Abstract
Objective
Determine the impact of cochlear implantation on quality of life (QOL) and determine the correlation between QOL and speech recognition ability
Study Design
Two authors independently searched PubMed, Medline, Scopus, and CINAHL to identify studies reporting hearing-specific or CI-specific QOL outcomes before and after cochlear implantation and studies reporting correlations between QOL and speech recognition after cochlear implantation. Data from the included articles were obtained independently by two authors. Standardized mean difference (SMD) for each measure and pooled effects were determined to assess improvement in QOL before and after cochlear implantation.
Results
From 14 articles with 679 CI patients that met inclusion criteria, pooled analyses of all hearing-specific QOL measures revealed a very strong improvement in QOL after cochlear implantation (SMD=1.77). Subset analysis of CI-specific QOL measures also showed very strong improvement (SMD=1.69). Thirteen articles with 715 patients met criteria to evaluate associations between QOL and speech recognition. Pooled analyses showed a low positive correlation between hearing-specific QOL and word recognition in quiet (r=0.213), sentence recognition in quiet (r=0.241), and sentence recognition in noise (r=0.238). Subset analysis of CI-specific QOL showed similarly low positive correlations with word recognition in quiet (r=0.213), word recognition in noise (r=0.241), and sentence recognition in noise (r=0.255).
Conclusions
Using hearing-specific and CI-specific measures of QOL, patients report significantly improved QOL after cochlear implantation. However, widely used clinical measures of speech recognition are poor predictors of patient-reported QOL with CIs.
Keywords: Cochlear implant, quality of life, speech recognition, word recognition, outcomes research
Introduction
Cochlear implantation is the standard treatment for severe-to-profound bilateral sensorineural hearing loss. Over 500,000 cochlear implants (CIs) have been implanted worldwide with this number expected to rise with an aging population and expanding indications.1,2 With rising health care costs, increased focus has been placed on comprehensive assessments of functional outcomes to ensure that procedures such as cochlear implantation are having a significant positive impact on patients' lives.3
Open-set word and sentence recognition are widely considered the standard CI outcome measures.4 However, speech recognition alone does not adequately represent the complex communication and other experiences that patients encounter on a daily basis. There is also a lack of consensus in the published literature about how accurately improvements in speech recognition scores represent the full impact of cochlear implantation on an individual's life.5-7 Cochlear implantation likely impacts an individual's quality of life (QOL) beyond speech recognition ability alone. Given that assessments of word and sentence recognition are the widely accepted standard outcome measures, it is of great importance to determine the extent to which QOL, as measured through patient-reported outcome measures (PROMs), correlates with speech recognition abilities.
Health-related quality of life (HRQOL) is a patient's perceived mental and physical health status that encompasses many aspects of their life. The Centers for Medicare and Medicaid Services Quality Strategy report has targeted QOL improvement as a primary outcome measure.8 Although several studies have evaluated QOL in the adult CI population, all have included relatively small numbers of patients from single institutions. To address these gaps, the current study reports the results of a meta-analysis that collates hearing-specific and CI-specific QOL measures to better understand the impact of cochlear implantation on individuals' QOL. A second meta-analysis reviewed associations among various measures of speech recognition and QOL.
Materials and Methods
Literature search
Search was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.9 Two authors independently searched the PubMed, Scopus, and OVID/Medline databases in June 2016 for the following search terms: ‘cochlear implant’ or ‘cochlear implantation’ and ‘quality of life’. This resulted in 591 unique articles that were reviewed by abstract for inclusion and exclusion criteria (Figure 1). After review by abstract, 231 articles underwent full-text review for inclusion. These articles were included in either the meta-analysis of QOL improvement or the meta- analysis of correlations (four articles satisfied criteria for both). Disagreements regarding the inclusion of a study were mediated with a third author to reach a mutual consensus. As best possible, articles were also reviewed to ensure that overlapping study populations were not included. All included subjects were CI candidates with bilateral severe to profound sensorineural hearing loss. When adding ‘patient reported outcome measures’ to search terms, no additional articles met inclusion criteria.
Figure 1.
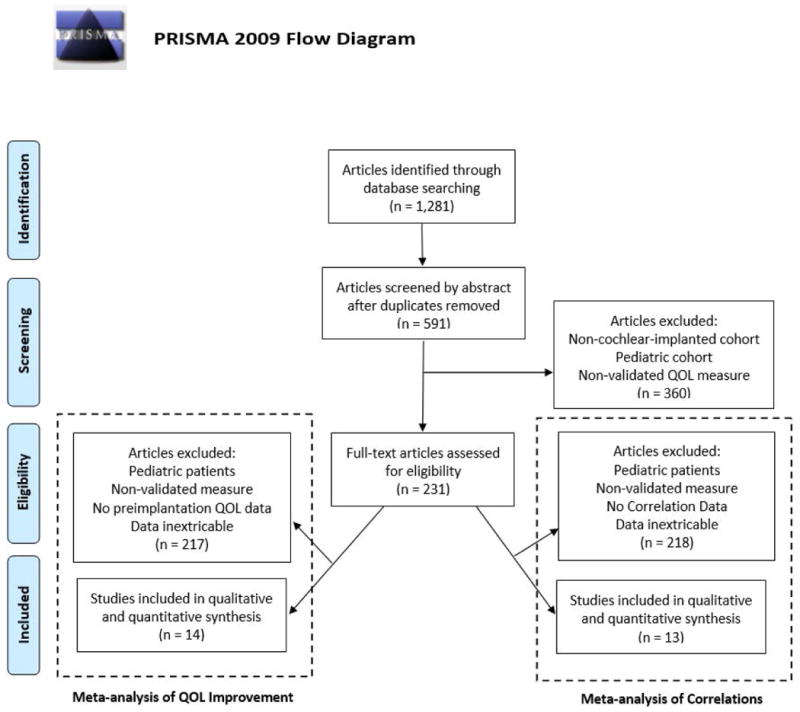
Literature review process utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) search method. The flowchart details the methods used to select articles for inclusion in the meta-analysis of quality of life (QOL) improvement and meta-analysis of correlations. Four articles satisfied criteria for both meta-analyses.
