Abstract
In this report, we review the relationship between succinate dehydrogenase (SDH) deficiency and the epigenome, especially with regards to two clinical conditions. Carney triad (CT) is a very rare disease with synchronous or metachronous occurrence of at least three different tumor entities; gastric gastrointestinal stromal tumor (GIST), paraganglioma (PGL), and pulmonary chondroma. This condition affects mostly females and it is never inherited. Another disease that shares two of the tumor components of CT, namely GIST and PGL is the Carney-Stratakis syndrome (CSS) or dyad. CSS affects both genders during childhood and adolescence. We review herein the main clinical features and molecular mechanisms behind those two syndromes that share quite a bit of similarities, but one is non-hereditary (CT) whereas the other shows an autosomal-dominant, with incomplete penetrance, inheritance pattern (CSS). Both CT and CSS are caused by the deficiency of the succinate dehydrogenase (SDH) enzyme. The key difference between the two syndromes is the molecular mechanism that causes the SDH deficiency. Most cases of CT show down-regulation of SDH through site-specific hyper-methylation of the SDHC gene, whereas CSS cases carry inactivating germline mutations within one of the genes coding for the SDH subunits A, B, C, or D (SDHA, SDHB, SDHC, and SDHD). There is only partial overlap between the two conditions (there are a few patients with CT that have SDH subunit mutations) but both lead to increased methylation of the entire genome in the tumors associated with them. Other tumors (outside CT and CSS) that have SDH deficiency are associated with increased methylation of the entire genome, but only in CT there is site-specific methylation of the SDHC gene. These findings have implications for diagnostics and the treatment of patients with these, often metastatic tumors.
Keywords: Carney triad, Carney-Stratakis syndrome, paraganglioma, gastrointestinal stromal tumor (GIST), methylation
1. Introduction
In this review, we report on recent studies of succinate dehydrogenase (SDH) deficiency and the latter’s effect on the epigenome. This is particularly relevant for two genetic conditions, Carney triad (CT) and Carney-Stratakis syndrome (CSS), but also for “wild type” gastric stromal tumors (GISTs), paragangliomas, phaeochromocytomas and a few other tumors with SDH deficiency.
CT (OMIM# 604287) was originally described by Dr. J. Aidan Carney as the association of three uncommon neoplasms – gastric leiomyosarcoma or GIST, functioning extra-adrenal paraganglioma (PGL) and pulmonary chondroma (CHO)- in unrelated patients (Carney et al. 1977). CT is a very rare condition and the occurrence of those three tumors can be synchronous or metachronous (Haller et al., 2014). Young females (median 18 years old) are mostly affected from this disease, for reasons that remain unknown (Carney, 2009; Zhang et al. 2010). Although a decrease in the SDH enzymatic activity was detected in tumors from patients with CT, Matyakhina et al. (2007) could not identify any mutations in the genes coding for the four SDH subunits (SDHA, SDHB, SDHC, and SDHD, respectively, collectively known as the ‘SDHx’ genes) in a cohort of 37 patients with CT. However, this investigation also led to the description of a related disease, the dyad of PGL/pheochromocytoma (PHEO) and GIST, also known as CSS (OMIM# 606864) that affects both genders during childhood and adolescence. CSS is inherited as an autosomal-dominant trait and is never associated with the third component of the triad, CHO (Carney and Stratakis, 2002). Although there are some common features between the CSS and the CT, the relative frequency of the shared tumors in the two conditions is reversed: paraganglioma is predominant in CSS whereas GISTs are predominant as a manifestation in CT. It is important to mention that in most patients who develop GIST the sarcoma is symptomatic before paragangliomas or pheochromocytomas (Carney and Stratakis, 2002).
