Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) has been implicated in the pathology of numerous diseases involving diabetes, stroke, cancer, or obesity. It is expressed in diverse cell types, including vessels, immune and glial cells, and neurons. PPARγ plays crucial roles in the regulation of cellular differentiation, lipid metabolism, or glucose homeostasis. PPARγ ligands also exert effects on attenuating degenerative processes in the brain, as well as in peripheral systems, and it has been associated with the control of anti-inflammatory mechanisms, oxidative stress, neuronal death, neurogenesis, differentiation, and angiogenesis. This review will highlight key advances in the understanding of the PPARγ-related mechanisms responsible for neuroprotection after brain injuries, both ischemia and traumatic brain injury, and it will also cover the natural and synthetic agonist for PPARγ, angiotensin receptor blockers, and PPARγ antagonists, used in experimental and clinical research. A better understanding of the pleiotropic mechanisms and applications of these drugs to improve the recovery and to repair the acute and chronic induced neuroinflammation after brain injuries will pave the way for more effective therapeutic strategies after brain deficits.
Keywords: Brain injury, Angiogenesis, Agonist, PPAR gamma, Inflammation, Angiotensin receptor blockers
Introduction
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that regulate genes essential on various metabolic processes and cell differentiation, but also exert anti-inflammatory properties after brain injury or neurodegenerative diseases (Kapadia et al. 2008; Yonutas and Sullivan 2013). PPARs are members of the nuclear hormone receptor superfamily of ligand-inducible transcription factors that heterodimerize with the retinoid X receptor (RXR), interact with cofactors, and act on specific DNA sequences to cause transcriptional activity (Moreno et al. 2004). After interaction with specific ligands, PPARs are translocated to the nucleus, where they change their structure and regulate gene transcription. In addition to transcriptional transactivation, PPARs can repress gene transcription by negatively interfering with other transcription factor pathways independent of DNA binding (Abdelrahman et al. 2005). PPARs are transcription factors that belong to the superfamily of nuclear receptors, and the members PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3) represent the family of PPARs (Ehrmann et al. 2002). They are expressed in different tissues and have central roles in the homeostasis and energy metabolism, regulating energy storage. PPAR-α is expressed highly in the liver, plays a role in fatty acids oxidation, which provides energy for peripheral tissues, lipoprotein metabolism, and has also a potential role in oxidant/antioxidant pathway. PPAR-δ/β promotes fatty acids metabolism and suppresses macrophage-derived inflammation. PPARγ is highly expressed in adipose tissue, where it is a regulator of adipogenesis, lipid metabolism, and insulin sensitivity (Tontonoz and Spiegelman 2008). Also, PPARγ activation plays a crucial role in the regulation of proliferation, metabolism, differentiation, development, and inflammatory responses of the central nervous system (CNS) (Gurley et al. 2008), in this way, PPARγ agonists have significant therapeutic potential in brain disorders.
The present review mainly discusses the effective neuroprotective role of PPARγ activation in the peripheral and brain inflammation, and the significant role for PPARγ agonist and antagonist in the regulation of neuroinflammatory processes following brain injuries. Also, its role in apoptosis, neurogenesis, differentiation, and angiogenesis after brain damage.
Role of PPARγ in Brain Inflammation
In the peripheral organs as well as in the CNS, the regulation of inflammatory processes conduces to the reduction of the brain damage and improvement of motor and cognitive outcome. The mediators responsible for this process are the resident microglia and infiltrated inflammatory cells originating from the blood (Morganti-Kossmann et al. 2007; Woodcock and Morganti-Kossmann 2013). Effects on inflammation are regulated through mechanistic signaling pathways where multiple factors interfere and can be modulated by PPARs. The expression of PPARs was analyzed by immunohistochemistry and in situ hybridization in several rodent tissues, including the CNS. PPARγ is present in most cell types, vessels, neurons, and astrocytes (Fig. 1), where it mediates multimodal function, whereas oligodendrocytes exclusively show PPAR-/ expression (Giannini et al. 2004; Moreno et al. 2004). PPARγ is also expressed in various immune-related cell types, particularly in adipocytes, macrophages, dendritic cells, and microglia (Yuan et al. 2015). PPARγ regulates the alternative activation of immune cells by increasing anti-inflammatory-related gene expression (Bouhlel et al. 2007), and down-regulation of pro-inflammatory mediators through their action on activated microglia/macrophages (Kapadia et al. 2008). PPARγ-mediated CD36 upregulation has been involved in the modulation of microglia activation and phenotype, promoting phagocytosis of apoptotic cells and thus contributing to the resolution of inflammation after ischemia (Ballesteros et al. 2014). Also, PPARγ has the ability mainly to inhibit transcription factors, such as the transcription factors activator protein-1, Stat 1, and nuclear factor-kB (NF-κB) (Ricote et al. 1998). PPARγ also mediates down-regulation of pro-inflammatory genes such as cyclooxygenase-2 (COX-2), metalloproteinase-9 (MMP-9), scavenger receptor A, inducible nitric oxide synthase (iNOS), as well as the production of pro-inflammatory cytokines, chemokines, and interleukins (Heneka et al. 2000; Kapadia et al. 2008; Lenglet et al. 2013) (Fig. 2). Thus, reducing PPARγ activation may contribute to the chronic inflammation. PPARγ agonists may modulate expression of inflammatory genes through PPARγ-independent mechanisms, as was demonstrated in PPARγ-null embryonic stem cells (Chawla et al. 2001; Moore et al. 2001). The convenience of PPARγ agonists as a tool for down-regulation of brain inflammation that occurs after brain damage is an important area to be developed in the future.
Fig. 1.
PPAR expression in different cell types. a Microglia/macrophages cells (Iba-1, green) express PPARγ (red) at the border of the lesion after brain injury in vacuolated cells with amoeboid morphology (high magnification images in A, right side) or hypertrophy microglia morphology (high magnification images in A, bottom side). b Blood vessels in the injured brain (Collagen IV (Colg IV), green) express PPARγ (red). A few PPARγ positive cell are extending processes around and along a capillary (Colg IV, green) in the cortex of a mouse (high magnification images in B). c Astrocytes (GFAP, green) collate around PPARγ positive cells (red) in the injured cortex, with a little co-localization with PPARγ. Nuclei (dapi, blue) (Color figure online)
Fig. 2.
