Abstract
Inhibition of monoacylglycerol lipase (MAGL), the primary enzyme that hydrolyzes the endocannabinoid 2-arachidonoylglycerol (2-AG) in the brain, produces profound anti-inflammatory and neuroprotective effects and improves synaptic and cognitive functions in animal models of Alzheimer’s disease (AD). However, the molecular mechanisms underlying the beneficial effects produced by inhibition of 2-AG metabolism are still not clear. The cannabinoid receptor type 2 (CB2R) has been thought to be a therapeutic target for AD. Here we provide evidence, however, that CB2R does not play a role in ameliorating AD neuropathology produced by inactivation of MAGL in 5XFAD APP transgenic mice, an animal model of AD. We observed that expression of APP and β-secretase as well as production of total Aβ and Aβ42 were significantly reduced in APP transgenic mice lacking CB2R (TG-CB2-KO) treated with JZL184, a selective and potent inhibitor for MAGL. Inactivation of MAGL also alleviated neuroinflammation and neurodegeneration in TG-CB2-KO mice. Importantly, TG-CB2-KO mice treated with JZL184 still exhibited improvements in spatial learning and memory. In addition, MAGL inhibition prevented deterioration in expression of important synaptic proteins in TG-CB2-KO mice. Our results suggest that CB2R is not required in ameliorating neuropathology and preventing cognitive decline by inhibition of 2-AG metabolism in AD model animals.
Keywords: Endocannabinoid, cannabinoid receptor, 2-arachidonoylglycerol, monoacylglycerol lipase, cyclooxygenase, arachidonic acid, prostaglandin
Introduction
The endocannabinoid system consists of endocannabinoids, cannabinoid receptors (CB1R and CB2R), the enzymes synthesizing and degrading endocannabinoids, and transporters. Growing evidence indicates that endocannabinoids display properties with anti-inflammation and neuroprotection in response to harmful insults [1–5]. The endocannabinoid 2-arachidonoylglycerol (2-AG) is the most abundant endogenous cannabinoid and a full agonist for both CB1R and CB2R [6]. 2-AG is primarily produced from diacylglycerol (DAG) by diacylglycerol lipases and hydrolyzed by monoacylglycerol lipase (MAGL) and α/β hydrolase domain-containing protein 6 and 12 (ABHD6/12). It is also oxidatively metabolized by cyclooxygenase-2 (COX-2) when expression and activity of COX-2 are excessively elevated during inflammation [4, 7]. It has been estimated that 85% of 2-AG in the brain is degraded by MAGL [8, 9].
The metabolites of 2-AG are glycerol and arachidonic acid (AA), which is a precursor of prostaglandins through the catalytic activity of COX-1/2 and leukotrienes through the enzyme arachidonate 5-lipoxygenase (LOX). Prostaglandins (i.e., PGE2) and leukotrienes are important inflammatory mediators and proinflammatory [10, 11], while 2-AG is anti-inflammatory and neuroprotective [1–5]. This suggests that inhibition of MAGL would be a promising strategy for alleviation of neuroinflammation-associated neuropathology in neurodegenerative diseases by enhancing anti-inflammatory and neuroprotective 2-AG signaling, while reducing proinflammatory and neurotoxic eicosanoid levels [4, 7, 12]. Indeed, previous studies demonstrate that pharmacological inactivation or genetic deletion of MAGL reduces Aβ formation and accumulation, neuroinflammation and neurodegeneration and improves synaptic and cognitive function in APP transgenic mice, animal models of Alzheimer’s disease (AD) and in the 1-methyl-4-phenyl-tetrahydropyridine (MPTP) mouse model of Parkinsonism [9, 13, 14]. However, the molecular mechanisms underlying the beneficial effects produced by inhibition of 2-AG metabolism are still not clear.
CB2R is expressed in both neurons and astroglial cells in the brain and plays an important role in neuroinflammatory responses [15–24]. Earlier studies show that CB2R is upregulated in microglia surrounding senile plaques in post-mortem brains of AD patients, suggesting the involvement of CB2R in AD neuropathology [25, 26]. However, increased expression of CB2R detected in postmortem cortical brain tissues from AD patients does not correlate with their cognitive status [26]. The studies using animal models of AD also exhibit diverse results. For instance, APP transgenic mice lacking CB2R display increased Aβ and plaque associated microglia [27, 28], while reactive microglia, proinflammatory cytokines, and Aβ are reduced in other studies. [29]. Moreover, inhibition of CB2R fails to block MAGL inactivation-produced decreases in inflammatory cytokines in APP transgenic animals or in animals-treated with the inflammagen lipopolysaccharides [9, 14]. Similarly, inhibition of 2-AG metabolism still reduces neuroinflammation in APP transgenic mice deficient in CB2R [13]. The results from these previous studies suggest that the role of CB2R in AD is complex. In particular, CB2R has been thought to be a promising therapeutic target for neurodegenerative diseases such as AD [16, 17, 30]. To further determine whether CB2R contributes to the MAGL inactivation-produced alleviation of AD neuropathology, we created 5XFAD APP transgenic mice lacking CB2R (TG-CB2-KO). Our results suggest that CB2R does not play an active role in ameliorating AD neuropathology and improving cognitive function produced by inhibition of 2-AG metabolism in an animal model of AD.
