Abstract
Osteocalcin (OC) is an abundant extracellular calcium-binding protein synthesized by osteoblasts. Although most OC is bound to hydroxyapatite mineral during bone formation, a consistent amount is released directly to circulation. Plasma OC (pOC) levels are highly sensitive to stressful stimuli that alter stress-responsive hormones, such as glucocorticoids (cortisol or corticosterone) and the catecholamines norepinephrine and epinephrine. To gain a better understanding of the apparent relationship of OC to the effects of ethanol (EtOH) and the stress responses, we compared mice that have OC (WT [OC+/+] and HET [OC+/−]) with OC null mutants (KO [OC−/−]), which have no OC in either plasma or in bone. One experiment included chronic unpredictable stress, a second was conducted in the absence of any known stressors other than EtOH, while a third imposed a more severe acute immobilization stress in addition to EtOH consumption. The data obtained confirmed significant differences in EtOH consumption in mice that previously experienced various stressful stimuli. We also determined that adrenal tyrosine-hydroxylase expression was inversely proportional to EtOH consumption and tended to be lower in KO than in WT. Data suggest that OC possesses the ability to modulate the adrenal gene expression of the catecholamine synthetic pathway. This modulation may be responsible for differences in EtOH consumption under stress.
Keywords: Two-bottle choice, Catecholamine, Gene expression, Adrenal, Sensitization
Introduction
Investigations of the relationship between stress and ethanol (EtOH) consumption have resulted in conflicting data, although the common belief is that stress elevates EtOH consumption (Pohorecky 1981; 1990; 1991; Anthenelli 2012; Keyes et al. 2012; Lopez et al. 2016). Several investigators found that stressors affected EtOH consumption differently, depending on the type of stressor (Roske et al. 1994; Lopez et al. 2016). A possible explanation for this variability may be found in the heterogeneity of hormonal responses to various stressful stimuli (Pacak et al. 1998; Kvetnansky 2004). Among the known effects of such stimuli are hypothalamic-pituitary-adrenocortical activation, which increases adrenal corticosteroid secretion, and sympathetic neural system (SNS) activation, which increases adrenomedullary epinephrine (Epi) secretion, and sympathetic ganglionic release of norepinephrine (NE) (Pacak et al. 1998). Both circulating corticosteroids and catecholamines may be reduced by small doses of ethanol prior to stressor (Pohorecky et al. 1980; Vogel et al. 1986). Studies of human subjects also show stress-dampening effects of ethanol consumption prior to stress, although there are significant variations depending on sex and family history of alcohol abuse (Sinha et al. 1998; Dai et al. 2002).
We previously reported that the rat adrenomedullary gene expression for enzymes of the catecholamine synthetic pathway (tyrosine-hydroxylase, TH; dopamine beta-hydroxylase, DBH; and phenylethanolamine-N-methyl transferase, PNMT) was increased by 7 weeks consumption of 6% w/v EtOH in drinking water, but significantly less so in EtOH-consuming rats subjected to 2-h daily restraint in wire mesh cylinders compared to non-drinking controls (Patterson-Buckendahl et al. 2004). In a second study we found that rats fed a liquid diet containing 5% w/v EtOH as the sole source of food and fluid for 8 days demonstrated a potentiated plasma catecholamine response to acute foot restraint immobilization (Immo) compared to rats given no EtOH (Patterson-Buckendahl et al. 2005). The elevated level of adrenomedullary gene expression suggested that EtOH itself is a stressful stimulus. These findings illustrate the conflicting data associated with investigations of the commonly accepted tension reduction hypothesis for EtOH consumption.
A protein that is also markedly responsive to stressful stimuli is osteocalcin (OC), an abundant extracellular calcium-binding protein synthesized by bone-forming osteoblasts. Although most is bound to hydroxyapatite bone mineral during formation, a relatively consistent amount (about 10%) is released directly to circulation (plasma OC, pOC) (Hauschka et al. 1989, Li et al. 2016). Osteocalcin synthesis is regulated by many hormones, including thyroid hormone (T3), parathyroid hormone (PTH), vitamin D, and glucocorticoids. Among these hormones, PTH and 1,25(OH)2D3 (the active form of vitamin D) are also the major endocrine regulators of plasma calcium homeostasis (Beresford et al. 1984). pOC levels are also very sensitive to altered stress-responsive hormones, such as the glucocorticoid corticosterone (CS), and the catecholamines NE and Epi (Patterson-Buckendahl et al. 1995; Patterson-Buckendahl et al. 2001; Patterson-Buckendahl et al. 2007).
Excessive ethanol consumption as well as chronic severe stress can have negative effects on bone. These effects are often monitored by quantification of plasma levels of pOC. In human studies, chronic high EtOH consumption has been linked to osteoporosis and is associated with decreased pOC, especially in males (Marrone et al. 2012; Mikosch 2014; Gaddini et al. 2016). Animal studies indicate that the reduction in bone mass and mineral density due to EtOH consumption reflects reduced osteoblast proliferation and a related decrease in pOC (Klein et al. 1996; Klein 1997; Maurel et al. 2012). For example, Peng et al. showed that a liquid diet containing 8% EtOH given to rats lowered pOC (Peng et al. 1991). Wezeman et al. on the other hand found that in growing bone, mRNA levels for OC increased in response to chronic EtOH consumption, while having no effect on the circulating levels of OC. They speculated that either the OC secretory pathway is inhibited by EtOH or that OC was degraded intracellularly due to the direct effect of EtOH on osteoblasts (Wezeman et al. 1999). We determined that moderate EtOH (6%) consumption with or without the imposition of 6 weeks daily restraint in wire mesh cages had little effect on young adult rat femur morphology or strength or on pOC, but that calorie-restricted rats pairfed to the EtOH-consuming group were negatively affected by restraint (Patterson-Buckendahl et al. 2008).