Schematic representation of the PPARγ signaling in nervous cells in the injured brain. PPARγ acts as an anti-inflammatory factor, pro-differentiating transcription factor, and antioxidant after brain injury. PPARγ activation also induces angiogenesis and glucose and lactate production in astrocytes. PPARγ ligands are known to inhibit or repress the activity of a number of transcription factors important in neuroinflammation. PPARγ also binds RXR and activates target gene expression through the recruitment of coactivators (PGC)-1a. PPARγ interacts with transcription factors such as NF-κB, Stat-1/-3/-6, or C/EBP, and represses their target genes transcription. A variety of endogenous (15dPGJ2) and exogenous (Thiazolidinediones (TDZs)) compounds have been identified as PPARγ ligands. 15dPGJ2 promotes direct binding of PPARγ to Stat-3, and TDZ induces repression of target genes, preventing transactivation of pro-inflammatory cytokines in a PPARγ-dependent manner
Role of PPARγ in Peripheral Organs
In addition to the CNS, PPARγ activation also occurs in the peripheral organs. PPARγ is predominantly detected in adipose tissue, liver, and intestine, regulating adipocyte differentiation and promotes lipid storage (Akiyama et al. 2005; Berger et al. 2005). Macrophages polarization switch was associated with the interaction between PPARγ and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signal pathway, and it was demonstrated that this disruption of PPARγ impaired alternative M2 macrophage activation/Kupffer cell polarization in a non-alcoholic fatty liver disease (Luo et al. 2017). PPARγ protein increases insulin sensitivity and decreases insulin resistance in adipose tissue, skeletal muscle, and liver (Heald and Cawthorne 2011; Hegarty et al. 2004; Lee et al. 2016; Odegaard et al. 2007; Wang et al. 2009). In the vascular system, PPARγ confers anti-atherosclerotic effects (Blaschke et al. 2006a, b). PPARγ antagonizes the metabolic syndrome by downregulating peripheral inflammatory processes, including the suppression of pro-inflammatory cytokines and adhesion molecules (Delerive et al. 2001). Besides, PPARγ activation is considered necessary for inhibiting an intestinal inflammatory response and defending cells oxidative damage (Serra et al. 2016). Recently, it was demonstrated how a novel PPARγ modulator, GED-0507-34, ameliorated intestinal fibrosis in a model of chronic colitis in mice and regulated the major profibrotic cellular and molecular mechanisms (Speca et al. 2016). Recently, studies have highlighted the PPARγ signaling association to the microbiota, a low grade of inflammation, and host metabolism (Sohn et al. 2015; Wang et al. 2016). However, microbiota-induced PPARγ has also a role beyond the gut (Angelakis et al. 2012; Couvigny et al. 2015; Karrout et al. 2015; Peyrin-Biroulet et al. 2010).
Role of PPARγ on Oxidative Stress and Neuronal Survival
The brain damage caused by oxidative stress induces a high rate of oxidative metabolic activity, and relatively low antioxidant capacity and insufficient neuronal cell repair activity. Overproduction of reactive oxygen species (ROS) results in oxidative damage, including lipid peroxidation, and DNA damage, which can lead to cell death (Lozano et al. 2015). Several PPARγ agonists have been shown to exert protective activity against oxidative damage, mitochondrial dysfunction, and apoptosis protecting neurons and glial cells in various animal models. Activation of PPARγ induces expression of antioxidant catalase and copper/zinc superoxide dismutase (SOD), two enzymes capable of alleviating oxidative stress, and inhibiting NADPH oxidase (Dunning et al. 2013; Eslami et al. 2014; Zarzuelo et al. 2013). The PPARγ antagonist GW9662, blocked the increase of PPARγ DNA binding activity and antioxidant enzymatic activities (SOD and CAT) abolishing the protection of PPARγ activation in OGD-exposed neurons (Zeng et al. 2012). Other mechanisms by which these PPARγ agonists prevent oxidative stress include a decrease in iNOS activity, NFκB blockade, inhibition of TNF-α release, or activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (Heneka and Landreth 2007; Park et al. 2004) (Fig. 2).
The Role of PPARγ in Neurogenesis and Differentiation
Neuronal stem cell (NSC) and progenitors following brain injury are thought to proliferate, migrate to, and differentiate at injury sites, affecting variable degrees of structural and functional recovery. Endogenous stem cells and stem cell transplant therapy supported by their local vasculature, are promising for new therapeutic strategies in the chronic neuroinflammatory environment that accompany brain damage, stroke, or other neurodegenerative diseases (Ormerod et al. 2013; Prakash and Kumar 2014; Qi et al. 2010). PPARγ is essential in regulating the early brain development and post-injury brain repair (Eriksson et al. 1998). PPARγ activation promotes neurite outgrowth in mature neurons significantly contributing to a proper neuronal connectivity in neuronal networks (Miglio et al. 2009). Also, it has been demonstrated that PPARγ-mediated pathways can be involved in the proliferation and differentiation of NSCs (Cimini and Ceru 2008; Wada et al. 2006). PPARγ activation by PPARγ agonists stimulated NSC proliferation and inhibited differentiation into neurons, furthermore abundant activation of PPARγ with higher levels of agonists resulted in cell death (Wada et al. 2006). Oligodendrocytes are required for myelin formation and maintenance (Griggs et al. 2017). PPARγ has a role in the differentiation and function of oligodendrocytes (Roth et al. 2003), being these effects blocked by the PPARγ antagonist GW9662 (Wan Ibrahim et al. 2013). It was demonstrated that GW9662 could also inhibit the differentiation towards neurons and astrocytes induced by pioglitazone and rosiglitazone in neurospheres from adult rat brains (Morales-Garcia et al. 2011). A transient immune response stimulated by lipopolysaccharide (LPS) compromised hippocampal neurogenesis and impaired hippocampus-dependent spatial memory, and PPARγ agonist activity protects neurogenesis and memory from the effects of LPS-produced transient illness (Ormerod et al. 2013). The blockade of PPARγ was able to significantly straighten cannabidiol effects on reactive gliosis and subsequently on neuronal damage. Moreover, cannabidiol-mediated activation of PPARγ is associated with a significant neurogenic activity in the granule cell layer of the hippocampus (Esposito et al. 2011).
Role of PPARγ in Angiogenesis
Angiogenesis is the formation of new blood vessels around the injured brain restoring the damaged regions and inducing neurovascular repair (Arai et al. 2009). PPARγ activation increases vascular endothelial growth factor (VEGF) expression in human vascular smooth muscle cells (Yamakawa et al. 2000). PPARγ coactivator, (PGC)-1α, is a transcriptional coactivator that powerfully regulates oxidative and mitochondrial metabolism, but also angiogenesis activities in the brain. (PGC)-1α is a known regulator of VEGF gene transcription (Arany et al. 2008), and it was found elevated in the cortex during the chronic hypoxic exposure (Ndubuizu et al. 2010).
Rosiglitazone was found to induce the endothelial cells proliferation, endothelial NOS expression benefiting angiogenesis, preserving the cerebral blood flow and limiting of neurological loss and functional recovery (Chu et al. 2006). By another hand, resveratrol was attributed to its role as an intracellular antioxidant, an anti-inflammatory agent, its ability to induce sirtuin 1 (SIRT1) activity, NOS expression, and angiogenesis (Annabi et al. 2012). It was also demonstrated how resveratrol, exerts pharmacological preconditioning by activating (PGC)-1α, reducing the extent of ischemia/reperfusion injury (Tan et al. 2008) (Fig. 2). Treatment with PPARγ agonists exerts direct protective action on cerebral glucose and glutamate metabolism, disrupting the regulation of neuronal glucose transporter (GLUT-3) expression and glial glutamate transporter EAAT-2 (Garcia-Bueno et al. 2007).