The SDH enzyme (also known as succinate- ubiquinone oxydoreductase) is a key enzyme in the mitochondrial citric acid cycle and electron transport chain. It is a highly conserved heterotetrameric protein that consists of two catalytic subunits that protrude into the mitochondrial matrix (SDHA and SDHB) anchored to the inner membrane by the other two subunits, SDHC and SDHD. The SDHC and SDHD subunits provide also the binding site for the ubiquinone (Fig. 1). The SDHx subunits are encoded by genes expressed in the nucleus. Following their import into the mitochondria, the SDHx subunits are further modified, folded and assembled into active forms. SDH, unlike most of the Krebs cycle enzymes, has no cytosolic analogue and comprises mitochondrial complex II, which is involved in the Krebs cycle and in electron transport chain (ETC). Complex II couples the oxidation of succinate to fumarate in the Krebs cycle with the electron transfer to the terminal acceptor ubiquinone in the ETC (Bardella et al., 2011; Scheffler, 1998).
Figure 1.
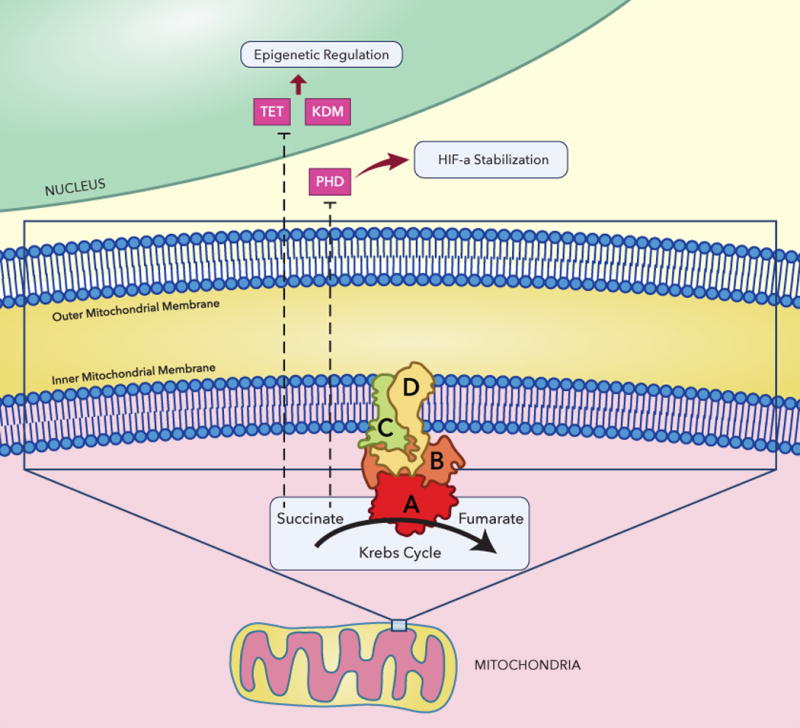
Succinate dehydrogenase (complex II) and epigenetic regulation. SDH is the only membrane-bound enzyme of the Krebs cycle, which is also a functional member (complex II) of the electron transport chain (ETC). SDH forms a complex of four different polypeptides (SDHA, SDHB, SDHC and SDHD) with several other prosthetic groups that include FAD, non-heme iron ubiquinone and heme; TET ten–eleven translocation, KDM lysine (K) demethylase, PHD prolyl hydroxylase
2. SDH subunit mutations in paragangliomas and pheochromocytomas
Germline mutations in SDHD, SDHB and SDHC genes, were identified in patients with hereditary paragangliomas and pheochromocytomas (Astuti et al., 2001; Baysal et al., 2000; Niemann and Muller, 2000) in the early 2000’s (Astuti et al., 2001; Gimm et al., 2000; van Nederveen et al., 2007). More recently, mutations in the genes encoding SDHA and SDHAF2 were also linked to hereditary paragangliomas and pheochromocytomas (HPGL/PCC) (Burnichon et al., 2010; Hao et al., 2009). The genetic defects in the SDH genes predisposing to the HPGL/PCC syndromes are germline heterozygous inactivating mutations that typically affect the protein function. Loss of heterozygosity (LOH) in the tumor leads to complete loss of the enzymatic function of the SDH; therefore, the SDH subunit genes are classified as classical tumor suppressor genes (Gimenez-Roqueplo et al., 2001; Gimenez-Roqueplo et al., 2002; Gimm et al., 2000).