RESULTS
Inhibition of MAGL reduces expression of BACE1 and production of Aβ in APP TG mice lacking CB2R
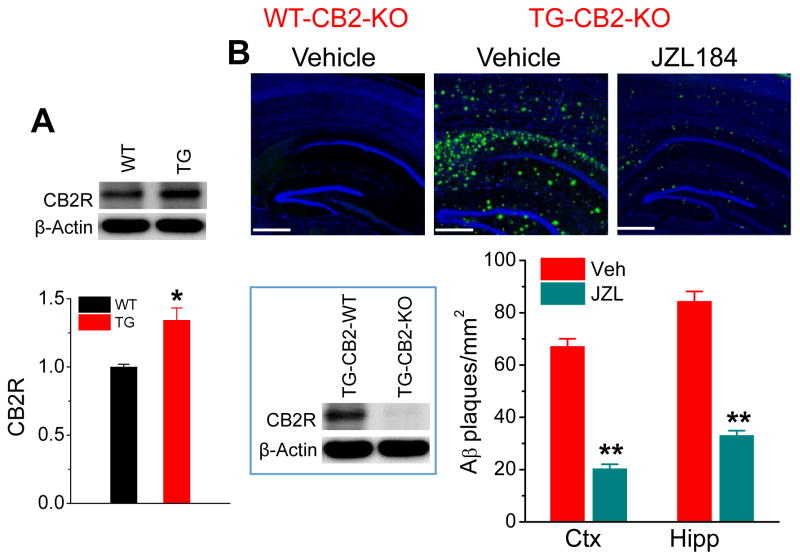
Previous studies demonstrate that pharmacological inhibition or genetic deletion of MAGL reduces expression of BACE1 and formation of Aβ in APP transgenic mice [13, 14, 31, 32]. Expression of CB2R is increased in AD [25] and CB2R has been implicated important in neuroinflammation and neurodegenerative dieses [16, 17, 27, 33]. We observed that expression of CB2R was increased in the brain of 6-month-old TG mice (Fig. 1A), suggesting that Aβ and inflammatory responses stimulate expression of CB2R. If CB2R is involved in MAGL inhibition-produced decreases in Aβ processing and formation, ablation of CB2R should diminish or attenuate these effects. In this regards, we generated 5XFAD transgenic mice lacking CB2R (TG-CB2R-KO), as described previously [13], to determine whether CB2R is important in alleviation of AD neuropathology induced by MAGL inactivation. To confirm that CB2R is knocked out in TG-CB2R KO mice, we validated the knockout of CB2R by detecting expression of CB2R using immunoblotting in addition to genotyping and confirmed that expression of CB2R was absent in the brain of TG-CB2R KO mice (inset, Fig. 1B). Interestingly, we still observed accumulation and deposition of a significant number of Aβ plaques in the brain sections of 6-month-old TG-CB2R-KO mice treated with vehicle, similar to that in TG mice reported previously [13]. However, treatment with the MAGL inhibitor JZL184 at a dose of 12 mg/kg (three times/week for 8 weeks) resulted in reduction of total Aβ accumulation and deposition in the brain (Fig. 1B). The rationale for this dosing regimen was largely based on our previous studies where JZL184 at a dose of 12 mg/kg three times a week for 8 consecutive weeks significantly reduces neuropathology and improves learning and memory in 5XFAD TG mice [13, 31].
Figure 1. MAGL inhibition reduces production and deposition of Aβ in 5XFAD APP transgenic (TG) mice lacking CB2R (TG-CB2R-KO).
A) Expression of CB2R is increased in 6-month-old TG mice. Western blot analysis of brain CB2R expression in TG mice and their age-matched wild-type controls. Data are means ±SEM. *P<0.05 compared with WT controls (ANOVA with Fisher PLSD test, n=3 mice per group). B) Number of Aβ plaques is reduced in TG-CB2R-KO mice treated with JZL184. Total Aβ plaques were detected by anti-4G8 antibody in the brain of 6 months old APP transgenic (TG) mice lacking CB2R (TG-CB2R-KO) and their age-matched WT mice lacking CB2R (WT-CB2R-KO) that received vehicle or JZL184 (12 mg/kg, i.p., three times per week for 8 weeks) starting at 4 months of age. Cell nuclei in the sections were stained with DAPI (Blue). Data are means ±SEM. **P<0.01 compared with the vehicle control (ANOVA with Bonferroni post-hoc test, n=4 to 5 mice per group). Scale Bars: 400 μm. Inset: Validation of CB2R knockout by immunoblotting detection of CB2R in TG-CB2R-WT and TG-CB2R-KO mice.