Natural or Physiological PPARγ Agonists
Fatty acids are natural modulators of PPARγ; however, their connection with the receptor does not always lead to PPARγ activation and target gene transcription. A physiological PPARγ agonist is the 15-Deoxy-Delta12,14-prostaglandin J2 (15dPGJ2), a reactive membrane lipid metabolite, and anti-inflammatory downstream product of prostaglandin D2. (Kimura et al. 2008). In basal conditions, physiological PGJ2 closes a negative feedback loop on COX-2, whereas, in stress conditions, COX-2 is activated by enhanced levels of PGJ2 (Behl et al. 2016; Liu et al. 2012; Napimoga et al. 2013). Thus, PGJ2’s anti-inflammatory effect is more potent in stressful conditions due to induction of endogenous PGJ2 production, when it is combined with exogenous 15dPGJ2 (Mouihate et al. 2004).
Pharmacological Agonists for PPARγ: Thiazolidinediones
PPARγ agonists have been demonstrated to show a benefit in multiple CNS injury models including spinal cord injury (SCI) (Park et al. 2007), TBI (Yi et al. 2008), and stroke (Collino et al. 2008). PPARγ agonist have been reported to be protective after experimental brain trauma in rodents, reduction mitochondrial dysfunction, cognitive impairment, tissue loss, and inflammation (Sauerbeck et al. 2011b; Yi et al. 2008). PPARγ possesses a high number of pharmacological or synthetic high-affinity ligands as thiazolidinediones (TZDs), which include troglitazone, rosiglitazone, pioglitazone, and ciglitazone (White and Murphy 2010). The kinetics of intraperitoneal TZDs are unknown. However, the half-life of oral TZDs is 4–9 h (Chapelsky et al. 2003), and so it is plausible that they are present during the alteration of PPARγ activation and expression after brain injury. The TZDs have the capacity to reduce the expression of proteins that contribute to the inflammatory damage observed in after brain injuries (Arai et al. 2009; Culman et al. 2007), such as the pro-inflammatory cytokine TNF-α and iNOS, gelatinase B (MMP-9) and COX-2 in LPS-stimulated macrophages, glial cells and neurons (Heneka and Landreth 2007) (Fig. 2). It was also found that PPARγ agonist attenuates ischemia-induced activation of microglia and neutrophil infiltration in mice (Tureyen et al. 2007). Troglitazone was the first drug approved by the Food and Drug Administration (FDA) for clinical use, followed by rosiglitazone and pioglitazone (Sood et al. 2000). They were introduced on the market in the early 1990s, and are currently in clinical use to regulate the blood glucose levels in patients with type II diabetes. PPARγ activation enhances the expression of proteins involved in glucose and lipid metabolism, improving insulin resistance by mitigating the effect of TNF-α in adipocytes (Tyagi et al. 2011). Besides decreasing insulin resistance, TZDs positively affect the vasculature, reducing the high blood pressure and its associated risks, such as atherosclerosis, cardiovascular diseases, and stroke. It was demonstrated that Pioglitazone mitigates the severity of radiation-induced cognitive impairment in a well-characterized rat model (Zhao et al. 2007). It also attenuates dopaminergic cell death in a Parkinson’s disease model (Breidert et al. 2002), induces upregulation of SOD (Shimazu et al. 2005), COX-2 and TNF-α expression (Zhao et al. 2006), and microglia and macrophage activation (Zhao et al. 2005) in a rat model of stroke, and reduces lesion volume and cerebral inflammation in a murine model of TBI (Thal et al. 2011). A single dose of Pioglitazone administered early following lateral fluid percussion injury (LFPI), decreased the cortical lipid and protein oxidative damage, edema, increased the GSH-Px activity, and reduced microglial activation (Pilipovic et al. 2015). Troglitazone reduces cell death in cultured cerebellar granule neurons following glutamate exposure, suggesting that it interferes with downstream consequences of glutamate activation (Uryu et al. 2002), and cell death in rat cerebellum exposed to bacterial LPS and interferon-γ (Heneka et al. 2000). Another PPARγ agonist, rosiglitazone, has been studied after cerebral ischemia in rats. Rosiglitazone induced brain repair promoting white matter restoration (Han et al. 2015), and found to decrease secondary neuronal damage, gliosis, myelin loss, and neuropathic pain in animal models of SCI while improving motor function recovery (Li et al. 2013; Zhang et al. 2010). It was also found to reduce neuroinflammation, inhibit pro-apoptotic caspase-3, and attenuate both intercellular adhesion molecule 1 (ICAM-1), myeloperoxidase (MPO) activity, and cytokine expression in mouse models of transient cerebral ischemia (Collino et al. 2006; Luo et al. 2006; Sundararajan et al. 2005; Victor et al. 2006a). Rosiglitazone has also been shown to interfere with NF-κB activation in an experimental model of autoimmune encephalomyelitis (Iruretagoyena et al. 2006).
Furthermore, two non-thiazolidinedione PPARγ agonists, L-796 and L-449, have been shown to decrease middle cerebral artery occlusion (MCAO)-induced infarct size, inhibit NF-κB signaling and improve neurological scores (Pereira et al. 2005). GW1929 treatment ameliorated cognitive deficits, cerebral ischemic-reperfusion, and hippocampal neuronal damage (Kaundal and Sharma 2011a, b).
PPARγ Agonist Activity of Angiotensin Receptors Blockers
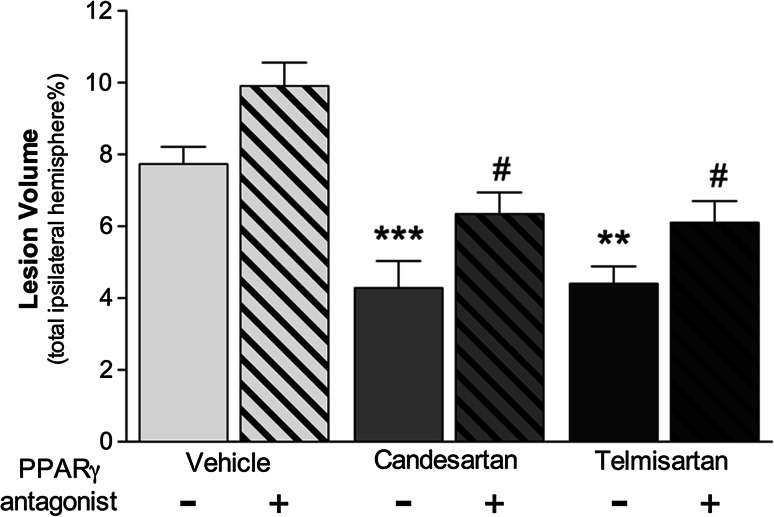
Angiotensin II type 1 (AT1R) receptor blockers (ARBs) have selective PPARγ agonist activity in the stress response to injury (Pang et al. 2012a, b). The net result of the AT1R blockade and PPARγ activation is to improve energy balance and blood flow to the brain with relevant neuroprotective properties after brain injuries (Villapol and Saavedra 2015). ARBs improve stroke outcome, at least in part, through activation PPARγ and blockade of the AT1R in cerebral ischemia models (Jung et al. 2007; Schmerbach et al. 2008). Candesartan and telmisartan are ARBs that induce activation of PPARγ and were studied in ischemic animal models showing neuroprotective features (Schmerbach et al. 2008; Zeng et al. 2013). Telmisartan was demonstrated that suppresses brain injury following ischemia and improves outcome, at least in part, through activation of PPARγ (Kasahara et al. 2010). We have previously demonstrated that candesartan treatment reduced lesion volume, apoptosis, and microglia activation, improving performance in the motor and learning and memory behavior test in a mouse model of traumatic brain injury (TBI) (Villapol et al. 2012), and both drugs, candesartan and telmisartan, decreased lesion volume, apoptosis, gliosis, and protected cerebral blood flow after TBI (Villapol et al. 2015). However, we have demonstrated the neurorestorative effects of both ARBs with dual AT1R blocking and PPARγ activation (Fig. 3) (Villapol et al. 2015).