Recent publications suggest that OC has hormonal functions and that bone has an endocrine function, secreting OC to regulate energy metabolism by stimulating pancreatic synthesis of insulin and fat cell synthesis of adiponectin (Lee et al. 2007; Ferron et al. 2008; Li et al. 2016). Given the evidence that OC has extraskeletal hormonal influence, and studies demonstrating that EtOH consumption lowers pOC in rats, we speculated that OC might somehow be associated with regulation of EtOH consumption. To test this hypothesis, we conducted three studies of voluntary EtOH consumption by wild-type (WT [OC+/+]) and heterozygous (Het [OC+/−]) mice that have pOC and compared them with knockout (KO [OC−/−]) mice that have no OC in either bone or plasma. In the first experiment, mice experienced unplanned, unpredictable stress concurrent with the quantification of EtOH preference and consumption. The second experiment was conducted under stable environmental conditions to determine whether results of the first were related solely to ethanol consumption or were influenced by stressors. The third imposed several planned stressors, including the acute, more severe stress of foot restraint immobilization in combination with EtOH consumption.
Methods and Materials
Subjects
All protocols were reviewed and approved by the Rutgers Institutional Animal Care and Use Committee and are consistent with guidelines specified by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Osteocalcin-null mutant mice (OC−/−), back-crossed to C57Bl/6J for more than 15 generations, were a generous gift from Drs. Gerard Karsenty and Patricia Ducy, Baylor University Medical Center (Ducy et al. 1996). The null mutation deleted the two genes (of three homologous genes described by Desbois et al. (Desbois et al. 1994) that are expressed almost exclusively in bone and are responsible for synthesis of OC deposited in bone and released to circulation. C57Bl/6 OC−/− males were mated with C57Bl/6J wild-type females to obtain heterozygous breeding stock. Because OC−/− are not available commercially, all experiments used male progeny from these matings. Animal genotypes were determined by PCR analysis of DNA from tail tissue. Animals were housed in a vivarium on 12/12 light/dark cycle, lights on at 6 AM. The temperature was maintained at 22 ± 2 °C, except as noted.
Experiment 1: Voluntary EtOH Consumption Plus Unpredictable Stress
This experiment was the final step in a series of comparisons of phenotypic differences among WT, Het, and KO mice. Up to the point when this experiment began, the only published phenotypic difference between KO and WT mice was increased bone mass and strength in fully adult mice at least 5 months of age. We chose to begin our testing at that age. Mice were evaluated for behavior in the light/dark box, open field, and on a 4 °C cold plate. These data were reported previously (Patterson-Buckendahl et al. 2012). Following these tests, we evaluated taste preferences for saccharin, capsaicin, and menthol, for which there were no significant genotypic differences (data not shown). We proceeded to determination of the preference for increasing concentrations of ethanol. At age 9 months, mice (11KO, 10HET and 13 WT) were housed two per cage, separated by a wire mesh divider.
All mice received rodent chow ad libitum (LabDiet #5010) and had continuous access to two drinking tubes. One tube contained tap water and the other a solution of EtOH in water, which was increased by 1% ethanol (w/v) every 3 to 4 days from 2 to 15% w/v. The position of the drinking tubes was switched every 2 days to prevent development of place preferences. Tubes were weighed initially and again every 2–3 days to determine consumption. Mice were weighed weekly to monitor health and to enable the calculation of g/kg consumption. During the course of this experiment, there were unexpected fluctuations in temperature, noise, and unfamiliar individuals coming through the animal colony due to renovation of the nearby laboratory space. Once the ethanol offered had reached 15%, and exterior noise levels increased, animals were maintained on 9% w/v ethanol for an additional 6 weeks before final termination of the study. At that time, mice were anesthetized with CO2 and exsanguinated by cardiac puncture. Serum was separated from cells and maintained at −20 °C until analyzed for blood alcohol concentration (BAC), CS and pOC levels. Adrenals were removed and placed into RNAlater, refrigerated for at least 24 h and subsequently stored frozen at −20 °C until analyzed for mRNA.
Experiment 2: Voluntary EtOH Consumption Under Stable Conditions
Seven-month old mice (11 WT, 11 Het, and 12 KO) were housed, fed, and fluid provided as in Experiment 1. In addition, 10 KO mice served as water-drinking controls. Concentration of EtOH was increased from 2.5 to 16% w/v. Each concentration was offered for a period of 2 days except for 11%, which was given for a period of 4 days (holiday weekend). Unfortunately, there were insufficient WT males to provide water-drinking controls. At the end of the experimental period, animals were killed by decapitation to minimize handling and possible effects of CO2, which we have determined will increase plasma CS. Adrenals were removed and placed into RNAlater, refrigerated for at least 24 h and subsequently stored frozen at −20 °C until analyzed for mRNA.
Experiment 3: Voluntary EtOH Consumption Plus Foot Restraint Immobilization Stress
11 KO and 10 WT mice were initially observed in a series of 6 open field behavior trials, during 4 of which they received intraperitoneal injections of isotonic saline or 1.5 g/kg EtOH. Although the data from that series were lost due to computer software difficulties, the description of treatment is retained because it may have affected later results. Subsequently, the mice were maintained undisturbed for 7 weeks prior to being assigned to housing, two per cage separated by a Plexiglas® divider, for evaluation of voluntary EtOH consumption, beginning at age 7 months. All mice received rodent chow (LabDiet #5010) and had access to two drinking tubes ad libitum as described for Experiment 2. Because we had established thresholds for preference and consumption in the previous experiments, we more rapidly increased EtOH concentrations of 3, 5, 7, 9, 12, and 15% w/v, every 24 h except for 9 and 15%, which were given for a 72-h period. All tubes were weighed daily to quantify consumption. After 3 days at 15% EtOH, five mice of each genotype were subjected to acute foot restraint immobilization (Immo) for a period of 2 h after which they were returned to their home cages and provided water and 15% EtOH solution for an additional 10 days. The remaining mice of each genotype served as non-Immo controls and continued to receive 15% EtOH. Two hours after lights off on day 6 following Immo, approximately 40 µl of blood was drawn from tail cuts into heparinized hematocrit tubes for the determination of BAC. Three days later, the same 5 mice of each genotype that were previously subjected to Immo were again Immo for 2 h before being killed. All mice were killed by decapitation and adrenals were removed and placed into RNAlater, refrigerated for at least 24 h, then stored frozen at −20 °C until analyzed for mRNA.