Case reports, letters to the editor, abstracts, articles not published or translated into English, and book chapters were excluded. No date range limitations were used. Studies with pediatric patients (less than 18 years old) in the study cohort were excluded. Studies using QOL PROMs translated to languages other than their native English format were included.
This study analyzed either hearing-specific or CI-specific PROMs that assessed the impact of cochlear implantation on QOL. Therefore, studies were excluded that used general health QOL instruments, such as the Health Utility Index (HUI-3)10 or Short Form-36 (SF-36)11, which were not validated on individuals with hearing loss. These general health QOL instruments were included in a previous meta-analysis by our group.12 We defined hearing-specific QOL PROMs as those instruments that focus on how hearing loss influences a patient's well-being, but have not been validated in the CI population. CI-specific PROMs are those that have been specifically created and validated for CI users.
Data Extraction
Articles selected for meta-analysis of QOL improvement met the following inclusion criteria: collection of either hearing-specific or CI-specific PROM data in an adult CI cohort before and after CI; sample size, mean, and standard deviation available for QOL PROM data; and post-implantation follow-up of at least 3 months. Data from the included articles were obtained independently from two authors including: author, year of publication, number of patients, patient demographics, speech recognition scores, and QOL PROM data obtained pre- and post-implantation (mean and standard deviation).
Articles selected for meta-analysis of correlations between speech recognition and QOL PROMs met the following inclusion criteria: correlation of speech recognition scores versus either hearing-specific or CI-specific PROM data in an adult cohort after CI; sample size and Spearman or Pearson correlation values available; and follow-up of at least 3 months. Data from the included articles were obtained independently from two authors including: author, year of publication, number of patients, patient demographics, speech recognition scores, and correlation values. Reporting of pre- and post-implantation QOL measures were not required for the meta-analysis of correlations, as we were evaluating correlations between speech recognition abilities and QOL at the latest time point following implantation.
Data reported in graphical plots were not extracted for meta-analysis unless numerical points were available and verifiable. We attempted to obtain complete details of published results from authors in the event of incomplete data in order to allow inclusion of their study. PROMs that use a reverse scale (lower scores represent a better QOL) had values multiplied by -1 for analysis. If the study followed patients after sequential implantation, only data obtained after the first implantation were included.
Statistical Methods: Meta-analysis of QOL Improvement
Meta-analysis evaluating the impact of CI on QOL with a continuous measure (comparison of means and standard deviations between pre- and post-implantation) was performed with Cochrane Review Manager (RevMan) version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, 2011, Copenhagen, Denmark). For this analysis, the null hypothesis was no difference between pre and post-implantation QOL using hearing-specific or CI-specific PROMs. Both the fixed effects model and the random effects model were used in this study. Under the fixed effects model, it is assumed that all studies come from a common population and that the effect size (standardized mean difference) is not significantly different among the different studies. This assumption is tested by the heterogeneity test or I2 statistic. If this test yields a low probability value (p < 0.05) then the fixed effects model is likely invalid. In this case, the random effects model is more appropriate, in which both the random variation within the studies and the variation between the studies are incorporated. Under the random effects model, the true effects are assumed to vary between studies and the summary effect is the weighted average of the effects reported in the different studies.13 The random effects model provides a more conservative estimate (i.e., with a wider confidence interval), but the results from the two models typically agree when there is no heterogeneity. For the current analyses, the random effects model was the preferred model when heterogeneity was present. Additionally, Sterne and Egger tests were performed for assessment of risk of publication bias.14,15 For this test, a low probability indicates a high likelihood that included articles were more likely to be published as their results were statistically significant.
Effect size is represented by SMD, a unitless numerical value also known as Cohen's d, which assesses the magnitude and certainty of benefit.16,17 Positive values indicate the treatment has a positive effect on outcome measures with the following thresholds for subjective interpretation being suggested by Cohen: 0.2 - small effect, 0.5 – medium effect, and 0.8 – large effect.16 The total SMD with 95% confidence interval is given for both the fixed effects model and the random effects model. Data are presented as SMD [95% confidence interval].
Statistical Methods: Meta-analysis of Subdomains from the Nijmegen Cochlear Implant Questionnaire (NCIQ)
For subdomain analysis, only studies that used the NCIQ18 met inclusion criteria. Each study's sample data were combined, with the weighted mean and weighted standard deviation determined. Differences were noted using the variable delta (Δ). Pre-implantation and post-implantation pooled means of each subdomain were compared using a comparison of weighted means test through the program MedCalc.