Fig. 3.
Angiotensin II receptor blocker effectiveness after brain injury is partially dependent on PPARγ activation. Effects on lesion volume at 3 days after traumatic brain injury, candesartan (0.1 mg/Kg) and telmisartan (1 mg/Kg) treatment, significantly reduced the lesion volume, and PPARγ antagonist (T0070907, 2 mg/Kg) administration alone, or combined with candesartan or telmisartan, reverses this effect. Data are mean ± SEM, n = 8–15. ***P < 0.001, **P < 0.01 candesartan, or telmisartan versus vehicle; # P < 0.05 groups versus PPARγ antagonist. Adapted from (Villapol et al. 2015, 2012)
PPAR Antagonists
PPAR antagonists were used in animal models of brain injury to test whether neuroprotection after damage is mediated by PPARγ activation (Victor et al. 2006b). This widely used pharmacologic antagonist has been shown to bind covalently to the Cys313 residue of PPARγ and induce conformational changes that block the recruitment of transcriptional cofactors to the PPARγ/RXR heterodimer (Lee et al. 2002). Contrarily to PPARγ agonist by TZDs, the PPARγ blockage increases lesion size after ischemia. PPARγ antagonist, GW9662, reduces the protective effects of LPS preconditioning against organ damage caused by endotoxemia or ischemia/reperfusion (Collin et al. 2006; Sivarajah et al. 2005). Other studies have shown how a novel PPARγ antagonist, T0070907, blocks and promotes recruitment of nuclear receptor corepressors to PPARγ (Lee et al. 2002). T0070907 is highly specific for PPARγ having an 800-fold preference for PPARγ, over PPARα and PPARδ (Lee et al. 2002). The beneficial neuroprotective effects of telmisartan were reduced by concomitant administration of GW9662, a PPARγ antagonist on ischemia/reperfusion injury (Kasahara et al. 2010), suggesting that PPARγ activation may contribute to part (or all) of the neuroprotective effects of candesartan or telmisartan after TBI (Villapol et al. 2015, 2012) (Fig. 3). In agreement with our studies, PPARγ agonist rosiglitazone reduced infarction volume around 75% in an MCAO rodent model and its protection was completely lost when T0070907 was given along with rosiglitazone (Sobrado et al. 2009).
PPARγ Activation After Brain Injury
Brain damage also induces detrimental secondary damage and neuroinflammatory response (Aronowski and Zhao 2011). There are multiple implicated pathways for inducing central or peripheral inflammation. Neuroprotection merely reduces cell death or lesion volume after brain injury. However, neurorestorative approaches can promote endogenous neurogenesis, axonal sprouting, synaptogenesis, oligodendrogenesis, or angiogenesis, which enhance neuroplasticity and improve repair and functional recovery (Xiong et al. 2009). PPARγ agonists confer neuroprotection on the injured brain and PPARγ antagonists reverse the PPARγ activation effects in animal models of ischemia or TBI (Fig. 4). PPARγ presents lower levels of expression in the normal adult brain, limited to the hippocampal dentate gyrus, thalamus, basal ganglia, the piriform cortex, and expression in the rat cerebral frontal cortex, mainly in the neurons of layer II (Moreno et al. 2004). Also, PPARγ is expressed in microglia and astrocytes, both important cell types involved in the neuroinflammatory activity in neurological diseases and brain damage (Bernardo and Minghetti 2006, 2008). PPARγ activation can simultaneously weaken or reprogram the immune response, death of neurons, and glia following CNS injury (Mandrekar-Colucci et al. 2013). Reduction of lesion volume might be associated with anti-inflammatory activities related to PPARγ agonist activity of TZDs in the ischemic region and improve neurological function (Sundararajan et al. 2005; Zhao et al. 2005). Recent studies indicate that PPARγ agonists attenuate ischemia-induced activation of microglia, expression of ICAM-1, and neutrophil infiltration in C57BL/6mice (Luo et al. 2014; Tureyen et al. 2007). However, PPARγ is also implicated in the differentiation of monocytes to macrophages in the periphery, and PPARγ agonist can inhibit the expression of iNOS, TNF-α, and IL-1ß from macrophages (Ricote et al. 1998). The PPARγ antagonist increased infarction size in the absence of exogenous agonist, suggesting that even the low level of PPARγ activation that occurs during ischemia is protective. There are several studies in murine brain ischemia, which showed neuroprotective effects of pioglitazone in transient focal ischemia (Tureyen et al. 2007; Victor et al. 2006a) and brain trauma (Sauerbeck et al. 2011b). However, PPARγ activation and expression increased following brain damage and when the inflammatory response is developing. Mainly, pioglitazone was showing protection of mitochondrial function, reducing inflammation and cortical lesion, and improving cognitive function following TBI (Sauerbeck et al. 2011b). Furthermore, the natural agonist 15dPGJ2 decreases the neurological deficits after experimental intracerebral hemorrhage. Both pioglitazone and rosiglitazone share similar protective efficacy after cerebral damage, and both were described that decrease the infarct volume and improve functional recovery from stroke in rats (Sundararajan et al. 2005; Sundararajan and Landreth 2004). However, pioglitazone passes through the blood–brain barrier (BBB), while rosiglitazone is known not to penetrate the BBB (Gemma et al. 2004; Maeshiba et al. 1997). Pioglitazone and troglitazone reduced the release of ROS, disrupting the BBB, microglia activation, damaging endothelial cells, and enhancing leukocyte infiltration (Culman et al. 2007; Ji et al. 2009; Lee et al. 2015; Sauerbeck et al. 2011a; Thal and Neuhaus 2014). Moreover, treatment with PPARγ agonists, either rosiglitazone or pioglitazone significantly reduced oxidative stress, COX-2 protein expression, and activation of p38 and p42/44 mitogen-activated protein kinases (MAPKs) and NF-κB, in a rat model of ischemia/reperfusion injury by inhibiting oxidative stress and excessive inflammatory response (Collino et al. 2006).