Foot Restraint Immobilization Stress
Mice were placed in the prone position on an H-shaped Plexiglas® restrainer 52 mm wide. The center section, 67 mm long, supported the torso. Front and hind limbs were secured with adhesive athletic tape to extensions of the center section. Forelimb extensions were 30 mm long; hind limb extensions were 15 mm long. Mice were Immo for 2 h after which they were quickly released and returned to their home cage for continued evaluation of EtOH drinking. After the second Immo at the end of the experiment, Immo were killed immediately on release; controls were killed during the time Immo mice were restrained.
Biochemical Measurements
Adrenals were removed from RNAlater, blotted to remove remaining liquid, and placed into TRI Reagent for immediate homogenization to isolate total RNA according to well-characterized procedures routinely used in the Kvetnansky lab as described in detail by Kubovcakova et al. (2002). Relative quantification of mRNA of the gene of interest was expressed as a ratio of specific gene mRNA to GAPDH mRNA. Repeated RT-PCR quantification of a standard tissue preparation indicated that intra-assay variation of this method is less than 5%.
Blood alcohol concentration was determined spectrophotometrically with NAD-ADH and buffer reagents obtained from Diagnostic Chemicals Ltd, Oxford, CT (kit # 229-29). This assay was adapted for use in a 96-well microplate, and was read at 340 nm on a BioTek Microplate reader. Intra- and inter-assay variations are less than 5%.
Serum CS concentration was determined by radioimmunoassay (#07-120103) from MPBiomedical Inc, Costa Mesa, CA. Intra- and inter-assay variations are less than 5%.
We thank Dr. Caren Gundberg, Yale University, New Haven, CT, for analysis of pOC levels in mice from Experiment 1 (Gundberg et al. 1992).
Data Analysis
Data were analyzed for statistical significance by analysis of variance (ANOVA) or ANOVA with repeated measures, with StatView software for Macintosh. Data sets yielding values for p < 0.05 were considered significantly different. Where appropriate, we used the Tukey test for post hoc analysis.
Results
Experiment 1: Voluntary EtOH Consumption, Unpredictable Stressful Conditions
The EtOH consumption portion of this experiment was preceded by a series of comparisons of behavioral characteristics among WT, Het, and KO mice. When this experiment began, the only published phenotypic difference between KO and WT mice was evident as increased bone mass and strength in fully adult mice at least 5 months of age. We chose to begin our testing at that age. Mice were evaluated for behaviors in the light/dark box, open field, and on a 4 °C cold plate, followed by evaluation of taste preferences for saccharin, capsaicin, and menthol and ultimately preference for increasing concentrations of ethanol. During the course of the experiment, a number of unavoidable and stressful environmental conditions were encountered by the animals. These included commotion from an adjacent laboratory clean-out prior to renovation, in which all personnel handling the animals were also involved, outside contractors inspecting the vivarium, wide temperature fluctuations and air flow problems. Temperature profiles and a rough estimate of when taste preferences were conducted are shown in Fig. 1. In the month preceding termination, noise from electrical saws, sledge hammers, and jack hammers occurred at unpredictable times.
Fig. 1.
Daily morning and afternoon colony temperatures are shown for 92 days of preference testing. Double line indicates when chemical taste preferences were conducted; bold solid line indicates EtOH consumption and preference portion of the experiment. Thin solid line indicates the period when especially noisy demolition occurred, and mice were maintained at 9% EtOH until termination day indicated by arrow. There are no temperature data available for the final 6 weeks
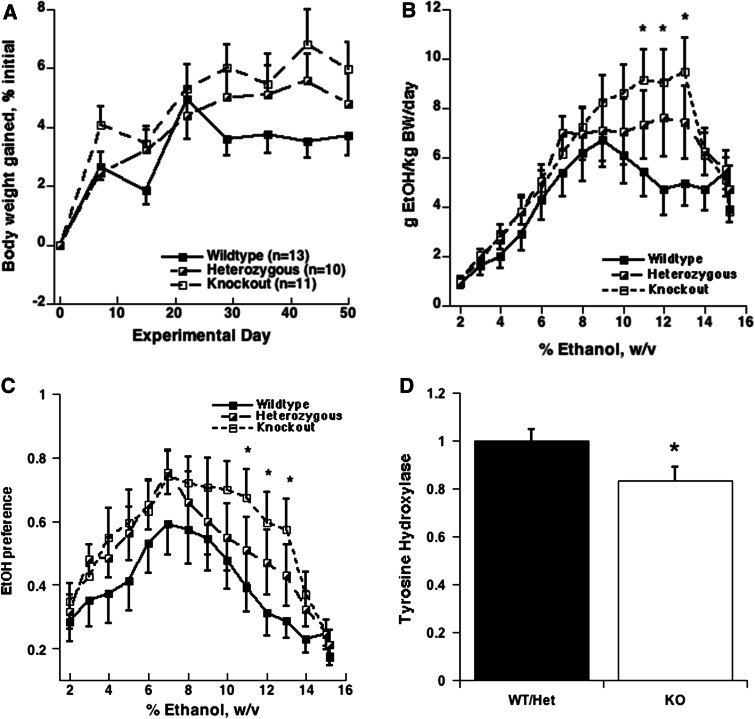
The concentration of alcohol given to the mice in Experiment 1 was increased by 1% every 3–4 days to 15% v/w. All three genotypes gained weight over the course of the study (Fig. 2a). Two factor repeated measures ANOVA of % weight gained from the initiation of the drinking showed no significant differences among the three genotypes (F [2, 217] = 2.202, p = 0.128).
