| OSA Categories (AHI) | ||||
|---|---|---|---|---|
|
|
||||
| AHI N |
Normal (<5) 849 |
Mild (5 to <15) 503 |
Moderate (15 to <30) 213 |
Severe (>30) 102 |
| Age, years | 62.0 (5.5) | 63.4 (5.3) | 63.6 (5.4) | 63.9 (5.4) |
| Female, % | 64.6 | 45.1 | 30.5 | 35.3 |
| Education level, % | ||||
| <High school | 8.9 | 13.3 | 14.1 | 9.8 |
| High school graduate | 47.7 | 45.5 | 43.7 | 52.0 |
| College/Graduate school | 43.4 | 41.2 | 42.2 | 38.2 |
| APOE genotype, % | ||||
| e4/e4 | 2.9 | 2.6 | 1.9 | 4.9 |
| e2/e4 or e3/e4 | 25.4 | 22.8 | 23.0 | 26.5 |
| Other | 66.4 | 69.2 | 71.8 | 63.7 |
| Missing | 5.2 | 5.4 | 3.3 | 4.9 |
| Smoking status, % | ||||
| Current | 13.8 | 6.6 | 7.0 | 6.9 |
| Former | 44.3 | 52.3 | 54.5 | 53.9 |
| Never | 41.9 | 41.1 | 38.5 | 39.2 |
| Body mass index, kg/m2 | 27.1 (4.3) | 29.3 (4.7) | 31.2 (5.5) | 34.1 (5.4) |
| Prevalent diabetes, % | 8.4 | 15.9 | 13.6 | 20.6 |
| C-reactive protein, mg/L* | 2.1 (3.6) | 2.2 (3.6) | 2.3 (3.7) | 3.4 (5.8) |
| Leisure index | 2.5 (0.5) | 2.4 (0.5) | 2.4 (0.5) | 2.3 (0.5) |
| Antihypertensive medications, % | 31.6 | 39.2 | 41.3 | 49.0 |
| Systolic blood pressure, mmHg | 123.2 (17.1) | 128.0 (18.5) | 126.6 (18.0) | 130.7 (17.0) |
Data shown as mean (SD) or percentage except for *geometric mean (interquartile range)
3.2. Obstructive sleep apnea and neurocognitive study-adjudicated dementia
Among the 1,083 who underwent comprehensive cognitive assessments as part of the neurocognitive study, we evaluated the association between OSA severity and 15-year risk of adjudicated dementia or MCI, dementia, MCI and dementia or MCI due to AD (Table 3). Of the 1,083 with cognitive testing, a total of 269 (24.8%) had dementia or MCI, with 5.3% having dementia and 19.6% having MCI. Among participants with severe OSA versus no OSA, after accounting for demographic factors (Model 1), the risk ratio (RR) (95% CI) was 1.43 (0.99, 2.07). This association was modestly attenuated with adjustment for behaviors and CVD risk factors [Model 3: 1.30 (0.87, 1.93)]. When the outcomes were evaluated separately, severe OSA versus no OSA was associated with greater risk of dementia [Model 1: 2.35 (1.06, 5.18), though there was attenuation with adjustment [Model 3: 2.19 (0.92, 5.19)]. Notably, these estimates are very imprecise as there were only 5 individuals with severe OSA who at the neurocognitive study had dementia. OSA severity was associated with little increase in risk of MCI [Model 1 RR = 1.36 (0.84, 2.18)].
Table 3.