3. Gastrointestinal stromal tumors (GISTS)
GISTs are the most common mesenchymal tumors of the gastrointestinal tract and they arise from the interstitial cells of Cajal (ICC) or from an ICC precursor (Rubin et al., 2005; Sommer et al., 2003). GISTs are divided into two categories based on the genetic defect they are harboring. The largest group includes GISTs that carry mutations in KIT (75% to 80% of the cases) and PDGFRA (5% to 15%) genes (Hirota et al., 1998; Huss et al., 2013; Rossi et al., 2015). The remaining ~10% of the gastrointestinal stromal tumors that are called “wild type” GISTs are classified into succinate dehydrogenase (SDH)-deficient and non-SDH-deficient groups; the latter group includes neurofibromatosis-1 (NF1)-mutant cases and others with mutations in the BRAF, KRAS, PIK3CA and in the ETV6-NTRK3 fusion gene (Fig. 2) (Agaram et al., 2008; Brenca et al., 2016; Hostein et al., 2010; Lasota et al., 2016; Miettinen et al., 2006; Miranda et al., 2012; Patil and Rubin, 2015; Weldon et al., 2016).
Figure 2.
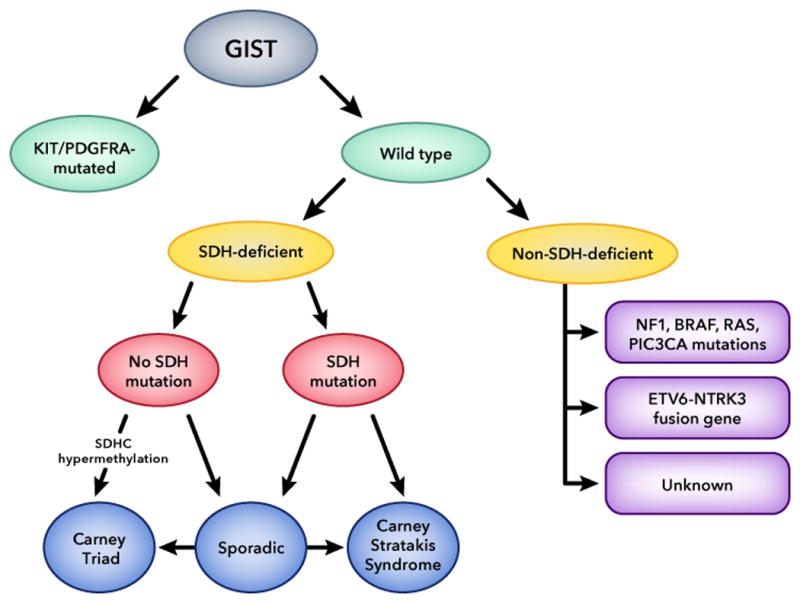
Classification of gastrointestinal stromal tumors (GIST) based on genetic and epigenetic background; SDH: succinate dehydrogenase. SDH defects lead to increased methylation of the entire genome in tumors caused by these defects; however, SDH methylation is a specific molecular signature for tumors associated with Carney Triad and a subgroup of pediatric “wild-type” GISTs.
3.1. Wild Type (WT) GISTs without SDHx mutation in CT
Patients with CT usually present with symptoms of gastric “wild type” GISTs. These tumors are usually multiple with variable sizes (Weldon et al., 2016). They consist of mostly epithelioid tumor cells with pleomorphism commonly noted (Zhang et al., 2010). Immunohistochemical determination in this type of GISTs is positive for KIT expression (normal Cajal cells and the infiltrating mast cells) (Wada et al., 2016; Zhang et al., 2010). GISTs in CT are classified as low risk, however in a quarter of the patients a lymph node metastasis has been found after their first surgery (Wada et al., 2016).
