Abstract
Protein phosphatase 2A (PP2A) is the first Ser/Thr phosphatase recognized to contribute to human and murine lupus immunopathology. PP2A expression in SLE is controlled both epigenetically and genetically, and it is increased in patients with SLE, which contributes to decreased IL-2 production, decreased CD3ζ and increased FcRγ expression on the surface of T cells, increased CREMα expression, hypomethylation of genes associated with SLE pathogenesis, and increased IL-17 production. B regulatory subunit of PP2A regulates IL-2 deprivation-induced T cell death and is decreased in SLE patients. A mouse overexpressing PP2Ac in T cells displays peripheral granulocytosis, elevated IL-17 production, and develops glomerulonephritis when challenged. A mouse which lacks PP2Ac only in regulatory T cells develops severe autoimmunity and multiorgan inflammation because of loss of restraint on mTORC1 and inability of Foxp3+ cells to regulate conventional T cells. Targeting PP2A in T cell subsets may be therapeutic for SLE and other autoimmune diseases.
1. Introduction
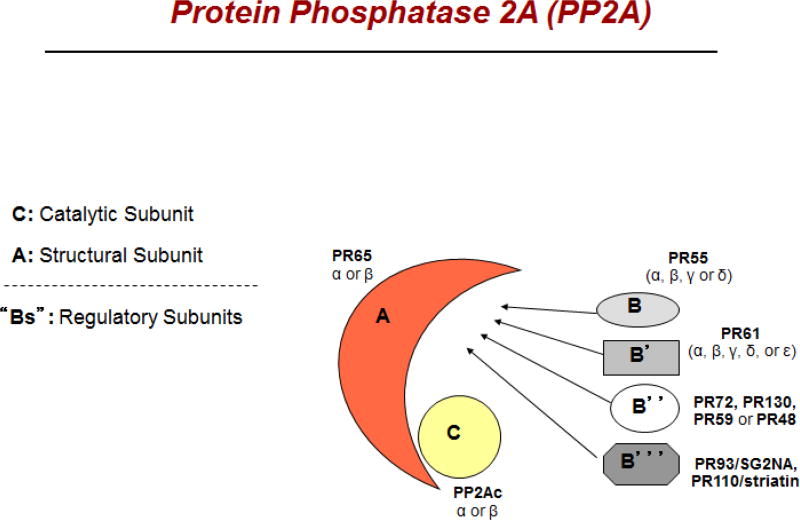
Protein phosphatase 2A (PP2A) is a highly conserved and ubiquitous serine-threonine phosphatase that is reviewed here. It is involved in essential cellular functions including cell cycle progression, cellular metabolism, migration and apoptosis [1]. PP2A is a heterotrimer composed of three distinct subunits —the scaffold A subunit (PP2AA), the regulatory B subunit (PP2AB) and the catalytic C subunit (PP2AC). The scaffold A subunit and the catalytic C subunit (PP2AA-PP2AC) forms the PP2A core enzyme that is responsible for dephosphorylation, and this catalytic core associates with one of the regulatory B subunits. In humans each PP2A subunit is located on separate chromosomes. The diversity in PP2A complex is dictated by the multiple isoforms for each subunit. PP2AC has two isoforms (PPP2CA, Cα and PPP2CB, Cβ), PP2AA has two isoforms (PPP2R1A, Aα and PPP2R1B, Aβ) and PP2AB has at least 17 different isoforms that are categorized into four families as B family (B55; gene symbol PPP2R2), B′ family (B56; gene symbol PPP2R5), B″ family (PR72/130; gene symbol PPP2R3), and Striatin family [2] (Fig. 1).
Figure 1.
Schematic structure of protein phosphatase 2A (PP2A). It is composed of three hetero-dimers, the scaffold subunit, the catalytic subunit, and the regulatory subunit (depicted as A, C, and B, respectively). There are four families of regulatory subunits, each having multiple isoforms as shown in the figure.
PP2Ac activity is N at its carboxy-terminal tail [3], wherein phosphorylation of the Tyr307 residue at the carboxy-terminal end of PP2Ac results in the inactivation of PP2A. Further, inhibition of PP2A activity occurs when alpha 4 associates with PP2AC [4]. When alpha 4 binds PP2Ac it also prevents its degradation [5]. PP2A is involved in the development of cancer, neurodegenerative diseases and systemic lupus erythematosus (SLE) [6,7]. In the last decade PP2A has been the focus of our research while studying autoimmune diseases. This review will focus on the role of PP2A in the development of autoimmunity and specifically SLE. In addition, it will exemplify the potential of the PP2AB diversity of subunits to affect the evolvement of pathogenic pathways that may cause autoimmune diseases.