Fig. 2.
a Weight gained by mice expressed as % of initial weight, mean ± SEM; b Mean EtOH consumption, g/kg BW/day, by mice provided with a continuous choice of water or an increasing concentration of EtOH; c EtOH preference (% EtOH/total fluid consumed) of KO, WT, and Het mice offered continuous two-bottle choice of water or increasing concentrations of EtOH; d Adrenomedullary gene expression for tyrosine-hydroxylase in mice given choice of water or varying concentrations of EtOH (mean ± SEM) for 3.5 months, mean ± SEM. Data are expressed relative to the presence (WT/Het) or absence (KO) of OC gene. Significant differences from WT are indicated by *p < 0.05
Total amount of fluid consumed by the mice ranged from about 4.5 to 5.2 ml per day as concentrations and body weight increased. Repeated measures ANOVA for main effect of genotype on consumption (excluding Hets) demonstrated marginal differences in consumption for all concentrations (F [1, 286] = 3.84, p = 0.0628). As can be seen in Fig. 2b, the consumption of EtOH for WT and KO diverged. There were significant differences in consumption from 9% EtOH up to 13%EtOH (F [1, 88] = 5.300, p = 0.0312). Significant differences between WT and KO in EtOH consumption were observed at 11% (F [1, 22] = 5.083, p = 0.0345), at 12% (F [1, 22] = 6.071, p = 0.022), and at 13% (F [1, 22] = 6.809, p = 0.016). This portion of the experiment was terminated when heavy equipment was brought into the neighboring laboratory space for demolition, and consumption of 14 and 15% EtOH was markedly reduced in all mice. KO mice reached a maximum consumption of 9.48 g/kg BW/day at 13% EtOH, while the WT mice reached a maximum consumption of 6.73 g/kg BW/day at 9% EtOH.
EtOH preference (the ratio of amount of EtOH consumed to total fluid consumption) also indicated significant genotypic differences. Repeated measures ANOVA for the effect of genotype on EtOH preference (excluding Hets) showed significant differences in preference (9% EtOH to 13% EtOH only) (F [1, 88] = 5.476, p = 0.0288) (Fig. 2c).
As disruption of laboratory activities began, the mice were placed on a maintenance level of 9% EtOH until sacrifice. During the first two weeks of 9% EtOH, noise and vibrations were the loudest, and consumption in each group fluctuated from a low of 3.5 to a high of 8.5 g/kg BW/day (data not shown) until eventually stabilizing at levels previously observed for the 9% concentration depicted in Fig. 2b, in spite of commotion occurring outside the colony area. This suggests that the animals had adapted to the disruptions. Consumption of EtOH during the 24 h prior to sacrifice and the BAC, CS and pOC levels in terminal blood are shown in Table 1. There were no significant differences in any of these parameters except for pOC, which in Het mice is about half that of WT, reflecting the expression of only one copy of the OC gene (Ducy et al. 1996).
Table 1.
Consumption of 9% w/v EtOH, g/kg BW/day, during the 24 h prior to sacrifice; blood alcohol concentration (BAC), % w/v; corticosterone, ng/ml; and osteocalcin, ng/ml in blood taken at termination of WT, Het, and KO mice
| WT | Het | KO | |
|---|---|---|---|
| EtOH, g/kg BW/day | 6.11 ± 1.16 | 7.76 ± 1.40 | 8.22 ± 1.10 |
| BAC % w/v | 0.0037 ± 0.0008 | 0.0068 ± 0.0027 | 0.0037 ± 0.0008 |
| Corticosterone, ng/ml | 29.8 ± 5.8 | 23.7 ± 7.4 | 27.8 ± 8.4 |
| Osteocalcin, ng/ml | 20.1 ± 2.2 | 12.1 ± 0.8 | NA |
All values are mean ± SEM. There were no significant differences in any of these parameters
Expression of genes for the enzymes of the catecholamine synthetic pathway (TH, DBH and PNMT) was measured in the adrenals harvested at the time the animals were killed. No significant differences in DBH and PNMT expression were observed between any of the groups (data not shown). Because there was no significant difference between Het and WT mouse expression of TH (F [1,20] = 0.0065, p = 0.94), values were combined to indicate presence of OC. However, there was a significant difference between WT/Het and KO expression of TH. As can be seen from Fig. 2d, the TH expression was attenuated in the absence of OC compared with animals (F [1, 32] = 4.69, p = 0.038) that were able to synthesize and secrete OC (WT/Het). There was also a significant inverse correlation between TH expression and ethanol consumption by all mice during the 24 h preceding sacrifice, r = 0.43716, p < 0.05.
Experiment 2: Voluntary EtOH Consumption, Stable Conditions
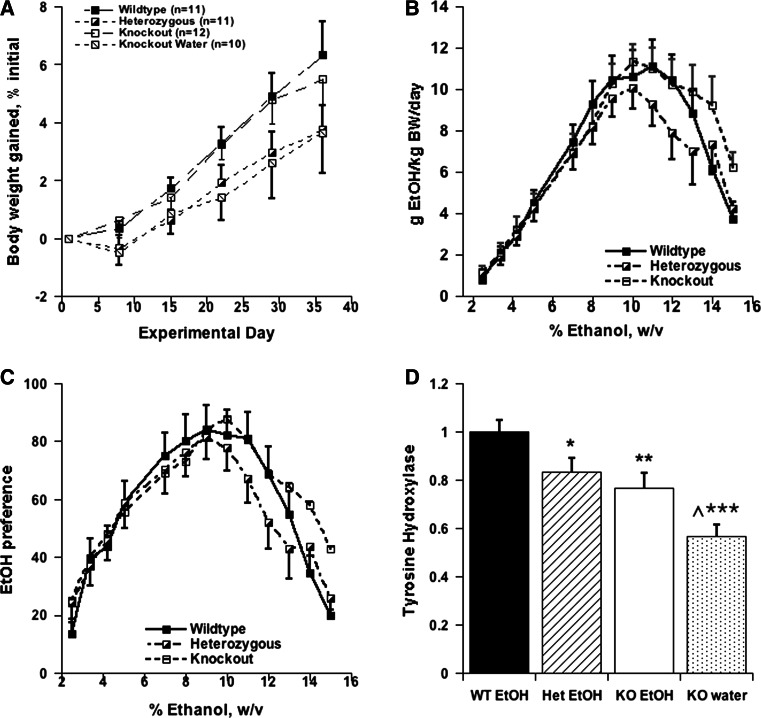
To evaluate the possible effects of environmental disruptions in Experiment 1, a second consumption study was conducted under stable vivarium conditions following completion of renovations to the adjacent laboratory. The concentration of EtOH was gradually increased from 2.5% w/v to 16% wt/vol. There were no significant differences in weight gained at any time during the study (Fig. 3a). Repeated measures ANOVA for the main effect of genotype did not indicate any significant differences in consumption (g/kg BW/day) (F [1273] = 0.306, p = 0.5857) (Fig. 3b) or preference (F[1273] = 0.302, p = 0.5886) (3C). Divergence of KO and WT occurred only above 13% and significant differences in consumption were observed only at 15% EtOH (F [1, 21] = 5.323, p = 0.0313).