Statistical Methods: Meta-analysis of Correlations
A meta-analysis of correlations was performed for correlations between speech recognition and QOL PROMs after cochlear implantation. The program MedCalc 16.8.4 (MedCalc Software, Oostende, Belgium) lists the results of the individual studies included in the meta-analysis, number of cases, and the mean correlation coefficient with the 95% confidence interval. These data were used to construct forest plots using Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA). The pooled correlation value with 95% confidence interval is given for either the fixed effects model or the random effects model. Model selection was performed as described in an earlier section. Each study was weighted according to the number of included patients. MedCalc uses the Hedges-Olkin method for calculating the weighted summary correlation coefficient under the fixed effects model, using a Fisher Z transformation of the correlation coefficients.19 Under the random effects model, the heterogeneity statistic is incorporated in order to calculate the summary correlation coefficient.20 For this analysis, the null hypothesis was that speech recognition ability and QOL following cochlear implantation are not correlated. The following thresholds were used for subjective assessment of correlation values (r): 0 - 0.3, negligible; 0.3 – 0.5, low; 0.5 – 0.7, medium; 0.7 – 0.9, high; 0.9 – 1.0, very high.21,22
Results
Meta-analysis of QOL Improvement
Fourteen articles met inclusion criteria for this analysis (Table 1).5,7,23-34 Although 37 articles had pre- and post-implantation data, 23 articles did not have extricable and complete data (sample size, mean, or standard deviation). A total of 697 subjects were included in the analysis. Of 510 patients identified by sex, 42% were male and 58% were female. The mean ages of patients for individual studies ranged from 32.0 to 82.9 years.
Table 1. Studies Included in Meta-analysis of QOL Improvement.
| Article | Level of Evidence | Patient Age | Follow-Up Period (Months) | |
|---|---|---|---|---|
| Mean, ± SD, Range | Male %/ Female % | |||
| Damen 200733 | 3 | 49.6 ± 10.9 | 54 / 46 | ≥ 12 |
| Hawthorne 200446 | 4 | 49 ± 13 | 47/53 | 6 |
| Klop 200837 | 4 | 54.7 ± 15.7 | 34 / 66 | 12 |
| Knopke 201647 | 4 | 82.9 ± 2.7 | NA | 6 |
| Krabbe 200030 | 4 | 51 ± 16 | 47 / 53 | ≥ 12 |
| Mo 200534 | 4 | 57.6 ± 14.5 (28-82) | 44 / 56 | 12 |
| Mosnier 201548 | 4 | 72 (65-85) | NA | 12 |
| Olze 201149 | 4 | 51.7 ± 16.9 (19-77) | 27 / 73 | ≥ 6 |
| Ottaviani 201538 | 4 | 50 ± 16 (26 – 76) | 46 / 54 | ≥ 6 |
| Park 201127 | 4 | 56 ± 15 | 39 / 61 | 12 |
| Sanchez-Cuadrado 201525 | 4 | 60 (24-85) | 46 / 54 | ≥ 6 |
| Tavora-Veira 201550 | 4 | 53.8 ± 11.6 | 46 / 54 | 24 |
| Van Dijkhuizen 201139 | 4 | 39 (20 – 62) | 52 / 48 | 12 |
| Vermeire 200528 | 4 | 58 | NA | ≥ 4 |
Articles satisfying inclusion criteria for meta-analysis of QOL improvement. Study design; patient age mean, standard deviation, and range (when available); male/female percentages; and follow-up time period in months.
Sterne and Egger testing (p < 0.000001) suggested a relationship between the sample size of these studies and their effect sizes indicating a high likelihood of publication bias. These data were significantly heterogeneous (I2 = 92%, p < 0.00001). Thus, meta-analysis was performed with a random effects model, including the subset analyses of CI-specific measures (I2 = 95%, p < 0.00001) and hearing-specific measures (I2 = 84%, p < 0.00001), which had similarly high heterogeneity.
Overall, a very large improvement in QOL from pre- to post-implantation was found when combining hearing-specific and CI-specific QOL data (SMD = 1.77 [1.28 – 2.26]) (Figure 2). Similar significant improvements were found when separately evaluating hearing-specific QOL (SMD = 1.82 [0.81 – 2.83]) and CI-specific QOL (SMD = 1.69 [1.24 – 2.14]). A negligible association was found between date of article publication and improvement in QOL.
Figure 2.
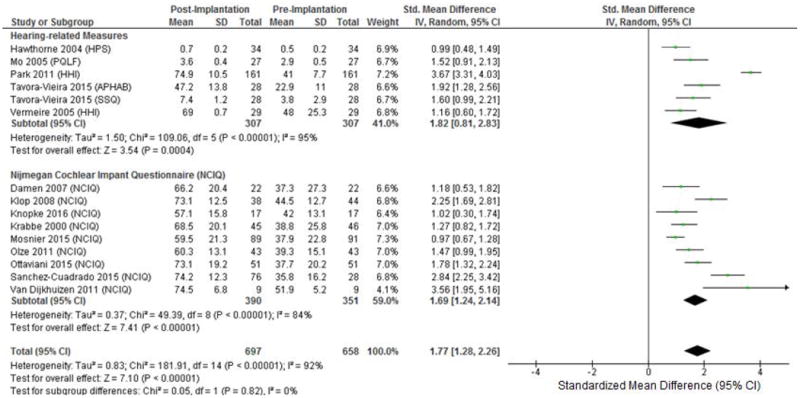
Forest plot of PROMS including subset analysis of hearing-specific and CI-specific QOL PROMs. CI-specific QOL PROMs included only the NCIQ as only this PROM met inclusion criteria for this subset analysis. HPS: Hearing Participation Scale; PQLF: Patient Quality of Life Form; HHI: Hearing Handicap Inventory; APHAB: Abbreviated Profile of Hearing Aid Benefit; SSQ: Speech, Spatial, and Qualities of hearing Questionnaire; NCIQ: Nijmegan Cochlear Implant Questionnaire; IV: Inverse Variance.