2. PP2A is elevated in patients with SLE
The levels (mRN A and protein) of the catalytic subunit and activity of protein phosphatase 2A (PP2Ac) is increased in T cells from SLE patients [8]. This heightened expression occurs regardless of the disease activity status or the treatment the patients received and therefore it is considered a disease-specific abnormality. The expression levels in T cells are determined both genetically and epigenetically.
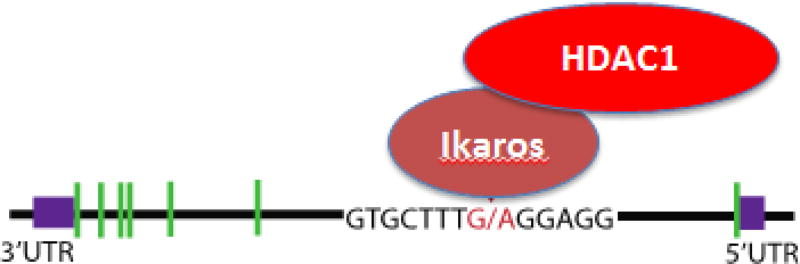
A CpG motif in the proximal promoter is involved in the transcription of the PP2Ac [9], whereas a SNP in the first exon was recognized in a GWAS study to confer susceptibility for SLE [10]. The SNP is located within the cis cite for the transcription Ikaros, a transcriptional repressor [11] that suppresses PP2A expression by modulating chromatin modifications. After binding Ikaros recruits histone deacetylase HDAC1 which leads to suppression of PP2A transcription. The susceptibility allele binds Ikaros with lower avidity and therefore its repressive activity may be limited. In addition, peripheral blood mononuclear cells from SLE patients were shown to have reduced levels of Ikaros mRNA [12], yet it is likely that proteins/factors in addition to Ikaros are involved in the regulation of PP2A levels (Fig. 2).
Figure 2.
Single nucleotide polymorphism (SNP) in PP2A from SLE T cells impairs the binding of repressor transcription factor Ikaros. A schematic representation of the gene IKZF1, wherein the green boxes represent the introns, and the red letters the sequence of the variant site to which Ikaros binding is impaired resulting in less histone deacetylase HDAC1 recruitment, and less suppression of PP2A transcription.
The expression of PP2Ac, in addition to the genetic factors, is epigenetically controlled. Specifically, in SLE T cells the catalytic subunit α isoform of PP2A (PP2Acα) activity (mRNA and protein) is increased, and this occured due to hypomethylation within the cAMP response element (CRE) motif in the gene promoter, and within the region in the gene that binds p-CREB [9]. Importantly, enhanced binding of p-CREB to the CRE site leads to increased expression of PP2Acα, which contributed to the decrease in IL-2 production in SLE T cells.
3. PP2A suppresses IL-2 production
The high levels of PP2Ac in T cells from SLE patients play a significant role in decreased IL-2 expression as IL-2 levels could be restored upon silencing of the mRNA expression of PP2Ac [8]. Normalization of IL-2 was shown to be mediated by elevating the levels of phosphorylated cAMP response element-binding (pCREB) protein, which enable its binding to the IL-2 and c-fos promoters. As a result, the activity of activator protein 1 (AP-1- cfos/c-jun heterodimer) increases and the reduced production of IL-2 becomes normal.