Weighted* risk ratios (RRs) and 95% confidence intervals (CI) of obstructive sleep apnea (OSA) categories with dementia, mild cognitive impairment (MCI), and dementia or MCI due to AD: The Atherosclerosis Risk in Communities (ARIC) study, 1996–2013
| OSA Categories (AHI) | ||||
|---|---|---|---|---|
|
|
||||
| N | Normal (<5) 581 |
Mild (5 to <15) 312 |
Moderate (15 to <30) 132 |
Severe (>30) 58 |
| Dementia or MCI, n | 126 | 83 | 41 | 19 |
| Model 1 | 1 | 1.10 (0.86, 1.39) | 1.17 (0.86, 1.57) | 1.43 (0.99, 2.07) |
| Model 2 | 1 | 1.09 (0.86, 1.39) | 1.14 (0.84, 1.56) | 1.35 (0.91, 2.01) |
| Model 3 | 1 | 1.08 (0.85, 1.37) | 1.16 (0.85, 1.59) | 1.30 (0.87, 1.93) |
| Dementia, n | 28 | 20 | 4 | 5 |
| Model 1 | 1 | 1.31 (0.73, 2.37) | 0.45 (0.16, 1.26) | 2.35 (1.06, 5.18) |
| Model 2 | 1 | 1.49 (0.83, 2.66) | 0.50 (0.18, 1.39) | 2.51 (1.09, 5.79) |
| Model 3 | 1 | 1.34 (0.76, 2.37) | 0.53 (0.18, 1.49) | 2.19 (0.92, 5.19) |
| MCI, n | 98 | 63 | 37 | 14 |
| Model 1 | 1 | 1.07 (0.80, 1.42) | 1.31 (0.95, 1.82) | 1.36 (0.84, 2.18) |
| Model 2 | 1 | 1.05 (0.78, 1.41) | 1.27 (0.90, 1.80) | 1.30 (0.78, 2.18) |
| Model 3 | 1 | 1.04 (0.78, 1.40) | 1.29 (0.91, 1.83) | 1.24 (0.74, 2.09) |
| AD dementia or MCI, n | 81 | 55 | 29 | 14 |
| Model 1 | 1 | 1.17 (0.85, 1.60) | 1.32 (0.90, 1.93) | 1.66 (1.03, 2.68) |
| Model 2 | 1 | 1.15 (0.83, 1.58) | 1.23 (0.82, 1.84) | 1.46 (0.86, 2.46) |
| Model 3 | 1 | 1.13 (0.82, 1.55) | 1.25 (0.83, 1.87) | 1.37 (0.82, 2.30) |
Inverse-probability weighting was used.
Model 1: Relative risk regression adjusted for age, sex, center, education level, and APOE
Model 2: Model 1 + additional adjustment for body mass index, smoking status, and leisure time physical activity
Model 3: Model 2 + additional adjustment for diabetes, antihypertensive medications, C-reactive protein, and systolic blood pressure
In our analytic sample, etiologic diagnoses were available for 91.7% of participants who underwent cognitive testing (993 of 1,083). Of these, 179 were classified as having dementia or MCI due to AD. Risk of dementia or MCI due to AD tended to be higher among those with severe OSA versus a normal sleep breathing pattern after adjustment for demographics [RR Model 1: 1.66 (1.03, 2.68)], though this was attenuated in the fully adjusted models [Model 3: 1.37 (0.82, 2.30)].
For analyses utilizing the data from the comprehensive cognitive assessments as part of the neurocognitive study, results from analyses without IPW were generally similar to the IPW analyses (Supplemental Table 2).
3.3. Sleep duration and incident dementia
Of the 1,653 participants included in the sleep duration analyses, 395 (23.9%) reported sleeping <7 hours per night, 627 (37.9%) reported 7 to ≤8 hours per night, 521 (31.5%) reported 8 to ≤9 hours per night, and 110 (6.7%) reported sleeping ≥9 hours per night. Relative to participants who reported sleeping 8 to <9 hours per night, those participants who slept <7 hours tended to be female, have lower educational attainment, and were more likely to have prevalent diabetes (Table 4). Relative to participants sleeping 8 to <9 hours/night, neither short nor long sleep duration were associated with higher hazards of incident dementia in demographic adjusted models: HR<7 vs. 8 to ≤9 hours/night: 1.16 (0.74–1.82); HR≥9 vs. 8 to ≤9 hours/night: 1.25 (0.65–2.38) (Supplemental Table 3).
Table 4.