Fig. 3.
a Weight gained by mice expressed as % of initial weight; b EtOH consumption, g EtOH/kg BW/day; c EtOH preference (%EtOH/total fluid); d Adrenal Tyrosine Hydroxylase gene expression. All data are expressed as mean ± SEM. Significant differences among the groups were evident as noted in figure, * p < 0.05; ** p < 0.01; *** p < 0.005 relative to WT drinker; ^ indicates significant difference between KO EtOH and KO water, p < 0.05. Environmental conditions in the colony were stable during the entire testing period
TH mRNA was measured in the adrenals harvested after the animals were killed. ANOVA indicated significant differences among the three groups of mice (F[3,39] = 10.41, p < 0.0001). As depicted in Fig. 3d, TH expression in WT was significantly higher than KO drinkers (p < 0.01) and KO non-drinkers (p < 0.0001). TH expression was also significantly higher in KO drinkers compared to KO non-drinkers (p < 0.05). Unlike in Experiment 1, there was no correlation between TH expression and amount of ethanol consumed during 24 h prior to sacrifice (r = 0.14213, p > 0.05). Although we did not have WT non-drinkers to compare with the drinkers in this study, TH expression did not differ significantly in a separate experiment with WT purchased from Jackson Labs (Non-drinkers 1.000 ± 0.107; drinkers 0.778 ± 0.076, mean ± SEM relative to non-drinkers; F[1,18] = 2.86, p > 0.1).
Experiment 3: Voluntary EtOH Consumption Plus Immobilization Stress
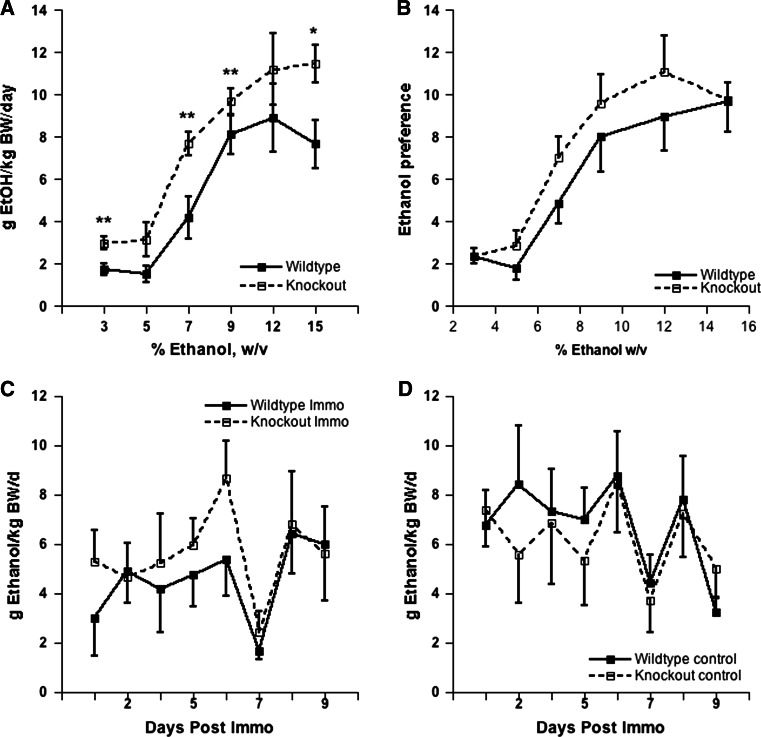
In this experiment, we tested WT and KO that had previously been used for behavioral observations of effects of saline and EtOH injection, after which they had remained undisturbed for 7 weeks. Repeated measures ANOVA for the main effect of genotype indicated significant effects on consumption (g/kg BW/day) (F [1, 90] = 9.408, p < 0.0.01) (Fig. 4a). Significant differences in consumption (g/kg BW/day) were observed at 3% (F [1, 18] = 9.687, p < 0.01), 7% (F [1, 18] = 9.216, p < 0.01), 9% (F [1, 18] = 8.428, p < 0.01), and 15% (F [1, 18] = 4.856, p < 0.05). Repeated measures ANOVA for the main effect of genotype on EtOH preference indicted no significant differences (F [1, 90] = 0.879, p > 0.1) (Fig. 4b).
Fig. 4.
a EtOH consumption by mice provided with a choice of water and an increasing concentration of EtOH; b EtOH preference ratios (%EtOH/total fluid) of mice offered continuous two-bottle choice of water or increasing concentrations of EtOH; Consumption of 15% EtOH by c Immo and d non-Immo controls for a period of 10 days post 2-h Immo stress. No significant difference between WT and KO animals was observed at any of the days post stress. Note distinct dip in both Immo and controls at day 7 post-Immo, after all were restrained for blood sampling from tail cuts. All values are expressed as mean ± SEM. *indicates significant genotypic difference, p < 0.05
Figure 4c and d show consumption and preference for 15% EtOH solution for 10 days following imposition of 2 h of Immo stress. No significant genotypic differences were observed in consumption or preference at any of the days post stress. On day 6 in this series, blood was obtained from animals via tail bleeding 2 h into the dark cycle for the purpose of BAC determination when EtOH consumption was likely highest. On the day following the blood collection, consumption decreased sharply for both genotypes. As shown in Table 1, blood alcohol concentrations in the samples did not differ between genotypes (p > 0.05).