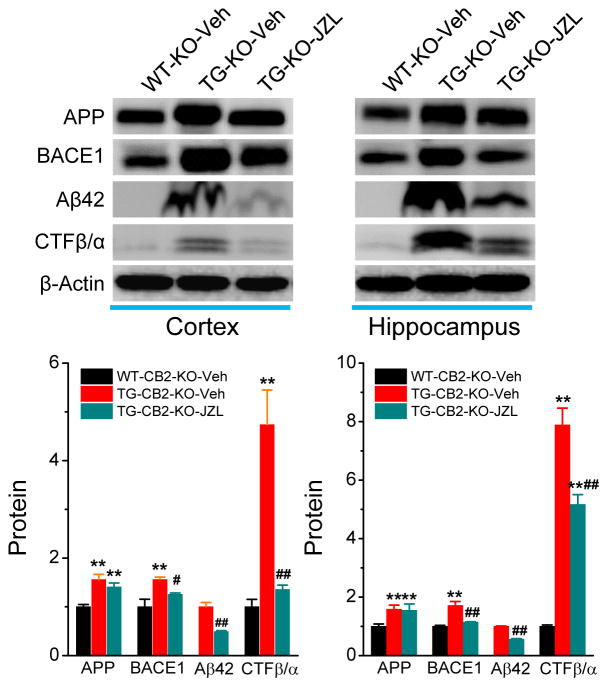
β-Site amyloid precursor protein cleaving enzyme 1 (BACE1) is a key enzyme responsible for formation of Aβ. We demonstrated previously that MAGL inhibition-reduced Aβ is associated with suppression of BACE1 [13]. To determine whether CB2R contributes to the reduced BACE1 by MAGL inactivation, we detected expression of APP and BACE1 in TG-CB2R-KO mice treated with vehicle or JZL184. As shown in Figure 2, expression of APP and BACE1 was significantly reduced in both the cortex and hippocampus of TG-CB2R-KO mice when compared with the vehicle-treated animals. Reduced APP and BACE1 by inactivation of MAGL also led to decreases in production of Aβ42 and the c-terminal fragments CTFβ/α. These results indicate that JZL184 still decreases expression of BACE1 and production of Aβ in TG-CB2R-KO, suggesting that CB2R does not play an important role in MAGL inhibition-induced decrease in Aβ processing.
Figure 2. JZL184 decreases expression of BACE1 and production of Aβ in TG-CB2R-KO mice.
Immunoblot analysis of APP, BACE1, Aβ42, and CTFβ/α in the cortex and hippocampus in 6 months old WT-CB2R-KO and TG-CB2R-KO mice treated with the vehicle or JZL184. **P<0.01 compared with the WT-CB2R-KO vehicle control; #P<0.05, ##P<0.01 compared with the TG-CB2R-KO vehicle control. (ANOVA with Fisher’s PLSD test, n=3 mice per group).
Inactivation of MAGL suppresses astrocytic reactivity and reduces neurodegeneration in TG-CB2R-KO mice
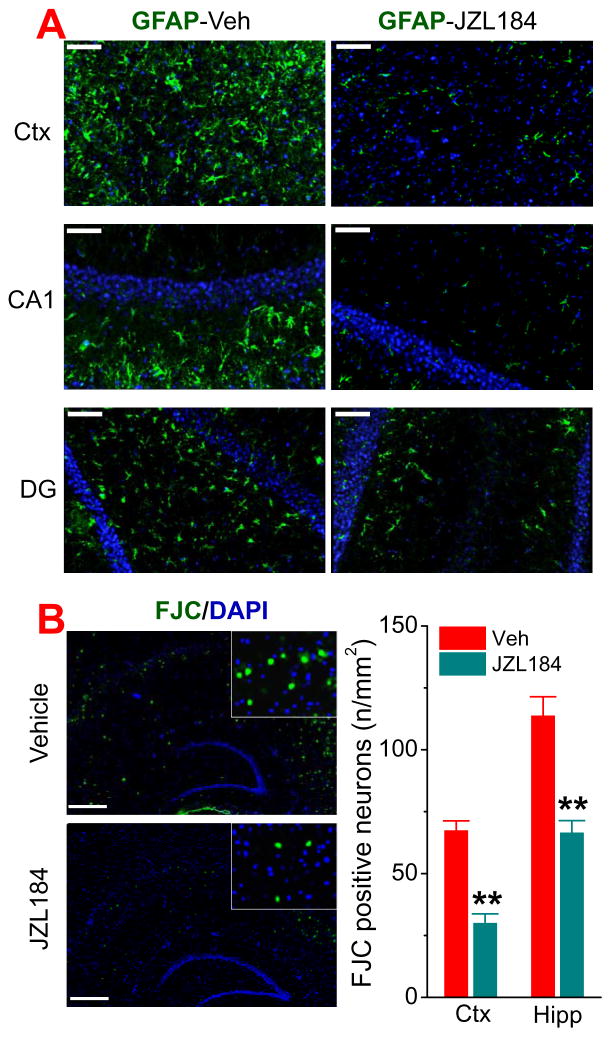
CB2R is expressed in both neurons and astroglial cells in the brain and plays an important role in neuroinflammatory responses [15–24]. However, previous studies demonstrate that CB2R does not play a role in resolving neuroinflammation by inhibition of 2-AG metabolism [9, 13, 14]. To confirm the anti-inflammatory effects of MAGL inhibition in the absence of CB2R, we assessed GFAP immunoreactivity, a marker for neuroinflammation, in the brain of TG-CB2R-KO mice treated with vehicle or JZL184 three times a week for 8 weeks. As shown in Figure 3A, GFAP immunoreactivity was decreased in the cortex and hippocampus of mice treated with JZL184. This is consistent with previous observations where pharmacological inhibition or genetic deletion of CB2R does not block MAGL inactivation-induced resolution of neuroinflammation in APP TG mice [13, 14].
Figure 3. MAGL inhibition reduces neuroinflammation and neurodegeneration in TG-CB2R-KO mice.