Immunohistochemistry (IHC) for SDHB is helpful in making the diagnosis of an SDH-deficient GIST: loss of SDHB expression indicates that the tumor belongs to the non-KIT, non-PDGFRA-mutant or “wild type” category (Janeway et al., 2011; Killian et al., 2013). In SDH-deficient GISTs that do not harbor SDHx DNA defects, SDH deficiency results from an SDHx epigenetic down-regulation (Szarek et al., 2015) and leads to downstream activation of the HIF signaling pathway. Although, CT is mainly caused from SDH epimutation there are some rare cases where CT is associated with germline SDH defects (Boikos et al., 2016).
Lasota et al. (2013) proposed that overexpression of type 1 insulin-like growth factor receptor (IGF1R) can be associated with SDH-deficient GISTs: the majority of SDH-deficient GISTs (89%) expressed high levels of IGF1R at the protein level. The exact molecular mechanism for IGF1R overexpression in these GISTs is currently unknown (Mahadevan et al. 2015). More recently, Killian et al. (2014) studied SDH deactivation through a genome-wide DNA methylation and expression study that included 59 SDH-deficient GISTs. They reported that 94% of their SDH-deficient GISTs that lacked actual SDH mutations showed SDHC promoter-specific CpG island hypermethylation and subsequent gene silencing. These results supported their hypothesis that SDHC epimutation could be the molecular mechanism that leads to succinate dehydrogenase enzyme dysfunction in most SDH-deficient GISTs that lack SDH mutations.
3.1.1. DNA methylation in CT
The promoter regions of the genes coding for SDH subunits harbor extensive CpG islands. We studied different types of tumors from patients with CT and CSS (Haller et al., 2014) and performed broad, high-resolution, and quantitative analysis of DNA methylation; we detected differential DNA methylation for the gene loci encoding the SDHB and SDHC subunits and no significant DNA methylation at the gene loci encoding for the SDHA and SDHD subunits. The SDHB gene was extensively methylated in 80% of the CpGs of the GISTs and PGLs of the patients with CT included in the study but it was not specific: methylation was also observed in CSS and in sporadic GIST. On the other hand, in the SDHC gene a different, consistent and unique hypermethylation pattern per case was observed up to 80% of the analyzed CpG islands of “wild type” GISTs from patients with CT. Interestingly, an unequal methylation load was observed in a metastatic GIST of a different CT patient with only four CpGs sites being hypermethylated (14–64%). In a recent study of Boikos et al. (2016) it was reported that 30% of the SDH deficient “wild type” GISTs showed SDHC promoter methylation that is in accordance with the previous literature. The cause of this specific SDHC hypermethylation pattern that is the main molecular characteristic of GIST in CT patients is still under investigation.
3.2. Wild Type GISTs in CSS
This disease is characterized by the presence of multifocal gastric GISTs and paragangliomas (Carney and Stratakis, 2002). The inheritance of this syndrome follows an autosomal dominant manner, with incomplete penetrance. More specifically, GISTs in this syndrome occur as a result of germline mutations or large deletions in the genes encoding the subunits B, C or D of the SDH enzyme. This fact leads to accumulation of succinate that inhibits dioxygenases ten-eleven translocation (TET) and histone lysine (K) demethylases (KDM) (Fig. 1) (Boikos and Stratakis, 2014). Succinate also inhibits hypoxia-inducible factor 1 alpha (HIF-1a)-prolyl hydroxylases in the cytosol that leads at the end into stabilization and activation of HIF-1a (Selak et al., 2005). Few years ago, Killian et al. (2013) showed that SDH-deficient GISTs might occur as a result of a failure in TET2 maintenance demethylation. It is known that increased levels of succinate inside the cells are toxic for the dioxygenase TET2 enzyme that is required to catalyze DNA demethylation by conversion of 5-methylcytosine to 5-hydroxymethylcytosine (Patil and Rubin, 2015). In CSS in contrast to CT, DNA methylation patterns have been identified only at a few of the CpGs located close to the SDHB gene (Haller et al., 2014). In these patients, the SDHC gene promoter was completely unmethylated in all screened CpG sites supporting the hypothesis that the CSS is a truly different entity from CT.