The two studies in our analysis with published subdomains of Hearing Handicap Inventory in Adults35/Elderly36 (HHIA/HHIE) improvement (emotional and social) showed similar improvements in both subdomains.27,28 These subdomains of the HHIE/HHIA gauge the emotional morbidity and social interaction function associated with hearing loss, respectively.36 No other measures in the current analysis included published domain or subdomain scores except for the NCIQ (discussed in a later section).
Meta-analysis of NCIQ Subdomains
Nine studies had published subdomain scores of the NCIQ before and after cochlear implantation, from which a meta-analysis of the NCIQ subdomain scores was performed.25,30,33,37-39 All subdomains showed improvement in QOL after implantation (p<0.0001), although a wide range of improvements was observed (from largest to smallest): Basic Sound Processing (Δ = 52.7), Advanced Sound Processing (Δ = 39.7), Activity (Δ = 30.3), Social (Δ = 24.8), Speech Production (Δ = 23.6), and Self-esteem (Δ = 22.2).
Meta-Analysis of Correlations
After applying inclusion and exclusion criteria, 13 articles were included in this analysis (Table 2).5-7,23-29,31-33 From the 13 articles, 715 patients were included. Of 553 patients identified by sex, 42% were male and 58% were female. The mean ages of patients for individual studies ranged from 40.0 to 67.0 years.
Table 2. Studies Included in Meta-analysis of Correlations.
| Article | Level of Evidence | Speech Recognition Task | Patient Age | Follow-Up Period (Months) | |
|---|---|---|---|---|---|
| Mean ± SD (Range) | Male %/ Female % | ||||
| Calvino 201524 | 4 | NS | 52.8 ± 14.0 | 45/55 | ≥ 6 |
| Capretta 20155 | 4 | Words in Quiet: CID Sentences in Quiet: AzBio Sentences in Noise: AzBio |
67 (53-88) | 35/65 | ≥ 9 |
| Cohen 200423 | 4 | Sentences in Noise: CID/ HINT | 67.0 ± 8.5 | 63/37 | ≥ 12 |
| Damen 200733 | 3 | Words in Quiet: Antwerp-Nijmegen Words in Quiet: NVA |
49.6 ± 10.9 | 54/46 | ≥ 12 |
| Fuller 201226 | 4 | NS | 65.6 ± 11.9 | 40/60 | ≥ 12 |
| Granco 20136 | 4 | Words in Quiet: NS Sentences in Quiet: HINT (quiet) Sentences in Noise: HINT |
40 (19-59) | NA | ≥ 12 |
| Maillet 199532 | 4 | NS | (30 - 80) | NA | 24 |
| Olze 2012 - 17 | 4 | Words in Quiet: Freiburg monosyllable Sentences in Quiet: HSM |
58.4 ± 17.0 | 36/64 | ≥ 6 |
| Olze 2012 - 231 | 4 | Words in Quiet: Freiburg monosyllable Sentences in Noise: HSM, Oldenburg |
53.8 ± 14.0 | 28/72 | ≥ 6 |
| Park 201127 | 4 | Sentences in Quiet: HINT (quiet) | 56 ± 15 | 39 / 61 | 12 |
| Sanchez-Cuadrado 201525 | 4 | NS | 60 (24-85) | 46/54 | ≥ 6 |
| Vermeire 200528 | 4 | Words in Quiet: NVA | 58 | NA | ≥ 4 |
| Vermeire 200629 | 4 | Words in Quiet: NVA | 62 (40-78) | NA | ≥ 3 |
Articles satisfying inclusion criteria for meta-analysis of correlations. Study design; speech recognition task; patient age mean, standard deviation, or range (when available); male/female percentages; and follow-up time period in months. NS: Not Specified; CID: Central Institute for the Deaf; HINT: Hearing in Noise Test; NVA: Dutch Audiological Society; HSM: Hochmair Schulz Moser
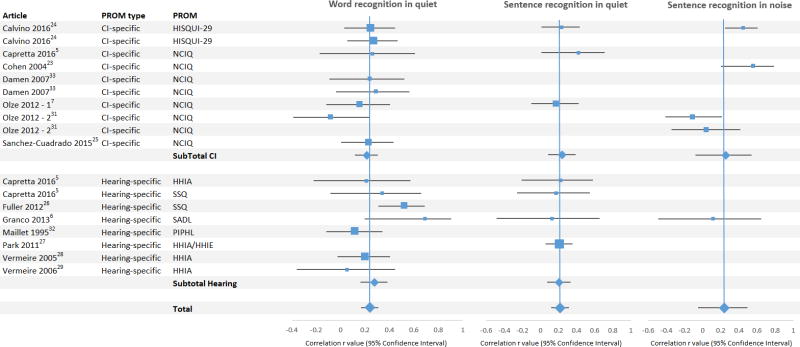
Pooled correlation values were low between hearing-specific QOL and speech recognition scores and between CI-specific QOL and speech recognition scores (Figure 3, Table 3). When pooling all QOL PROMs, negligible positive correlations with QOL were observed with word recognition in quiet (r = 0.239 [0.166 – 0.309]), sentence recognition in quiet (r = 0.219 [0.118 – 0.316]), and sentence recognition in noise (r = 0.238 [-0.054 – 0.493]). Subset analysis of hearing-specific QOL revealed low correlations with word recognition in quiet (r = 0.276 [0.142 – 0.367]) and sentence recognition in quiet (r = 0.204 [0.070 – 0.330]); only one study met criteria for analysis correlating hearing-specific QOL and sentence recognition in noise, so this subset analysis could not be performed. Subset analysis of CI-specific QOL revealed low correlations with QOL and word recognition in quiet (r = 0.213 [0.117 – 0.304]), sentence recognition in quiet (r = 0.241 [0.083 – 0.386]), and sentence recognition in noise (r = 0.255 [0.078 – 0.537]).