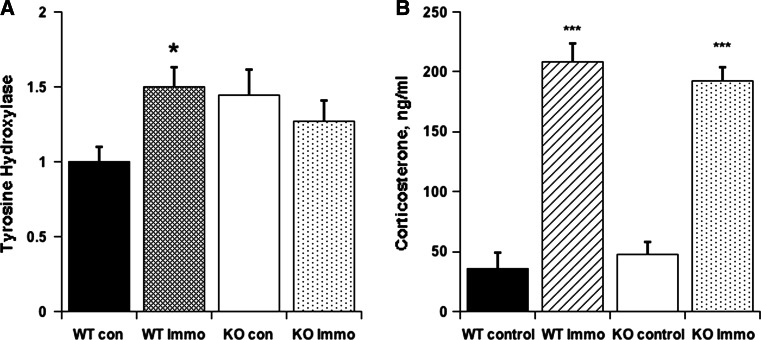
Figure 5a shows TH mRNA data from adrenals harvested from 5 KO and 5 WT control, non-stressed mice and from 5 KO and 5 WT mice that had been subjected to Immo for 2 h. All mice had access to 15% EtOH for a period of 10 days between the first and second Immo. The data demonstrate that WT Immo mice had significantly higher TH gene expression than WT control, while the response in KO was attenuated. ANOVA indicated only a marginal difference (p = 0.0552) between WT and KO immobilized mice. Similar to Experiment 1, there was a significant inverse correlation between TH expression and ethanol consumption prior to sacrifice in the Immo animals (r = 0.38773, p < 0.05. Non-stressed control mice did not show this relationship.
Fig. 5.
a Adrenomedullary gene expression (mean ± SEM relative to WT controls) for Tyrosine-hydroxylase and b corticosterone levels (mean ± SEM) in mice in Experiment 3 given a choice of water or EtOH solution in increasing concentrations. IMMO cohort was subjected to foot restraint immobilization stressor 10 days and again just prior to termination and extraction of adrenals
Figure 5b depicts CS levels in blood taken by decapitation from controls and from Immo immediately after release. Both WT and KO controls had essentially basal levels of CS. However, both responded 2-h Immo with a fourfold elevation of CS, p < 0.001.
Table 2 illustrates that in all three experiments, including 5 days after Immo stress in Experiment 3 when both WT and KO were consuming 15% EtOH, KO mice consumed more ethanol than WT at the peak of consumption. In experiments 1 and 3, this peak was reached by KO at a higher ethanol concentration. However, in Experiment 2, peak consumption and concentration at peak were nearly identical for all three genotypes. Blood alcohol levels in both controls and Immo mice of both genotypes exceeded what is considered intoxication in humans, but did not differ significantly by group.
Table 2.
Concentration of ethanol at which highest level of consumption was reached by wild type and knockout mice in three experiments, mean ± SEM
| Wild type | Knockout | |||
|---|---|---|---|---|
| % EtOH w/v | g/kg BW/day | % EtOH w/v | g/kg BW/day | |
| Expt 1 | 9 | 6.7 ± 1.1 | 13 | 9.5 ± 1.3 |
| Expt 2 | 11 | 11.1 ± 1.3 | 10 | 11.3 ± 0.8 |
| Expt 3 | 12 | 8.3 ± 1.6 | 15 | 11.8 ± 0.7 |
| Expt 3, post-Immo | 15 | 5.4 ± 1.5 | 15 | 8.7 ± 1.5 |
| Expt 3, 5 d post-Immo controls | 15 | 8.8 ± 1.8 | 15 | 8.5 ± 2.1 |
| Blood alcohol (mg/dl) | ||||
|---|---|---|---|---|
| WT control | WT Immo | KO control | KO Immo | |
| Blood alcohol (mg/dl) | 0.118 0.007 | 0.109 ± 0.005 | 0.135 ± 0.008 | 0.120 ± 0.006 |
For Experiment 3, post-Immo values are for consumption 5 days after the first immobilization. Blood alcohol level was determined from tail cut blood taken on day 6 post-immobilization
Discussion
Three experiments to test the relationship between ethanol consumption and complete removal of osteocalcin from the mouse genome yielded results that differed with respect to stressful stimuli and genotype. We acknowledge the fact that conditions present during Experiment 1 are far from the norm when conducting planned chronic stress experiments, but data were interesting enough to attempt to find explanations for the obvious differences in ethanol consumption and preference observed. The effects of environmental perturbation are too often ignored by animal care personnel and by researchers when analyzing data. In the natural environment, extreme temperature fluctuations, thunderstorms, hurricanes, tornadoes, earthquakes, and volcanic eruptions occur at random. These may have long-lasting effects on fauna in the vicinity, as well as those in a research animal facility (McBride 2017). Even building construction blocks away from an animal facility can disrupt the stress response axes (Dallman et al. 1999).
In our experiments, both the unpredictable environmental stressors experienced by the mice during Experiment 1, including noise, temperature fluctuations, human traffic through the colony, and vibrations due to moving equipment in the nearby laboratory, and the prior injection stress experienced by mice in Experiment 3 appeared to influence ethanol consumption and preference differently in WT and KO mice, as opposed to Experiment 2 where no significant differences were observed. As can be seen from Figs. 2b, c, KO drank more EtOH at each concentration up to 13% than their WT counterparts, while Het mice were intermediate. These data are consistent with observations of Lopez and colleagues in mice exposed to chronic variable stress (Lopez et al. 2011). McCaul and colleagues observed that in humans, anxiety and perceived stress were strongly associated with alcohol abuse (McCaul et al. 2017).