Fig. 4.
Multiple roles of PPARγ agonist and antagonist effects in the injured brain. PPARγ agonists confer neuroprotection on the injured brain at several operational levels, such as at the anti-inflammatory response, differentiation, or stabilization of vascular processes levels. PPARγ antagonists reverse the PPARγ activation effects such as increasing lesion volume or neuroinflammation in animal models of ischemia or traumatic brain injury
Conclusion
Brain damage is associated with secondary injury, oxidative stress, and inflammation, generating neurodegeneration and neuropathology (Nizamutdinov and Shapiro 2017). These phenomena could be prevented, mitigated, or treated by a combination of therapeutic approaches that involve a neurorestorative process. However, brain injuries have no effective treatment at the present. For this reason, development of effective treatments for brain injuries is a pressing medical necessity. Current therapies, designed to target single pathogenic mechanisms, or on a singular cell type, have not been effective and are likely to fail in clinical trials. PPARγ agonist activity acts as a powerful agent for inducing antioxidant/anti-inflammatory-mediated pathways (Mandrekar-Colucci et al. 2013). This beneficial propriety of PPARγ agonist has the potential for rapid transfer to clinical therapies for brain injuries and peripheral organs damaged. There is a strong rationale to consider novel neuroprotective treatments using pleiotropic drugs that target several neuropathologies. In conclusion, it is essential we continue to search for novel neuroprotective and neurorestorative treatments for brain injuries with high clinical impact; the pleiotropic effects of PPARγ activation are a promising efficacious candidate. Given the findings presented in this review, the field should continue to focus on elucidating novel targets and therapies that directly, or indirectly, can restore the damaged brain.
Acknowledgements
This work was supported by R03NS095038 (SV) from the National Institute for Neurological Disorders and Stroke (NINDS), and by the Department of Neuroscience at Georgetown University Medical Center.
Compliance with Ethical Standards
Conflict of interest
The author declares no conflict of interest for this manuscript.
References
- Abdelrahman M, Sivarajah A, Thiemermann C (2005) Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res 65:772–781. doi:10.1016/j.cardiores.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Akiyama TE, Meinke PT, Berger JP (2005) PPAR ligands: potential therapies for metabolic syndrome. Curr Diabetes Rep 5:45–52 [DOI] [PubMed] [Google Scholar]
- Angelakis E et al (2012) An evaluation of the effects of Lactobacillus ingluviei on body weight, the intestinal microbiome and metabolism in mice. Microb Pathog 52:61–68. doi:10.1016/j.micpath.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Annabi B, Lord-Dufour S, Vezina A, Beliveau R (2012) Resveratrol targeting of carcinogen-induced brain endothelial cell inflammation biomarkers MMP-9 and COX-2 is Sirt1-independent. Drug Target Insights 6:1–11. doi:10.4137/DTI.S9442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Jin G, Navaratna D, Lo EH (2009) Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J 276:4644–4652. doi:10.1111/j.1742-4658.2009.07176.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z et al (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451:1008–1012. doi:10.1038/nature06613 [DOI] [PubMed] [Google Scholar]
- Aronowski J, Zhao X (2011) Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42:1781–1786. doi:10.1161/STROKEAHA.110.596718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros I et al (2014) Rosiglitazone-induced CD36 up-regulation resolves inflammation by PPARgamma and 5-LO-dependent pathways. J Leukoc Biol 95:587–598. doi:10.1189/jlb.0613326 [DOI] [PubMed] [Google Scholar]
- Behl T, Kaur I, Goel H, Kotwani A (2016) Implications of the endogenous PPAR-gamma ligand, 15-deoxy-delta-12, 14-prostaglandin J2, in diabetic retinopathy. Life Sci 153:93–99. doi:10.1016/j.lfs.2016.03.054 [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, Meinke PT (2005) PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci 26:244–251. doi:10.1016/j.tips.2005.03.003 [DOI] [PubMed] [Google Scholar]
- Bernardo A, Minghetti L (2006) PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des 12:93–109 [DOI] [PubMed] [Google Scholar]
- Bernardo A, Minghetti L (2008) Regulation of glial cell functions by PPAR-gamma natural and synthetic agonists. PPAR Res 2008:864140. doi:10.1155/2008/864140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke F, Caglayan E, Hsueh WA (2006a) Peroxisome proliferator-activated receptor gamma agonists: their role as vasoprotective agents in diabetes. Endocrinol Metab Clin N Am 35:561–574. doi:10.1016/j.ecl.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Blaschke F, Takata Y, Caglayan E, Law RE, Hsueh WA (2006b) Obesity, peroxisome proliferator-activated receptor, and atherosclerosis in type 2 diabetes. Arterioscler Thromb Vasc Biol 26:28–40. doi:10.1161/01.ATV.0000191663.12164.77 [DOI] [PubMed] [Google Scholar]
- Bouhlel MA et al (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6:137–143. doi:10.1016/j.cmet.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC (2002) Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem 82:615–624 [DOI] [PubMed] [Google Scholar]
- Chapelsky MC, Thompson-Culkin K, Miller AK, Sack M, Blum R, Freed MI (2003) Pharmacokinetics of rosiglitazone in patients with varying degrees of renal insufficiency. J Clin Pharmacol 43:252–259 [DOI] [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM (2001) PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 7:48–52. doi:10.1038/83336 [DOI] [PubMed] [Google Scholar]
- Chu K et al (2006) Peroxisome proliferator-activated receptor-gamma-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res 1093:208–218. doi:10.1016/j.brainres.2006.03.114 [DOI] [PubMed] [Google Scholar]
- Cimini A, Ceru MP (2008) Emerging roles of peroxisome proliferator-activated receptors (PPARs) in the regulation of neural stem cells proliferation and differentiation. Stem Cell Rev 4:293–303. doi:10.1007/s12015-008-9024-2 [DOI] [PubMed] [Google Scholar]
- Collin M, Murch O, Thiemermann C (2006) Peroxisome proliferator-activated receptor-gamma antagonists GW9662 and T0070907 reduce the protective effects of lipopolysaccharide preconditioning against organ failure caused by endotoxemia. Crit Care Med 34:1131–1138. doi:10.1097/01.CCM.0000206472.63040.6D [DOI] [PubMed] [Google Scholar]
- Collino M et al (2006) Modulation of the oxidative stress and inflammatory response by PPAR-gamma agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur J Pharmacol 530:70–80. doi:10.1016/j.ejphar.2005.11.049 [DOI] [PubMed] [Google Scholar]
- Collino M, Patel NS, Thiemermann C (2008) PPARs as new therapeutic targets for the treatment of cerebral ischemia/reperfusion injury Ther Adv. Cardiovasc Dis 2:179–197. doi:10.1177/1753944708090924 [DOI] [PubMed] [Google Scholar]