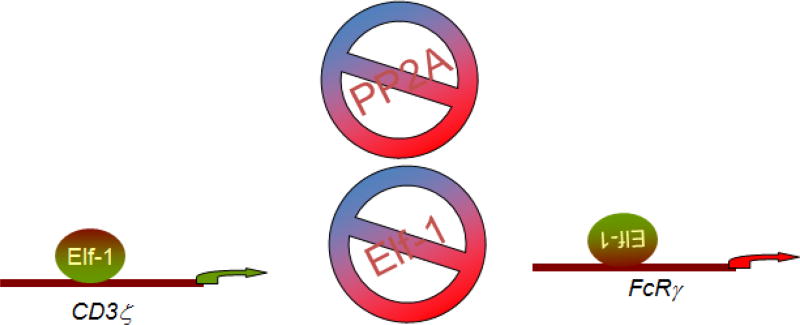
PP2A controls the wiring of the CD3/TCR complex by controlling the expression of. CD3ζ- and FcRγ genes at the transcriptional level [13]. The increased PP2Ac activity results in aberrant signaling of the CD3 complex that contributes to the abnormal T cell function. CD3ζ- and FcRγ chains are affected antithetically by the transcription factor Elf-1, and PP2Ac dephosphorylates Elf-1 (at Thr-231), yielding limited binding of Elf-1 to the CD3ζ and FcRγ promoters. Consequently, the content of CD3ζ-chain is decreased and that of FcRγ-chain is increased within the CD3 complex. This aberrant TCR-initiated signaling is not propagated through the normally used CD3ζ-chain but rather through the FcRγ-chain and corroborates decreased production of IL-2 [14]. Replenishment of the CD3ζ -chain expression in SLE T cells also results in increased production of IL-2 [15] (Fig. 3). Notably, although the effect of PP2A is counterbalanced by PKC, in SLE T cells it probably remains unopposed through this pathway because the expression and activity of PKC were reported to be low [8].
Figure 3.
CD3ζ - and FcRγ chains are components of CD3/TCR complex, and the transcription factor Elf-1 affects them antithetically. The increased PP2Ac activity results in Elf-1 dephosphorylation, and impairs Elf-1 binding to the CD3ζ and FcRγ promoters suppressing and enhancing their transcriptional activity respectively.
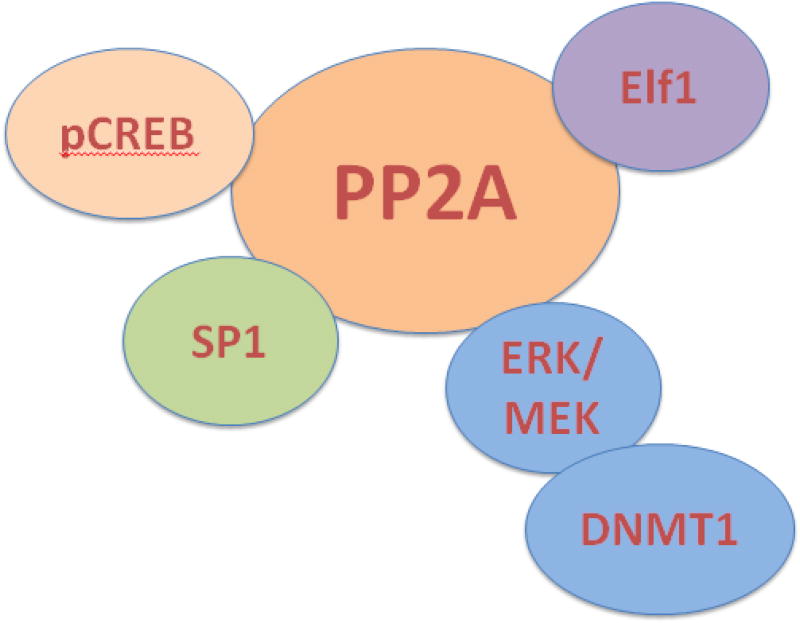
Another pathway that mediates the reduction in IL-2 levels in SLE is through PP2A-driven dephosphorylation of the transcription factor spec ificity protein-1 (SP-1) at Ser59 [16]. Consequently, SP-1 binds strongly to the basic leucine zipper transcription factor cAMP-responsive element modulator (CREM)α. CREMα is abnormally increased in SLE T cells and it directly binds to IL-2 and T cell receptor ζ chain promoters to suppress their transcription (Fig. 4).
Figure 4.
PP2A controls a number of pathways in the SLE T cell.
4. PP2A and pathogenesis of SLE
The steadily enhanced levels of PP2Ac in SLE T-cells mediates dephosphorylation of MEK and ERK upon T cell activation, which results in decreasing the enzyme activity of DNA methyltransferase (DNMT). Consequently, DNA hypomethylation becomes predominant and in SLE it is considered a characteristic feature [17]. Among the methylation-sensitive genes that are highly express is CD70 gene, whose protein product is expressed on SLE-T cells and it is a costimulatory ligand for B cells thereby mechanistically participating in immunoglobulin overproduction.