Baseline characteristics by habitual sleep duration categories: The Atherosclerosis Risk in Communities (ARIC) study, 1996–1998
| Average Sleep Duration (hours) | ||||
|---|---|---|---|---|
|
|
||||
| N | <7 395 |
7 to <8 627 |
8 to <9 521 |
>9 110 |
| Age, years | 62.4 (5.3) | 61.8 (5.5) | 63.6 (5.5) | 64.3 (5.0) |
| Female, % | 54.2 | 51.5 | 51.1 | 57.3 |
| Education level, % | ||||
| <High school | 15.4 | 7.3 | 11.1 | 11.8 |
| High school graduate | 46.1 | 46.7 | 45.7 | 56.4 |
| College/Graduate school | 38.5 | 45.9 | 43.2 | 31.8 |
| APOE genotype, % | ||||
| e4/e4 | 2.3 | 3.7 | 1.9 | 3.6 |
| e2/e4 or e3/e4 | 24.0 | 23.3 | 23.8 | 36.4 |
| Other | 69.1 | 68.2 | 69.1 | 52.7 |
| Missing | 4.6 | 4.8 | 5.2 | 7.3 |
| Smoking status, % | ||||
| Current | 11.7 | 8.4 | 10.8 | 11.8 |
| Former | 50.1 | 45.8 | 48.8 | 59.1 |
| Never | 38.2 | 45.8 | 40.4 | 29.1 |
| Body mass index, kg/m2 | 29.0 (5.2) | 28.6 (4.9) | 28.7 (5.0) | 28.5 (6.1) |
| Prevalent diabetes, % | 14.4 | 10.7 | 11.0 | 17.3 |
| C-reactive protein, mg/L* | 2.3 (3.9) | 2.1 (3.9) | 2.3 (3.6) | 2.0 (2.7) |
| Leisure index | 2.4 (0.5) | 2.5 (0.5) | 2.5 (0.5) | 2.4 (0.6) |
| Antihypertensive medications, % | 35.2 | 33.7 | 36.9 | 48.2 |
| Systolic blood pressure, mmHg | 126.4 (19.2) | 123.6 (16.9) | 126.1 (17.2) | 131.8 (19.9) |
Data shown as mean (SD) or percentage except for *geometric mean (interquartile range)
3.4. Sleep duration and neurocognitive study-adjudicated dementia
Among the 1,081 participants who had cognitive assessments as part of the neurocognitive exam, we also evaluated the association between habitual sleep duration and risk of dementia or MCI, dementia, MCI, and MCI or dementia due to AD (Table 5). Relative to participants sleeping 8 to <9 hours/night, the RR of dementia or MCI associated with sleeping <7 hours in the demographic-adjusted model (Model 1) was 1.28 (0.98, 1.66), while for participants sleeping ≥9 hours/night the RR was 0.89 (0.56, 1.40). Additional adjustment for behaviors and CVD risk factors (Model 2) had little effect on these estimates. When solely assessing objectively measured dementia as the outcome, sleeping <7 hours per night was associated with higher risk of dementia across all models: Model 1: 2.00 (1.03, 3.86); Model 3: 1.89 (1.01, 3.51). For long sleep duration the RR for dementia was 1.69 (0.75, 3.78) in Model 1 and 1.53 (0.69, 3.39) in Model 3. There were only 4 dementia cases among participants reporting >9 hours per night. Sleep duration was not associated with risk of MCI, or MCI or dementia due to AD. Analyses which did not include IPW yielded similar results (Supplemental Table 4).
Table 5.
Weighted* risk ratios (RRs) and 95% confidence intervals (CI) of habitual sleep duration categories with dementia, mild cognitive impairment (MCI), and dementia or MCI due to AD: The Atherosclerosis Risk in Communities (ARIC) study, 1996–2013
| Average Sleep Duration (hours) | ||||
|---|---|---|---|---|
|
|
||||
| N | <7 257 |
7 to <8 435 |
8 to <9 330 |
>9 59 |
| Dementia or MCI, n | 73 | 100 | 81 | 13 |
| Model 1 | 1.28 (0.98, 1.66) | 1.09 (0.85, 1.40) | 1 | 0.89 (0.56, 1.40) |
| Model 2 | 1.28 (0.99, 1.66) | 1.08 (0.84, 1.38) | 1 | 0.90 (0.57, 1.42) |
| Model 3 | 1.26 (0.97, 1.63) | 1.08 (0.84, 1.39) | 1 | 0.86 (0.54, 1.36) |
| Dementia, n | 17 | 21 | 15 | 4 |
| Model 1 | 2.00 (1.03, 3.86) | 1.39 (0.73, 2.66) | 1 | 1.69 (0.75, 3.78) |
| Model 2 | 2.04 (1.08, 3.85) | 1.33 (0.69, 2.56) | 1 | 1.56 (0.67, 3.67) |
| Model 3 | 1.89 (1.01, 3.51) | 1.42 (0.75, 2.70) | 1 | 1.53 (0.69, 3.39) |
| MCI, n | 56 | 79 | 66 | 9 |
| Model 1 | 1.18 (0.86, 1.63) | 1.01 (0.75, 1.35) | 1 | 0.74 (0.40, 1.34) |
| Model 2 | 1.18 (0.86, 1.63) | 1.00 (0.75, 1.34) | 1 | 0.74 (0.40, 1.36) |
| Model 3 | 1.19 (0.86, 1.63) | 1.00 (0.75, 1.34) | 1 | 0.73 (0.40, 1.32) |
| AD dementia or MCI, n | 50 | 65 | 55 | 8 |
| Model 1 | 1.25 (0.88, 1.76) | 1.01 (0.73, 1.41) | 1 | 0.83 (0.44, 1.58) |
| Model 2 | 1.25 (0.89, 1.76) | 1.01 (0.73, 1.41) | 1 | 0.80 (0.42, 1.52) |
| Model 3 | 1.26 (0.89, 1.77) | 1.01 (0.73, 1.41) | 1 | 0.75 (0.40, 1.43) |
Inverse-probability weighting was used.