- Couvigny B et al (2015) Commensal Streptococcus salivarius modulates PPARgamma transcriptional activity in human intestinal epithelial cells. PLoS ONE 10:e0125371. doi:10.1371/journal.pone.0125371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culman J, Zhao Y, Gohlke P, Herdegen T (2007) PPAR-gamma: therapeutic target for ischemic stroke. Trends Pharmacol Sci 28:244–249. doi:10.1016/j.tips.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Delerive P, Fruchart JC, Staels B (2001) Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 169:453–459 [DOI] [PubMed] [Google Scholar]
- Dunning S et al (2013) Glutathione and antioxidant enzymes serve complementary roles in protecting activated hepatic stellate cells against hydrogen peroxide-induced cell death. Biochim Biophys Acta 1832:2027–2034. doi:10.1016/j.bbadis.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Ehrmann J Jr, Vavrusova N, Collan Y, Kolar Z (2002) Peroxisome proliferator-activated receptors (PPARs) in health and disease. Biomed Pap 146:11–14 [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317. doi:10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Eslami H, Sharifi AM, Rahimi H, Rahati M (2014) Protective effect of telmisartan against oxidative damage induced by high glucose in neuronal PC12 cell. Neurosci Lett 558:31–36. doi:10.1016/j.neulet.2013.10.057 [DOI] [PubMed] [Google Scholar]
- Esposito G et al (2011) Cannabidiol reduces Abeta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS ONE 6:e28668. doi:10.1371/journal.pone.0028668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bueno B, Caso JR, Perez-Nievas BG, Lorenzo P, Leza JC (2007) Effects of peroxisome proliferator-activated receptor gamma agonists on brain glucose and glutamate transporters after stress in rats. Neuropsychopharmacology 32:1251–1260. doi:10.1038/sj.npp.1301252 [DOI] [PubMed] [Google Scholar]
- Gemma C, Stellwagen H, Fister M, Coultrap SJ, Mesches MH, Browning MD, Bickford PC (2004) Rosiglitazone improves contextual fear conditioning in aged rats. NeuroReport 15:2255–2259 [DOI] [PubMed] [Google Scholar]
- Giannini S, Serio M, Galli A (2004) Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J Endocrinol Invest 27:982–991. doi:10.1007/BF03347546 [DOI] [PubMed] [Google Scholar]
- Griggs RB, Yermakov LM, Susuki K (2017) Formation and disruption of functional domains in myelinated CNS axons. Neurosci Res 116:77–87. doi:10.1016/j.neures.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Gurley C, Nichols J, Liu S, Phulwani NK, Esen N, Kielian T (2008) Microglia and astrocyte activation by toll-like receptor ligands: modulation by PPAR-gamma agonists. PPAR Res 2008:453120. doi:10.1155/2008/453120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L et al (2015) Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke 46:2628–2636. doi:10.1161/STROKEAHA.115.010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald M, Cawthorne MA (2011) Dual acting and pan-PPAR activators as potential anti-diabetic therapies. Handb Exp Pharmacol. doi:10.1007/978-3-642-17214-4_2 [DOI] [PubMed] [Google Scholar]
- Hegarty BD, Furler SM, Oakes ND, Kraegen EW, Cooney GJ (2004) Peroxisome proliferator-activated receptor (PPAR) activation induces tissue-specific effects on fatty acid uptake and metabolism in vivo—a study using the novel PPARalpha/gamma agonist tesaglitazar. Endocrinology 145:3158–3164. doi:10.1210/en.2004-0260 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Landreth GE (2007) PPARs in the brain. Biochim Biophys Acta 1771:1031–1045. doi:10.1016/j.bbalip.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL (2000) Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J Neurosci 20:6862–6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruretagoyena MI et al (2006) Inhibition of nuclear factor-kappa B enhances the capacity of immature dendritic cells to induce antigen-specific tolerance in experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther 318:59–67. doi:10.1124/jpet.106.103259 [DOI] [PubMed] [Google Scholar]
- Ji S, Kronenberg G, Balkaya M, Farber K, Gertz K, Kettenmann H, Endres M (2009) Acute neuroprotection by pioglitazone after mild brain ischemia without effect on long-term outcome. Exp Neurol 216:321–328. doi:10.1016/j.expneurol.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Jung KH et al (2007) Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J Pharmacol Exp Ther 322:1051–1058. doi:10.1124/jpet.107.120097 [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R (2008) Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci 13:1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrout Y et al (2015) In vivo efficacy of microbiota-sensitive coatings for colon targeting: a promising tool for IBD therapy. J Control Release 197:121–130. doi:10.1016/j.jconrel.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Kasahara Y et al (2010) Telmisartan suppresses cerebral injury in a murine model of transient focal ischemia. Brain Res 1340:70–80. doi:10.1016/j.brainres.2010.03.101 [DOI] [PubMed] [Google Scholar]
- Kaundal RK, Sharma SS (2011a) Ameliorative effects of GW1929, a nonthiazolidinedione PPARgamma agonist, on inflammation and apoptosis in focal cerebral ischemic-reperfusion injury. Curr Neurovasc Res 8:236–245 [DOI] [PubMed] [Google Scholar]
- Kaundal RK, Sharma SS (2011b) GW1929: a nonthiazolidinedione PPARgamma agonist, ameliorates neurological damage in global cerebral ischemic-reperfusion injury through reduction in inflammation and DNA fragmentation. Behav Brain Res 216:606–612. doi:10.1016/j.bbr.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Kimura H et al (2008) A natural PPAR-gamma agonist, 15-deoxy-delta 12,14-prostaglandin J2, may act as an enhancer of PAI-1 in human proximal renal tubular cells under hypoxic and inflammatory conditions. Nephrol Dial Transplant 23:2496–2503. doi:10.1093/ndt/gfn139 [DOI] [PubMed] [Google Scholar]
- Lee G et al (2002) T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J Biol Chem 277:19649–19657. doi:10.1074/jbc.M200743200 [DOI] [PubMed] [Google Scholar]
- Lee CH, Yi MH, Chae DJ, Zhang E, Oh SH, Kim DW (2015) Effect of pioglitazone on excitotoxic neuronal damage in the mouse hippocampus. Biomol Ther 23:261–267. doi:10.4062/biomolther.2014.146(Seoul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KC, Hsieh YC, Yang YY, Chan CC, Huang YH, Lin HC (2016) Aliskiren reduces hepatic steatosis and epididymal fat mass and increases skeletal muscle insulin sensitivity in high-fat diet-fed mice. Sci Rep 6:18899. doi:10.1038/srep18899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglet S, Montecucco F, Mach F (2013) Role of matrix metalloproteinases in animal models of ischemic stroke. Curr Vasc Pharmacol 13(2):161–166 [DOI] [PubMed] [Google Scholar]
- Li X, Du J, Xu S, Lin X, Ling Z (2013) Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reduces secondary damage in experimental spinal cord injury. J Int Med Res 41:153–161. doi:10.1177/0300060513476601 [DOI] [PubMed] [Google Scholar]