In addition to the human data, the study of PP2A in mice has significantly elaborated on mechanistic pathways in the pathogenesis of SLE. Microarray analyses demonstrated that the increased expression of PP2Ac in T cells enables the production of an array of proinflammatory effector molecules, including IL-17 [18]. PP2Ac regulates the Il17 locus by enhancing histone 3 acetylation in T cells from both PP2Ac transgenic mice and patients with SLE, and it involved the activation of interferon regulatory factor 4.
5. PP2A and the control of autoimmunity
We found that Tregs require PP2A for keeping their suppressive capabilities in vivo [19]. Mice whose Tregs are deficient in PP2A develop a severe lymphoproliferative and autoimmune disorder with spontaneous activation of the immune system and autoantibody production that were also against lupus-associated nuclear autoantigens. These mice manifest wasting, dermatitis, scaly tails and ears, skin rash and ulcerations, all of which showed similarities to the phenotype of scurfy mice [20]. Mice with PP2A-deficient Tregs had greater activation of both CD4+ T cells and CD8+ T cells and they produced significantly larger amounts of IL-17 and IL-2, IFN-γ, and TNF-α.
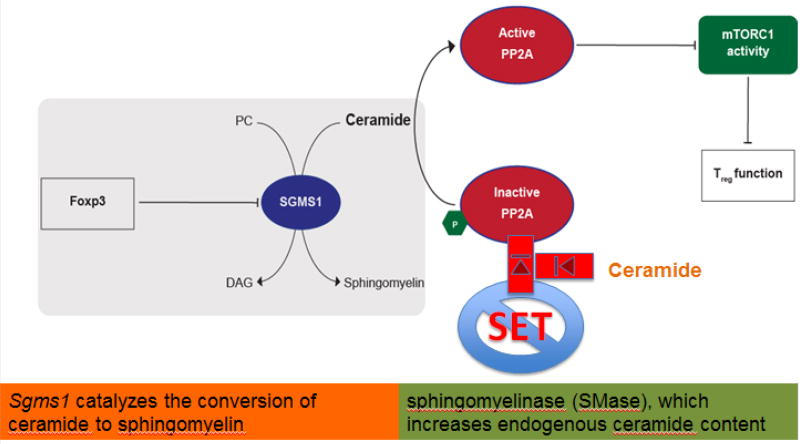
Potent Tregs maintain PP2Ac activity in contrast to Tconv wherein PP2Ac is inactivated by Tyr307-phosphorylation in response to TCR stimulation. Although both Tregs and Tconv have equal levels of SET that phosphorylates at Tyr307- [21], only Tregs had higher amounts of ceramide, which inhibits SET, thus allowing increased activity of PP2A in Tregs despite the high levels of SET. This difference between the two cell types is explained by the action of the Treg master gene Foxp3 that targets and represses Sgms1 encoding the enzyme catalyzing ceramide, eventually leading to ceramide accumulation in Tregs. Ceramide-driven activation of PP2Ac in Tregs restrains the mTORC1 complex (Fig 5).
Figure 5.
Treg cells inhibit mTO RC1 through ceramide-mediated activation of PP2A. PC: Phosphatidylcholine, DAG: diacyl-glycerol.
6. PP2A B subunits
The PP2A family of phosphatases comprises about 100 heterotrimeric holoenzymes. It is achieved due to the combinatorical association between the different subunits that compose PP2A. The regulatory PP2A B subunit families are composed of 4 with up to five family members that have been identified, resulting in a large diversity of PP2A holoenzymes [22]. The regulatory subunits confer substrate specificity and subcellular localization. The PP2A trimer is assembled when the PP2A core enzyme (the A and C dimer) gets associate with the appropriate B subunit, after which the specificity and regulation of PP2A is then determined [23]. In the literature most of the B subunits are described in relation to cancer and only a few of them are linked to the autoimmune diseases.