4. Epigenetic mechanisms in GIST tumorigenesis and prognosis
Among different types of “wild-type” GISTs, there is a huge variation in clinical behavior. It has been postulated that some of this variation is due to epigenetic changes. Epigenetic regulation by covalent modification of cytosines in CpG dinucleotides silences gene expression by disturbing or promoting the recruitment of regulatory proteins to DNA (Sharma et al., 2010). DNA methylation may prevent transcriptional activation by blocking transcription factors from accessing target-binding sites. Or, alternatively, it can mediate gene downregulation through interactions of histone deacetylases (HDACs) with methyl-binding domain proteins (Sharma et al., 2010). There are limited data regarding epigenetic mechanisms in GISTs and their prognosis (Sioulas et al., 2013). Igarashi et al. (2010) demonstrated that hypomethylation of LINE-1 gene repeats may correlate with high-risk GISTs; these same tumors may also have higher DNA copy number arbnormalities. Promoter methylation of the CDK inhibitor p16INK4A gene also correlates with more aggressive tumors (Schneider-Stock et al., 2003) and metastasis (Ricci et al. (2004). This may extend to other genes such as the REC8 and PAX3 which showed a unique methylation pattern in small vs. malignant GISTs (Okamoto et al., 2012). A more recent study demonstrated that SPP1 methylation levels relate to the progression of the disease and can be an excellent prognostic factor in GISTs (Haller et al., 2015). The above-mentioned studies provide great insight into GIST tumorigenesis and prognosis, but they did not identify a unique molecular background.
5. Summary
CT, CSS, and the so-called “wild type” GISTs share the molecular phenotype of increased methylation of the whole genome. This is due to SDH deficiency that is common among CT, CSS and “wild-type” GISTs regardless of the underlying molecular defect. This has implications for patients with CT, CSS, and “wild type” GISTs who may be candidates for treatment with medications that reverse genomic methylation. Finally, SDHC-specific methylation is a molecular signature of CT only, and may be used for the purposes of establishing the diagnosis of this rare condition.
Acknowledgments
This study was supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), Bethesda, Md 20892, USA. We thank Jeremy A. Swan, NICHD, Computer Support Services Core, and Nichole C. Swan, NICHD, Computer Support Services Core, for the development of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agaram NP, Wong GC, Guo T, Maki RG, Singer S, Dematteo RP, Besmer P, Antonescu CR. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853–9. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti D, Douglas F, Lennard TW, Aligianis IA, Woodward ER, Evans DG, Eng C, Latif F, Maher ER. Germline SDHD mutation in familial phaeochromocytoma. Lancet. 2001;357:1181–2. doi: 10.1016/S0140-6736(00)04378-6. [DOI] [PubMed] [Google Scholar]
- Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, Skoldberg F, Husebye ES, Eng C, Maher ER. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardella C, Pollard PJ, Tomlinson I. SDH mutations in cancer. Biochim Biophys Acta. 2011;1807:1432–43. doi: 10.1016/j.bbabio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW, 3rd, Cornelisse CJ, Devilee P, Devlin B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–51. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, Trent JC, von Mehren M, Wright JA, Schiffman JD, Raygada M, Pacak K, Meltzer PS, Miettinen MM, Stratakis C, Janeway KA, Helman LJ. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016;2:922–8. doi: 10.1001/jamaoncol.2016.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boikos SA, Stratakis CA. The genetic landscape of gastrointestinal stromal tumor lacking KIT and PDGFRA mutations. Endocrine. 2014;47:401–408. doi: 10.1007/s12020-014-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boikos SA, Xekouki P, Fumagalli E, Faucz FR, Raygada M, Szarek E, Ball E, Kim SY, Miettinen M, Helman LJ, Carney JA, Pacak K, Stratakis CA. Carney triad can be (rarely) associated with germline succinate dehydrogenase defects. Eur J Hum Genet. 2016;24:569–73. doi: 10.1038/ejhg.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenca M, Rossi S, Polano M, Gasparotto D, Zanatta L, Racanelli D, Valori L, Lamon S, Dei Tos AP, Maestro R. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol. 2016;238:543–9. doi: 10.1002/path.4677. [DOI] [PubMed] [Google Scholar]
- Burnichon N, Briere JJ, Libe R, Vescovo L, Riviere J, Tissier F, Jouanno E, Jeunemaitre X, Benit P, Tzagoloff A, Rustin P, Bertherat J, Favier J, Gimenez-Roqueplo AP. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–20. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132–9. doi: 10.1002/ajmg.10235. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P, Rotig A, Jeunemaitre X. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet. 2001;69:1186–97. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Kerlan V, Plouin PF, Rotig A, Jeunemaitre X. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab. 2002;87:4771–4. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- Gimm O, Armanios M, Dziema H, Neumann HP, Eng C. Somatic and occult germ-line mutations in SDHD, a mitochondrial complex II gene, in nonfamilial pheochromocytoma. Cancer Res. 2000;60:6822–5. [PubMed] [Google Scholar]
- Haller F, Moskalev EA, Faucz FR, Barthelmess S, Wiemann S, Bieg M, Assie G, Bertherat J, Schaefer IM, Otto C, Rattenberry E, Maher ER, Strobel P, Werner M, Carney JA, Hartmann A, Stratakis CA, Agaimy A. Aberrant DNA hypermethylation of SDHC: a novel mechanism of tumor development in Carney triad. Endocr Relat Cancer. 2014;21:567–77. doi: 10.1530/ERC-14-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller F, Zhang JD, Moskalev EA, Braun A, Otto C, Geddert H, Riazalhosseini Y, Ward A, Balwierz A, Schaefer IM, Cameron S, Ghadimi BM, Agaimy A, Fletcher JA, Hoheisel J, Hartmann A, Werner M, Wiemann S, Sahin O. Combined DNA methylation and gene expression profiling in gastrointestinal stromal tumors reveals hypomethylation of SPP1 as an independent prognostic factor. Int J Cancer. 2015;136:1013–23. doi: 10.1002/ijc.29088. [DOI] [PubMed] [Google Scholar]
- Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, Devilee P, Cremers CW, Schiffman JD, Bentz BG, Gygi SP, Winge DR, Kremer H, Rutter J. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–42. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- Hostein I, Faur N, Primois C, Boury F, Denard J, Emile JF, Bringuier PP, Scoazec JY, Coindre JM. BRAF mutation status in gastrointestinal stromal tumors. Am J Clin Pathol. 2010;133:141–8. doi: 10.1309/AJCPPCKGA2QGBJ1R. [DOI] [PubMed] [Google Scholar]