- Liu X, Yu H, Yang L, Li C, Li L (2012) 15-Deoxy-Delta(12,14)-prostaglandin J(2) attenuates the biological activities of monocyte/macrophage cell lines. Eur J Cell Biol 91:654–661. doi:10.1016/j.ejcb.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV (2015) Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat 11:97–106. doi:10.2147/NDT.S65815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y et al (2006) Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem 97:435–448. doi:10.1111/j.1471-4159.2006.03758.x [DOI] [PubMed] [Google Scholar]
- Luo Y, He Q, Kuang G, Jiang Q, Yang J (2014) PPAR-alpha and PPAR-beta expression changes in the hippocampus of rats undergoing global cerebral ischemia/reperfusion due to PPAR-gamma status. Behav Brain Funct 10:21. doi:10.1186/1744-9081-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Xu Q, Wang Q, Wu H, Hua J (2017) Effect of modulation of PPAR-gamma activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep 7:44612. doi:10.1038/srep44612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S (1997) Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung 47:29–35 [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Sauerbeck A, Popovich PG, McTigue DM (2013) PPAR agonists as therapeutics for CNS trauma and neurological diseases. ASN Neuro 5:e00129. doi:10.1042/AN20130030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglio G, Rattazzi L, Rosa AC, Fantozzi R (2009) PPARgamma stimulation promotes neurite outgrowth in SH-SY5Y human neuroblastoma cells. Neurosci Lett 454:134–138. doi:10.1016/j.neulet.2009.03.014 [DOI] [PubMed] [Google Scholar]
- Moore KJ et al (2001) The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nat Med 7:41–47. doi:10.1038/83328 [DOI] [PubMed] [Google Scholar]
- Morales-Garcia JA, Luna-Medina R, Alfaro-Cervello C, Cortes-Canteli M, Santos A, Garcia-Verdugo JM, Perez-Castillo A (2011) Peroxisome proliferator-activated receptor gamma ligands regulate neural stem cell proliferation and differentiation in vitro and in vivo. Glia 59:293–307. doi:10.1002/glia.21101 [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP (2004) Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123:131–145 [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T (2007) Modulation of immune response by head injury. Injury 38:1392–1400. doi:10.1016/j.injury.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Mouihate A, Boisse L, Pittman QJ (2004) A novel antipyretic action of 15-deoxy-Delta 12,14-prostaglandin J2 in the rat brain. J Neurosci 24:1312–1318. doi:10.1523/JNEUROSCI.3145-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napimoga MH, Demasi AP, Bossonaro JP, de Araujo VC, Clemente-Napimoga JT, Martinez EF (2013) Low doses of 15d-PGJ2 induce osteoblast activity in a PPAR-gamma independent manner. Int Immunopharmacol 16:131–138. doi:10.1016/j.intimp.2013.03.035 [DOI] [PubMed] [Google Scholar]
- Ndubuizu OI, Tsipis CP, Li A, LaManna JC (2010) Hypoxia-inducible factor-1 (HIF-1)-independent microvascular angiogenesis in the aged rat brain. Brain Res 1366:101–109. doi:10.1016/j.brainres.2010.09.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizamutdinov D, Shapiro LA (2017) Overview of traumatic brain injury: an immunological context. Brain Sci. doi:10.3390/brainsci7010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI et al (2007) Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447:1116–1120. doi:10.1038/nature05894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod BK, Hanft SJ, Asokan A, Haditsch U, Lee SW, Palmer TD (2013) PPARgamma activation prevents impairments in spatial memory and neurogenesis following transient illness. Brain Behav Immun 29:28–38. doi:10.1016/j.bbi.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang T, Benicky J, Wang J, Orecna M, Sanchez-Lemus E, Saavedra JM (2012a) Telmisartan ameliorates lipopolysaccharide-induced innate immune response through peroxisome proliferator-activated receptor-gamma activation in human monocytes. J Hypertens 30:87–96. doi:10.1097/HJH.0b013e32834dde5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang T, Wang J, Benicky J, Sanchez-Lemus E, Saavedra JM (2012b) Telmisartan directly ameliorates the neuronal inflammatory response to IL-1beta partly through the JNK/c-Jun and NADPH oxidase pathways. J Neuroinflammation 9:102. doi:10.1186/1742-2094-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EY, Cho IJ, Kim SG (2004) Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-gamma and retinoid X receptor heterodimer. Cancer Res 64:3701–3713. doi:10.1158/0008-5472.CAN-03-3924 [DOI] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R (2007) Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther 320:1002–1012. doi:10.1124/jpet.106.113472 [DOI] [PubMed] [Google Scholar]
- Pereira MP et al (2005) The nonthiazolidinedione PPARgamma agonist L-796,449 is neuroprotective in experimental stroke. J Neuropathol Exp Neurol 64:797–805 [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L et al (2010) Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc Natl Acad Sci USA 107:8772–8777. doi:10.1073/pnas.0905745107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipovic K, Zupan Z, Dolenec P, Mrsic-Pelcic J, Zupan G (2015) A single dose of PPARgamma agonist pioglitazone reduces cortical oxidative damage and microglial reaction following lateral fluid percussion brain injury in rats. Prog Neuropsychopharmacol Biol Psychiatry 59:8–20. doi:10.1016/j.pnpbp.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Prakash A, Kumar A (2014) Role of nuclear receptor on regulation of BDNF and neuroinflammation in hippocampus of beta-amyloid animal model of Alzheimer’s disease. Neurotox Res 25:335–347. doi:10.1007/s12640-013-9437-9 [DOI] [PubMed] [Google Scholar]
- Qi L, Jacob A, Wang P, Wu R (2010) Peroxisome proliferator activated receptor-gamma and traumatic brain injury. Int J Clin Exp Med 3:283–292 [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391:79–82. doi:10.1038/34178 [DOI] [PubMed] [Google Scholar]
- Roth AD, Leisewitz AV, Jung JE, Cassina P, Barbeito L, Inestrosa NC, Bronfman M (2003) PPAR gamma activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J Neurosci Res 72:425–435. doi:10.1002/jnr.10596 [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG (2011) Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol 227:128–135. doi:10.1016/j.expneurol.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmerbach K et al (2008) Comparison between single and combined treatment with candesartan and pioglitazone following transient focal ischemia in rat brain. Brain Res 1208:225–233. doi:10.1016/j.brainres.2008.02.032 [DOI] [PubMed] [Google Scholar]
- Serra D, Almeida LM, Dinis TC (2016) Anti-inflammatory protection afforded by cyanidin-3-glucoside and resveratrol in human intestinal cells via Nrf2 and PPAR-gamma: comparison with 5-aminosalicylic acid. Chem Biol Interact 260:102–109. doi:10.1016/j.cbi.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Shimazu T et al (2005) A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke 36:353–359. doi:10.1161/01.STR.0000152271.21943.a2 [DOI] [PubMed] [Google Scholar]
- Sivarajah A, McDonald MC, Thiemermann C (2005) The cardioprotective effects of preconditioning with endotoxin, but not ischemia, are abolished by a peroxisome proliferator-activated receptor-gamma antagonist. J Pharmacol Exp Ther 313:896–901. doi:10.1124/jpet.104.080598 [DOI] [PubMed] [Google Scholar]