Figure 3.
Forest plots pertaining to meta-analysis of correlations for articles reporting CI-specific (top) and hearing-specific (bottom) QOL measures. Pooled correlations are represented by diamonds. PROM: patient-reported outcome measure; CI: cochlear implant; HISQUI-29: Hearing Implant Sound Quality Index; PIPHL: Performance Inventory for Profound Hearing Loss
Table 3. Meta-analysis of Correlations Results.
| r | 95% CI | I2 | p | |
|---|---|---|---|---|
| Subtotal: CI-specific QOL | ||||
| Word recognition in quiet | 0.213 | 0.117 – 0.304 | 0.00% | 0.7679 |
| Sentence recognition in quiet | 0.241 | 0.0830 – 0.386 | 0.00% | 0.5840 |
| Sentence recognition in noise | 0.255 | -0.0783 – 0.537 | 75.61% | 0.0064 |
| Subtotal: Hearing-specific QOL | ||||
| Word recognition in quiet | 0.276 | 0.142 – 0.367 | 48.09% | 0.0726 |
| Sentence recognition in quiet | 0.204 | 0.0701 – 0.330 | 0.00% | 0.9925 |
| Sentence recognition in noise | NA | NA | NA | NA |
| Total | ||||
| Word recognition in quiet | 0.239 | 0.166 – 0.309 | 14.58% | 0.2902 |
| Sentence recognition in quiet | 0.219 | 0.118 – 0.316 | 0.00% | 0.9716 |
| Sentence recognition in noise | 0.238 | -0.0535 – 0.493 | 68.19% | 0.0135 |
Pooled correlation values (r and 95% confidence interval [CI]) and heterogeneity statistics (I2 and p) for meta-analysis of correlations. NA: Not Available
Speech, Spatial, and Qualities of Hearing Scale
Two studies utilized the Speech, Spatial, and Quality (SSQ) PROM.3,18 Capretta et. al.5 found an overall low correlation of SSQ with word recognition in quiet (r = 0.34) and negligible correlation with sentence recognition in quiet (r = 0.17). In evaluating the individual domains of the SSQ, Capretta et al.3 found a medium correlation in the Speech domain with speech recognition ability (word recognition in quiet, r = 0.56; sentence recognition in quiet, r = 0.61). However, correlations between the Spatial and Quality domains and word and sentence recognition in quiet were negligible or low (0.09, 0.13; 0.33, 0.11, respectively). The other study utilizing the SSQ26 found an overall medium correlation of the SSQ and word recognition in quiet (r = 0.52) with individual correlations in the Speech, Spatial, and Quality domains of 0.59, 0.483, and 0.516, respectively.26
Satisfaction with Amplification in Daily Life
Granco et. al.6 found a medium correlation (r = 0.69 [0.196 - 0.906]) between the Satisfaction with Amplification in Daily Life (SADL)40 PROM and word recognition in quiet. Medium to high correlations with word recognition in quiet were found in the SADL subdomains of Positive Effect (r = 0.71) and Personal Image (r = 0.50), which includes hearing performance, personal satisfaction, and social interaction benefit.40 The SADL subdomains of Service and Cost (r = 0.39) and Negative Effects (r = 0.40) showed low correlations with word recognition in quiet. The SADL had overall negligible correlations with sentence recognition in quiet (r = 0.126) and sentence recognition in noise (r = 0.116).
Hearing Handicap Inventory in Adults/Elderly
Four studies utilized the HHIA/HHIE to correlate QOL with speech recognition scores. All 4 studies of the HHIA/HHIE found a negligible correlations either with word recognition in quiet (r = 0.05, 0.20, 0.21) or sentence recognition in quiet (r = 0.21, 0.22). One study published domain correlations of the HHIA/HHIE (emotional and social). Within these domains, negligible correlations were observed with word recognition in quiet and sentence recognition in quiet (r = 0.18 – 0.26).
Nijmegen Cochlear Implant Questionnaire
Studies using the NCIQ reported negligible correlations of QOL with word recognition in quiet (-0.08 – 0.29), negligible to low correlations with sentence recognition in quiet (0.173 – 0.42), and negligible to medium correlations with sentence recognition in noise (-0.11 – 0.56). When investigating the NCIQ subdomains of QOL, Olze et al.7,49 found negligible or low correlations of QOL with word recognition in quiet (-0.34 – 0.30) and sentence recognition in quiet (-0.29 – 0.24). Capretta et al.5 found a medium correlation with word recognition in quiet and the Advanced Sound Perception subdomain of QOL (r = 0.55), but negligible or low correlations with word recognition in quiet and sentence recognition in quiet and the other subdomains of QOL (0.11 – 0.47).
Discussion
We report the first comprehensive meta-analysis of QOL improvements in an adult CI population using the standardized PRISMA methods. This is also the first meta-analysis of correlations between CI patient self-report QOL and speech recognition scores. Two previous meta-analyses of QOL PROMs after cochlear implantation have been reported but neither performed a meta-analysis of correlations of QOL and speech recognition.41,42 Gaylor et al.41 included many studies that reported pre-implantation QOL using a retrospective question format, that is, asking patients after implantation about their health status prior to implantation. This retrospective approach to data gathering is limited by recall bias and, therefore, studies that included data gathered in this manner were excluded in the current analysis. In contrast to Gaylor et al., we did not include the Glasgow Benefit Index (GBI)43 in our meta-analysis of QOL improvement, as this measure requires patients to subjectively assess their improvement following implantation.43 The review by Loeffler et al.42 included qualitative analyses of QOL improvement and correlations and did not perform a quantitative analysis.