- Huss S, Kunstlinger H, Wardelmann E, Kleine MA, Binot E, Merkelbach-Bruse S, Rudiger T, Mittler J, Hartmann W, Buttner R, Schildhaus HU. A subset of gastrointestinal stromal tumors previously regarded as wild-type tumors carries somatic activating mutations in KIT exon 8 (p.D419del) Mod Pathol. 2013;26:1004–12. doi: 10.1038/modpathol.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi S, Suzuki H, Niinuma T, Shimizu H, Nojima M, Iwaki H, Nobuoka T, Nishida T, Miyazaki Y, Takamaru H, Yamamoto E, Yamamoto H, Tokino T, Hasegawa T, Hirata K, Imai K, Toyota M, Shinomura Y. A novel correlation between LINE-1 hypomethylation and the malignancy of gastrointestinal stromal tumors. Clin Cancer Res. 2010;16:5114–23. doi: 10.1158/1078-0432.CCR-10-0581. [DOI] [PubMed] [Google Scholar]
- Janeway KA, Kim SY, Lodish M, Nose V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M, Lai AH, Kelly L, Hornick JL, O’Sullivan M, de Krijger RR, Dinjens WN, Demetri GD, Antonescu CR, Fletcher JA, Helman L, Stratakis CA. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011;108:314–8. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, Quezado M, Smith WI, Jr, Jahromi MS, Xekouki P, Szarek E, Walker RL, Lasota J, Raffeld M, Klotzle B, Wang Z, Jones L, Zhu Y, Wang Y, Waterfall JJ, O’Sullivan MJ, Bibikova M, Pacak K, Stratakis C, Janeway KA, Schiffman JD, Fan JB, Helman L, Meltzer PS. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648–57. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian JK, Miettinen M, Walker RL, Wang Y, Zhu YJ, Waterfall JJ, Noyes N, Retnakumar P, Yang Z, Smith WI, Killian MS, Lau CC, Pineda M, Walling J, Stevenson H, Smith C, Wang Z, Lasota J, Kim SY, Boikos SA, Helman LJ, Meltzer PS. Recurrent epimutation of <em>SDHC</em> in gastrointestinal stromal tumors. Science Translational Medicine. 2014;6:268ra177–268ra177. doi: 10.1126/scitranslmed.3009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasota J, Felisiak-Golabek A, Wasag B, Kowalik A, Zieba S, Chlopek M, Wang ZF, Coates T, Kopczynski J, Gozdz S, Sarlomo-Rikala M, Miettinen M. Frequency and clinicopathologic profile of PIK3CA mutant GISTs: molecular genetic study of 529 cases. Mod Pathol. 2016;29:275–82. doi: 10.1038/modpathol.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasota J, Wang Z, Kim SY, Helman L, Miettinen M. Expression of the receptor for type i insulin-like growth factor (IGF1R) in gastrointestinal stromal tumors: an immunohistochemical study of 1078 cases with diagnostic and therapeutic implications. Am J Surg Pathol. 2013;37:114–9. doi: 10.1097/PAS.0b013e3182613c86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyakhina L, Bei TA, McWhinney SR, Pasini B, Cameron S, Gunawan B, Stergiopoulos SG, Boikos S, Muchow M, Dutra A, Pak E, Campo E, Cid MC, Gomez F, Gaillard RC, Assie G, Fuzesi L, Baysal BE, Eng C, Carney JA, Stratakis CA. Genetics of carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab. 2007;92:2938–43. doi: 10.1210/jc.2007-0797. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90–6. doi: 10.1097/01.pas.0000176433.81079.bd. [DOI] [PubMed] [Google Scholar]
- Miranda C, Nucifora M, Molinari F, Conca E, Anania MC, Bordoni A, Saletti P, Mazzucchelli L, Pilotti S, Pierotti MA, Tamborini E, Greco A, Frattini M. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18:1769–76. doi: 10.1158/1078-0432.CCR-11-2230. [DOI] [PubMed] [Google Scholar]
- Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–70. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Sawaki A, Ito S, Nishida T, Takahashi T, Toyota M, Suzuki H, Shinomura Y, Takeuchi I, Shinjo K, An B, Ito H, Yamao K, Fujii M, Murakami H, Osada H, Kataoka H, Joh T, Sekido Y, Kondo Y. Aberrant DNA methylation associated with aggressiveness of gastrointestinal stromal tumour. Gut. 2012;61:392–401. doi: 10.1136/gut.2011.241034. [DOI] [PubMed] [Google Scholar]