In Experiment 2, when colony conditions were stable and mice had not experienced any known prior stressful stimuli, EtOH consumption was highest for both WT and KO at 11% and 10%, respectively, and then declined steadily for both genotypes with increasing concentrations of EtOH. There was a slight divergence after a high concentration (15% w/v) of EtOH was reached that may be related to genotypic differences in taste sensitivity at the higher concentration of ethanol. Ichikawa and colleagues reported that OC immunoreactive neurons in glossopharyngeal sensory ganglia may have chemoreceptive functions in the tongue (Ichikawa et al. 2005).
Consumption by KO and WT animals in Experiment 3 diverged from the beginning. In this experiment, concentration of EtOH was rapidly escalated every 24 h with the result that KO animals consistently drank more EtOH than their WT counterparts (Fig. 3). Although this study was conducted under stable vivarium conditions, it is important to note that prior treatment of these animals included acute doses of EtOH or saline via intraperitoneal injections and behavioral testing in open field. It is possible that the animals were sensitized by prior manipulation and colony disruption and were still exhibiting the effects of those stressful events and that both KO and WT were affected by the stressors. Furthermore, KO could be less sensitive to those prior conditions such that their drinking was less affected, or they might be less sensitive to intoxication resulting from the EtOH consumption. We have previously documented decreased sensitivity by KO in response to various physical stimuli, including injections (Patterson-Buckendahl et al. 2012).
The lack of divergence of consumption in Experiment 3 following Immo stress and the pronounced dip in drinking following blood sampling from tail cuts confirm a prior finding that stressful stimuli are not indentical in the responses they generate (Anthenelli 2012). This suggests that acute stress reduces EtOH consumption but the effect may be only temporary. Yang et al. reported that C57BL/6 mice subjected to repeated restraint stress showed no significant increase in EtOH preference and consumption (Yang et al. 2008), while Lopez and colleagues reported that severe stressors such as restraint, forced swim, and social defeat all tended to reduce ethanol intake in mice (Lopez et al. 2016).
In all three experiments, adrenomedullary expression of tyrosine hydroxylase was inversely related to amount of ethanol consumed during the 24 h prior to sacrifice. In Experiment 2, unlike Experiment 1, there were KO non-drinkers serving as controls, TH expression was elevated in EtOH-consuming KO mice compared to water-consuming KO (Fig. 4). This confirmed that EtOH consumption was a possible stressor, as seen previously in rats (Patterson-Buckendahl et al. 2005), because it elevated TH expression in KO drinkers; however, the TH response was still attenuated in the absence of OC as compared to WT EtOH-drinking animals, even though their EtOH consumption was greater. This suggests that the effect of EtOH on catecholamine gene expression might be mediated through pOC. Researchers in the Karsenty laboratory that supplied us with the KO breeding stock have reported that OC influences pancreatic beta cell production of insulin and fat cell production of adiponectin (Lee et al. 2007, Ferron et al. 2008). We believe that OC also provides input to the nervous system.
Some limitations to these studies must be mentioned. We have yet to compare adolescent or young adults of either sex, and we have only one limited study of female drinkers and non-drinkers, in which EtOH consumption and preference were similar to that of males in Experiment 2, and did not differ by genotype (data not shown). We did not have TH analysis from the female study. We hope to conduct these studies in the future. Our experiments indicate that pOC is not just a passive component of plasma, but rather plays an active role in modulating EtOH consumption and possibly responses to a variety of stressors through interaction with the sympathetic nervous system.
Acknowledgements
The authors would like to express their gratitude to Dr. Richard Kvetnansky for all his advice, encouragement, and friendship. Without his assistance and that of his laboratory staff, this work would not have been possible. This work was supported by grants from the National Science Foundation, SGER #0343515 and the National Institute on Alcohol Abuse and Alcoholism R21 AA 14399-01A2 (PP-B), and by funds from the Aresty Foundation for Undergraduate Research to MS and AS in partial fulfillment of requirements for Henry Rutgers Scholar awards to each.
Authors Contributions
PP-B designed the experiments, prepared and wrote the manuscript. MS and AS performed all experiments, collected and analyzed data, and assisted in preparation of manuscript. LAP advised on experimental design and interpretation of manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest.
Research Involving Animal Rights
All protocols were reviewed and approved by the Rutgers Institutional Animal Care and Use Committee and were consistent with guidelines specified by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
References
- Anthenelli RM (2012) Overview: stress and alcohol use disorders revisited. Alcohol Res 34(4):386–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford JN, Gallagher JA, Poser JW, Russell RG (1984) Production of osteocalcin by human bone cells in vitro. Effects of 1,25(OH)2D3, 24,25(OH)2D3, parathyroid hormone, and glucocorticoids. Metab Bone Dis Relat Res 5(5):229–234 [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C (2002) Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology 27(3):442–452 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bell ME, Bhatnagar S, Choi S, Chu A, Gomez F, Laugero K, Soriano L, Viau V (1999) Warning! Nearby construction can profoundly affect your experiments. Endocrine 11(2):111–113 [DOI] [PubMed] [Google Scholar]
- Desbois C, Hogue DA, Karsenty G (1994) The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem 269(2):1183–1190 [PubMed] [Google Scholar]
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382(6590):448–452 [DOI] [PubMed] [Google Scholar]
- Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105(13):5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddini GW, Turner RT, Grant KA, Iwaniec UT (2016) Alcohol: a simple nutrient with complex actions on bone in the adult skeleton. Alcohol Clin Exp Res 40(4):657–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundberg CM, Clough ME, Carpenter TO (1992) Development and validation of a radioimmunoassay for mouse osteocalcin: paradoxical response in the Hyp mouse. Endocrinology 130(4):1909–1915 [DOI] [PubMed] [Google Scholar]