In the current study, we found that patients reported significant improvements in QOL following cochlear implantation when measured using hearing-specific or CI-specific QOL PROMs. Although analysis of the published literature showed heterogeneity in outcomes, as noted by the I2 values, a consistent improvement in QOL was seen. This is in contrast to relatively modest improvements in QOL reported (SMD= 0.61) 12 when using general health-related QOL measures, such as HUI-3 and SF-36, which do not typically include questions that assess communication abilities and focus on subdomains that may be unrelated to cochlear implantation. Given that economic benefits of CIs are often determined using general health-related QOL measures not validated in the CI population, these QOL instruments are likely to greatly underestimate the quality adjusted life year and other economic impacts of cochlear implantation.
The NCIQ was the only validated CI-specific QOL PROM that reported individual domain data. Evaluation of the domains revealed that Basic Sound Processing and Advanced Sound Processing were the major drivers of QOL improvement with Self Esteem, Speech Production, and Social domains having much less impact. Two factors may explain these differences. First, cochlear implantation may simply have a greater impact on sound processing than other non-hearing domains. Second, the questions in the QOL instrument in the other domains may not reflect the concerns that contribute most to QOL in CI patients. To date, no CI-specific QOL instrument has been developed using modern standards as developed by the NIH's Patient Reported Outcomes Measurement Information System (PROMIS), including the involvement of patients in item development (as discussed in a later section). Therefore, current QOL instruments may not be sensitive to concerns most important to CI patients.
In the analyzed articles, speech recognition was measured using words in quiet, sentences in quiet, and sentences in noise. A narrow range of low correlations was consistently found between these speech recognition categories and QOL (r = 0.20 - 0.26). Correlation values <0.3 are considered negligible by most statisticians.21,22 The coefficient of determination (R2) provides a means to understand the proportion of variance explained by the independent variable (speech recognition). In this study, the corresponding R2 range of 0.048 to 0.058 signifies that only 4.8% to 5.8% of the variance of patient-reported QOL can be attributed to speech recognition scores, with the vast majority of the variation in QOL unexplained. Unfortunately, only a small number of published studies reported domain specific correlation data, which precluded subset analysis. The few studies that reported communication-related domain data showed medium correlations with speech recognition (r=0.55-0.61).
Speech recognition ability is the gold standard and required reporting outcome for cochlear implantation despite the well-established weak association of speech recognition and patient self-reported benefits and QOL with CIs.44 Although important and easily measured in a clinic setting, how a person listens, communicates and interacts with his or her environment is far more complex than currently available speech recognition tasks in which patients repeat lists of words or sentences, even tasks that include background noise. Additionally, individual dependent factors not limited to age, duration of deafness, pre-operative outcome expectations, and functional ability may impact QOL in ways unrelated to results from speech recognition testing. Patient reported QOL instruments having become increasingly important in assessing the impact of an intervention in a patient's life. Recently, the Centers for Medicare and Medicaid Services (CMS) identified QOL improvement as a primary outcome measure and the FDA now requires PROMs to be included in all clinical trials where an intervention seeks FDA approval.8,45 These measures are especially important when the intervention does not alter quantity of life (i.e., survival), but rather QOL. In such situations, the use of PROMs allows the population of interest to provide the individual's perspective of their ability or functional level beyond standard clinical metrics.
The meta-analysis results provide further support for the need for more regular use of QOL instruments to assess CI outcomes. Although significant improvements in QOL were reported after cochlear implantation, all of the instruments used were either not specifically developed for CI patients or do not meet the rigorous development and reporting standards, as described in the PROMIS guidelines44,45. The need for a CI-specific QOL instrument that meets these standards has been recognized in the Minimal Reporting Standards for Cochlear Implantation of the American Academy of Otolaryngology-Head and Neck Surgery and the 2017-2021 Strategic Plan of the National Institute on Deafness and Other Communication Disorders of the NIH (NIDCD). One of the most significant limitations in current CI-specific QOL instruments is the lack of CI patient involvement in developing the instruments' item banks. Using focus groups of CI patients to develop the item bank provides a better understanding of the factors that significantly impact CI patient QOL. This may greatly alter our understanding of the health utility of cochlear implantation, and specific questions such as the impact of a second implant and an implant combined with a hearing aid.
Our study is limited by biases inherent to all systematic reviews, as authors and journals are biased to publish statistically significant findings. In addition, the speech recognition tasks varied widely among the included studies. We attempted to minimize the effect of these differences by performing separate analyses for word recognition in quiet, sentence recognition in quiet, and sentence recognition in noise. These study differences are common in meta-analyses and error related to these differences is accounted for through the use of pooled estimates.
Individual patient data such as age, sex, hearing and CI related information, were not adequately reported to allow for a multivariable analysis. The impact of patient follow up period on QOL improvement could not be determined, primarily due to the way the data were reported. In addition, the results of this study are limited to the impact of unilateral implantation on QOL because we did not include results with sequential implantation. With respect to correlations, many studies excluded from our analysis stated that no significant correlations were found, but did not cite numerical data; therefore, these studies could not be included in our analysis. Many studies were also excluded from our analysis due to incomplete statistical data, particularly standard deviations.
Conclusions
A meta-analysis of QOL improvement showed a very large positive effect of cochlear implantation on QOL using hearing-specific or CI-specific PROMs. However, a meta-analysis of correlations showed negligible pooled correlations between speech recognition scores and hearing-specific or CI-specific QOL. Systematic assessment of the published literature also revealed that no CI-specific QOL PROMs meets modern development and reporting standards.