- Patil DT, Rubin BP. Genetics of Gastrointestinal Stromal Tumors: A Heterogeneous Family of Tumors? Surg Pathol Clin. 2015;8:515–24. doi: 10.1016/j.path.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Ricci R, Arena V, Castri F, Martini M, Maggiano N, Murazio M, Pacelli F, Potenza AE, Vecchio FM, Larocca LM. Role of p16/INK4a in gastrointestinal stromal tumor progression. Am J Clin Pathol. 2004;122:35–43. doi: 10.1309/MJ4X-N2M5-7HNC-8X5H. [DOI] [PubMed] [Google Scholar]
- Rossi S, Gasparotto D, Miceli R, Toffolatti L, Gallina G, Scaramel E, Marzotto A, Boscato E, Messerini L, Bearzi I, Mazzoleni G, Capella C, Arrigoni G, Sonzogni A, Sidoni A, Mariani L, Amore P, Gronchi A, Casali PG, Maestro R, Dei Tos AP. KIT, PDGFRA, and BRAF mutational spectrum impacts on the natural history of imatinib-naive localized GIST: a population-based study. Am J Surg Pathol. 2015;39:922–30. doi: 10.1097/PAS.0000000000000418. [DOI] [PubMed] [Google Scholar]
- Rubin BP, Antonescu CR, Scott-Browne JP, Comstock ML, Gu Y, Tanas MR, Ware CB, Woodell J. A knock-in mouse model of gastrointestinal stromal tumor harboring kit K641E. Cancer Res. 2005;65:6631–9. doi: 10.1158/0008-5472.CAN-05-0891. [DOI] [PubMed] [Google Scholar]
- Scheffler IE. Molecular genetics of succinate:quinone oxidoreductase in eukaryotes. Prog Nucleic Acid Res Mol Biol. 1998;60:267–315. doi: 10.1016/s0079-6603(08)60895-8. [DOI] [PubMed] [Google Scholar]
- Schneider-Stock R, Boltze C, Lasota J, Miettinen M, Peters B, Pross M, Roessner A, Gunther T. High prognostic value of p16INK4 alterations in gastrointestinal stromal tumors. J Clin Oncol. 2003;21:1688–97. doi: 10.1200/JCO.2003.08.101. [DOI] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioulas AD, Vasilatou D, Pappa V, Dimitriadis G, Triantafyllou K. Epigenetics in gastrointestinal stromal tumors: clinical implications and potential therapeutic perspectives. Dig Dis Sci. 2013;58:3094–102. doi: 10.1007/s10620-013-2785-8. [DOI] [PubMed] [Google Scholar]
- Sommer G, Agosti V, Ehlers I, Rossi F, Corbacioglu S, Farkas J, Moore M, Manova K, Antonescu CR, Besmer P. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci U S A. 2003;100:6706–11. doi: 10.1073/pnas.1037763100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek E, Ball ER, Imperiale A, Tsokos M, Faucz FR, Giubellino A, Moussallieh FM, Namer IJ, Abu-Asab MS, Pacak K, Taieb D, Carney JA, Stratakis CA. Carney triad, SDH-deficient tumors, and Sdhb+/− mice share abnormal mitochondria. Endocr Relat Cancer. 2015;22:345–52. doi: 10.1530/ERC-15-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nederveen FH, Korpershoek E, Lenders JW, de Krijger RR, Dinjens WN. Somatic SDHB mutation in an extraadrenal pheochromocytoma. N Engl J Med. 2007;357:306–8. doi: 10.1056/NEJMc070010. [DOI] [PubMed] [Google Scholar]
- Wada R, Arai H, Kure S, Peng WX, Naito Z. “Wild type” GIST: Clinicopathological features and clinical practice. Pathol Int. 2016;66:431–7. doi: 10.1111/pin.12431. [DOI] [PubMed] [Google Scholar]
- Weldon CB, Madenci AL, Boikos SA, Janeway KA, George S, von Mehren M, Pappo AS, Schiffman JD, Wright J, Trent JC, Pacak K, Stratakis CA, Helman LJ, La Quaglia MP. J Clin Oncol. 2016. Surgical Management of Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Pediatric and Wildtype GIST Clinic. Jco2016686733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Smyrk TC, Young WF, Jr, Stratakis CA, Carney JA. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol. 2010;34:53–64. doi: 10.1097/PAS.0b013e3181c20f4f. [DOI] [PMC free article] [PubMed] [Google Scholar]