- Hauschka PV, Lian JB, Cole DE, Gundberg CM (1989) Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev 69(3):990–1047 [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Jin HW, Fujita M, Nagaoka N, Sugimoto T (2005) Osteocalcin-immunoreactive neurons in the vagal and glossopharyngeal sensory ganglia of the rat. Brain Res 1031(1):129–133 [DOI] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS (2012) Stress and alcohol: epidemiologic evidence. Alcohol Res 34(4):391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RF (1997) Alcohol-induced bone disease: impact of ethanol on osteoblast proliferation. Alcohol Clin Exp Res 21(3):392–399 [DOI] [PubMed] [Google Scholar]
- Klein RF, Fausti KA, Carlos AS (1996) Ethanol inhibits human osteoblastic cell proliferation. Alcohol Clin Exp Res 20(3):572–578 [DOI] [PubMed] [Google Scholar]
- Kubovcakova L, Sabban EL, Kvetnansky R, Krizanova O (2002) Comparative study of catecholamine synthesizing enzymes in adrenal medulla of CRH knock-out mice, their CRH (+/+) mates and Sprague-Dawley rats. Endocr Regul 36(3):107–113 [PubMed] [Google Scholar]
- Kvetnansky R (2004) Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenylethanolamine N-methyltransferase. Ann N Y Acad Sci 1032:117–129 [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130(3):456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang H, Yang C, Li Y, Dai Z (2016) An overview of osteocalcin progress. J Bone Miner Metab 34(4):367–379 [DOI] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC (2011) Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol 45(4):355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC (2016) Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol 51:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone JA, Maddalozzo GF, Branscum AJ, Hardin K, Cialdella-Kam L, Philbrick KA, Breggia AC, Rosen CJ, Turner RT, Iwaniec UT (2012) Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause 19(9):974–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel DB, Boisseau N, Benhamou CL, Jaffre C (2012) Cortical bone is more sensitive to alcohol dose effects than trabecular bone in the rat. Joint Bone Spine 79(5):492–499 [DOI] [PubMed] [Google Scholar]
- McBride EA (2017) Small prey species’ behaviour and welfare: implications for veterinary professionals. J Small Anim Pract 58(8):423–436 [DOI] [PubMed] [Google Scholar]
- McCaul ME, Hutton HE, Stephens MA, Xu X, Wand GS (2017) Anxiety, anxiety sensitivity, and perceived stress as predictors of recent drinking, alcohol craving, and social stress response in heavy drinkers. Alcoholism 41(4):836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikosch P (2014) Alcohol and bone. Wien Med Wochenschr 164(1–2):15–24 [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS (1998) Heterogeneous neurochemical responses to different stressors: a test of Selye’s doctrine of nonspecificity. Am J Physiol 275(4 Pt 2):R1247–R1255 [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Kvetnansky R, Fukuhara K, Cizza G, Cann C (1995) Regulation of plasma osteocalcin by corticosterone and norepinephrine during restraint stress. Bone 17(5):467–472 [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Rusnak M, Fukuhara K, Kvetnansky R (2001) Repeated immobilization stress reduces rat vertebral bone growth and osteocalcin. Am J Physiol Regul Integr Comp Physiol 280(1):R79–R86 [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Blakley G, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R (2004) Alcohol alters rat adrenomedullary function and stress response. Ann N Y Acad Sci 1018:173–182 [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R (2005) Ethanol consumption increases rat stress hormones and adrenomedullary gene expression. Alcohol 37(3):157–166 [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Pohorecky LA, Kvetnansky R (2007) Differing effects of acute and chronic stressors on plasma osteocalcin and leptin in rats. Stress 10(2):163–172 [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Pohorecky LA, Kubovcakova L, Krizanova O, Martin RB, Martinez DA, Kvetnansky R (2008) Ethanol and stress activate catecholamine synthesis in the adrenal: effects on bone. Ann N Y Acad Sci 1148:542–551 [DOI] [PubMed] [Google Scholar]
- Patterson-Buckendahl P, Sowinska A, Yee S, Patel D, Pagkalinawan S, Shahid M, Shah A, Franz C, Benjamin DE, Pohorecky LA (2012) Decreased sensory responses in osteocalcin null mutant mice imply neuropeptide function. Cell Mol Neurobiol 32(5):879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng TC, Lian JB, Hirsch PF, Kusy RP (1991) Lower serum osteocalcin in ethanol-fed rats. J Bone Miner Res 6(2):107–115 [DOI] [PubMed] [Google Scholar]
- Pohorecky LA (1981) The interaction of alcohol and stress. A review. Neurosci Biobehav Rev 5(2):209–229 [DOI] [PubMed] [Google Scholar]
- Pohorecky LA (1990) Interaction of ethanol and stress: research with experimental animals—an update. Alcohol Alcohol 25(2–3):263–276 [DOI] [PubMed] [Google Scholar]
- Pohorecky LA (1991) Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res 15(3):438–459 [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Rassi E, Weiss JM, Michalak V (1980) Biochemical evidence for an interaction of ethanol and stress: preliminary studies. Alcohol Clin Exp Res 4(4):423–426 [DOI] [PubMed] [Google Scholar]
- Roske I, Baeger I, Frenzel R, Oehme P (1994) Does a relationship exist between the quality of stress and the motivation to ingest alcohol? Alcohol 11(2):113–124 [DOI] [PubMed] [Google Scholar]
- Sinha R, Robinson J, O’Malley S (1998) Stress response dampening: effects of gender and family history of alcoholism and anxiety disorders. Psychopharmacology 137(4):311–320 [DOI] [PubMed] [Google Scholar]
- Vogel WH, DeTurck K, Miller JM (1986) Differential effects of ethanol on plasma catecholamine levels in rats. Biochem Pharmacol 35(22):3983–3987 [DOI] [PubMed] [Google Scholar]
- Wezeman FH, Emanuele MA, Emanuele NV, Moskal SF 2nd, Woods M, Suri M, Steiner J, LaPaglia N (1999) Chronic alcohol consumption during male rat adolescence impairs skeletal development through effects on osteoblast gene expression, bone mineral density, and bone strength. Alcohol Clin Exp Res 23(9):1534–1542 [PubMed] [Google Scholar]
- Yang X, Wang S, Rice KC, Munro CA, Wand GS (2008) Restraint stress and ethanol consumption in two mouse strains. Alcohol Clin Exp Res 32(5):840–852 [DOI] [PMC free article] [PubMed] [Google Scholar]