A) Immunoreactivity of astrocytic marker GFAP (green) in the cortex and hippocampus is reduced in TG-CB2R-KO mice that received JZL184. Scale bars: 50 μm. B) JZL184 decreases number of FJC-positive neurons (green) in the cortex and hippocampus in TG-CB2R-KO mice. **P<0.01 compared with the vehicle control (ANOVA with Bonferroni post-hoc test, n=4 mice per group). Scale bars: 400 μm
Neurodegeneration is one of the important neuropathological hallmarks in AD. To determine whether inactivation of MAGL by JZL184 reduces neurodegeneration in TG-CB2R-KO mice, we used Fluoro-Jade C (FJC, a neurodegenerative marker) staining to detect degenerating neurons [13, 31]. As shown in Figure 3B, the number of FJC-positive neurons was significantly reduced in TG-CB2R-KO mice treated with JZL184. These results suggest that resolution of neuroinflammation and protection of neurons produced by inhibition of 2-AG metabolism are independent on CB2R.
Inhibition of 2-AG metabolism prevents cognitive decline in TG-CB2R-KO mice
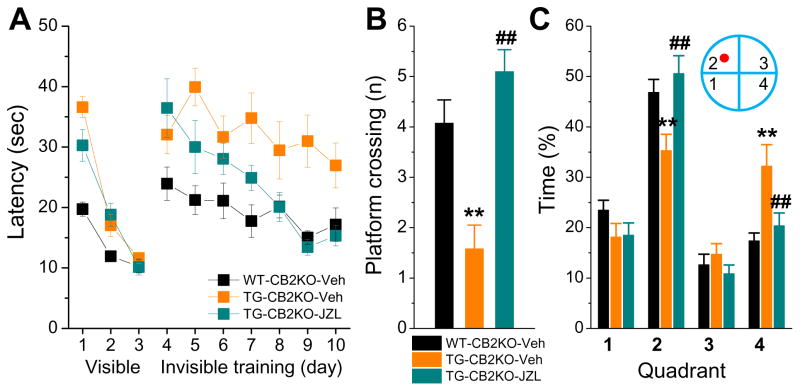
Inactivation of MAGL improves spatial learning and memory in both normal and APP transgenic mice [13, 31, 34]. To determine whether CB2R participates in the improvement of cognitive function produced by inhibition of MAGL, we assessed spatial learning and memory using the Morris water maze test in TG-CB2R-KO mice treated with JZL184 for 8 weeks. As shown in Figure 4, TG-CB2R-KO mice treated with vehicle still displayed impaired learning acquisition and memory retention, similar to 5XFAD TG mice [13]. However, the TG-CB2R-KO mice treated with JZL184 exhibited improved learning acquisition and memory retention, similar to that in non-transgenic CB2R knockout (WT-CB2R-KO) mice. These results suggest that CB2R is not required in the MAGL inactivation-induced improvement in learning and memory in AD animals.
Figure 4. Inactivation of MAGL improves spatial learning and memory in TG-CB2R-KO mice.
A) The Morris water maze test was performed in 6-month-old WT-CB2R-KO and TG-CB2R-KO mice that received vehicle or JZL184. The test was conducted one week following the cessation of the treatment. Mice received visible platform training for 3 days followed by 7 days of invisible platform training. B) Number of platform crossing during the probe test. The probe trial was conducted 24 hrs after 7 days of acquisition training. During the probe test, the platform was removed from the water tank. Number of times crossing the target zone was recorded. C) The percentage of time spent in search of the target zone in each quadrant during the probe trial. **<0.01, compared with the WT-CB2R-KO vehicle control; ##P<0.01 compared with the TG-CB2R-KO vehicle (ANOVA with Bonferroni post-hoc test, n=10 to 13 mice/group).
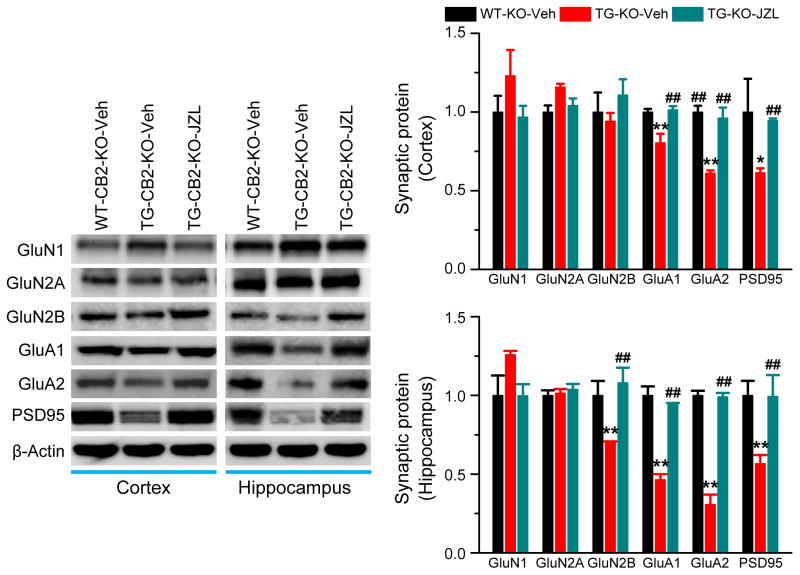
Inhibition of 2-AG metabolism prevents deterioration in expression of synaptic proteins in TG-CB2R-KO mice
Deficits in structure and function of synapses are primary events in the early stages of AD [35]. Expression and function of glutamate receptors are crucial for normal synaptic transmission and plasticity. Previous studies demonstrate that improvements in synaptic and cognitive functions by inactivation of MAGL are likely associated with a recovery of downregulated expression of glutamate receptor subunits in the brain [13]. To determine whether CB2R plays a role in prevention of deterioration in expression of synaptic proteins in AD animals, we detected expression of PSD95, a postsynaptic marker, and glutamate receptor subunits, including GluA1, GluA2, GluN1, GluN2A and GluN2B, in TG-CB2R-KO mice treated with vehicle or JZL184. As shown in Figure 5, expression of PSD95, AMPA receptor subunits GluA1 and GluA2, and NMDA receptor subunit GluN2B (but not in the cortex) was significantly reduced in TG-CB2R-KO mice treated with vehicle. However, the expression of these synaptic proteins in mice treated with JZL184 was returned to the levels in WT-CB2R-KO mice, suggesting that inhibition of 2-AG metabolism is still able to prevent deteriorating expression of important synaptic proteins in in AD animals deficient in CB2R.