6.1 B subunit - B55 alpha (PPP2R2A)
B55 alpha isoform was shown to affect cell cycle and stress survival protein. In cancer cells it affected apoptosis through survival signaling conveyed by dephosphorylation of AKT at threonine 308 [24], and it was also capable to affect PKC alpha [25]. Thus, B55 alpha is likely to share some of these pathways in autoreactive cells to affect their survival and proliferative status. In the context of autoimmunity, the B55α-PP2Ac complex was demonstrated to control autophagy [26]. Autophagy is induced following the formation of autophagy-related proteins that produce complexes such as ULK1 [27]. The latter was found to have at least two mTOR sites and during amino acid starvation of the cells when autophagy is initiated, these sites are dephosphorylated by PP2Ac-B55α. In SLE it is suggested that autophagy may play a pathogenic role. B55α was shown in patients and in lupus-prone mice to regulate the survival of autoreactive B cells and to promote differentiation of plasma cells [28]. Further, autophagy-related gene 5 (ATG5) was linked to SLE in genome-wide association studies [29,30].
6.2 B subunit - B55 beta (PPP2R2B)
PP2A supports the survival of autoreactive T cells. In healthy subjects, the Bβ subunit of PP2A (e.g. PPP2R2B) mediates apoptosis of resting T cells that is due to IL-2 deprivation [31]. Activated T cells are protected from apoptosis, however, as IL-2 declines it leads to the induction of PPP2R2B and then the cells are no longer protected from apoptosis. In patients with SLE, the expected induction of PP2A Bβ in T cells upon IL-2 deprivation did not occur in 50% of the patients, and indeed this defect was accompanied by the cell resistance to apoptosis, which might accounted, at least in part, for the longer survival of autoreactive T cells.
6.3 B’ subunit - B56γ - (PPP2R5C)
The PP2A regulatory subunit B56 was reported as suppressor of NF-κB in TCR signaling [32]. NF-κB is essential for activation of normal T cells [33]. Human T cells that were stimulated via their TCR had increased the expression of B56. Silencing of B56 led to increased IKK and IκBa phosphorylation, enhanced NF-κB activity, which resulted in increased NF-κB target gene expression, including IL-2 that was strongly enhanced on mRNA and protein level. Moreover, T cell proliferation was increased upon B56 silencing.
6.4 B” subunit - PR130 - (PPP2R3A)
The regulatory B subunit PR130 of the PP2A is the ubiquitous spliced variant of the PPP2R3A gene (PR72 is another spliced variant of this gene). The biological function of PP2A-PR130 complex was demonstrated to affect EGFR degradation and EGF-mediated signaling [34]. PR130 interacts with SHIP2 and prevents EGF-induced EGFR degradation, thereby constituting a positive regulator of EGF signaling.
EGFR signaling regulates lipocalin 2 (LCN-2) that is a biomarker for renal injury including in lupus nephritis [35]. Polymorphism of the EGFR Bsr I gene was related to SLE [36]. Further, autoantibodies to the recombinant extra cellular domain of EGFR were detected in sera from lupus prone mice in patients with scleroderma and SLE [37]. Given that PR130-PP2A complex controls EGFR degradation it is possible that it may play a pathogenic role in the development of these autoantibodies.
7. Concluding Remarks
Tyrosine phosphatases have been implicated in the expression of autoimmunity [38]. Ser/Thr phosphatases however, have not been considered in the pathogenesis of autoimmunity. The work which was discussed here presents the role of PP2A in the regulation of signaling pathways in SLE T cells and assigns to it a central role. Study of PP2A in genetically engineered mice has shown that PP2A may control opposing functions in various T cell subsets. Moreover, study of the PP2A regulatory subunits has revealed that each subunit may control distinct lymphocyte functions. Detailed knowledge on the role PP2A plays in T cell subsets and the unique contribution of the regulatory subunits will enable targeting PP2A with inhibitors or stimulators in T cell subsets and the consideration of developing enhancers or inhibitors of regulatory subunits in the treatment of SLE and other autoimmune disease.
It remains to be seen whether PP2A controls the function of other cells of the immune system including B cells, neutrophils, monocytes and innate lymphoid cells. Complete understanding or the role of the PP2A in the control of the immune response should allow us to better design PP2A modulators to treat autoimmune disease and cancer where the opposite exactly effect is desired.