Model 1: Relative risk regression adjusted for age, sex, center, education level, and APOE
Model 2: Model 1 + additional adjustment for body mass index, smoking status, and leisure time physical activity
Model 3: Model 2 + additional adjustment for diabetes, antihypertensive medications, C-reactive protein, and systolic blood pressure
3.5. Additional sensitivity analyses
There were no statistically significant interactions between the APOE ε4 risk allele and OSA or sleep duration categories on neurocognitive outcomes. However, precision was low for these analyses due to small cell sizes within APOE ε4 risk allele and sleep categories. Percent sleep time with oxygen saturation <90% was also explored as an exposure; results were similar to those observed for OSA (Supplemental Table 5).
4. Discussion
OSA and habitual short sleep duration are common, with severe OSA afflicting approximately 15% of adults[36] and short sleep duration 34%[37]. These conditions are amenable to correction through behavioral changes and/or clinical intervention. Therefore, were either OSA or short sleep duration causally related to dementia and MCI, there would be important opportunities for intervention to prevent the development of dementia and MCI. In our analysis of nearly 1,700 participants who were followed for more than 15 years, OSA was not associated with incidence of dementia as defined by a broad endpoint that included hospitalization diagnosis codes. In analyses restricted to those who underwent a comprehensive neurocognitive assessment, severe OSA was associated with greater risk of all-cause and AD dementia after accounting for demographics, but the associations were attenuated with additional adjustment for behaviors and CVD risk factors. This attenuation suggests that the effect of OSA on dementia may be mediated through cardiovascular pathways (e.g. hypertension, diabetes). Results when percent time in oxygen saturation <90% was the exposure followed a pattern similar to those observed for OSA. For habitual sleep duration, a similar pattern was observed whereby there was no association with dementia as ascertained by hospitalization diagnosis codes. However, when restricted to participants with a comprehensive assessment of cognitive status, sleeping less than 7 hours per night was associated with 2-fold higher risk of all-cause dementia.
The 15 year time-span between the sleep studies and the neurocognitive exam is an important strength of our study since, for many dementia risk factors, stronger associations have been observed when the risk factors were measured at middle-age than when they were measured later in life[4–11]. However, this time-span also creates challenges in the interpretation of our results. Of the 1,667 participants eligible for this analysis, 21.5% died prior to the neurocognitive study, and 13.5% did not participate for other reasons. For these individuals we did not conduct the full neurocognitive battery, but do have information on dementia hospitalization ICD codes, and some also have TICSm and informant interviews. Importantly, sensitivity of hospitalization ICD codes is poor[30, 38], and we invariably missed numerous cases of dementia that occurred among individuals who did not attend the neurocognitive exam. As the ARIC neurocognitive study has previously reported, the sensitivity of hospital and death diagnostic codes for dementia was 25% (95% CI: 22%–29%) and the specificity 99% (95% CI: 99%–99%)[30]. The poor sensitivity of hospitalization codes may explain why, in the present analysis, associations with sleep characteristics were stronger for analyses restricted to the neurocognitive study participants than for analyses that also utilized the ICD hospitalization codes. Through use of IPW, we attempted to account for selection bias that could have occurred as a result of individuals who participated in the neurocognitive study being different than those who did not. However, some bias may have remained, and the true cognitive status of individuals who did not attend the neurocognitive study is unknown.