Figure 5. Inactivation of MAGL prevents deterioration in expression of synaptic molecules in TG-CB2R-KO mice.
Immunoblot analysis of hippocampal expression of PSD95, AMPA (GluR1 and GluR2), and NMDA (NR1, NR2A and NR2B) receptor subunits in 6 months old WT-CB2R-KO and TG-CB2R-KO mice treated with vehicle or JZL184. *P<0.05; **P<0.01 compared with the WT-CB2R-KO vehicle control, ##P<0.01 compared with the TG-CB2R-KO vehicle control (ANOVA with Fisher’s PLSD test, n=3 mice per group).
DISCUSSION
CB2R has been thought to play an important role in neuroinflammatory responses and proposed as a therapeutic target for neurodegenerative diseases such as AD [15–17, 30]. Previous studies demonstrate that MAGL inactivation, which augments 2-AG signaling and reduces eicosanoid levels [8, 9], alleviates neuropathology and prevents synaptic and cognitive decline in animal models of AD. 2-AG is a full agonist for both CB1R and CB2R [6]. Ablation of CB2R would diminish or attenuate the beneficial effects produced by inactivation of MAGL if the enhanced 2-AG level plays a role in these events mediated through CB2R. In the present study, however, we observed that expression of APP and BACE1 as well as accumulation and deposition of Aβ were significantly reduced in TG-CB2-KO mice treated with JZL184, a potent and highly selective MAGL inhibitor [9, 36, 37]. Also astrocytic reactivity and number of degenerating neurons were decreased by inhibition of MAGL in TG-CB2-KO mice. Importantly, TG-CB2-KO mice treated with JZL184 still displayed improved spatial learning and memory, similar to that in APP TG mice [13]. The improvement in cognitive function by inactivation of MAGL in TG-CB2-KO mice is likely associated with a restoration of downregulated expression of PSD95 and glutamate receptor subunits. Our results suggest that CB2R does not play a role in ameliorating AD neuropathology produced by inactivation of MAGL in AD animals.
Several lines of evidence suggest that 2-AG is an important endogenous signaling molecule maintaining brain homeostasis against harmful insults [1–5, 38–40]. Therefore, augmentation of 2-AG signaling would be a promising approach to resolve neuroinflammation and protect neurons in neurodegenerative diseases. Since 2-AG is unstable and degraded immediately by enzymes or oxidation upon its synthesis, direct application of 2-AG would not be a good strategy for augmentation of 2-AG signaling. It is estimated that 85% of 2-AG in the brain is hydrolyzed by MAGL [8, 36, 37]. Therefore, inhibition or inactivation of MAGL would likely achieve better effects by preventing 2-AG hydrolysis, while reducing its metabolites eicosanoids, which are primarily inflammatory and neurotoxic [10, 11]. Previous studies provide evidence showing that pharmacological or genetic inactivation of MAGL produces profound anti-inflammatory and neuroprotective effects and improves synaptic and cognitive functions in animal models of AD and MPTP model of Parkinson’s disease [9, 13, 14, 32]. However, pharmacological inhibition or genetic deletion of CB1R or CB2R fails to block the anti-inflammatory effects of MAGL inactivation in response to LPS or in AD animals, suggesting that neither CB1R nor CB2R plays a role in suppression of neuroinflammation produced by inactivation of MAGL [9, 13, 14]. Since neuroinflammation has long been believed to contribute to the pathogenesis and neuropathology of AD and CB2R is important in resolving neuroinflammatory responses, in this study, we reexamined the role of CB2R in alleviation of AD neuropathology by inhibition of 2-AG metabolism in APP transgenic mice deficient in CB2R. In particular, it is not clear whether CB2R contributes to MAGL inhibition-induced decrease in neurodegeneration and improvement in learning and memory in AD animals. Previous studies demonstrated that Rimonabant, a CB1R antagonist, together with AM630, a CB2R antagonist, do not block JZL184-induce reduction of inflammatory cytokines in APP TG mice [14]. In the present study, we provided further evidence that JZL184 reduced Aβ plaques, neuroinflammation, and neurodegeneration in APP TG mice lacking CB2R. Importantly, we provided evidence for the first time that treatment of APP TG mice deficient in CB2R with JZL184 still improved learning and memory, confirming that CB2R is essentially ineffective in reducing neuroinflammation and AD neuropathology by MAGL inhibition in APP TG mice.