Acknowledgments
Work was supported by NIH grants RO1 AI68787 and T32 74549 to GCT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Lambrecht C, Haesen D, Sents W, Ivanova E, Janssens V. Structure, regulation, and pharmacological modulation of PP2A phosphatases. Methods Mol. Biol. 2013;1053:283–305. doi: 10.1007/978-1-62703-562-0_17. [DOI] [PubMed] [Google Scholar]
- 3.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Kong M, Fox CJ, Mu J, Solt L, Xu A, Cinalli RM, Birnbaum MJ, Lindsten T, Thompson CB. The PP2A-associated protein alpha4 is an essential inhibitor of apoptosis. Science. 2004;306:695–698. doi: 10.1126/science.1100537. [DOI] [PubMed] [Google Scholar]
- 5.Jiang L, Stanevich V, Satyshur KA, Kong M, Watkins GR, Wadzinski BE, Sengupta R, Xing Y. Structural basis of protein phosphatase 2A stable latency. Nat Commun. 2013;4:1699. doi: 10.1038/ncomms2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sontag JM, Sontag E. Protein phosphatase 2A dysfunction in Alzheimer's disease. Front Mol Neurosci. 2014;7:16. doi: 10.3389/fnmol.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 8.Katsiari CG, Kyttaris VC, Juang YT, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J Clin Invest. 2005;115:3193–3204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunahori K, Juang YT, Kyttaris VC, Tsokos GC. Promoter hypomethylation results in increased expression of protein phosphatase 2A in T cells from patients with systemic lupus erythematosus. J Immunol. 2011;186:4508–4517. doi: 10.4049/jimmunol.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan W, Sunahori K, Zhao J, Deng Y, Kaufman KM, Kelly JA, Langefeld CD, Williams AH, Comeau ME, Ziegler JT, Marion MC, Bae SC, Lee JH, Lee JS, Chang DM, Song YW, Yu CY, Kimberly RP, Edberg JC, Brown EE, Petri MA, Ramsey-Goldman R, Vilá LM, Reveille JD, Alarcón-Riquelme ME, Harley JB, Boackle SA, Stevens AM, Scofield RH, Merrill JT, Freedman BI, Anaya JM, Criswell LA, Jacob CO, Vyse TJ, Niewold TB, Gaffney PM, Moser KL, Gilkeson GS, Kamen DL, James JA, Grossman JM, Hahn BH, Tsokos GC, Tsao BP, Alarcón GS. BIOLUPUS Network.; GENLES Network.. Association of PPP2CA polymorphisms with systemic lupus erythematosus susceptibility in multiple ethnic groups. Arthritis Rheum. 2011;63:2755–2763. doi: 10.1002/art.30452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagpal K, Watanabe KS, Tsao BP, Tsokos GC. Transcription factor Ikaros represses protein phosphatase 2A (PP2A) expression through an intronic binding site. J Biol Chem. 2014;289:13751–1377. doi: 10.1074/jbc.M114.558197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W, Sun L, Gao J, Li Y, Wang P, Cheng Y, Pan T, Han J, Liu Y, Lu W, Zuo X, Sheng Y, Yao S, He C, Yu Z, Yin X, Cui Y, Yang S, Zhang X. Down-regulated expression of IKZF1 mRNA in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Rheumatol Int. 2011;31:819–822. doi: 10.1007/s00296-010-1576-1. [DOI] [PubMed] [Google Scholar]
- 13.Tsokos GC, Nambiar MP, Tenbrock K, Juang YT. Rewiring the T-cell: signaling defects and novel prospects for the treatment of SLE. Trends Immunol. 2003;24:259–263. doi: 10.1016/s1471-4906(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 14.Juang YT, Wang Y, Jiang G, Peng HB, Ergin S, Finnell M, Magilavy A, Kyttaris VC, Tsokos GC. PP2A dephosphorylates Elf-1 and determines the expression of CD3zeta and FcRgamma in human systemic lupus erythematosus T cells. J Immunol. 2008;181:3658–3664. doi: 10.4049/jimmunol.181.5.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nambiar MP, Fisher CU, Warke VG, Krishnan S, Mitchell JP, Delaney N, Tsokos GC. Reconstitution of deficient T cell receptor zeta chain restores T cell signaling and augments T cell receptor/CD3-induced interleukin-2 production in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1948–1955. doi: 10.1002/art.11072. [DOI] [PubMed] [Google Scholar]