To date, the strongest evidence suggesting a prospective relation between OSA and incident dementia comes from the Study of Osteoporotic Fractures, which using data from 298 women (mean age 82 years) followed for a mean of 4.7 years, found that women with sleep disordered breathing (AHI ≥15 events/hour) were 85% (95% CI: 11%–208%) more likely to develop MCI or dementia as compared to women with an AHI <15[14]. Sleep duration was not associated with MCI/dementia risk in this sample. However, in a recent publication from the Women’s Health Initiative Memory Study, self-reported short and long sleep duration were associated with higher risk of MCI and dementia among older women followed from 1995–2008[39]. Long sleep duration was also associated with greater risk of incident dementia over 3 years of follow-up in the Neurological Disorders in Central Spain Study, but there was no relation between short sleep duration and dementia risk[40]. These findings are also consistent with the majority of cross-sectional studies of OSA and cognition, and prospective studies of change in cognitive test scores[41–44]. Notably, in a prior analysis of this ARIC sample, there was no association between OSA or sleep duration and change in scores on cognitive tests that were administered at baseline and at the time of the neurocognitive exam[45]. For that analysis, similar to the results presented herein, it is possible that issues of selection bias may have masked a true association. Also, there may have been “floor effects”, whereby a factor doesn’t affect the rate of decline after a threshold is reached. For example, in a prior ARIC publication low education was associated with dementia risk but not with cognitive decline.[35]
One limitation of our study was that despite the relatively large overall sample size, the number of cases with severe OSA and severe reductions in sleep duration were small. As such, our results were imprecise for estimations of future dementia risk among participants at the extreme ends of the exposure spectrum – specifically individuals with severe OSA or who slept less than 7 hours per night. Furthermore, precision was poor when looking at dementia of AD etiology, and we were unable to evaluate relations of sleep with other etiology subtypes (e.g. vascular dementia). Also, as noted above, selection bias related to non-attendance at the neurocognitive exam is an important limitation of this study. Results of analyses which attempted to account for selection bias by using IPW models were similar to those from standard analyses. However, this does not necessarily mean that there is no selection bias; it is possible that we did not have appropriate information to correct for bias. Additional limitations include a single assessment of sleep, self-reported sleep duration, no comprehensive neurocognitive assessment at baseline, lack of biomarker tests to verify AD dementia, residual confounding, and the testing of multiple comparisons may have led to spurious associations. The generalizability of our findings is also uncertain, as the prevalence of OSA and sleep duration were lower than often observed in generally healthy populations[36, 46]. The present ARIC data also have important strengths including a relatively large sample size, 15 years of follow-up, a multifactorial comprehensive cognitive assessment, objective information on CVD risk factors, and representation of both men and women.
In sum, in this community-based sample, OSA and short sleep duration were associated with greater risk of all-cause and AD dementia when employing carefully phenotyped outcome definitions. Our findings highlight the need for studies with rigorous adjudication of dementia, and additional research to understand whether the observed associations are causal.
Supplementary Material
Research in Context.
Systematic review
We identified relevant studies in PubMed. Though prior research suggests that obstructive sleep apnea (OSA) and short and long sleep duration may be linked to dementia, no studies have considered OSA in midlife.
Interpretation
Neither OSA nor short or long sleep duration were associated with dementia incidence when a non-adjudicated dementia definition was used. However, when adjudicated outcomes were used, there was some evidence that midlife OSA and short sleep duration may be linked to all-cause and Alzheimer’s disease dementia later in life.
Future directions
There may be value in additional studies of sleep quantity and quality in relation to subclinical and clinical markers of dementia and Alzheimer’s disease dementia. It is unknown whether improving midlife sleep quality and/or quantity would reduce the likelihood of developing dementia.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Dr. Knopman serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by Biogen, TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study. Numerous authors receive research support from the NIH. Dr. Gottesman is Associate Editor for Neurology.