It has been shown previously that expression of CB2R is upregulated in the brains of AD patients [25, 26]. We also observed in the present study that expression of CB2R is increased in TG animals. However, increased expression of CB2R detected in postmortem cortical brain tissues from AD patients does not correlate with their cognitive status [26]. Inactivation of CB2R in APP transgenic mice also show contradictory outcomes in Aβ formation and neuroinflammation [27–29] and these different outcomes do not correlate with their memory impairment [27, 29]. These divergent results from previous studies suggest that the role of CB2R in the pathogenesis and neuropathology of AD is complex.
While inactivation of MAGL produces significant anti-inflammatory and neuroprotective effects, the mechanisms underlying these beneficial effects remain unclear. Arachidonic acid, the main 2-AG metabolite, is a precursor of prostaglandins and leukotrienes, which are proinflammatory and neurotoxic [10, 11]. Reduced formation of prostaglandins may play an important role in resolving inflammation and protecting neurons when 2-AG metabolism is inhibited by inactivation of MAGL [9]. It is also possibly that anti-inflammatory effects by MAGL inactivation are associated with elevated 2-AG, which activates transient receptor potential vanilloid 1 receptors (TRPV1) [41, 42]. Recent studies reveal that peroxisome proliferator-activated receptor γ (PPARγ) is a target of endocannabinoids [43]. PPARγ is an important nuclear receptor that displays significant anti-inflammatory properties through suppression of NF-κB [44], an important transcription factor that regulates expression of genes involved in many physiological and pathological processes. The anti-inflammatory and neuroprotective effects of 2-AG is likely mediated by activation of PPARγ via CB1/2-depednet and/or -independent pathways [2, 43]. In particular, it has been demonstrated earlier that PPARγ can be activated by 2-AG independently of CB1R or CB2R [45]. Our recent study also shows that MAGL inactivation-induced suppression of BACE1 in TG mice is through upregulation of the non-coding RNA miR-188-3p, which targets BACE1, via 2-AG-mediated expression and activity of PPARγ [31]. As such, more studies are needed to illustrate PPARγ signaling in mediating the beneficial effects produced by inactivation of MAGL. In particular, it would be of great interest to identify cell type-specific MAGL in alleviation of AD neuropathology.
In summary, the results from the present study, which show reduced neuropathology (including expression of APP and BACE1, production of Aβ, neuroinflammation, and neurodegeneration), improved spatial learning and memory, and rescued expression of important synaptic proteins by inhibition of MAGL in TG-CB2R-KO mice, suggest that while MAGL is a promising therapeutic target for AD, CB2R is not required in MAGL inactivation-produced beneficial effects in AD.
MATERIALS AND METHODS
Animals
Both 5XFAD transgenic (B6SJL-Tg(APPSwFlLon, PSEN1*M146L*L286V)6799Vas/Mmjax, stock number: 006554) and CB2R knockout (B6.129P2-Cnr2tm1Dgen/J, stock number: 005786) mice were obtained from the Jackson Lab[13, 31]. APP transgenic mice deficient in CB2R (TG-CB2-KO) were generated by 5XFAD mice crossing with CB2R KO mice, as reported previously [13]. Female TG-CB2-KO and age-matched non-transgenic wild-type littermates lacking CB2R (WT-CB2-KO) were used in the present study. All the experiments were performed in compliance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals. The care and use of the animals reported in this study were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center. 4-Nitrophenyl-4-[bis (1,3-benzodioxol-5-yl)(hydroxy)methyl]piperidine-1-carboxylate (JZL184, provided by the NIH Mental Health Institute Chemical Synthesis and Drug Supply Program), a potent and selective inhibitor of MAGL, was dissolved in the vehicle containing Tween-80 (10%), DMSO (10%), and saline (80%). Animals were treated with either vehicle or JZL184 (12 mg/kg, i.p.) three times per week starting at 4 months of age for 8 weeks, as described previously [13, 31]. All the observations and assessments in mice were made at 6 months of age.
Immunoblot
Western blot assay was conducted to determine Aβ42 and expression of APP, BACE1, PSD95, and glutamate receptor subunits in the brains from animals that received vehicle or JZL184. Brain tissue was extracted and immediately homogenized in RIPA lysis buffer and protease inhibitors, and incubated on ice for 30 min, then centrifuged for 10 min at 10,000 rpm at 4°C. Supernatants were fractionated on 4–15% SDS-PAGE gels and transferred onto PVDF membranes (Bio-Rad). A special 16.5% Criterion™ Tris-Tricine Gelforgel (Bio-Rad) was used for detecting Aβ42 and CTFβ/α, immunoreactivity. The membrane was incubated with anti-Aβ42 (1:1,000, Life Technologies, Cat# 44-344, RRID:AB_2313572), anti-BACE1 (1:1000, Covance, Inc., Cat# PRB-617C-100 RRID:AB_10063987), anti-GluA1 (1:1,000, EMD Millipore, Cat# AB1504 RRID:AB_2113602), GluA2 (1:1,000, EMD Millipore, Cat# MAB397 RRID:AB_2113875), GluN1 (1:500, EMD Millipore, Cat# MAB363 RRID:AB_94946), GluN2A (1:1,000, EMD Millipore, Cat# AB1555P RRID:AB_90770), GluN2B (1:1,000, Abcam, Cat# ab73001 RRID:AB_1269571), and PSD95 (1:1,000, Abcam, Cat# ab99009) at 4°C overnight. The blots were washed and incubated with a secondary antibody (goat anti-rabbit 1:2,000, Cell Signaling Technology, Cat# 7074 RRID: AB_2099233) at room temperature for 1 hr. Proteins were visualized by enhanced chemiluminescence (Amersham Biosciences, UK). The densities of specific bands were quantified by densitometry using FUJIFILM Multi Gauge software (version 3.0). Band densities were quantified and converted to the total amount of protein loaded in each well as determined by mouse anti β-actin (1:4000, Sigma-Aldrich, Cat# A2228 RRID:AB_476697) as described previously [13, 31, 46].