Acknowledgments
The authors would like to thank Adrian Ong for his help with this project. We would also like to thank Aaron Moberly and Heidi Olze for providing additional data from their published manuscripts for this study.
This publication was supported by a K12 award through the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant Number UL1TR001450 and a grant from the Doris Duke Foundation.
Footnotes
Presented at COSM, April 2017, in San Diego, CA
The authors have no conflicts of interest to declare.
The authors have no financial disclosures to make.
References
- 1.NIDCD. Cochlear Implants. [Accessed 05/01/17];2016 https://www.nidcd.nih.gov/health/cochlear-implants. 2017.
- 2.Hochmair I. Cochlear Implant: Facts. [Accessed 05/01/17];2013 http://www.medel.com/cochlear-implants-facts/ 2017.
- 3.O'Leary TJ, Slutsky JR, Bernard MA. Comparative effectiveness research priorities at federal agencies: the view from the Department of Veterans Affairs, National Institute on Aging, and Agency for Healthcare Research and Quality. J Am Geriatr Soc. 2010;58(6):1187–1192. doi: 10.1111/j.1532-5415.2010.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luxford WM Ad Hoc Subcommittee of the Committee on H, Equilibrium of the American Academy of O-H, Neck S. Minimum speech test battery for postlingually deafened adult cochlear implant patients. Otolaryngol Head Neck Surg. 2001;124(2):125–126. doi: 10.1067/mhn.2001.113035. [DOI] [PubMed] [Google Scholar]
- 5.Capretta NR, Moberly AC. Does quality of life depend on speech recognition performance for adult cochlear implant users? Laryngoscope. 2016;126(3):699–706. doi: 10.1002/lary.25525. [DOI] [PubMed] [Google Scholar]
- 6.Granco FS, Fernandes NF, Morettin M, Filho OA, Bevilacqua MC. The relationship between the speech perception and the degree of satisfaction among adult users of cochlear implants. Int Arch Otorhinolaryngol. 2013;17(2):202–207. doi: 10.7162/S1809-97772013000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olze H, Grabel S, Forster U, et al. Elderly patients benefit from cochlear implantation regarding auditory rehabilitation, quality of life, tinnitus, and stress. Laryngoscope. 2012;122(1):196–203. doi: 10.1002/lary.22356. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services: Quality Strategy. 2016 https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/CMS-Quality-Strategy.pdf.
- 9.Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;21:339b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40(2):113–128. doi: 10.1097/00005650-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 12.McRackan T, Bauschard M, Hatch J, Nguyen S, Dubno J. Cochlear implantation outcomes evaluated using health related quality of life. Otol and Neurotol under review. doi: 10.1097/MAO.0000000000001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borenstein M. Introduction to meta-analysis. Chichester, U.K: John Wiley & Sons; 2009. [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- 17.Faraone SV. Interpreting estimates of treatment effects: implications for managed care. PT. 2008;33(12):700–711. [PMC free article] [PubMed] [Google Scholar]
- 18.Hinderink JB, Krabbe PF, Van Den Broek P. Development and application of a health-related quality-of-life instrument for adults with cochlear implants: the Nijmegen cochlear implant questionnaire. Otolaryngol Head Neck Surg. 2000;123(6):756–765. doi: 10.1067/mhn.2000.108203. [DOI] [PubMed] [Google Scholar]
- 19.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee Rodgers J, Nicewander WA. Thirteen ways to look at the correlation coefficient. The American Statistician. 1988;42(1):59–66. [Google Scholar]
- 22.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen SM, Labadie RF, Dietrich MS, Haynes DS. Quality of life in hearing-impaired adults: the role of cochlear implants and hearing aids. Otolaryngol Head Neck Surg. 2004;131(4):413–422. doi: 10.1016/j.otohns.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Calvino M, Gavilan J, Sanchez-Cuadrado I, et al. Using the HISQUI29 to assess the sound quality levels of Spanish adults with unilateral cochlear implants and no contralateral hearing. Eur Arch Otorhinolaryngol. 2016;273(9):2343–2353. doi: 10.1007/s00405-015-3789-0. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Cuadrado I, Gavilan J, Perez-Mora R, Munoz E, Lassaletta L. Reliability and validity of the Nijmegen Cochlear Implant Questionnaire in Spanish. Eur Arch Otorhinolaryngol. 2015;272(7):1621–1625. doi: 10.1007/s00405-014-2983-9. [DOI] [PubMed] [Google Scholar]
- 26.Fuller C, Free R, Maat B, Baskent D. Musical background not associated with self-perceived hearing performance or speech perception in postlingual cochlear-implant users. J Acoust Soc Am. 2012;132(2):1009–1016. doi: 10.1121/1.4730910. [DOI] [PubMed] [Google Scholar]
- 27.Park E, Shipp DB, Chen JM, Nedzelski JM, Lin VY. Postlingually deaf adults of all ages derive equal benefits from unilateral multichannel cochlear implant. J Am Acad Audiol. 2011;22(10):637–643. doi: 10.3766/jaaa.22.10.2. [DOI] [PubMed] [Google Scholar]