Funding Sources
This was not an industry supported study. The ARIC portion of the SHHS was supported by National Heart, Lung, and Blood Institute cooperative agreements U01HL53934 (University of Minnesota) and U01HL64360 (Johns Hopkins University).The ARIC is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 from the NHLBI and the National Institute of Neurological Disorders and Stroke, and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. This study was additionally supported by grant R21 HL121412 to Dr. Pamela Lutsey. Ms. Ogilvie was supported by a National Heart Lung and Blood Training Grant (T32 HL007779). Statistical analyses took place at the University of Minnesota. All authors reviewed the manuscript at their respective institutions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AD – Alzheimer’s disease; AHI – apnea-hypopnea index; ARIC – Atherosclerosis Risk in Communities; CI – confidence interval; CDR – clinical dementia rating; CVD – cardiovascular disease; FAQ – functional activities questionnaire; HR – hazard ratio; IPW – inverse probability weighting; MCI – mild cognitive impairment; NPI – neuropsychiatric inventory; OSA – obstructive sleep apnea; MRI – magnetic resonance imaging; PSG – polysomnography; RR – relative risk; SHHS – Sleep Heart Health Study; TICSm – modified telephone interview for cognitive status
Conflicts
The authors have indicated no financial conflicts of interest.
References
- 1.Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 2.Ortman JM, Velkoff VA, Hogan H. Population Estimates and Projections: Current Population Reports. U.S. Census Bureau; 2014. An aging nation: The older population in the United States. [Google Scholar]
- 3.World Population Aging. ST/ESA/SERA/348. United Nations; 2013. United Nations - Department of Economic and Social Affairs - Population Division. [Google Scholar]
- 4.Staessen JA, Richart T, Birkenhager WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49:389–400. doi: 10.1161/01.HYP.0000258151.00728.d8. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–27. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso A, Mosley TH, Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80:1194–201. doi: 10.1136/jnnp.2009.176818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias PK, Elias MF, D'Agostino RB, Cupples LA, Wilson PW, Silbershatz H, et al. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20:1388–95. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- 8.Gregg EW. Is Diabetes Associated With Cognitive Impairment and Cognitive Decline Among Older Women? Archives of Internal Medicine. 2000;160:174. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 9.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70–81 years. BMJ. 2004;328:548. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–93. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernan MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19:448–50. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 12.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93:1778–94. doi: 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- 13.Amlander C, Fuller P. Basics of Sleep Guide. Westchester, Illinois: Sleep Research Society; 2009. [Google Scholar]
- 14.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bliwise DL. Sleep apnea, APOE4 and Alzheimer's disease 20 years and counting? J Psychosom Res. 2002;53:539–46. doi: 10.1016/s0022-3999(02)00436-1. [DOI] [PubMed] [Google Scholar]
- 16.Durgan DJ, Bryan RM., Jr Cerebrovascular consequences of obstructive sleep apnea. J Am Heart Assoc. 2012;1:e000091. doi: 10.1161/JAHA.111.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 20.Munoz R, Duran-Cantolla J, Martinez-Vila E, Gallego J, Rubio R, Aizpuru F, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 21.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadotani H. Association Between Apolipoprotein E ε4 and Sleep-Disordered Breathing in Adults. Jama. 2001;285:2888. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb DJ, DeStefano AL, Foley DJ, Mignot E, Redline S, Givelber RJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63:664–8. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 24.Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE epsilon4 carriers. Sleep. 2013;36:873–80. doi: 10.5665/sleep.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 26.O'Hara R, Schroder CM, Kraemer HC, Kryla N, Cao C, Miller E, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005;65:642–4. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 27.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 28.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 29.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 30.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Alzheimers Dement (Amst) 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 34.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–28. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–66. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcantara C, et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–88. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Consensus Conference P. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11:591–2. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin YP, Gatz M, Johansson B, Pedersen NL. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology. 2004;63:739–41. doi: 10.1212/01.wnl.0000134604.48018.97. [DOI] [PubMed] [Google Scholar]
- 39.Chen JC, Espeland MA, Brunner RL, Lovato LC, Wallace RB, Leng X, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement. 2016;12:21–33. doi: 10.1016/j.jalz.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol. 2009;16:990–7. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 41.Potvin O, Lorrain D, Forget H, Dube M, Grenier S, Preville M, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 43.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 44.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 45.Lutsey PL, Bengtson LG, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, et al. Obstructive Sleep Apnea and 15-Year Cognitive Decline: The Atherosclerosis Risk in Communities (ARIC) Study. Sleep. 2015 doi: 10.5665/sleep.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:137–41. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.