Immunohistochemistry
Immunohistochemical analyses were performed to determine total Aβ and astrocytic marker GFAP in coronal brain sections, as described previously [13, 31, 47]. Animals were anesthetized with ketamine/Xylazine (200/10 mg/kg) and subsequently transcardially perfused with PBS followed by 4% paraformaldehyde in phosphate buffer. The brains were quickly removed from the skulls and fixed in 4% paraformaldehyde overnight, and then transferred into the PBS containing 30% sucrose until sinking to the bottom of the small glass jars. Cryostat sectioning was made on a freezing Vibratome at 40 μm and series sections (every 10th section from each animals) were collected in 0.1M phosphate buffer. Free floating sections were immunostained using specific antibodies for total Aβ (4G8, 1:2,000, Covance, Inc., Cat# SIG-39220-200 RRID: AB_10174824) and GFAP (1:200, Sigma-Aldrich, Cat# G3893 RRID: AB_477010) followed by incubation with the corresponding fluorescent-labeled secondary antibody. 4′-6-Diamidino-2-phenylindole (DAPI), a fluorescent stain that binds strongly to DNA, was used it to detect cell nuclei in the sections. The sections were then mounted on slides for immunofluorescence detection using a Zeiss deconvolution microscope. The numbers and the area of total Aβ in the hippocampal area in each image were analyzed and quantified using SlideBook 6.0, as described previously [13, 31].
Histochemistry
Degenerating neurons were detected using Fluoro-Jade C (FJC), which is an anionic dye that specifically stains the soma and neurites of degenerating neurons and thus is unique as a neurodegenerative marker. Cryostat sectioning was made on a freezing Vibratome at 25 μm and sections were incubated in the solution with FJC (0.0001% solution, EMD Millipore) and DAPI (0.5 μg/ml) for 10 min, followed by 3× 1-min wash with distilled water. Slices were dried naturally at room temperature without light. The images were taken using a Zeiss deconvolution microscope with SlideBook 6.0 software, as described previously [13, 47].
Morris water Maze
The classic Morris water maze (MWM) test was used to determine spatial learning and memory, as described previously [13, 31, 46, 47]. Animals were initially randomized grouped to receive different treatments. The test was performed 24 hrs after last cessation of treatments. A circular water tank (diameter 120 cm) was filled with water and the water was made opaque with non-toxic white paint. A round platform (diameter 15 cm) was hidden 1 cm beneath the surface of the water at the center of a given quadrant of the water tank. Before hidden platform training, the mice were given 3 days of non-spatial training (4 trials per day) to find the platform above the water. Animals that failed to find the platform were gently guided to the platform and allowed to stay on it for 10 s. Invisible platform training was performed continuously for 7 days (7 sessions), and each session consisted of 4 trials. For each trial, the mouse was released from the wall of the tank and allowed to search, find, and stand on the platform for 10 seconds within the 60-second trial period. Animals that failed to find the hidden platform during the 7 days of training were excluded from the experiments. For each training session, the starting quadrant and sequence of the four quadrants from where the mouse was released into the water tank were randomly chosen so that it was different among the separate sessions for each animal and was different for individual animals. The mice in the water pool were recorded by a video-camera using an EthoVision video tracking system (Noldus). A probe test to assess memory retention was conducted 24 hours after the completion of the training acquisition. During the probe test, the platform was removed from the pool, and the task performances were recorded for 60 seconds.
Data analysis
Data are presented as mean ± S.E.M. Unless stated otherwise, analysis of variance (ANOVA) followed by post-hoc tests were used for statistical comparison when appropriate. Differences were considered significant when P< 0.05.
Acknowledgments
This work was supported by National Institutes of Health Grants NS076815. We thank National Institutes of Health Mental Health Institute Chemical Synthesis and Drug Supply Program for providing JZL184.
Footnotes
Author contribution
C.C. and J.Z. designed and performed the experiment. C.C. and J.Z. analyzed the data. C.C. wrote the manuscript. All authors reviewed the manuscript.