- 28.Vermeire K, Brokx JP, Wuyts FL, Cochet E, Hofkens A, Van de Heyning PH. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol. 2005;26(2):188–195. doi: 10.1097/00129492-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Vermeire K, Brokx JP, Wuyts FL, et al. Good speech recognition and quality-of-life scores after cochlear implantation in patients with DFNA9. Otol Neurotol. 2006;27(1):44–49. doi: 10.1097/01.mao.0000187240.33712.01. [DOI] [PubMed] [Google Scholar]
- 30.Krabbe PF, Hinderink JB, van den Broek P. The effect of cochlear implant use in postlingually deaf adults. Int J Technol Assess Health Care. 2000;16(3):864–873. doi: 10.1017/s0266462300102132. [DOI] [PubMed] [Google Scholar]
- 31.Olze H, Grabel S, Haupt H, Forster U, Mazurek B. Extra benefit of a second cochlear implant with respect to health-related quality of life and tinnitus. Otol Neurotol. 2012;33(7):1169–1175. doi: 10.1097/MAO.0b013e31825e799f. [DOI] [PubMed] [Google Scholar]
- 32.Maillet CJ, Tyler RS, Jordan HN. Change in the quality of life of adult cochlear implant patients. Ann Otol Rhinol Laryngol Suppl. 1995;165:31–48. [PubMed] [Google Scholar]
- 33.Damen GW, Beynon AJ, Krabbe PF, Mulder JJ, Mylanus EA. Cochlear implantation and quality of life in postlingually deaf adults: long-term follow-up. Otolaryngol Head Neck Surg. 2007;136(4):597–604. doi: 10.1016/j.otohns.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 34.Mo B, Lindbaek M, Harris S. Cochlear implants and quality of life: a prospective study. Ear Hear. 2005;26(2):186–194. doi: 10.1097/00003446-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Newman CW, Weinstein BE, Jacobson GP, Hug GA. The Hearing Handicap Inventory for Adults: psychometric adequacy and audiometric correlates. Ear Hear. 1990;11(6):430–433. doi: 10.1097/00003446-199012000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Ventry IM, Weinstein BE. The hearing handicap inventory for the elderly: a new tool. Ear Hear. 1982;3(3):128–134. doi: 10.1097/00003446-198205000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Klop WM, Boermans PP, Ferrier MB, van den Hout WB, Stiggelbout AM, Frijns JH. Clinical relevance of quality of life outcome in cochlear implantation in postlingually deafened adults. Otol Neurotol. 2008;29(5):615–621. doi: 10.1097/MAO.0b013e318172cfac. [DOI] [PubMed] [Google Scholar]
- 38.Ottaviani F, Iacona E, Sykopetrites V, Schindler A, Mozzanica F. Cross-cultural adaptation and validation of the Nijmegen Cochlear Implant Questionnaire into Italian. Eur Arch Otorhinolaryngol. 2016;273(8):2001–2007. doi: 10.1007/s00405-015-3765-8. [DOI] [PubMed] [Google Scholar]
- 39.van Dijkhuizen JN, Beers M, Boermans PP, Briaire JJ, Frijns JH. Speech intelligibility as a predictor of cochlear implant outcome in prelingually deafened adults. Ear Hear. 2011;32(4):445–458. doi: 10.1097/AUD.0b013e31820510b7. [DOI] [PubMed] [Google Scholar]
- 40.Cox RM, Alexander GC. Measuring Satisfaction with Amplification in Daily Life: the SADL scale. Ear Hear. 1999;20(4):306–320. doi: 10.1097/00003446-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Gaylor JM, Raman G, Chung M, et al. Cochlear implantation in adults: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(3):265–272. doi: 10.1001/jamaoto.2013.1744. [DOI] [PubMed] [Google Scholar]
- 42.Loeffler C, Aschendorff A, Burger T, Kroeger S, Laszig R, Arndt S. Quality of life measurements after cochlear implantation. The Open Otorhinolaryngology Journal. 2010;4:47–54. [Google Scholar]
- 43.Robinson K, Gatehouse S, Browning GG. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann Otol Rhinol Laryngol. 1996;105(6):415–422. doi: 10.1177/000348949610500601. [DOI] [PubMed] [Google Scholar]
- 44.MSTB: The new minimum speech test battery. 2011 http://auditorypotential.com/MSTB.html.
- 45.Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10(Suppl 2):S125–137. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 46.Hawthorne G, Hogan A, Giles E, et al. Evaluating the health-related quality of life effects of cochlear implants: a prospective study of an adult cochlear implant program. Int J Audiol. 2004;43(4):183–192. doi: 10.1080/14992020400050026. [DOI] [PubMed] [Google Scholar]
- 47.Knopke S, Grabel S, Forster-Ruhrmann U, Mazurek B, Szczepek AJ, Olze H. Impact of cochlear implantation on quality of life and mental comorbidity in patients aged 80 years. Laryngoscope. 2016;126(12):2811–2816. doi: 10.1002/lary.25993. [DOI] [PubMed] [Google Scholar]
- 48.Mosnier I, Bebear JP, Marx M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg. 2015;141(5):442–450. doi: 10.1001/jamaoto.2015.129. [DOI] [PubMed] [Google Scholar]
- 49.Olze H, Szczepek AJ, Haupt H, et al. Cochlear implantation has a positive influence on quality of life, tinnitus, and psychological comorbidity. Laryngoscope. 2011;121(10):2220–2227. doi: 10.1002/lary.22145. [DOI] [PubMed] [Google Scholar]
- 50.Tavora-Vieira D, Marino R, Acharya A, Rajan GP. The impact of cochlear implantation on speech understanding, subjective hearing performance, and tinnitus perception in patients with unilateral severe to profound hearing loss. Otol Neurotol. 2015;36(3):430–436. doi: 10.1097/MAO.0000000000000707. [DOI] [PubMed] [Google Scholar]