- 16.Juang YT, Rauen T, Wang Y, Ichinose K, Benedyk K, Tenbrock K, Tsokos KC. Transcriptional activation of the cAMP-responsive modulator promoter in human T cells is regulated by protein phosphatase 2A-mediated dephosphorylation of SP-1 and reflects disease activity in patients with systemic lupus erythematosus. J Biol Chem. 2011;286:1795–1801. doi: 10.1074/jbc.M110.166785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunahori K, Nagpal K, Hedrich CM, Mizui M, Fitzgerald LM, Tsokos GC. The catalytic subunit of protein phosphatase 2A (PP2Ac) promotes DNA hypomethylation by suppressing the phosphorylated mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)/phosphorylated ERK/DNMT1 protein pathway in T-cells from controls and systemic lupus erythematosus patients. J Biol Chem. 2013;288:21936–21944. doi: 10.1074/jbc.M113.467266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispín JC. Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling. J Biol Chem. 2013;288:26775–26784. doi: 10.1074/jbc.M113.483743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apostolidis SA, Rodríguez-Rodríguez N, Suárez-Fueyo A, Dioufa N, Ozcan E, Crispín JC, Tsokos MG, Tsokos GC. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat Immunol. 2016;17:556–564. doi: 10.1038/ni.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 22.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283:1882–18892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 25.Ruvolo PP, Ruvolo VR, Jacamo R, Burks JK, Zeng Z, Duvvuri SR, Zhou L, Qiu Y, Coombes KR, Zhang N, Yoo SY, Pan R, Hail N, Jr, Konopleva M, Calin G, Kornblau SM, Andreeff M. The protein phosphatase 2A regulatory subunit B55α is a modulator of signaling and microRNA expression in acute mye loid leukemia cells. Biochim Biophys Acta. 2014;1843:1969–1977. doi: 10.1016/j.bbamcr.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong PM, Feng Y, Wang J, Shi R, Jiang X. Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Nat Commun. 2015;6:8048. doi: 10.1038/ncomms9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong PM, Puente C, Ganley IG, Jiang X. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 2013;9:124–137. doi: 10.4161/auto.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke AJ, Ellinghaus U, Cortini A, Stranks A, Simon AK, Botto M, Vyse TJ. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann Rheum Dis. 2015;74:912–920. doi: 10.1136/annrheumdis-2013-204343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou XJ, Lu XL, Lv JC, Yang HZ, Qin LX, Zhao MH, Su Y, Li ZG, Zhang H. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis. 2011;70:1330–1337. doi: 10.1136/ard.2010.140111. [DOI] [PubMed] [Google Scholar]
- 30.International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN) Harley JB, Alarcón-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crispín JC, Apostolidis SA, Finnell MI, Tsokos GC. Induction of PP2A Bβ, a regulator of IL-2 deprivation-induced T-cell apoptosis, is deficient in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2011;108:12443–12448. doi: 10.1073/pnas.1103915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breuer R, Becker MS, Brechmann M, Mock T, Arnold R, Krammer PH. The protein phosphatase 2A regulatory subunit B56γ mediates suppression of T cell receptor (TCR)-induced nuclear factor-κB (NF-κB) activity. J Biol Chem. 2014;289:14996–15004. doi: 10.1074/jbc.M113.533547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zwaenepoel K, Goris J, Erneux C, Parker PJ, Janssens V. Protein phosphatase 2A PR130/B"alpha1 subunit binds to the SH2 domain-containing inositol polyphosphate 5-phosphatase 2 and prevents epidermal growth factor (EGF)-induced EGF receptor degradation sustaining EGF-mediated signaling. FASEB J. 2010;24:538–547. doi: 10.1096/fj.09-140228. [DOI] [PubMed] [Google Scholar]
- 35.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CM, Tsai CH, Chen CL, Chang CP, Lai CC, Tsai FJ. Epidermal growth factor receptor (EGFR) gene Bsr I polymorphism is associated with systemic lupus erythematosus. Lupus. 2004;13:773–776. doi: 10.1191/0961203304lu1081oa. [DOI] [PubMed] [Google Scholar]
- 37.Planque S, Zhou YX, Nishiyama Y, Sinha M, O'Connor-Mccourt M, Arnett FC, Paul S. Autoantibodies to the epidermal growth factor receptor in systemic sclerosis, lupus, and autoimmune mice. FASEB J. 2003;17:136–143. doi: 10.1096/fj.01-0847com. [DOI] [PubMed] [Google Scholar]
- 38.Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]