- Sobrado M et al (2009) Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci 29:3875–3884. doi:10.1523/JNEUROSCI.5529-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W, Jun DW, Lee KN, Lee HL, Lee OY, Choi HS, Yoon BC (2015) Lactobacillus paracasei induces M2-dominant Kupffer Cell Polarization in a mouse model of nonalcoholic steatohepatitis. Dig Dis Sci 60:3340–3350. doi:10.1007/s10620-015-3770-1 [DOI] [PubMed] [Google Scholar]
- Sood V, Colleran K, Burge MR (2000) Thiazolidinediones: a comparative review of approved uses. Diabetes Technol Ther 2:429–440 [DOI] [PubMed] [Google Scholar]
- Speca S et al (2016) Novel PPARgamma Modulator GED-0507-34 Levo Ameliorates Inflammation-driven Intestinal Fibrosis. Inflamm Bowel Dis 22:279–292. doi:10.1097/MIB.0000000000000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan S, Landreth GE (2004) Antiinflammatory properties of PPARgamma agonists following ischemia. Drug News Perspect 17:229–236 [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE (2005) Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience 130:685–696. doi:10.1016/j.neuroscience.2004.10.021 [DOI] [PubMed] [Google Scholar]
- Tan L, Yu JT, Guan HS (2008) Resveratrol exerts pharmacological preconditioning by activating PGC-1alpha. Med Hypotheses 71:664–667. doi:10.1016/j.mehy.2008.06.031 [DOI] [PubMed] [Google Scholar]
- Thal SC, Neuhaus W (2014) The blood-brain barrier as a target in traumatic brain injury treatment. Arch Med Res 45:698–710. doi:10.1016/j.arcmed.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Thal SC, Heinemann M, Luh C, Pieter D, Werner C, Engelhard K (2011) Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR-gamma-independent mechanisms. J Neurotrauma 28:983–993. doi:10.1089/neu.2010.1685 [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77:289–312. doi:10.1146/annurev.biochem.77.061307.091829 [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R (2007) Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem 101:41–56. doi:10.1111/j.1471-4159.2006.04376.x [DOI] [PubMed] [Google Scholar]
- Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S (2011) The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res 2:236–240. doi:10.4103/2231-4040.90879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu S, Harada J, Hisamoto M, Oda T (2002) Troglitazone inhibits both post-glutamate neurotoxicity and low-potassium-induced apoptosis in cerebellar granule neurons. Brain Res 924:229–236 [DOI] [PubMed] [Google Scholar]
- Victor NA et al (2006) Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci 24:1653–1663. doi:10.1111/j.1460-9568.2006.05037.x [DOI] [PubMed] [Google Scholar]
- Villapol S, Saavedra JM (2015) Neuroprotective effects of Angiotensin receptor blockers. Am J Hypertens 28:289–299. doi:10.1093/ajh/hpu197 [DOI] [PubMed] [Google Scholar]
- Villapol S, Yaszemski AK, Logan TT, Sanchez-Lemus E, Saavedra JM, Symes AJ (2012) Candesartan, an angiotensin II AT(1)-receptor blocker and PPAR-gamma agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology 37:2817–2829. doi:10.1038/npp.2012.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Balarezo MG, Affram K, Saavedra JM, Symes AJ (2015) Neurorestoration after traumatic brain injury through angiotensin II receptor blockage. Brain 138:3299–3315. doi:10.1093/brain/awv172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K et al (2006) Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem 281:12673–12681. doi:10.1074/jbc.M513786200 [DOI] [PubMed] [Google Scholar]
- Wan Ibrahim WN, Tofighi R, Onishchenko N, Rebellato P, Bose R, Uhlen P, Ceccatelli S (2013) Perfluorooctane sulfonate induces neuronal and oligodendrocytic differentiation in neural stem cells and alters the expression of PPARgamma in vitro and in vivo. Toxicol Appl Pharmacol 269:51–60. doi:10.1016/j.taap.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Wang H et al (2009) Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes 58:116–124. doi:10.2337/db07-1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y et al (2016) Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure. Eur J Nutr 55:821–831. doi:10.1007/s00394-015-0904-3 [DOI] [PubMed] [Google Scholar]
- White AT, Murphy AN (2010) Administration of thiazolidinediones for neuroprotection in ischemic stroke: a pre-clinical systematic review. J Neurochem 115:845–853. doi:10.1111/j.1471-4159.2010.06999.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC (2013) The role of markers of inflammation in traumatic brain injury. Front Neurol 4:18. doi:10.3389/fneur.2013.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M (2009) Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs 14:67–84. doi:10.1517/14728210902769601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K, Hosoi M, Koyama H, Tanaka S, Fukumoto S, Morii H, Nishizawa Y (2000) Peroxisome proliferator-activated receptor-gamma agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem Biophys Res Commun 271:571–574. doi:10.1006/bbrc.2000.2665 [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R (2008) PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res 1244:164–172. doi:10.1016/j.brainres.2008.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonutas HM, Sullivan PG (2013) Targeting PPAR isoforms following CNS injury. Curr Drug Targets 14:733–742 [DOI] [PubMed] [Google Scholar]
- Yuan G, Chen X, Li D (2015) Modulation of peroxisome proliferator-activated receptor gamma (PPAR gamma) by conjugated fatty acid in obesity and inflammatory bowel disease. J Agric Food Chem 63:1883–1895. doi:10.1021/jf505050c [DOI] [PubMed] [Google Scholar]
- Zarzuelo MJ et al (2013) SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 85:1288–1296. doi:10.1016/j.bcp.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Xie K, Dong H, Zhang H, Wang F, Li Y, Xiong L (2012) Hyperbaric oxygen preconditioning protects cortical neurons against oxygen-glucose deprivation injury: role of peroxisome proliferator-activated receptor-gamma. Brain Res 1452:140–150. doi:10.1016/j.brainres.2012.02.063 [DOI] [PubMed] [Google Scholar]
- Zeng XC, Li XS, Wen H (2013) Telmisartan protects against microvascular dysfunction during myocardial ischemia/reperfusion injury by activation of peroxisome proliferator-activated receptor gamma. BMC Cardiovasc Disord 13:39. doi:10.1186/1471-2261-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Hu W, Meng B, Tang T (2010) PPARgamma agonist rosiglitazone is neuroprotective after traumatic spinal cord injury via anti-inflammatory in adult rats. Neurol Res 32:852–859. doi:10.1179/016164110X12556180206112 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J (2005) The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci 22:278–282. doi:10.1111/j.1460-9568.2005.04200.x [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J (2006) Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J 20:1162–1175. doi:10.1096/fj.05-5007com [DOI] [PubMed] [Google Scholar]
- Zhao W, Payne V, Tommasi E, Diz DI, Hsu FC, Robbins ME (2007) Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys 67:6–9. doi:10.1016/j.ijrobp.2006.09.036 [DOI] [PubMed] [Google Scholar]