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- 1.Chen X, Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons against beta-amyloid insults. Neuroscience. 2011;178:159–68. doi: 10.1016/j.neuroscience.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du H, Chen X, Zhang J, Chen C. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-gamma. Br J Pharmacol. 2011;163:1533–49. doi: 10.1111/j.1476-5381.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J Biol Chem. 2008;283:22601–11. doi: 10.1074/jbc.M800524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu JY, Chen C. Endocannabinoids in Synaptic Plasticity and Neuroprotection. Neuroscientist. 2015;21:152–168. doi: 10.1177/1073858414524632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shohami E, Cohen-Yeshurun A, Magid L, Algali M, Mechoulam R. Endocannabinoids and traumatic brain injury. Br J Pharmacol. 2011;163:1402–10. doi: 10.1111/j.1476-5381.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–46. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci. 2014;35:358–67. doi: 10.1016/j.tips.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–56. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–13. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hein AM, O’Banion MK. Neuroinflammation and memory: the role of prostaglandins. Mol Neurobiol. 2009;40:15–32. doi: 10.1007/s12035-009-8066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salmon JA, Higgs GA. Prostaglandins and leukotrienes as inflammatory mediators. Br Med Bull. 1987;43:285–96. doi: 10.1093/oxfordjournals.bmb.a072183. [DOI] [PubMed] [Google Scholar]
- 12.Chen C. Endocannabinoid metabolism in neurodegenerative diseases. Neuroimmunol Neuroinflamm. 2016;3:268–270. doi: 10.20517/2347-8659.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, Teng Z, Chen C. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep. 2012;2:1329–39. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piro JR, Benjamin DI, Duerr JM, Pi Y, Gonzales C, Wood KM, Schwartz JW, Nomura DK, Samad TA. A dysregulated endocannabinoid-eicosanoid network supports pathogenesis in a mouse model of Alzheimer’s disease. Cell Rep. 2012;1:617–23. doi: 10.1016/j.celrep.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atwood BK, Straiker A, Mackie K. CB(2): therapeutic target-in-waiting. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:16–20. doi: 10.1016/j.pnpbp.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front Neurosci. 2017;11:30. doi: 10.3389/fnins.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brusco A, Tagliaferro P, Saez T, Onaivi ES. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008;62:944–9. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- 19.den Boon FS, Chameau P, Schaafsma-Zhao Q, van Aken W, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A. 2012;109:3534–9. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–67. doi: 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Racz I, Ponomarenko A, Xi ZX, Zimmer A, Schmitz D. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A. 2014;111:E5007–15. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–13. doi: 10.1523/jneurosci.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solas M, Francis PT, Franco R, Ramirez MJ. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol Aging. 2013;34:805–8. doi: 10.1016/j.neurobiolaging.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Aso E, Andres-Benito P, Carmona M, Maldonado R, Ferrer I. Cannabinoid Receptor 2 Participates in Amyloid-beta Processing in a Mouse Model of Alzheimer’s Disease but Plays a Minor Role in the Therapeutic Properties of a Cannabis-Based Medicine. J Alzheimers Dis. 2016;51:489–500. doi: 10.3233/jad-150913. [DOI] [PubMed] [Google Scholar]
- 28.Koppel J, Vingtdeux V, Marambaud P, d’Abramo C, Jimenez H, Stauber M, Friedman R, Davies P. CB2 receptor deficiency increases amyloid pathology and alters tau processing in a transgenic mouse model of Alzheimer’s disease. Mol Med. 2014;20:29–36. doi: 10.2119/molmed.2013.00140.revised. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmole AC, Lundt R, Ternes S, Albayram O, Ulas T, Schultze JL, Bano D, Nicotera P, Alferink J, Zimmer A. Cannabinoid receptor 2 deficiency results in reduced neuroinflammation in an Alzheimer’s disease mouse model. Neurobiol Aging. 2015;36:710–9. doi: 10.1016/j.neurobiolaging.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Aso E, Ferrer I. CB2 Cannabinoid Receptor As Potential Target against Alzheimer’s Disease. Front Neurosci. 2016;10:243. doi: 10.3389/fnins.2016.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Hu M, Teng Z, Tang YP, Chen C. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188–3p in a mouse model of Alzheimer’s disease. J Neurosci. 2014;34:14919–33. doi: 10.1523/jneurosci.1165-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pihlaja R, Takkinen J, Eskola O, Vasara J, Lopez-Picon FR, Haaparanta-Solin M, Rinne JO. Monoacylglycerol lipase inhibitor JZL184 reduces neuroinflammatory response in APdE9 mice and in adult mouse glial cells. J Neuroinflammation. 2015;12:81. doi: 10.1186/s12974-015-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bisogno T, Oddi S, Piccoli A, Fazio D, Maccarrone M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol Res. 2016;111:721–30. doi: 10.1016/j.phrs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–30. doi: 10.1523/jneurosci.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 36.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–53. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–31. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 39.Chen C. Homeostatic regulation of brain functions by endocannabinoid signaling. Neural Regen Res. 2015;10:691–2. doi: 10.4103/1673-5374.156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Y, Zhang J, Chen C. Fine-tuning of synaptic upscaling at excitatory synapses by endocannabinoid signaling is mediated via the CB1 receptor. Sci Rep. 2015;5:16257. doi: 10.1038/srep16257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchalant Y, Brothers HM, Norman GJ, Karelina K, DeVries AC, Wenk GL. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol Dis. 2009;34:300–7. doi: 10.1016/j.nbd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Petrosino S, Schiano Moriello A, Cerrato S, Fusco M, Puigdemont A, De Petrocellis L, Di Marzo V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br J Pharmacol. 2016;173:1154–62. doi: 10.1111/bph.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173:1899–910. doi: 10.1111/bph.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–7. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 45.Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–11. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- 46.Chen R, Zhang J, Fan N, Teng ZQ, Wu Y, Yang H, Tang YP, Sun H, Song Y, Chen C. Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell. 2013;155:1154–65. doi: 10.1016/j.cell.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Teng Z, Song Y, Hu M, Chen C. Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. J Cereb Blood Flow Metab. 2015;35:443–53. doi: 10.1038/jcbfm.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]